HAL Id: hal-01148710
https://hal.archives-ouvertes.fr/hal-01148710
Submitted on 5 May 2015
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
PEDOT-modified integrated microelectrodes for the
detection of ascorbic acid, dopamine and uric acid
Fadhila Sekli-Belaidi, Aurélie Civélas, Valentina Castagnola, Aliki Tsopela,
Laurent Mazenq, Pierre Gros, Jérôme Launay, Pierre Temple-Boyer
To cite this version:
Fadhila Sekli-Belaidi, Aurélie Civélas, Valentina Castagnola, Aliki Tsopela, Laurent Mazenq, et al..
PEDOT-modified integrated microelectrodes for the detection of ascorbic acid, dopamine and uric
acid. Sensors and Actuators B: Chemical, Elsevier, 2015, 214, pp. 1-9. �10.1016/j.snb.2015.03.005�.
�hal-01148710�
O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 13875
To link to this article :
DOI:10.1016/j.snb.2015.03.005
URL :
http://dx.doi.org/10.1016/j.snb.2015.03.005
To cite this version :
Sekli-Belaidi, Fadhila and Civélas, Aurélie and Castagnola,
Valentina and Tsopela, Aliki and Mazenq, Laurent and Gros, Pierre
and Launay, Jérôme and Temple-Boyer, Pierre PEDOT-modified
integrated microelectrodes for the detection of ascorbic acid,
dopamine and uric acid. (2015) Sensors and Actuators B: Chemical,
214. pp. 1-9. ISSN 0925-4005
Any correspondance concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
PEDOT-modified
integrated
microelectrodes
for
the
detection
of
ascorbic
acid,
dopamine
and
uric
acid
F.
Sekli
Belaidi
a,b,c,d,
A.
Civélas
a,b,
V.
Castagnola
a,b,
A.
Tsopela
a,b,
L.
Mazenq
a,b,
P.
Gros
c,d,
J.
Launay
a,b,
P.
Temple-Boyer
a,b,∗aCNRS,LAAS,7avenueducolonelRoche,F-31400Toulouse,France bUniversitédeToulouse,UPS,LAAS,F-31400Toulouse,France cUniversitédeToulouse,UPS,INPT,LGC,F-31062Toulouse,France dCNRS,LGC,F-31062Toulouse,France Keywords: Integratedmicroelectrode Electrochemicalmicrocell PEDOT Ascorbicacid Dopamine Uricacid
a
b
s
t
r
a
c
t
Integrated(Pt/PEDOT–Pt–Ag/AgCl)and(Au/PEDOT–Pt–Ag/AgCl)electrochemicalmicrocells(ElecCell) wereelaboratedforthedetectionofascorbicacid,dopamineanduricacidbydifferentialpulse voltam-metry.Specificattentionwasbroughttotheintegrationofpoly(3,4-ethylenedioxythiophene)(PEDOT) filmbyelectropolymerization.Goldandplatinumworkingmicroelectrodeswereinvestigatedwhile usingethylenedioxythiophene(EDOT)electrodepositionprocessesinwateroracetonitrilesolvents.For thethreeantioxidantspecies,best(multi-)detectionpropertieswereobtainedforacetonitrile-based PEDOTfilmsdepositedongoldworkingelectrode.Thus,usingintegrated(Au/PEDOT–Pt–Ag/AgCl) Elec-Cellmicrodevices,analyticalperformancesweredeterminedforascorbicacid,dopamineanduricacid, exhibitinghighselectivity(oxidationpotential:−40,150and280mV,respectively),linear concentra-tionrangefrom0.1to300mM,highsensitivities(0.85,1.65and3.06mA/mMcm2,respectively)andlow detectionlimit(0.2mM,0.1mMand0.05mM,respectively).
1. Introduction
During thelasttwo decades,theareaofsensorshasgreatly
benefited from the development of micro/nanotechnologies in
termofdesign,fabricationanddetectionperformances.Thiswas
alsotrueforchemicalmicrosensorsandelectrochemicalanalysis
forbiosensing applications.Consequently, integrated
microelec-trodeshavebecomewell-acceptedtoolsforclinical,environmental,
chemicaland pharmaceuticalapplicationswithhighspatialand
temporalresolution[1,2].Indeed,theypresentmanyadvantages:
specificity, highsensitivity,fastresponse time, small capacitive
currents,enhancedmasstransport,lowohmicdropallowingtheir
useinlowconductingandhighlyviscousmedia,aswellas
versa-tility.Moreover,comparedtoultra-microelectrodes(UME)sealed
intoglass-capillaries[3–6],theytakeadvantageofmassfabrication
atlowcostthankstotheuseofsilicon-basedmicrotechnologies
[7–9],addressingmanybioanalyticalapplications[10–14].
Never-theless,torealizeasimpleandfunctionalelectrochemicalsensor,
∗ Correspondingauthorat:CNRS,LAAS,7avenueducolonelRoche,F-31400 Toulouse,France.Tel.:+33561336954.
E-mailaddress:temple@laas.fr(P.Temple-Boyer).
microfabricationstrategieshavetoaddresstheproblemsrelatedto
theanalysisofrealsamples,emphasizingonsensitivity,
selectiv-ity,stability,reproducibilityandreliability.Wehaveselectedthis
approachtodevelopanintegratedelectrochemicalmicrosensorfor
thesimultaneousdetectionofascorbicacid(AA),dopamine(Dop)
anduricacid(UA)intheframeofantioxidantspeciesanalysis.
Thedetectionofthesethreeanalytesisofparticularinterestin
clinical,chemical,pathology,foodanalysisandmanyotherfields
[15–17].AAisavitalvitaminpopularlyknownforitsantioxidant
properties and is present in mammalian brain along with
sev-eralneurotransmitteraminessuchasdopamine.Ascorbicacidhas
beenusedforpreventionandtreatmentofcommoncold,
men-talillness,infertilityandcancer[18].Dopamineisanimportant
neurotransmitterformessagetransferincentralnervoussystem
[19].AbnormallevelsofDopleadtoneurologicaldisorderssuchas
ParkinsonismandSchizophrenia[20].Meanwhile,uricacidisthe
primaryfinalproductofpurinemetabolism.Theextreme
abnor-malitiesofUAlevelsleadtosomediseases,suchashypertension,
hyperuricaemia,goutandLesch-Nyandiseases[21].
Inrealbiologicalsamples,AA,DopandUAusuallycoexist,so
thedevelopmentofaccurate,selectiveandsimultaneous
determi-nationmethodsforthesethreeanalytesishighlydesiredespecially
inbiomedicalchemistryandmedicaldiagnostics.AA,DopandUA
areelectroactivecompoundsandcanbedetectedusing
electroan-alyticaltechniques.Unfortunately,withbareunmodifiedmetallic
electrodes,theyareoxidizedatnearlysamepotentialsandtheir
voltammetricresponsesoverlapmakestheirdiscriminationinreal
samplesverydifficult[22,23].Besides,bareelectrodesoften
suf-ferfromapronouncedfoulingaffectduetotheaccumulationof
oxidizedproductsonelectrode surface.Furthermore,the
modi-fiedelectrodemustbeinsensitivetointerferingchemicalspresent
inbiological media.To overcomethis problem, many
modifica-tionstrategieshavebeenadoptedtolowertheoverpotential,to
increase detectionsensitivity and toimproveselectivity. In the
frameofantioxidant detection,theyhaveledtotherealization
of various modified (micro)electrodes based on quantum dots
[24],nanoparticles[25–27],carbonnanotubes[28–30],graphene
[25–27,29,31–33] and conductive polymers [28,34–36]. Among
them,poly(3,4-ethylenedioxythiophene)(PEDOT)wasoneofthe
widelyusedconductingpolymersforthedetectionofAA,Dopand
UA[37–42].Ithasalowoxidationpotentialandmoderateband
gapwithgoodstabilityandtransparencyintheoxidizedstate,high
electricalconductivity[43],excellentthermalstability,intrinsically
lowthermalconductivityandlowprice[44,45].Inparallel,
elec-tropolymerizationisoneofthemethodsusedforthepreparation
ofpolymerfilmwithgoodquality.Itallowsthereproducible
for-mationoforganicpolymerfilmswithprecisespatialresolution.
Moreover,filmthicknessesareeasilycontrolledbythedeposition
chargeandthepolymerisdirectlyobtainedinhisconductingstate
[46].Thus,electrodepositionprotocolofPEDOTiseasiercompared
toothersstrategiesofelectrodemodifications.Finally,
ethylene-dioxythiophene(EDOT)isacommerciallyavailablemonomerthat
eliminatessynthesissteps.
In theframe of thedetection of antioxidantspecies, PEDOT
acts as a redox mediator responsible for oxidation catalysis.
Sinceascorbicanduric acidsareintheiranionicform(HA−)at
physiological pH,the occurringcatalytic mechanism is globally
givenby[47]:
PEDOTox+HA−→ PEDOTred−+A
PEDOTred−→PEDOTox+H++2e−
Inthecaseofdopaminknowntobeinitscationicformat
phys-iologicalpH,theglobalcatalyticmechanismis[37]:
PEDOTox+Dop→ PEDOTred+DoQ
PEDOTred→PEDOTox+2H++2e−
Our previous works illustrated that PEDOT deposited on
hand-mademicroelectrodeshasgoodcatalyticpropertiesforthe
electrochemicaloxidationof ascorbicanduric acidsandcanbe
usedfortheirsimultaneousdetection[39].Thisworkgoesfurther
towardstechnologicalintegrationandmassfabricationof
PEDOT-basedmicroelectrodes,focusingonthreemaingoals:(i)tostudy
the electropolymerization of PEDOT on thin-film-based
micro-electrodes, (ii) to integrate fully PEDOT-based electrochemical
microcells(ElecCell),and(iii)toanalyzePEDOT-basedElecCell
per-formancesfortheselectivedetectionofantioxidantspecies.Thus,
combiningtheadvantageousfeaturesofsilicon-based
microtech-nologies [23,48] and catalytic properties of PEDOT [46], we
presentedheretheanalyticperformances ofintegrated
electro-chemicalmicrodevicesmodifiedwithPEDOTelectrodepositedin
differentconditionsofpolymerizationforasimultaneousassayof
AA,Dop,andUA.
2. Experimental
2.1. Chemicals
3,4-Ethylenedioxythiophene(EDOT)monomer,poly(sodium
4-styrenesulfonate)(NaPSS),ascorbicacid(AA),dopamine(Dop)and
uricacid(UA)werepurchasedfromSigmaAldrich.
Tetrabutylamm-onium perchlorate (TBAPC), potassium dihydrogenophosphate
KH2PO4,di-potassiumhydrogenophosphateK2PHO4,sodium
chlo-rideNaClandacetonitrileCH3CNwerepurchasedfromAcros.All
reagentswereofanalyticalgradeandusedasreceived.Theaqueous
solutionswerepreparedwithhigh-qualitywater(MilliQgradient
A10system,Millipore,Bedford,MA).Highpurenitrogenwasused
fordeaeration.
2.2. Materials
ElectrochemicalImpedanceSpectroscopy(EIS)measurements
weremadein0.1MNaClsolutionbyapplyinga5mVRMSsine
wavewithfrequenciesrangingfrom10Hzto10kHz.Scanning
elec-tronmicroscopy(SEM)studieswerecarriedoutusingafocused
ionbeam(FIB) HELIOS600iequipmentoperatingat 3kV.
Sam-plesweremountedonadouble-sidedadhesivecarbonandoptical
microscope imageswere then made using a Hirox Microscope
(HI-SCOPEadvancedKH-3000).PEDOTelectropolymerizationand
electrochemicalexperimentswereperformedusingaVMP3
poten-tiostat (Biologic) interfaced to a microcomputer and using the
EC-Labsoftware.
2.3. Electrochemicalmicrocell(ElecCell)fabrication
Integrated(Pt–Pt–Ag/AgCl)and(Au–Pt–Ag/AgCl)
electrochem-ical microcells (ElecCell) were fabricatedon silicon chip using
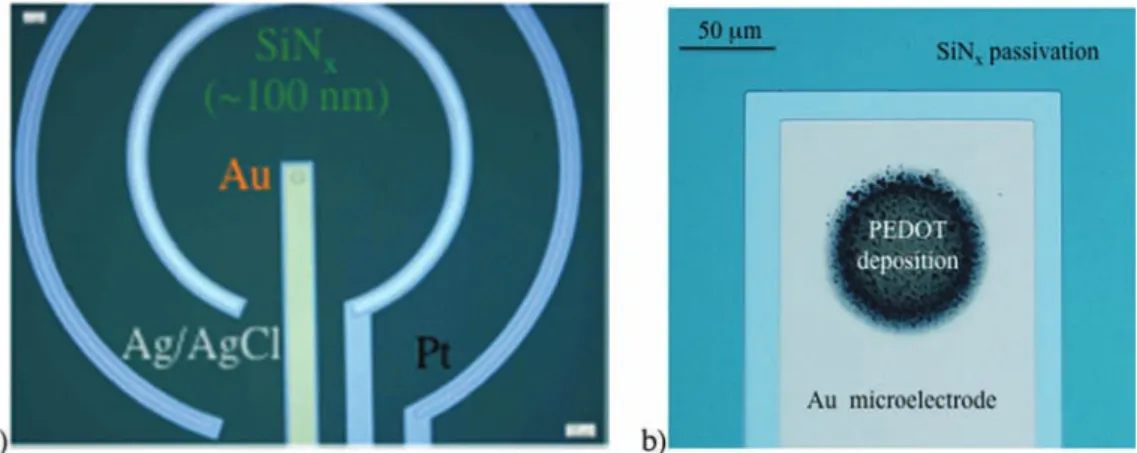
silicon-based microtechnologies (Fig. 1a) [23]. Oxidized silicon
waferswereusedinordertoensureelectricalinsulationbetween
thedifferentmicroelectrodes(oxidethickness:∼1mm).Then,the
differentthin metallic layers weredeposited byevaporation in
conventional physical vapour deposition(PVD) equipment, and
patternedusingabilayerlift-offprocessinordertoimprove
fab-ricationreproducibility.ThreePVDprocesseswereperformedina
row:firstly,a200nmplatinumlayerwasdepositedona 20nm
titanium underlayer in order to ensure platinum adhesion on
siliconoxide,followedbya800nmgoldanda400nmsilver
lay-ers. Finally,a biocompatible Si3N4 passivation layer(thickness:
100nm)wasdepositedatthewaferlevelandpatternedusing
pho-tolithographytechniques[48].Accordingtothisfinalwafer-level
passivationprocess,thedifferentmetallic layerswereinsulated
electricallyandtheiractivesurfacesweredefinedprecisely.The
goldandplatinumworkingmicroelectrodesweredefinedasdisks
and theirelectroactive areawasapproximately 4.9×10−4mm2
(diameter: 25mm). In contrast, very large silver/silver chloride
referencemicroelectrode(0.02mm2)andplatinumcounter
micro-electrode(1mm2)werefabricated.Afterthesiliconwaferdicing,
(Pt–Pt–Ag)and(Au–Pt–Ag)electrochemicalmicrocellswere
man-ufacturedonsiliconchip(Fig.1a).Thewholechipwasthenplaced
and gluedby an epoxyinsulating glue on a specificallycoated
printedcircuit,wirebondedandpackagedatthesystemlevelusing
asiliconeglop-topinordertobefullycompatiblewithliquidphase
measurement.
Foreachmicrodevice,thesilver/silverchlorideAg/AgCl
pseudo-reference was finally obtained by oxidizing the silver-based
microelectrode in a 0.01M KCl solution. This was performed
bylinearvoltammetry(potentialscan rate:1mV/sbetween0.1
and 0.25V/SCE) using a standard saturated calomel electrode
(SCE) Hg/Hg2Cl2/KClsat as reference. Thus, (Pt–Pt–Ag/AgCl) and
Fig.1. Opticalmicroscopeimagesof(a)theintegrated(Au-Pt-Ag/AgCl)electrochemicalmicrocell(ElecCell)deviceand(b)theelectrodepositedPEDOTfilmonthegold workingelectrode(solvent:acetonitrile)
2.4. PreparationandcharacterizationofPEDOTmodified
electrode
PEDOT electropolymerization processes were carried out in
organic,i.e.acetonitrile-based,orinorganic,i.e.water-based,
solu-tions.
Fortheorganicacetonitrile-basedprocess,theintegrated
work-ingmicroelectrodesurfacewasmodifiedinadeaeratedacetonitrile
solutioncontaining2.5mMEDOTmonomerand0.1MTBAPCas
supportingelectrolyte[39].Then,polymerizationwasperformed
bycyclicvoltammetryatascanrateof250mV/sbetween0.88and
1.5V.
Fortheinorganicwater-basedprocess,electropolymerization
experiment was performedfrom EDOT(0.1%W/V, 0.01M) and
NaPSS(0.7%W/V)inaqueousdeaeratedsolutions.Such
concentra-tionwaslowerthanEDOTsolubilityinwater(estimatedaround
15mM at 25◦C)to ensureits complete dissolving.Then, cyclic
voltammetrywascarriedbetween−0.9and1.2Vatascanrate
of25mV/s[49].
In bothcases, i.e.acetonitrileorwater solvents, theamount
of PEDOTsynthesizedcorrespondedto thesameanodic charge
of12mC/cm2.Aftertheelectropolymerization,themodified
elec-trodeswererinsedwithacetonitrileand/ordeionizedwaterina
rowtoremoveanyphysicallyadsorbedmonomer(Fig.1b).
2.5. ElectrochemicalexperimentsofPEDOT-basedElecCell
integratedmicrodevice
For the quantitative determination of AA, Dop and UA,
dif-ferential pulse voltammetry (DPV) was investigated since it is
moresensitivethancyclicvoltammetry.Differentialpulse
voltam-mogramswerecollectedinthepotentialrangebetween0.2and
0.4V, witha 50mVamplitude, a 6mVpotentialstep, a 119ms
pulsetime,a 1sinterval timeand a6mV/spotentialscan rate.
Integrated (Au/PEDOT–Pt–Ag/AgCl) and (Pt/PEDOT–Pt–Ag/AgCl)
electrochemicalmicrocellswereusedfortheseDPVexperiments.
For eachof them,goldor platinumPEDOT-modified
microelec-trodes were used as workingelectrodes whereas the platinum
and silver/silverchloridemicroelectrodes wereused ascounter
andpseudo-referenceelectrodes,respectively.Allelectrochemical
experimentswereperformedinaglasscellcontaining100mLof
0.1Mdeaeratedphosphatebuffersolution(PBS,pH=7.0)with
dif-ferentconcentrationsofAA,Dop,andUA.Thestandardaddition
methodwasappliedfordrawingthecalibrationcurvesforeach
specie.Freshlyconcentrated solutionsofAA,Dop, andUAwere
preparedandstoredat4◦C.Thenasmallknownconcentrationof
desiredelementisincreasinglyaddedtoPBSsolutions.Currents
werethenplottedagainsttheaddedconcentrations.Thelimitof
detectionwasestimatedforasignal-tonoise-ratioequaltothree.
3. Resultsanddiscussion
3.1. EffectofEDOTsolvent
Thesolventusedduringtheelectropolymerizationstephasa
keyinfluenceontheconductingpolymersultimateproperties.It
shouldleadtoahighelectricalconductivityandgood
electrochem-icalstabilityagainstdecompositionathighpotentialsrequiredto
oxidizethe monomer.Thus, electrosynthesisof PEDOTis often
performedinorganicsolvent[37,39].Nevertheless,evenifwater
hassomedrawbackssuchashighnucleophilicity,narrow
poten-tialwindowforelectrochemicalstabilityandhighEDOToxidation
potential(higherthantheacetonitrileone),itwasalsousedas
sol-vent forPEDOTelectrodepositioneveniftheEDOTmonomeris
slightlysolubleinaqueoussolution[49,50].Aboveallthese
prob-lems,theselectionofwater asthesynthesismediumwouldbe
self-evidentmerelyfromenvironmental,economicand
biocom-patibilityreasons.
Fig.2aandbshowsthecyclicvoltammogramsrecordedduring
PEDOTelectrogenerationonagoldintegratedmicroelectrode,in
water-basedorinacetonitrile-basedsolutions,respectively.
Simi-larelectrochemicalbehaviourswereobservedforbothsolvents.In
water(Fig.2a),theEDOTmonomeroxidationstartsat0.6Vandthe
anodiccurrentincreasesfromcycletocycleindicatingthepolymer
growth.Then,thePEDOTredoxpropertiesareevidencedat−0.1V.
Theelectropolymerizationpotentialdecreasewasattributedtothe
strongelectrostaticinteractionsbetweenEDOT•+ cationradicals
andPSS−species,facilitatingthepolymerizationprocess[51].
In acetonitrile (Fig. 2b), it is clearly visible that the EDOT
monomeroxidationstarsat1.2Vwhereastheredoxpotentialof
PEDOTisobtainedaround−0.25V.Itisknownthatpeaksposition
ofthepolymerredoxactivityisrelativetop-dopingprocess,leads
todifferencesinconductivityproperties[52],andmightindicate
thatahighermolecularmasspolymerisobtainedwhen
electrosyn-thesisis performed in organicmedium. Thus, even ifa similar
anodicchargeof12mC/cm2waschosenforthePEDOTsynthesis,
thisshouldalsoberesponsibleforsomethicknessandmorphology
discrepanciesforthedifferentPEDOTlayers.
To have further information on PEDOT depositions, they
were characterized by impedancemetry and scanning electron
microscopy(SEM).Comparedtowatersolvent,acetonitrileleadsto
lowerimpedancemodulusandthereforetohigherelectrical
con-ductivity(datanot shown).Such differenceintermofelectrical
Fig.2.Cyclicvoltammogrammsofelectropolymerizationatgoldworkingmicroelectrodeindeaerated0.1mol/LTBAPCand2.5mmol/LEDOT(a)water-based(potentialscan rate:25mV/s)and(b)acetonitrile-basedsolutions(potentialscanrate:250mV/s).
film.Indeed,theuseofTBAPC,andespeciallytheperchlorateion
ClO4−,aschargecompensationwasshowntogivePEDOTfilmswith
higherdopinglevelandbetterstability[53].
Nevertheless,moresignificantresultswereobtainedbySEM.
Fig. 3a and b presents the different surface morphologies of
PEDOTlayerselectrodepositedongoldmicroelectrodewhileusing
waterandacetonitrilesolvents.Incontrasttowater-basedPEDOT
that forms a cauliflower-type, compact structure,
acetonitrile-based ones show a porous complex structure. To the best of
ourknowledge,theeffectsofsolventonmorphologicalfeatures,
andthecorrelationbetweenthemorphologyof
electropolymer-izedfilmsandtheircatalyticpropertieswereneversystematically
investigated.To explain thesignificantdifferencesbetweenthe
morphologicalpropertiesofPEDOTfilmspreparedinwaterorin
acetonitrile,wecanspeculatethatthesechangesareattributedto
thedifferentintrinsic propertiesofeachsolventthatcontribute
todifferentsolute–solventand/orpolymer–solvent interactions.
Thebestsolventswerefoundtohavehighdipolemoments,low
polarizabilityandhighcapacitytodonateelectrons[54].
Further-more,higherdielectricconstants(∼80forwatercomparedto∼36
foracetonitrile)leadtolowerelectropolymerizationrateandto
morecompactfilms[55].Meanwhile,wecannotexcludethe
fac-torthatthesolubilityofEDOToligomersproducedatinitialstages
ofelectropolymerizationinbothsolventsmightberesponsibleof
suchmorphologicalstructures[56].Certainly,inthevery
begin-ningstageofpolymerization,oxidationofmonomersandcoupling
ofradicalcationstakeplace.Whenthechainlengthofoligomers
is highenough, theyprecipitate onto theelectrode, generating
thefirstpolymernuclei.Atthispoint, thePEDOTdepositionon
theelectrodestarts,i.e.nucleationbegins,andsubsequentlythe
propagationofpolymerchainsandpolymerprecipitationarethe
mainprocesses.Inwater,thepresenceofpoly-styrenesulphonate
(PSS),whichisagoodsolubilizingagentforbothEDOTmonomer
and PEDOTpolymer, facilitates theformation ofrelatively long
polymericchains onsolutionand consequentlysmootherfilms
areobserved.Inacetonitrile,shortoligomersaredepositedonthe
electrode,leadingtoahighnumberofnucleationcentres,which
yield tomoreheterogeneous andvery roughfilmsas observed
inSEM.Finally,sinceitwasshownthatthesurfacemorphology
is influenced by thepolymerization potential[57],
electropoly-merizationathigheroxidationpotential(1.2–1.5V)inacetonitrile
shouldproducesrougherPEDOTfilms.
Themodifiedmicrodeviceswerethereforetestedinan
equimo-larsolutionofAA,DopandUA1mmol/LpH7.0.Resultsareshown
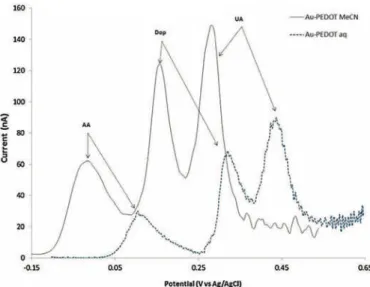
in Fig. 4. It is clear that the PEDOT grown in acetonitrilehas
muchbetterperformancesthanthePEDOTgrowninwater. For
acetonitrile-basedPEDOTlayers,theoxidationpeaksofAA,Dop
andUAappearat−0.04,0.15and0.28V,respectively,andhigher
sensitivitiesareevidenced.Forwater-basedones,oxidationofAA,
DopandUAoccursatmorepositive potentials,i.e.0.125,0.335
and 0.45V, inducing lower sensitivities.Such resultsshouldbe
associated tothe differences betweenPEDOTfilms in terms of
structure, morphologyand electricalconductivity(asshown by
SEMand impedancemetriccharacterizations, seebelow).Inthe
caseofacetonitrile,rougherandmoreporousmorphologiesaswell
ashigherelectricalconductivityprovidelargerelectroactive
sur-face,fasterdiffusionphenomenainandoutthepolymernetwork,
and betteraccessto electroactivesites,enhancingPEDOTfilms
Fig.3. Scanningelectronmicroscopy(SEM)picturesofPEDOTfilmsdepositedon(a)ongoldsurfaceusingwaterassolvent,(b)ongoldsurfaceusingacetonitrileassolvent and(c)onplatinumsurfacewhileusingacetonitrileassolvent
Fig.4.Differentialpulsevoltammograms(DPV)of(Au/PEDOT–Pt–Ag/AgCl)ElecCell in0.1MPBSpH7.0solutioncontaininganequimolarAA/Dop/UA(1mmol/L):PEDOT electrodepositedinacetonitrilesolution(plainline)orinaqueoussolution(dashed line).
Fig.5.Differentialpulsevoltammograms(DPV)of(Au–Pt–Ag/AgCl)(plainline)and (Pt–Pt–Ag/AgCl)(dashedline)ElecCellin0.1MPBSpH7.0solutioncontainingan equimolarAA/Dop/UAmixture(1mmol/L).
electrocatalytic properties and improving further antioxidant
detectionproperties[47].
Finally, even if water was successfully developed and gave
acceptable results, acetonitrile appears to be the best solvent
forintegratingPEDOT-modifiedelectrochemicalmicrosensorsand
Fig.6.Differentialpulsevoltammograms(DPV)of(Au/PEDOT–Pt–Ag/AgCl)(plain line)and(Pt/PEDOT–Pt–Ag/AgCl)(dashedline)ElecCellin0.1MPBSpH7.0solution containinganequimolarAA/Dop/UAmixture(1mmol/L).
improving PEDOT-based detection performances of antioxidant
speciesintermsofsensitivityandselectivity.
3.2. Effectofworkingelectrodenature
Asdescribed previously in section2.3, theintegrated
work-ingmicroelectrodecanbemadefromplatinumorgold.Previous
worksshowedthatthephysico-chemicalpropertiesoftheanode
metallic material could determinethe natureand the strength
of the bond between the electropolymerizedpolymer and the
electrode,impactingitsresultingproperties[46].So,westudied
the influence of the metal nature on the PEDOT-based
detec-tionproperties.Inthisview,acetonitrilesolventwasusedforthe
electrodepositionofPEDOTfilmsongoldandplatinumworking
surfaces(seesection3.1).Then,theelectrochemicalperformances
ofthePEDOT-modifiedworkingmicroelectrodeswereevaluated
in an equimolarsolution of AA, Dop and UA 1mmol/L pH 7.0.
For comparison, bare gold and platinum integrated
microelec-trodeswerealsostudiedinthesameway.Resultsareshownin
Figs.5and6.
Forbare goldandplatinum microelectrodes,abadlydefined
peakandlowcurrentvaluesareobserved(Fig.5).Such
amperomet-ricresponseswererelatedtocompetitiveoxidationphenomena
betweenAA,DopandUA.Indeed,bystudyingseparatelyeach
ana-lyte(resultnotshown),theirrespectiveoxidationpotentialsappear
at0.26,0.42and0.47Vongoldmicroelectrode,andat0.32,0.28
and0.52Vonplatinummicroelectrode,inagreementwithprevious
results[22,23].
OnPEDOT-basedmicroelectrodesmadefromgoldorplatinum,
threewell-definedoxidationpeaksareobservedcorrespondingto
Table1
Comparisonoftheanalyticalperformancesofdifferentelectrochemical,PEDOT-modified,electrodesforthesimultaneousdetectionofAA,Dop,andUA. Ref. EpvsSCE(mV) Limitofdetection(mM) Linearrange(mM)
AA Dop UA AA Dop UA AA Dop UA
[37] −50 150 365 – 1 1 – 1–30 1–20 [38] −80 120 275 7.4 – – 500–3500 20–80 20–130 [39] −94 – 308 2.5 – 1.5 5–300 – 2–600 [40] 100 250 320 – – – 100–500 100–500 100–500 [41] 3 210 360 10 1.5 2.7 20–1400 12–48 36–216 [42] 69 232 364 400 6 2 400–8000 6–75 2–40 Thiswork −40 150 280 0.2 0.1 0.05 0.5–300 0.2–300 0.1–300
theoxidationofAA,DopandUA,respectively(Fig.6).Compared
tothebroad and overlapped amperometricresponsesobtained
withbareelectrodes,allaboveresultsclearlyvalidatethecatalytic
activityofPEDOTfortheelectrochemicaloxidationofAA,Dopand
UAby loweringtheoxidation potentialandincreasingthe
cur-rent[37,38,47]. Nevertheless,electrochemical performancesare
stillslightlyloweronplatinumPEDOTmodifiedmicroelectrode:
thepeakpotentialsareshiftedtomorepositivevalues,andmore
preciselyat0.01,0.215and0.34V,respectively(comparedto−0.04,
0.15and0.28V,seesection3.1),andwithlowersensitivities.
Ear-lier,bystudyingtheexperimentalconditionsofpolymerization,we
haveobservedthatthemorphologicalpropertiesofPEDOTfilms
determinetoalargeextentthecatalyticbehaviourfortheassayof
AAandUA[39].So,thiselectrochemicalperformancesdiscrepancy
couldbealsoduetotheelectrical,morphologicalandstructural
propertiesoftheresultingpolymers.Indeed,through
impedance-metriccharacterization,PEDOTsynthesizedongoldisconfirmedto
havethehigherelectricalconductivitycomparedtoplatinumone.
Thesedifferencescanbedueeithertotheintrinsicconductivities
ortotheroughnessofPEDOTfilms[49].Furthermore,SEM
charac-terizationsshowthatacetonitrile-basedPEDOTfilmsdepositedon
platinumsurfaceshowlessporousstructurethanthosedeposited
ongoldsurface(Fig.3bandc).Ontheotherhand,PEDOT
adhe-sionisbestongoldsurfaceduetothestronginteractionsbetween
goldandsulphuratoms[58,59].Thus,comparedtoplatinum-based
ones, (Au/PEDOT–Pt–Ag/AgCl) ElecCell integrated microdevices
are more suitable for the simultaneous electrochemical
deter-mination of antioxidant species at millimolar concentration
levels.
3.3. Analyticalperformances
Accordingtoourpreviousresultsandoptimizations(see
sec-tions3.1and3.2),acetonitrile-basedPEDOTelectrodepositionwas
performedongoldmicroelectrode.Sincesilicon-basedintegration
enablesmassfabrication,theseinvestigationswereperformedfor
fivedifferent(Au/PEDOT–Pt–Ag/AgCl)electrochemicalmicrocells.
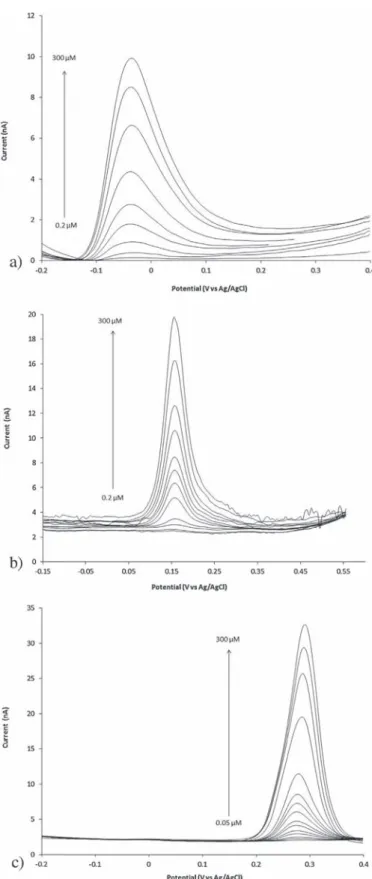
Fig.7a–crepresents theDPVresponsesofthePEDOT-modified
microelectrodes to various concentrations of AA, Dop and UA,
respectively. Calibration plots indicatean excellent linearity of
the amperometric responses with AA, Dop and UA
concentra-tions at−0.04, 0.15and 0.28V, respectively(Fig.8).ForAA, an
excellent linear relationship (sensitivity: 0.85mA/mMcm2) was
obtainedin theconcentration range from 0.5 to 300mM, with
a limit of detection estimated at 0.2mM for a signal to noise
ratio of 3. Then, the calibration for dopamin was also found
tobe linear in therange of 0.2–300mM. In this case, a higher
slope(1.65mA/mMcm2)valueandalimitofdetectionof0.1mM
were evidenced. Finally, in the case of UA, a linear
relation-ship was found again in the range of 0.1–300mM with a still
higher sensitivity(3.06mA/mMcm2)and a limit of detection of
0.05mM.Alltheseanalyticalresponsescanberesumedasfollowing
(R2>0.998):
Ascorbic acid detection (oxidation potential: −0.04V):
j(mA/cm2)=24+0.85C
AA(mM);
Dopamine detection (oxidation potential: 0.15V):
j(mA/cm2)=9.7+1.65C
Dop(mM);
Uric acid detection (oxidation potential: 0.28V):
j(mA/cm2)=25+3.06C
UA(mM).
Intermofconcentrationranges,theseresultswerewellsuited
totheassayoftheseanalytesinmedicalfields[60,61].Compared
toworksreportedinliteratureforthesimultaneousdetermination
ofAA,Dop,andUAonPEDOT-modifiedelectrodes,itisworthto
notethatourresultswerebetterorcomparabletomostofthese
Fig.7.Differentialpulsevoltammogramsof(Au/PEDOT-Pt-Ag/AgCl)elecCellin 0.1MPBS(pH7.0) containingdifferentconcentrationsof(a)ascorbicacid,(b) dopamineand(c)uricacid.
electrodes(Table1),althoughanalyteswereusedinexcessformost
ofthem.Finally,withtheintegratedelectrochemicalmicrodevice,it
appearsthatasignificantimprovementinlimitsofdetectionwas
obtainedcomparedtoourpreviousresults[39],makingitmore
Fig.8.Calibrationcurvesforthethreeanalytes:ascorbicacid,dopamineanduric acid.
3.4. Reproducibilityandstability
Thereproducibilityandstabilityofthesensorwereinvestigated
bysensingstudies.Ternarymixtureofanequimolarsolutionof
AA,DopandUA100mMwasusedforthereproducible
examina-tionsoffivedifferent(Au/PEDOT–Pt–Ag/AgCl)ElecCell.Therelative
standarddeviation(RSD)wasfoundtobelowerthan4.2%forAA,
4.5%forDopand3.2%forUA,suggestingthattheElecCell
technol-ogyreproducibilitywassufficientlygoodtodealwithcalibration.
Thestabilityofoursensorswasexaminedinternarymixtureafter
beingstoredtwoweeksinairorinphosphatebuffersolution(PBS).
Thus,PEDOTmodifiedmicrodevicesretained90%oftheirinitial
sensitivitiestothedifferentantioxidantspeciesstudies(datanot
shown).
4. Conclusion
We have developed fully integrated, PEDOT-based,
electro-chemical microcells (ElecCell) allowing the selective detection
of ascorbic acid, dopamine and uric acid in aqueous media.
PEDOThasbeensuccessfullysynthesizedonintegratedgoldand
platinum microelectrodeswhile usingwater andacetonitrileas
solvent.AccordingtoDPVcharacterization,resultsshowimproved
detectionperformancesintermofsensitivityandselectivityfor
electrodeposited PEDOTlayers, emphasizing good resultsusing
waterassolvent,betterresultsusingacetonitrileassolventand
best results on gold surfaces compared to platinum ones. For
this last and best case, detection properties of ascorbic acid,
dopamineanduricacidwerestudied,exhibitingwell-separated
oxidationphenomena(oxidationpotential:−0.04,0.15and0.28V,
respectively), linear current variations, high sensitivities (0.85,
1.65 and3.06mA/mMcm2,respectively)andlow detectionlimit
(0.2mM,0.1mMand0.05mM,respectively).Asaresult,theElecCell
technologicalplatformisadaptedtothemassfabricationof
PEDOT-modifiedelectrochemicaldevices fortheanalysisofantioxidant
species.Itwasappliedtomodelsolutionsuptonow,butshouldbe
extendedtorealsamplesofbloodseraand/orurinesintheframe
ofclinicaldiagnosisand/orenvironmentalapplications.
Acknowledgements
TheauthorswouldliketothankProfessorMauriceComtat(LGC,
Toulouse)for helpfuldiscussionsandadvices. Thetechnological
realizationsandassociatedresearchworkswerepartlysupported
bytheFrenchRENATECHnetwork.
References
[1]V. Beni, D.W.M. Arrigan, Microelectrode arrays and microfabricated devicesinelectrochemicalstripping analysis,Curr. Anal. Chem.4(2008) 229–241.
[2]O.A.Sadik,A.O.Aluoch,A.Zhou,Statusofbiomolecularrecognitionusing elec-trochemicaltechniques,Biosens.Bioelectron.24(2009)2749–2765.
[3]J.W.Schultze,V.Tsakova,Electrochemicalmicrosystemtechnologies:from fundamentalresearch to technical systems, Electrochim. Acta 44 (1999) 3605–3627.
[4]C. Amatore, S. Arbault, C. Bouton, K. Coffi, J.C. Drapier, H. Ghandour, Y. Tong, Monitoring in real time with a microelectrode the release of reactive oxygen and nitrogen species by a single macrophage stimula-tionbyitsmembranemechanicaldepolarization,ChemBioChem7(2006) 653–661.
[5]A.Ruffien-Ciszak, P.Gros, M.Comtat,A.M.Schmitt,E. Questel,C. Casas, D.Redoules,Explorationoftheglobalantioxidantcapacityofthestratum corneum by cyclic voltammetry, J. Pharm. Biomed. Analysis 40 (2006) 162–167.
[6]J.G.Roberts,J.V.Toups,E.Eyualem,G.S.McCarty,L.A.Sombers,Insituelectrode calibrationstrategyforvoltammetricmeasurementsinvivo,Anal.Chem.85 (2013)11568–11575.
[7]R.S.Pai, K.M. Walsh, M.M. Crain,T.J. Roussel, D.J. Jackson,R.P. Baldwin, R.S.Keynton,J.F.Naber,Fully integratedthree-dimensionalelectrodesfor electrochemicaldetectionin microchips:fabrication,characterization and applications,Anal.Chem.81(2009)4762–4769.
[8]Y.P.Chen,Y.Zhao,J.Chu,S.Y.Liu,W.W.Li,G.Liu,Y.C.Tian,Y.Xiong,H.Q.Yu, Fabricationandcharacterizationofaninnovativesolid-statemicroelectrode, Electrochim.Acta55(2010)5984–5989.
[9]K.Dawson,A.Wahl,S.Barry,C.Barrett,N.Sassiat,Fully-integratedon-chip nano-electrochemicaldevicesforelectroanalyticalapplications,Electrochim. Acta115(2014)239–246.
[10]N.Pereira-Rodriguez,Y.Sakai,T.Fujii,Cell-basedmicrofluidicbiochipforthe electrochemicalreal-timemonitoringofglucoseandoxygen,Sens.Actuators B132(2008)608–613.
[11]M.Miyashita,N.Ito,S.Ikeda,T.Murayama,K.Oguma,J.Kimura,Development ofurineglucosemeterbasedonmicro-planaramperometricbiosensorandits clinicalapplicationforself-monitoringofurineglucose,Biosens.Bioelectron. 24(2009)1336–1340.
[12]S.BenAmor,E.Vanhove,F.SékliBelaïdi,S.Charlot,D.Colin,M.Rigoulet,A. Devin,J.Launay,P.Temple-Boyer,S.Arbault,Enhanceddetectionofhydrogen peroxidewithplatinizedmicroelectrodearraysforanalysesofmitochondria activities,Electrochim.Acta126(2014)171–178.
[13]O.Frey,P.D.vanderWal,S.Spieth,O.Brett,K.Seidl,O.Paul,P.Ruther,R. Zengerle,N.F.deRooij,Biosensormicroprobeswithintegratedmicrofluidic channelsforbi-directionalneurochemicaldetection,J.NeuralEng.8(2011) 1–9.
[14]A.Altuna,L.MenendezdelaPrida,E.Bellistri,G.Gabriel,A.Guilera,J.Berganzo, R.Vila,L.J.Fernandez,SU-8basedmicroprobeswithintegratedplanar elec-trodesforenhancedneuraldepthrecording,Biosens.Bioelectron.37(2012) 1–5.
[15]J.Dawson,P.Jeemon,L.Hetherington,C.Judd,C.Hastie,C.Schulz,W.Sloan,S. Muir,A.Jardine,G.McInnes,D.Morrison,A.F.Dominiczak,S.Padmanabhan,M. Walters,Serumuricacidlevel,longitudinalbloodpressure,renalfunction,and long-termmortalityintreatedhypertensivepatients,Hypertension62(2013) 105–111.
[16]S.S.Rodriguez,K.A.Salazar,N.A.Jara,M.A.Garcia-Robles,F.Perez,L.E.Ferrada, E.Luciano,F.Martinez,F.J.Nualart,Superoxide-dependentuptakeofvitaminC inhumangliomacells,J.Neurochem.127(2013)793–804.
[17]S.D.Cekic,A.Cetinkaya,A.N.Avan,R.Apak,Correlationoftotalantioxidant capacitywithreactiveoxygenspecies(ROS)consumptionmeasuredby oxida-tiveconversion,J.Agric.FoodChem.61(2013)5260–5270.
[18]O.Orrigoni,M.C.DeTullio,Ascorbicacid:muchmorethanjustanantioxidant, Biochim.Biophys.Acta1569(2002)1–9.
[19]R.M.Wightman,L.J.May,A.C.Michael,Detectionofdopaminedynamicsinthe brain,Anal.Chem.60(1988)769A–793A.
[20]C.Martin,TheParkinson’spuzzle:newdevelopmentsinourunderstandingof Parkinson’sdiseasehavegeneratedanumberofpromisingnewtreatmentsfor thisdisablingcondition,Chem.Britain34(1998)40–42.
[21]V.V.S.EswaraDutt,H.A.Mottola,Determinationofuricacidatthemicrogram levelbyakineticprocedurebasedonapseudo-inductionperiod,Anal.Chem. 46(1974)1777–1781.
[22]H.Etnet,M.Knoll,Electrochemicalcharacterizationofuricacidandascorbic acidataplatinumelectrode,Anal.Chim.Acta449(2001)129–134.
[23]C.Christophe,F.SékliBelaïdi,J.Launay,P.Gros,E.Questel,P.Temple-Boyer, Elaborationofintegratedmicroelectrodesforthedetectionofantioxidant species,Sens.ActuatorsB77(2013)350–356.
[24]M.Roushani,M.Shamsipur,H.R.Rajabi,Highlyselectivedetectionofdopamine inthepresenceofascorbicacidanduricacidusingthioglycolicacidcapped CdTequantumdotsmodified electrode,J. Electroanal.Chem.712 (2014) 19–24.
[25]B.Kaur,T.Pandiyan,B.Satpati,R.Srivastava,Simultaneousandsensitive deter-minationofascorbicacid,dopamine,uricacid,andtryptophanwithsilver nanoparticules-decoratedreducedgrapheneoxidemodifiedelectrode,Colloid Surf.B111(2013)97–106.
[26]X.Wang,M.Wu,W.Tang,Y.Zhu,L.Wang,P.He,Y.Fang,Simultaneous elec-trochemicaldeterminationofascorbicacid,dopamineanduricacidusinga palladiumnanoparticle/graphene/chitosanmodifiedelectrode,J.Electroanal. Chem.695(2013)10–16.
[27]T.Q.Xu,Q.L.Fhang,J.N.Zheng,Z.Y.Lv,J.Wei,A.J.Wang,J.J.Feng,Simultaneous determinationofdopamineanduricacidinthepresenceofascorbicacidusing Ptnanoparticlessupportedonreducedgrapheneoxide,Electrochim.Acta115 (2014)109–115.
[28]E. dePieriTroiani,E.R.Pereira-Filho, R. CensiFaria,Chemometric strate-giestodevelopananocompositeelectrodeforsimultaneousdetermination of ascorbic acid, dopamine, and uric acid, Electroanalysis 23 (2011) 2822–2831.
[29]H.Li,Y.Wang,D.Ye,J.Luo,B.Su,S.Zhang,J.Kong,Anelectrochemicalsensorfor simultaneouslydeterminationofascorbicacid,dopamine,uricacidand trypto-phanbasedonMWNTsbridgedmesocellulargraphenefoamnanocomposite, Talanta127(2014)255–261.
[30]J.Zhan,Z.Zhu,J.Zhu,K.Li,S.Hua,Selectivedeterminationofdopamine,ascorbic acidanduricacidatSDS-MWCNTsmodifiedglassycarbonelectrode,Int.J. Electrochem.Soc.9(2014)1264–1272.
[31]P.Manivel,M.Dhakshnamoorthy,A.Balamurugan,N.Ponpandian,D. Man-galaraj, C. Viswanathan, Conducting polyaniline-graphene oxide fibrous nanocomposites: preparation, characterization and simultaneous electro-chemicaldetectionofascorbicacid,dopamineanduricacid,RSCAdv.3(2013) 14428–14437.
[32]J.Du,R.Yue,F.Ren,Z.Yao,F.Jiang,P.Yang,Y.Du,Novelgrapheneflowers mod-ifiedcarbonfibersforsimultaneousdeterminationofascorbicacid,dopamine anduricacid,Biosens.Bioelectron.53(2014)220–224.
[33]D. Wu,Y. Li,Y.Zhang,P.Wang, Q.Wei,B.Du,Sensitiveelectrochemical sensorforsimultaneousdeterminationofdopamine,ascorbicacid,anduric acidenhancedbyamino-groupfunctionalizedmesoporousFe3O4@graphene sheets,Electrochim.Acta116(2014)244–249.
[34]Y.Li,X.Lin,Simultaneouselectroanalysisofdopamine,ascorbicacidanduric acidbypoly(vinylalcohol)covalentlymodifiedglassycarbonelectrode,Sens. ActuatorsB115(2006)134–139.
[35]M.Mazloum-Ardakani,M.A.Sheikh-Mohseni,A.Benvidi, Electropolymeriza-tionofthinfilmconductingpolymeranditsapplicationforsimultaneous determinationofascorbicacid,dopamineanduricacid,Electroanalysis23 (2011)2822–2831.
[36]J.Samseya,R.Srinivasan,Y.T.Chang,C.W.Tsao,V.S.Vasantha,Fabrication and characterisation of high performance polypyrrole modified microar-ray sensorforascorbic aciddetermination, Anal.Chim.Acta 793 (2013) 11–18.
[37]S.S.Kumar,J.Mathiyarasu,K.L.N.Phani,V.Yegnaraman,Simultaneous deter-minationofdopamineandascorbicacidonpoly(34-ethylenedioxythiophene) modified glassy carbon electrode, J. Solid State Electrochem. 10 (2006) 905–913.
[38]J. Mathiyarasu,S. Senthilkumar, K.L.N. Phani, V. Yegnaraman,PEDOT-Au nanocomposite film for electrochemical sensing, Mater. Lett. 62 (2008) 571–573.
[39]F.SekliBelaidi,P.Temple-Boyer,P.Gros,Voltammetricmicrosensorusing PEDOTmodifiedgoldelectrodeforthesimultaneousassayofascorbicanduric acids,J.Electroanal.Chem.647(2010)159–168.
[40]K.C. Lin, C.Y. Yin, S.M. Chen, Simultaneous determination of AA, DA, and UA based on bipolymers by electropolymerization of luminol and 3,4-ethylenedioxythiophene monomers, Int. J. Electrochem. Sci. 6 (2011) 3951–3965.
[41]S.Yu,C.Luo,L.Wang,H.Peng,Z.Zhu,Poly(3 4-ethylenedioxythiophene)-modified Ni/silicon microchannel plate electrode for the simultaneous determinationofascorbicacid,dopamineanduricacid,Analyst138(2013) 1149–1155.
[42]K.C.Lin,J.Y.Huang,S.M.Chen,Simultaneousdeterminationofascorbicacid, dopamine,uricacidandhydrogenperoxidebasedonco-immobilizationof PEDOTandFADusingmulti-walledcarbonnanotubes,Anal.Methods6(2014) 8321–8327.
[43]L.B.Groenendaal,F.Jonas,D.Freitag,H.Pielartzik,J.R.Reynolds, Poly(3,4-ethylenedioxythiophene)anditsderivatives:past,present,andfuture,Adv. Mater.12(2000)481–494.
[44]R.Jolly,S.Pairis,C.Petrescu,Comparativeageingofthreeelectroconductive polymers,J.Chim.Phys.95(1998)1400–1405.
[45]K.Lerch,F.Jonas,M.Linke,PropertiesandapplicationsofBaytron(PEDT),J. Chim.Phys.95(1998)1506–1509.
[46]J.Roncali,A.Yassar,F.Garnier,Electrosynthesisofhighlyconducting poly(3-methylthiophene)thinfilms,J.Chem.Soc.Chem.Commun.9(1988)581–582.
[47]C.P.Andrieux,J.M.Dumas-Bouchiat,J.M.Savéant,Kineticsofelectrochemical reactionsmediatedbyredoxpolymersfilms,J.Electranal.Chem.169(1984) 9–21.
[48]E.Vanhove,A.Tsopéla,L.Bouscayrol,A.Desmoulin,J.Launay,P.Temple-Boyer, Finalcappingpassivationlayersforlong-lifemicrosensorsinrealfluids,Sens. ActuatorsB178(2013)350–358.
[49]V.Castagnola,C.Bayon,E.Descamps,C.Bergaud,Morphologyandconductivity ofPEDOTlayersproducedbydifferentelectrochemicalroutes,Synth.Metals 189(2014)7–16.
[50]E. Tamburri, S.Orlanducci, F. Toschi,M.L. Terranova,D. Passeri, Growth mechanisms,morphologyandelectroactivityofPEDOTlayersproducedby dif-ferentelectrochemicalroutesinaqueousmedium,Synth.Metals159(2009) 406–414.
[51]J.Bobacka,A.Lewenstam,A.Ivaska,Electrochemicalimpedancespectroscopy ofoxidizedpoly(34-ethylenedioxythiophene)filmelectrodesinaqueous solu-tions,J.Electroanal.Chem.489(2000)17–27.
[52]L.Pigani,B.Zanfrognini,R.Seeber,PEDOT-modifiedmicroelectrodes, prepara-tion,characterisationandanalyticalperformances,Electroanalysis24(2012) 1340–1347.
[53]J.C.Gustafsson,B.Ledberg,O.Inganäs,Insituspectroscopicinvestigations ofelectrochromismandiontransportinapoly(34-ethylendioxythiophene) electrodeinasolidstateelectrochemicalcell,SolidStateIonics69(1994) 145–152.
[54]T.F.Otero,I.Cantero,H.Grande,Solventeffectsonthechargestorageabilityin polypyrrole,Electrochim.Acta44(1999)2053–2059.
[55]R.Kiefer,G.A.Bowmaker,R.P.Cooney,P.A.Kilmartin,J.Travas-Sejdic,Cation drivenactuation forfreestandingPEDOTfilmspreparedfrompropylene carbonate electrolytescontainingTBACF3SO3,Electrochim.Acta53(2008) 2593–2599.
[56]H.J.Ahonen,J.Lukkari,T.Hellstrom,J.Mattila,J.Kankare,Characterisationof poly(34-ethylenedioxythiophene)filmspolymerisedinaqueousmedia,Synth. Metals119(2001)119–120.
[57]S.Patra,K.Parai,N.Munichandraiah,Scanningelectronmicroscopystudiesof PEDOTpreparedbyvariouselectrochemicalroutes,Synth.Metals158(2008) 430–435.
[58]G.E.Poirier,M.J.Tarlov,Molecularorderingandgoldmigrationobservedin butanethiolself-assembledmonolayersusingscanningtunnellingmicroscopy, J.Phys.Chem.A99(1995)10966–10970.
[59]Y.C.Yang,Y.P.Yen,L.Y.Yan,S.L.Yau,K.Itaya,Elucidationofthedeposition pro-cessesandspatialstructuresofalkanethiolandarylthiolmoleculesadsorbed onPt(111)electrodeswithinsituscanningtunnellingmicroscopy,Langmuir 20(2004)10030–10037.
[60]P.Boulanger,J.,Polonovski,F.,Tayeau,P.MandelandG.Biserte,Biochimie Médicale,8thEdition,Masson,Paris,1971.
[61]M.C.Polidori,W.Stahl,O.Eichler,I.Niestroj,H.Sies,Profilesofantioxidantsin humanplasma,FreeRadic.Biol.Med.30(2001)456–462.
Biographies
FadhilaSekliBelaïdiwasbornonFebruary221980.ShereceivedherMaster’s Degreeinprocessandenvironmentalengineeringfromthe“InstitutNationaldes SciencesAppliquéesdeToulouse”(France)in2006.Shejoinedthe“Laboratoire deGénieChimique”(LGC)fromtheUniversityofToulouse(France)in2007.She isworkingonthedevelopmentofelectrochemicalmicrosensorsforchemicaland biochemicaldetection.
AurélieCivélaswasborninAix-en-Provence,France,onJanuary121989.Shejoined the“Laboratoired’Analyseetd’ArchitecturedesSystèmes”(LAAS)ofthe“Centre NationaldelaRechercheScientifique”(CNRS)ofToulousein2012foraoneyear trainingcourse.Sheworkedonthedevelopmentofelectrochemicalmicrosensors forchemicalandbiochemicaldetection.Shereceivedthedegreeinelectronic Engi-neeringfromthein“Chimie-Physique–Electronique”school(Lyon–France)in2014. ValentinaCastagnolawasborninBologna,Italyin1986.Shereceivedthemaster degreeinphotochemistryandmaterialchemistryfromtheUniversityofBologna,in 2011.Shejoinedthe“Laboratoired’Analyseetd’ArchitecturedesSystèmes”ofthe “CentreNationaldelaRechercheScientifique”(LAAS-CNRS),in2011asPhDStudent. Sheiscarryingoutherexperimentalresearchconcerningimplantablemicrodevices fortherecordingoftheneuralactivity.
A.TsopelawasborninAthens,Greecein1988.Shereceivedthemasterdegree inchemicalengineeringfromtheNationalTechnicalUniversityofAthens(NTUA -Greece),in2011.Shejoinedthe“Laboratoired’Analyseetd’Architecturedes Sys-tèmes”ofthe“CentreNationaldelaRechercheScientifique”(LAAS-CNRS),in2011 asPhDStudent.Sheiscarryingoutherexperimentalresearchinthedevelopment ofmicrosensorswithenvironmentalapplications.
LaurentMazenqwasbornonMay30,1982.HejoinedtheLaboratoired’Architecture etd’AnalysedesSystèmesoftheFrenchCentreNationaldelaRechercheScientifique (LAAS-CNRS)in2002.Sincethen,hehasbeenworkingonphotolithographyandon micro/nanotechnologiesprocessrealization.
PierreGroswasbornin1970.Hegraduatedinphysicalchemistryin1992and receivedhisPhDdegreeinChemicalEngineeringin1996attheUniversityPaul SabatierinToulouse.HeisnowProfessorinElectroanalyticalEngineeringinthe ChemicalEngineeringLaboratory(Toulouse-France).Heiscurrentlyworkingonthe developmentofelectrochemical(bio)sensors.
JérômeLaunaywasbornonMarch11,1975.Hereceivedthedegreein elec-tronicengineeringfromtheInstitutNationaldesSciencesAppliquéesdeToulouse” (France)in1998.Hejoinedthe“Laboratoired’Analyseetd’Architecturedes Sys-tèmes”fromtheFrench“CentreNationaldelaRechercheScientifique”(LAAS-CNRS) in1998andreceivedthePhDdegreefromthe“InstitutNationaldesSciences AppliquéesdeToulouse”(France)in2001.In2002,hebecamelectureratthe
UniversityofToulouse(France).Hisresearchactivitiesincludethedevelopment ofelectrochemicalmicrosensorsforthedetectioninliquidphase.
Pierre Temple-Boyer was born onOctober 25, 1966. He received his Engi-neer’sDegreeinelectronicengineeringfromthe“EcoleSupérieured’Electricité” (Paris–France)in 1990and his Master’sDegree inmicroelectronics from the
UniversityofToulouse (France)in1992.Hejoinedthe“Laboratoired’Analyse etd’ArchitecturedesSystèmes”(LAAS)fromtheFrench“CentreNationaldela RechercheScientifique”(CNRS)in1992andreceivedthePhDdegreefromthe “Insti-tutNationaldesSciencesAppliquéesdeToulouse”(France)in1995.Sincethen,as aCNRSresearcher,hehasworkedatLAASonthedevelopmentofphysicaland chemicalmicrosensors.