Publisher’s version / Version de l'éditeur:
Journal of Fire and Flammability, 4, 1, pp. 15-22, 1973-02-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Combustion products of polymeric materials containing nitrogen in
their chemical structure
Sumi, K.; Tsuchiya, Y.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=8b6c7061-c680-466c-b161-c1a02c883daf
https://publications-cnrc.canada.ca/fra/voir/objet/?id=8b6c7061-c680-466c-b161-c1a02c883daf
Ser
THl
N21r2
no.
553
c.
2
NATIONAL RESEARCH COUNCIL OF CANADA
BLDG
CONSEIL NATIONAL DE RECHERCHES DU CANADA
COMBUSTION PRODUCTS OF POLYMERIC MATERIALS
CONTAINING NITROGEN IN THEIR CHEMICAL STRUCTURE
Kikuo Sumi and Yoshio Tsuchiya
Reprinted from
Journal of Fire and Flammability
Vol.
4,
January
1973
pp. 15
-
22
Research Paper No. 553 of the
Division of Building Research
OTTAWA
February 1973
LES PRODUITS DE COMBUSTION DE SUBSTANCES
POLYMERES CONTENANT DE L'AZOTE DANS
LEUR STRUCTURE CHIMIQUE
La combustion dans un flacon
A
800'~
de cinq substances contenant de I'azote a donne du cyanure d'hydroghne, de I'oxyde de carbone et de I'anhydride carbonique. On a soumis ces produitsA
une analyse quanti- tative par chromatographie en phase gazeuse et on en a Qvaluh les effets nocifs dans I'air. Les resultats montrent bien clairement le danger du cyanure d'hydrogine dans un incendie comportant des substances qui contiennent de I'azote.National Research Council of Canada Division of Building Research
COMBUSTION PRODUCTS OF POLYMERIC MATERIALS
CONTAINING NITROGEN IN THEIR CHEMICAL STRUCTURE
(Received January 31, 1972)
ABSTRACT: Five materials that contain nitrogen were burned in a flask at
800°C. and the amounts of hydrogen cyanide, carbon monoxide and
carbon dioxide produced were quantitatively analyzed b y gas chroma- tography. The harmful effects of these products t o the resulting a t -
mosphere were then evaluated. T h e results vividly illustrate the potential danger of H C N t o a fire environment when materials containing nitrogen are involved in fire.
INTRODUCTION
A
L A R G E NUMBER of fire deaths are caused by inhalation of toxic gases and vapors produced by thermal decomposition and oxidation reactions. Informa- tion on toxic compounds produced by the combustion of various materials that are found in buildings is of importance in determining the products that are responsible for fire deaths and in assessing the potential danger due to the formation of these harmful substances. A t present, experimental data on the quantities of toxic gases and vapors produced by combustion are still lacking for many organic materials. For example, little information i s available on the amount of hydrogen cyanide, (HCN) produced from the decomposition of materials containing nitrogen in their
chemical structure. Hydrogen cyanide i s a highly toxic compound. The concen- tration of HCN that i s fatal t o man in
30
minutes is reported to be135
ppm[1],
while that of CO that i s fatal in exposures of less than one hour is reported to be
4000
ppm[2]
.
Although the lethal concentration of gases and vapors estimated by various toxicologists are not in complete agreement, these examples show that HCNi s much more toxic than CO. The above data suggest that HCN is thirty times as toxic as CO on a volume basis.
I n
1933,
Olsen et a1[3]
analyzed the combustion products of a number of materials including wool and silk. A weighed sample in a porcelain boat was in- serted into a heated silica tube through which sufficient air was drawn to burn the sample with a flame. The combustion of wool yielded0.5
t o5.0%
CO,
1.26
t o2.52%
HCN,1.25
t o3.70%
ammonia (NH,),0.02
to0.41%
hydrogen sulfide (H,S) and0.05
to0.12%
sulfur dioxide (SO,); and the combustion of silk yielded3.0
to4.4% CO,
2.22
to6.80%
HCN, and3.09
to3.58%
NH,. These quantitative data onK. Sumi and Y. Tsuchiya
HCN and CO indicate that the greatest toxic hazard from the combustion of wool or silk i s HCN poisoning.
I n 1963, Dufour [4] made a comprehensive literature survey of data concerning the combustion products of building materials, and included experimental data on polymers containing nitrogen from unpublished reports by Hobbs and Patten [5] and McDermott and Critchfield [ 6 ] . Hobbs and Patten analyzed the combustion products of several materials. The combustion apparatus consisted of a furnace and a "Vycor" tube maintained a t 8 0 0 ' ~ . Either compressed air or a mixture con- taining 11.7% O2 and 88.3% N2 was passed through the tube containing the sample. The combustion of one gram of wool yielded 0.446 g of CO and 0.007 g of HCN; one gram of silk yielded 0.634 g of CO, 0.036 g of HCN and 0.053 g of NH,; and one gram of nylon yielded 0.304 g of CO, 0.0076 g of HCN and 0.032 g of NH,. The results are in conflict with those obtained in the early work of Olsen [ 3 ] , and suggest that the hazard due t o CO i s far greater than that due to HCN when wool, silk or nylon are involved in fire.
McDermott and Critchfield [6] investigated the amount of HCN produced by the combustion of wool, nylon and flexible urethane foam, but did not determine the amount of CO produced. Nagao et al [7] investigated the amount of HCN produced by the combustion of wool, silk and polyacrylonitrile. They, too, did not determine CO produced. Thus, two sets of conflicting data are available on the amounts of HCN and CO produced by materials containing nitrogen.
The purpose of the present investigation was to quantitatively analyze HCN, CO and C02 produced by the combustion of five organic materials containing nitrogen, and to assess the relative importance of these products in creating a dangerous atmosphere.
EXPERIMENTAL
1. Materials
(a) Acrylic fiber (b) Nylon-6 (c) Wool
(d) Urea-formaldehyde foam (e) Rigid urethane foam
2. Combustion
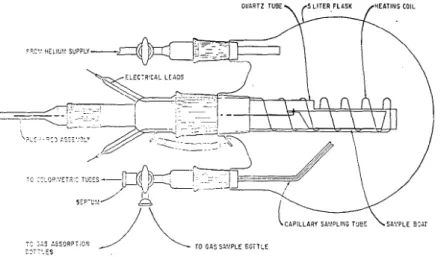
The combustion experiments were conducted in a 3-neck flask, having a capacity of 5 liters, shown in Figure 1. The air in the flask (open to the atmosphere) was heated by a nichrome wire wound around a half section of a quartz tube. The air was heated for 15 minutes to raise the temperature and maintain the hot zone a t
about 8 0 0 ' ~ . A weighed specimen was then pushed into the hot zone and a stopper was placed in the appropriate neck of the flask. Heating was continued for 2.5
Combustion Products of Polymeric Materials
OUARTZ TUBE, (5 LITER FLASK /qEATINPI COIL
Figure 1 . Combustion apparatus.
minutes, and the combustion flask was allowed to cool for 30 minutes. During the cooling period the pressure in the flask was maintained a t atmospheric by allowing helium to flow into the flask.
3. Analysis
A t the end of the cooling period, a sample of gas was taken in an evacuated bottle, and quantitatively analyzed for CO, C02 and HCN. Gas chromatography was the main method used in this analysis. Carbon monoxide and carbon dioxide were analyzed using a thermal conductivity detector, with a molecular sieve 5A packed column for CO and a Porapak N packed column for C02. Hydrogen cyanide was analyzed using a thermal conductivity detector and a Porapak S packed column. For low concentrations of HCN (<0.3% by volume), colorimetric detector tubes were used by pumping the products directly from the combustion flask. The detector tubes were also used to explore the presence of nitrogen dioxide.
RESULTS AND DISCUSSIONS
The experimental conditions that were adopted for the quantitative determina- tion of toxic products require some explanation. The procedure adopted was that favorable for the formation of toxic combustion products in order to find out under extreme conditions if HCN i s a potential hazard at fires. A combustion temperature of 800°C was selected to study the products formed from flaming combustion. At this temperature, flaming started soon after the specimen was inserted in the combustion flask and continued for 30 to 60 seconds, for all five samples. Preliminary experiments conducted a t various temperatures indicated that the quantities of toxic substances formed were influenced by the relative thermal
K .
Sumi andY .
Tsuchiyastability of the samples, and the highest amounts of toxic combustion products from given weights of materials were produced at the maximum working tem- perature of the apparatus of 8 0 0 " ~ . Preliminary experiments also indicated that HCN that i s produced by combustion reactions diminishes i f the heating time i s
extended for an appreciable period after flaming ceases. A series of experiments was therefore conducted with materials containing nitrogen t o find the amount of HCN produced as a function of heating time ranging from 0.5 t o 5.0 minutes. The maximum amount of HCN was found when the heating period was 2 t o 3 minutes. A heating time of 2.5 minutes was therefore adopted.
The experimental results showing the amounts of HCN, CO and COz produced at various sample weights ranging from 400 to 2400 mg are presented in Table 1.
They are reported in grams per gram of sample, and are based on means of two or more repeat tests. The standard deviation of the determination of each component was about 20% of the values reported in this table. The weight and hence the samplefair ratio was varied t o find the relative contribution of HCN and CO t o the toxicity of the resulting atmosphere over a wide range of conditions that may exist at fires. The possible yield of NOz was investigated by using colorimetric tubes having a range of 1 t o 1000 ppm. Nitrogen dioxide was not detected with any of the five materials following combustion of 800 mg of specimen.
The toxicity of a combustion product, based on both the nature of the com- pound and the quantity evolved, was evaluated by a relationship suggested in earlier papers [8, 91. According t o this relationship,
where
T i s a toxicity index
ce i s the experimentally determined concentration of a toxic product expressed as volume of product when one gram of material i s decomposed (or burned) and the products are diffused in a volume of one cubic meter.
cf i s the concentration of the same product that i s fatal (or dangerous) t o man in a 30-minute exposure.
The experimental data were translated into concentration for one gram burned in one cu.m. t o provide a convenient method for evaluating not only the present results but also those obtained by other investigators under different experimental conditions. The toxicity index of a material with two or more toxic combustion products i s determined by assuming that the effect of two or more harmful products is additive; that i s
Combustion Products o f Polymeric Materials
Table I. Combustion Producrs
M a t e r i a l
S a m p l e Weight of product per weight of sample (gig)
W e i g h t ( m g ) H C N C O
co2
R e s i d u e Acrylic fiber Nylon Wool Urea- formaldehyde foam Rigid urethane foamThe additive assumption may be conservative because of possible synergistic effects. Synergism may have to be considered in the future for certain combinations of toxic products, if i t i s found to be significant.
The toxicity indexes of combustion products were determined from the present experimental data and cf values given in Table 2. This information, presented in Table 3, vividly illustrates the importance of HCN as a potential contributor to the
K. Sumiand Y .
Tsuchiyatoxicity of the fire atmosphere. This Table shows that the toxicity due to HCN could be 55 times as great as that due to CO for acrylic fiber, 5 times as great for nylon, 8 times as great for wool, 26 times as great for urea-formaldehyde and twice as great for rigid urethane foam.
Table 2. Dangerous Concentrations of Combustion Products
Combustion product
Dangerous concentration for a 30.
minute exposure (ppm)
Reference
H C N
co
c o z
Table 3. Toxicity Index of Combustion Products
Sample Toxicity index due to: Weight (mg) H C N C O COz T o t a l Material Acrylic fiber N y l o n Wool Urea- forrnalde- hyde foam Rigid urethane foam
Combustion Products o f Polymeric Materials
The maximum toxicity index obtained for the five samples by varying the sample weights i s useful in assessing the toxic gas producing potential of materials. The data obtained at 8 0 0 ' ~ are presented in Table 4 for the five samples used in the present study as well as earlier results on white pine and polystyrene [ l o ]
.
The maximum toxicity indexes of some of the nitrogen-containing materials were much greater than the values for white pine and polystyrene.Table 4. Maximum Toxicity Index
T o x i c i t y index d u e t o : Material H C N CO CO, Total Acrylic fiber N y l o n Wool Urea-formaldehyde f o a m R i g i d urethane foam White Pine
-
0.09 0.003 0.09 Polystyrene - 0.09 0.01 0.10Kishitani [ I llconducted animal tests in which mice were exposed to the gaseous combustion products of a number of materials including urethane. Pathological examination of the mice that died from exposure to the combustion products of
.
urethane showed that the carboxyhemoglobin content in the blood was much lower than that required to kill these animals. Thus, deaths were probably due to the combined effect of CO and other toxic combustion products.CONCLUSIONS
Hydrogen cyanide, carbon monoxide and carbon dioxide formed by the com- bustion of five materials that contain nitrogen were quantitatively analyzed, and the harmful effects of these products to the resulting atmosphere were evaluated. The present results vividly illustrate the potential hazard of HCN produced when combustible materials containing nitrogen are involved in fire.
ACKNOWLEDGMENT
The authors wish to thank
J.
Boulanger and D. W. Morwick for assistance in conducting the experiments.K.
Sztrni andY.
TsuchiyaResearch Council of Canada, and i s published with the approval of the Director of the Division.
REFERENCES
1. Documentation o f Threshold L i m i t Values. American Conference o f Governmental In- dustrial Hygienists. 2 2 3 pp. (1966).
2. Y . Henderson and H. W. Haggard. Noxious Gases and the Principles o f Respiration In- fluencing Their Action. 212 pp. (1927). The Chemical Catalog Co., Inc., New Y o r k .
3. J. C. Olsen, G. E. Ferguson and L. Scheflan. Gases f r o m Thermal Decomposition o f
C o m m o n Combustible Materials. Ind. and Eng. Chem. 25, 599 (1933).
4. R. E. Dufour. Survey o f Available I n f o r m a t i o n o n t h e T o x i c i t y o f t h e Combustion and Thermal Decomposition Products o f Certain Building Materials under Fire Conditions. Underwriters' Laboratories, Inc. Bulletin o f Research No. 53, 5 2 pp. (July 1963).
5. A. P. Hobbs and G. A. Patten. Products o f Combustion o f Plastics and Other C o m m o n Solids. D o w Chemical Co. (March 1, 1962). Unpublished.
6. W. H. M c D e r m o t t and F. E. C r ~ t c h f i e l d . Thermal Degradation o f Polyurethane t o Hydrogen
Cyanide. U n i o n Carbide Plastics Co. (Feb. 24, 1961 ). Unpublished.
7. H. Nagao, M . Uchida and A. Yamaguchi. ( I n Japanese) Thermal Decomposition o f Poly- acrylonitrile. J. Chem. Soc. Japan, 59, 9 4 0 ( 1 9 5 6 ) .
8. Y . Tsuchiya and K . Sumi. Thermal Decomposition Products o f Polyvinyl Chloride. J. Appl.
Chem. 17, 3 6 4 ( 1 9 6 7 ) .
9. Y . Tsuchiya and K. Sumi. Evaluation o f the T o x i c i t y o f Combustion Products., J. Fire and Flammability 3, 4 6 ( 1 972).
10. K . Sumi and Y. Tsuchiya. T o x i c Combustion Products o f Wood and Polystyrene. Building Research Note No. 76, Division o f Building Research, National Research Council o f Canada, 3 p. (Sept. 1971 ).
11. K. Kishitani. Study on Injurious Properties o f Combustion Products o f Building Materials
at Initial Stage o f Fire. J. Faculty of Engineering, Univ. o f T o k y o (B), 31, No. 1, 1 (1971).
Kikuo Surni
Kikuo Sumi i s a Research Officer of the Division of Building Research, National Research Council of Canada. He received his B.A.Sc. degree in Applied Science from the University of Toronto and his Ph.D. degree in Chemical Engineering from the University of London. He joined the National Research Council in 1951 and has experience i n various aspects of fire research such as thermal decomposition of poly mers, fire extinguishment and fire prevention regulations.
Yoshio Tsuchiya
Yoshio Tsuchiya i s a Research Officer of the Division of Building Research, National Research Council of Canada. He received his Bachelor of Engineering and Doctor of Engineering degrees in Applied Chemistry from the University of Tokyo in 1953 and 1962 respectively. He joined the National Research Council in 1965. He has experience in the fields of industrial explosives, organic peroxides and fire research.
This publication i s being distributed by the Division of Building Research of the National Research Council of Canada. It should not be reproduced in whole or in part without permission of the original publisher. The Division would be glad to be of assistance in obtaining such permission.
Publications of the Division may be obtained by mailing the appro- priate remittance ( a Bank, Express, or Post Office Money Order, or a cheque, made payable to the Receiver General of Canada, credit NRC) to the National Research Council of Canada, Ottawa. KlAOR6. Stamps are not acceptable.
A list of all publications of the Division i s available and may be ob- tained from the Publications Section, Division of Building Research, National Research Council of Canada, Ottawa, KIAOR6.