READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Written discussion of "Structures and physical properties of cement
paste" by George J. Verbeck and Richard H. Helmuth (Portland Cement
Association, Skokie, Ill, U.S.A.)
Feldman, R. F.; Sereda, P. J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=31795901-d56e-4e7a-8658-392bfccd99ad https://publications-cnrc.canada.ca/fra/voir/objet/?id=31795901-d56e-4e7a-8658-392bfccd99ad
SESSION 111-1 STRUCTURES AND PHYSICAL
PROPERTIES
OF
CEMENT PASTE
Principal Paper Structures and Physical Properties of Cement Paste
George J. Verbeck and Richard H. Helmuth
Synopsis
Pseudomorphs of the original cement grains in hardened pastes result from differences in hydration products deposited within and between the original grain boundaries. Structures of the various hydration products, as seen in the electron microscope are described. Pore size distributions for cement pastes obtained by a variety of methods are consistent. Surface area values measured by nitrogen adsorption decrease with the water-cement ratio. The distinctions between capillary, gel, and non-evaporable water are reviewed. The gel water resembles interlayer water in clays.
Strength depends primarily on the capillary porosity. The uniformity of distribution of the hydration products explains the effects of gypsum content and temperature.
Initial drying shrinkage is relatively independent of clinker composition at "optimum" gypsum content. The dependence of this shrinkage o n water loss suggests independent effects from different classes of water in the paste. The dependence of this shrinkage o n water-cement ratio and duration both of curing and of subsequent drying is discussed. Carbonation shrinkage varies with the ambient relative humidity. Releases of equal amounts of water by carbonation o r drying result in equal shrinkages. Cycles of wetting and drying reveal that volume changes have reversible and irreversible components.
Thermal expansivity depends on moisture content, rate of temperature change, and the nature of the solid phases. Thermal conductivity changes little with water-cement ratio o r temperature.
The elastic properties are primarily related t o capillary porosity. Inelastic properties are discussed. The relationship between permeability and capillary porosity applies even to bleeding fresh pastes. Available data on moisture diffusion are sufficiently consistent to permit determination of the activation energy. The importance of osmotic and electrokinetic effects in studies of liquid flow is discussed.
Written Discussion
Rolf
F.
Feldman and Peter J. SeredaI t is appreciated t h a t t h e a u t h o r s faced a f o r m i d a b l e however, t h a t t h e a u t h o r s o m i t t e d an appreciable task in writing 011 the b r o a d subject o f t h e structure b o d y o f published i n f o r m a t i o n (1, 6, 8, 9, 10, 11, 16)
a n d physical properties o f cement pastes, a n d in this a n d , having accepted a new c o n c e p t f o r t h e "state" regard they a r e to be complimented. I t m u s t be noted, o f t h e w a t e r i n c e m e n t gel, d i d n o t a t t e m p t t o reas-
sess the significance of this on much of the previously published information.
It is readily accepted and the authors adequately support the idea that in hydrated cement paste the physical and mechanical properties are intimately dependent and profoundly affected by the "adsorbed" water. This being the case, it has long been recognized that one of the key questions in the understanding of this system was the "state" of the water. In this regard two schools of thought have existed: one proposing that the "gel" water was physically adsorbed, and the other that at least part of this water was interlayer. The existence of these two divergent ideas in this field of science has not been documented by the authors.
The importance of these concepts pertaining to the state of the water cannot be overemphasized be- cause they served as the basis for a model to explain the physical and mechanical properties. Most of the literature deals with these properties of hydrated cement on the basis that the "gel" water was physically adsorbed. The proposal that part of the "gel" water was interlayer water was first made by Kalousek and substantiated by extensive research at the Division of Building Research of the National Research Council of Canada.
It is gratifying to note that the authors of the princi- pal paper under discussion are now supporting the idea that much of the "gel" water is interlayer based on the results from nuclear magnetic resonance (N.
M. R.) measurements by Seligmann. The authors, however, have not discussed the profound implication of this fact on the understanding of the whole nature of hydrated portland cement and its properties.
A reassessment of much previous work is certainly necessary. The work at the Division of Building Re- search of the National Research Council of Canada, dating from 1963, makes available a body of infor- mation and discussion on this vital question, culminat- ing in a proposal of a new model for hydrated port- land cement. The publications containing the results of these studies have not been cited 2s references by the authors of this principal paper, although we realize that they may not have had access to our most recent papers (12, 14, 17). This discussion, therefore, attempts to summarize the information that has been omitted from the paper under review.
We fully recognized the complex nature of cement paste and the simplified model proposed is merely another step in the development of the understanding of this system. Much has yet to be done to provide full understanding.
The development of the present model of hydrated Portland cement has been largely based on surface
chemical considerations; this involved the establish- ment of criteria to distinguish between chemically- combined and physically adsorbed water. The deter- mination of the stoichiometry of the tobermorite gel, its water surface area, and its porosity were based on the above criteria.
Water adsorption experiments have been used to measure the porosity and surface area of hydrated portland cement; results of the latter have yielded values in excess of 200 m2/g. This value was of great importance and was used to estimate the number of layers in the tobermorite gel "crystallites"; it also provided the basis for calculating other physical parameters.
Kalousek ( I ) observed that the surface area using N, as adsorbate yielded very low values compared with those of water; the N, value was shown by Mik- hail et a1 (2) to decrease with the water-to-cement ratio to values below 30 m2/g, and, as reported by the authors, by Kantro to values below l m2/g. Similar results were obtained when organic vapours such as CH,-OH were used as adsorbates (3).
The determination of surface area by adsorption of N,, together with a calculation based on the B. E. T. equation, has become a standard method in the realm of surface chemistry. Carbons with very high surface areas and very small pores have yielded nitrogen sur- face areas that approach 1000 m2/g. Yet in the case of hydrated portland cement, the "water area" has been the one generally held "true and valid." It was considered that the N2 molecule could not enter small pores and be adsorbed on the whole surface because of its large size (2). The diameters of the water, nitrogen, and methanol molecules are approximately 3.25, 4.05 and 4.4
A
respectively; it seems difficult to accept the situation where over 90 per cent of the area is excluded to N, because of the small difference in diameters. If the holes were part of the cyrstal struc- ture as in certain "molecular sieves" that are cage- like molecules with small entrances, it would be realistic, but hydrated cement is not of this nature.Several years ago, in the laboratories of the Divi- sion of Building Research of the National Research Council of Canada, it was decided to undertake a careful study of the water-hydrated portland cement sorption system. This study included measurement of both length change and weight change with changing relative humidity. The measurements were done simultaneously in carefully controlled conditions and in a high-vacuum apparatus. We believe, therefore, that we are correct in stating that the Division of Building Research of the NRC of Canada was the first laboratory to carry out this type of study on hydrated
portland cement.
In light of this, it is indeed surprising that the authors of this principal paper state,
".
.
.
in most of the investigations of this matter during the past several decades, pertinent details regarding either the nature of the materials used, o r the environment to which the specimens were actually exposed, have been omitted from the published reports, Therefore, the present authors will draw largely upon their own studies that are believed t o have taken into conside- ration at least most of the important factors." This would seem t o confine unduly the scope of the dis- cussion presented in this principal paper.Before these experiments could be started, it was necessary t o understand the adsorption and length- change process. The project bega,n, therefore, with studies of the adsorption of water on materials less complicated than hydrated portland cement, but related t o it in some way. These materials included an alumino-silicate (molecular sieves), calcium sulphate hemihydrate, precipitated calcium carbonate, finely divided silica, porous glass and calcium hydroxide. A series of papers were published on this work (4 t o 8). The results of the early experiments o n bottle- hydrated cement showed that it was possible t o detect characteristic differences in the type of water that was being removed on drying (9). These characteristics provided the base for a hypothesis on the expansion and contraction of hydrated portland cement on sorption and desorption of water (10, 11). It must be emphasized that this hypothesis did not relate to the phenomena that operate during first drying. T h e hy- pothesis emphasized the role of the Gibbs and Bang- ham equations in the intermediate pressure regions
and it was concluded that the expansion o n sorption was initiated by the decrease in surface energy of the solid. It was not necessary t o introduce the concept that individual crystallites could move back and forth with respect to each other. As will be shown later, other evidence also disproves this concept.
From the length change and weight change charac- teristics and the sorption isotherm (10, 11) it was recognized that the exit and entry of the interlayer water between the sheets of the tobermorite gel played an important role in expansion and contraction. At this stage n o real quantitative estimate could be made as we have now been able to do.
The effect of interlayer water rehydration on water sorption properties may be easily illustrated. First, however, a brief discussion of some cases of sorption hysteresis will clarify the point.
Fig. I illustrates three fofms of hysteresis that have been observed on various materials. A loop of type I is formed over a limited range of pressures and is generally considered to be associated with the for- mation of a meniscus. Scanning of the loop may be performed by desorbing after reaching X 011 the ascending boundary curve; thus, one reaches Y on the descending curve and may return to X by readsorp- tion. Type I1 hysteresis, where the loop extends over the whole pressure range, has been observed for water o n cellulose o r protein fibres and also for polar adsorb- ates o n montmorillonite. In type 111, which combines the characteristics of the other types, the ascending and descending branches only join at very low o r zero pressure. T h e dotted lines show the pattern followed if adsorption recommences at X. Type I11 ioops are found with some graphites, clay minerals and, as has
P I P
0been shown (lo), with hydrated portland cement. This low-pressure hysteresis phenomenon is usually attributed to irreversible intercalation of adsorbate within the structure of the solid. Quite often the solids have a layer structure and a change in the spacing of these layers has been observed. Unfortunately. because of the nature of the tobermorite gel with regard to the c axis, it has not been possible to con- firm the interlamellar penetration by X-ray methods. It is clear, however, that somewhere along the ascend- ing branch of the isotherm water is sorbed irreversibly. This would affect the over-all mechanism for the sorption-expansion phenomena, and the use of the ascending curve for B. E. T. calculations if interlayer water re-enters in the 0 to 35 per cent relative humidity range.
The importance of these results was realized at an early stage in our laboratories. Both the classification and role of the various forms of water became a prime objective of our activities; it was suspected that the entry of interlayer water might affect many mechan- ical properties. The effect of physically-adsorbed water on the bond between crystallites (and the nature of this bond) was also considered to be of critical importance. As a result, further work in our labora- tories was planned to re-examine some areas in greater detail and to extend it in other areas.
Experiments at DBR/NRC can be divided into two categories :
i) measurement of the variation of adsorbed water, length, Young's modulus, flexural strength, and microhardness with relative humidity;
ii) measurement of the variation of Young's mo- dulus, flexural strength, microhardness at constant conditions of relative humidity as a function of poros- ity, and method of fabrication.
The methods of fabrication involved were as follows: (a) compaction of hydrated portland cement powder at different pressures to form rigid bodies of different porosity;
(b) cast samples at different water-cement ratios; (c) recompacted cast samples to lower effective
porosites than (b) above.
All these samples were made with small dimensions (= 30 mm diameter and 1 mm thick) to avoid stresses due to moisture gradients and to facilitate equili- brium.
These experiments were also performed on porous glass and some other materials of which more is known with regard to their properties and behaviour. The results from these were used to aid interpretation.
A discussion follows of the results of the various experiments.
Length and Sorption Isotherms and Scanning Loops
Some of this work has been presented in paper No. 111-23 (12) of this Symposium and only those conclu- sions that contribute to this discussion will be restated. We would like to draw attention to Fig. 1 to 4 of the above paper. It was concluded that interlayer water was entering simultaneously as adsorption occurs up the isotherm.
From the scanning loops and from some assump- tions that later provided to be justified, we were able to separate quantitatively the interlayer and physically adsorbed water and construct isotherms for both [Fig. 5 and 6 of paper No. 111-23 (12)l. From these calculations we were able to make the following conclusions that completely supported this approach. 1. Surface areas from N2 and H 2 0 adsorption are very similar.
2. The total interlayer water found by calculation from the scanning isotherms was equal to the differ- ence between the total water sorbed and the total nitrogen or methanol sorbed for many samples. This had previously been ascribed to small pores that prevented nitrogen but not water from entering.
3. A plot of length change versus weight change for the reversible isotherms produced a linear plot up to a relative humidity of about 45 per cent. This type of linear plot has been observed for water adsorption on many relatively inert materials.
4. A plot of length change versus the surface-free energy change was constructed, using the Gibbs and Bangham equations :
where AL/L is the length change, A F is the surface- free energy change, a is the surface area (cm2/g),
n is the number of moles adsorbed per unit weight of material. This plot produced a straight line through zero, and thus the equations were obeyed (see Fig. 5, paper No. 111-23).
5. From these calculations, Young's modulus for the solid material was calculated to be 4.35
x
lo6 Ib/in2. Helmuth and Turk (13) extrapolated from porosity E plots to get E of "gel phase" as 4.5 and of the "solid phase" as 10.8 Ib/in2. This latter value was similar to the extrapolation of Soroka and Sereda (14) (paper No. 111-34 of this Symposium). Both these values suffer from including as porosity the interlayer water removed during "d-drying". Considering the assumptions, the value found for the modulus of the solid phases is very good and is considered as further evidence of the validity of this approach.6. Further calculations from the scanning isotherms showed that less than 20 per cent of the total expan- sion along the isotherm was due to physically adsorb- ed water. On the desorption branch of the curve at 29 per cent R. H. only 15 per cent of the evaporable water is physically adsorbed.
This is consistent qualitatively with recent N. M. R. work (1 5) which suggests that no physically adsorbed water is present at 70 pel- cent R. H. and that the water is similar to the interlayer water in montmorillonite. It is difficult to agree with the authors' statement however, that no physically adsorbed water is present since nitrogen adsorption measurements do show a fairly significant surface area even at a water-to- cement ratio of 0.25.
Sorption and the Variation of Young's Modulus
In measuring Young's modulus as a function of relative humidity two types of responses have been observed for surface active materials:
(a) no change for rigid porous bodies (this has been observed with porous glass);
(b) a continuous decrease (this has been observed largely with cellulosic materials (see Fig. 2). Hydrated portland cement gave results which partly conformed with the first type but in addition presented
5 1 0 15 C%
C o n c e n t r a l i o n , 96
Fig. 2. Ej/hct of ~porious gases 011 Y O I I I I ~ ' S t r ~ o d u l ~ ~ s of ocetnte silk; I-tlietlryl olcohol, 2-ethyl nlcohol, 3 - b ~ t y l nlcollol, 4- ocetot~e, 5-wnter
certain features which at first we c o ~ ~ l d not explain. These results were published in 1966 (16) and at that time were explained generally by interlayel- dehydra- tion and rehydration. Fig. 3 is a representative sketch of some of the results.
As is shown, there was very little change in Young's modulus from 100 per cent R. H. to low humidites on drying and then on "d-drying" a large decrease in E. On sorption an increase in E took place just above 50 per cent R. H.
In the irreversible isotherms presented as Fig. 6 of paper No. 111-23, for ALIL and AW/W versus relative humidity, it will be noted that there is a simi- larity between them and the E versus relative humidity plot. The hysteresis and effects beyond 50 per cent R. H. are the same, and the AL/L versus AW/W plot shows a sharp break just above 50 per cent R. H.
We were able to incorporate these results in a simple model which can explain most of the properties de- scribed. This model has already been described (17) and is referred to briefly in paper No. 111-23.
The main points are that interlayer water re-enters between the layers from the outer edges, causing expansion by some opening of the layers (see Fig. 7 in paper No. 111-23); the water acts as webs or cross- links; there will be no real increase in E until the middle starts to fill, similar to sandwich-type construction; when this filling takes place, E goes up markedly. The length-to-weight change results indicate a rapid in- crease of interlayer water with a not so great length change beyond 50 per cent R. H. The model shows that most of the expansion has already taken place from the edges. Emptying now starts from the edges but only at low pressures; this explains the hysteresis shown in these plots. "E" does not decrease until the water has left the middle of the layers, and this occurs only at
0 I C e l n c n I P 2 s I c z W I C
-
0. 30 q ---,---.s
._---..----*-p.,,-" -,.-.\.=-?.. S a t . 0 5 0 1 0 0 % n.11.very low vapour pressures. In addition, since desorp- tion starts from the edges when the middle is still full, the AL/L versus AWIW characteristics will be changed and a hysteresis must be expected-in this plot also.
We must emphasize that this model is simplified and much work remains to be done to confirm it. The role of the hydrate water from the aluminates and sulpho- aluminates will have to be taken into account.
Flexural Strength and Microhardness as a Function of Relative Humidity
On Fig. 4 is shown, relative to 0 relative humidity, the variation of strength or microhardness of porous silica glass, hydrated portland cement paste, and fused quartz. It will be noted that there is a great similarity. The results for hydrated portland cement were published in 1966 (16). In contrast, results for cellulosic products show a linear decrease with the amount sorbed, similar to Fig. 2 which shows results for the variation of E with amount'sorbed.
These results are important; the decrease in flexural strength of cellulosic materials has been shown (8) to be due to the attenuation of hydrogen bonds be- tween fibres by adsorbed water. These hydrogen bonds hold individual fibres together. A previous model for swelling of hydrated portland cement which visualizes a separation of individual crystallite units (18) is similar to the model for cellulosic materials and would require similar "E" and flexural strength variations with change in R. H. As this is not borne out, because of this and other ev'idence the old model for swelling and shrinking must be discarded.
The similarity in behaviour of hydrated cement and
-0- C h a n g e i n M i c r o h a r d n e s s o l P o r o u s S i l i c a Clq:s C h a n g e i n S l r e n g t h o f P o r t l a n d C e m e n t P a s t e C h a n g e i n S t r e n g t h 0 1 F u s e d Q u a r t z " 0 . 9 10 20 30 40 50 6 0 70 80 90 100 R E L A T I V E H U M I D I T Y , PER C E N T I glass can be fully explained (8). Strained -di-O-si- bonds at the apex of cracks yield at lower stresses'in the presence of water vapour; this results in the forma- tion of (-OH) groups. This process is regarded as a form of stress corrosion. Thus the change in flexural strength is due to the change in environment rather than a change in structure of the material.
The experiments under category ii) variation of mechanical properties as a function of porosity and method of fabrication, will now be discussed.
Interrelation of Hardness, Modulus of Elasticity and Porosity in Various
Gypsum Systems
Generally, for otherwise identical conditions, me- chanical properties such as strength and modulus of elasticity are related to porosity. The relation usually takes the form of an exponential expression
S = Soe-blP Or E =. EOe-bzp
where S and E are the strength and modulus of elasti- city of the specimen respectively, So and Eo are these parameters at zero porosity; and p is the porosity (8). This type of equation was found to hold for various gypsum systems (19).
Study of the gypsum systems which are distinctly crystalline and in which intergrowth of crystals during hydration is a possibility was considered useful for comparison with the cement systems.
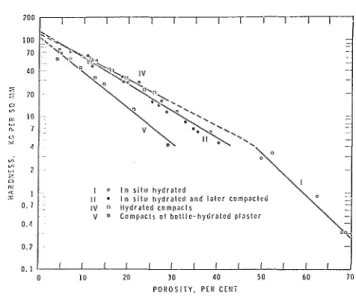
Results may be summarized by Figs. 5 and 6 where " E and hardness versus porosity are plotted for the several systems.
1. Hardness and modulus of elasticity are related to porosity. This relation can be described by the
-
I 0 I n s i t u hydratedI I I n r l l u hydrated and l a t e r compacted - IV 0 Hydrated compaclr -
V
.
Compacts o l boltla hydrated plaster --
-
'\ - 8 I 0 10 20 30 40 50 60 70 P O R O S I T Y , PER CENTP O R O S I T Y , PLR C C N i
Fig. 6 . Horclness vs porosity for the gypsmn systems
exponential expression already discussed.
2. Intergrowth and interlocking of crystals formed during hydration significantly affect mechanical properties. Depending 011 the porosity, hardness values were as much as four times higher and modulus of elasticity LIP to ten times higher than corresponding values in otherwise identical systclns in which crystal intergrowth is not very likely to occur. These latter syslems were type V (Figs. 5 and 6), which had been prepared by compaction of bottle-hydrated plaster, and type 11, which was in situ hydrated and recompact- ed.
Variation of Microhardness, Strength and Modulus of Elasticity of Hydrated
Porltand Cement
A detailed study of the above is presented to this Symposium (paper No. 111-34); this study was insti- gated, however, by results obtained some time ago and published in 1966 (16). These results showed the then surprising fact that bottle-hydrated cement compacts showed almost identical porosity vs flexural strength and porosity vs E characteristics.
The results presented in paper No. 111-34 to this Symposi~~m have expanded this work and the following C O I I C ~ L I S ~ O I ~ S C O L I ~ ~ be made:
1. The results for both hardness and modulus of elasticity could be plotted according to the following equations:
E = E O e - b ? p
H H O e - b l P
2. The values for the m o d ~ ~ l ~ ~ s of the compacts of
bottle-hydrated cement fit very closely to those of the paste and compacted paste when plotted against porosity (Fig. 2, paper No. 111-34).
3. The results of micro-hardness versus porosity lead to the same conclusions as the result for modulus (Fig. 3, paper No. 111-34).
These results enabled the conclusion to be made that pressure produced either by compaction or hydration would yield the same type of solid-solid bonds be- tween crystallites. This is, of course, in sharp contrast to the effect observed from the gypsum systems studied. In the gypsum system, the intergrowth of crystals seems to occur during hydration and forms "chemical" bonds which produce greater strength than do the bonds formed during compaction. Similarly, some recent work involving compacts of Ca(OH), during ageing (20) and during carbonation (21) shows how these interparticle bonds can be increased because of recrystallization of the material or because of new products formed as the result of the reaction.
The further contrasting results between the gypsum systems and the hydrated cement system produces convincing evidence for the nature of the interparti- cle bond in hydrated cement. The experiments where hydrated cement paste was compacted to lower poros- ities involving a reduction of porosity varying from as little as 5 per cent to as much as 45 per cent showed that i n all cases the ~nodulus values fitted the same relation as for the hydrated paste samples. If a "chenii- cal" bond were to be formed during hydration, such a bond would be broken during subsequent compac- tion and would not be remade, resulting in a lower modulus for compacted paste. This was not the case, which is evidence of the absence of "chemical" bonds and leads to the conclusion (mentioned previously) that the interparticle bonds are solid-to-solid which are responsible for the stable structure of cement paste after first drying. These bonds appear to be of such a nature that they can be broken and remade without suffering permanent decrease in modulus.
Before discussing some of the features of the inter- particle bonds in hydrated cement, perhaps a more general discussion of interparticle bonds may be made by way of definition.
It is recognized that the terms "chemical" or "pri- mary" and "secondary" bonds have been used in describing the structure of hydrated cement systems without a strict definition of their meaning. This has led to considerable midunderstanding. It is believed that what was meant by the "chemical" bond between the particles was a solid-to-solid contact similar to that of a grain boundary in a polycrystalline material where some atoms approach the spacing and arrange-
ment in the crystal. Such "chemical" bonds could be formed during a crystallization process accompany- ing a chemical reaction where the mobility of atoms allows for a regular arrangement resulting in an inter- growth of crystals. It follows that these bonds would be stong and when broken would not be remade in normal circumstances.
It is believed that the term "secondary" bonds as applied to the hydrated cement system arose from the assumption that physically adsorbed water was a constituent part of the interparticle bond; thus, the general term of van der Waals forces was considered appropriate. It is suggested, therefore, that a different interparticle bond be postulated which would involve a solid-to-solid contact resulting from the bringing together of surfaces (by pressure externally or inter- nally generated by the hydration reaction) forming a particle boundary having little or no regular atomic arrangement or spacing. Atoms at such a boundary would engage a varying proportion of the long-range and short-range forces depending on the degree of disorder and the average spacing. It can be visualized that this interparticle bond can be broken and sub- sequently remade under appropriate loading condi- tions. This type of bond differs from the "chemical" bond just defined, which has a more regular atomic arrangement and the spacing of more of the atoms may approach that of the lattice spacing in the crystal. If water is involved in such a bond (it will be either chemisorbed, hydrated or interlayer), it is considered a constituent part of the crystal.
A
-
I n t e r p a r t i c l e BoncisX
-
l n t e r l a y e r t l y d r a t e W a t e r B-
T o b e r m o r i t e S h e e t s0
-
P l i y s i c a l l y A d s o r b e d Waie.rFig. 7 . Simplified model for liydratedportland cement
Of all the various experiments leading to the under- standing of hydrated portland cement, and the bond- ing system involved in the structure, perhaps the most conclusive in support of the idea that interparticle bonds do not have adsorbed water at the boundary comes from the measurements of elastic modulus and strength at different conditions of relative humidity (16). If the adsorbed water were entering the inter- particle bond area, the net result should be a decrease in modulus or strength as occurs in the case of cellulosic materials (8).
A very simplified and tentative model for the struc- ture of hydrated portland cement is presented as Fig. 7 which shows the layerd structure of hydrated port- land cement. The role of the types of water and possible bonding between sheets is shown. The irreversible
shri~kage during first drying might involve formation
of more solid-to-solid bonds or further aggregation of layers. First drying would involve great energy changes, and the structure may be subject to forces which demand new positions of stability.
Creep Phenomena
The establishment of a new model for hydrated cement enables a fresh approach in understanding some of its properties. A discussion of some possibil- ities with regard to creep follows:
1. The importance of interlayer water in hydrated portland cement is now recognized, and this interlayer water may be removed at low relative humidities. Under higher stress levels creep may be associated with a slow decomposition of the interlayer hydrates. This effect is influenced by relative humidity and may result in both reversible and irreversible creep.
2. The nature of the interparticle bonds which show the capacity for breaking and remaking may be in- volved in irreversible creep.
3. A large part of creep, when measured before first drying, might be related to the irreversible shrink- age phenomena.
Concluding Statement
It is hoped that the interpretation of experimental work described in this discussion, and the model evolved, may lead to further understanding and sub- sequently a more advanced model for hydrated port- land cement.
References
1. G. L. Kalousek, "Fundamental factors in the dryingshrinkage of cement block," J. A m Concrete Inst.,
26, 233-248 (1954).
2. R. Sh. Mikhail, L. E. Copeland and S. Brunauer, "Pore structures and surface areas of hardened portland cement pastes by nitrogen adsorption," Can. J. Chem., 42, 426-438 (1964).
3. R. Sh. Mikhail and S. A . Selim, "Adsorption of orga- nic vapours in relation t o the hardened portland cement pastes," Highway Research Board Special Report 90, p. 123-134 (1966).
4. P. J. Sereda and R. F. Feldman, "Compacts of pow- dered materials as porous bodies for use in sorption studies," J. Appl. Chem., 13, 150-158 (1964). 5. R. F. Feldman and P. J. Sereda, "The use of compacts
t o study the sorption characteristics of powdered plaster of paris," J. Appl. Chem., 13, 158-167 (1963). 6. V. S. Ramachandran and R. F. Feldman, "Length
change characteristics of Ca(OH), compacts o n exposure t o water vapour," J. Appl. Chem., 17,
328-332 (1967).
7. R. F. Feldman and P. J. Sereda, "Moisture content, its significance and interaction in a porous body," Proc., Internat. Symposium on Humidity Measure- ments, Washington, C. D., 4, Ch. 28, 233-243 (1 963).
8. P. J. Sereda and R. F. Feldman, "Mechanical proper- ties and the solid gas interface." I n The Solid G a s Interface, Edited by E. A . Flood, Vol. 2, Ch. 24, 729-764, Marcel Dekker Inc., New York (1967). 9. R. F. Feldman and P. J. Sereda, "A datum point for
estimating the adsorbed water in hydrated portland cement," J. Appl. Chem., 13, 375-382 (1963). 10. R. F. Feldman and P. J. Sereda, "Sorption of water on
bottle-hydrated cement, I ; The sorption and length- change isotherms," J. Appl. Che ., 14,87-93 (1964). 11. R. F. Feldman and P. J. Sereda, "Sorption of wster on
compacts of bottle-hydrated cement, I1 ; thermody- namic considerations and theory of volume change," J. Appl. Cliem., 14, 93-104 (1964).
12. R. F. Feldman, "Sorption and length change scanning
isotherms of methanol and water o n hydrated port- land cement," Submitted t o the Fifth International Sympositum o n thL Chemistry of Cement, Tokyo, October 1968.
13. R. A. Helmuth and D . H . Turk, "Elastic moduli of hardened portland cement and tricalcium silicate pastes: effect of porosity, Highway Research Board, Special Report No. 90, 135-144 (1966).
14. I. Snroka and P. J. Sereda, "The structure of cement- stone and the use of compacts as structural models," Submitted to the Fifth International Symposium o n the Chemistry of Cement, Tokyo, October, 1968. 15. P. Seligmann, "Nuclear magnetic resonance studies
of the water in hardened cement paste," J. Res. Develop, Lab., Portland Cement ASSOC., 10, (I), 52-65 (1968).
16. P. J. Sereda, R. F. Feldman and E. G. Swenson, "Effect of sorbed water o n some mechanical pro- perties of hydrated cement pastes and compacts," Highway Research Board, Special Report No. 90, 58-73 (1966).
17. R. F. Feldman and P. J. Sereda, "A model for hydrated portland cement paste as deduced from sorption length change and mechanical properties," Pre- sented a t the RILEM Colloquium: The physical and chemical cause of creep and shrinkage of concrete, Munich, April 1968.
18. T. C. Powers, "Mechanism of shrinkage and reversible creep of hardened paste," Proc. Int. Conf. o n Struc- ture of Concrete, London, 1965.
19. I. Soroka and P. J. Sereda, "Interrelation of hardness, modulus of elasticity and porosity in various gyp- sum systems," J. Am. Cer. Soc., 51, 337-340 (1 968).
20. E. G. Swenson and P. J. Sereda, "Some ageing charac- teristics of lime," J. Appl. Chem., 17, 198-202 (1967).
21. E. G . Swenson and P. J. Sereda, "A mechanism for carbonation shrinkage of lime and hydrated ce- ment," J. Appl. Chem., in press.