HAL Id: hal-03219659
https://hal.archives-ouvertes.fr/hal-03219659
Submitted on 6 May 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
A multisensory perspective onto primate pulvinar
functions
Mathilda Froesel, Céline Cappe, Suliann Ben Hamed
To cite this version:
Mathilda Froesel, Céline Cappe, Suliann Ben Hamed. A multisensory perspective onto primate
pulv-inar functions. Neuroscience & Biobehavioral Reviews, Oxford: Elsevier Ltd., 2021, 125, pp.231-243.
�10.1016/j.neubiorev.2021.02.043�. �hal-03219659�
Neuroscience and Biobehavioral Reviews 125 (2021) 231–243
Available online 1 March 2021
0149-7634/© 2021 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
A multisensory perspective onto primate pulvinar functions
Mathilda Froesel
a,*
, C´eline Cappe
b, Suliann Ben Hamed
a,*
aInstitut des Sciences Cognitives Marc Jeannerod, CNRS UMR 5229, Universit´e Claude Bernard Lyon I, 67 Boulevard Pinel, 69675, Bron Cedex, France bCentre de Recherche Cerveau et Cognition, Universit´e Paul Sabatier, Universit´e de Toulouse, 31062, Toulouse Cedex 9, France
A R T I C L E I N F O Keywords: Pulvinar Cortex Multisensory Visual Auditory Somatosensory Anatomy fMRI A B S T R A C T
Perception in ambiguous environments relies on the combination of sensory information from various sources. Most associative and primary sensory cortical areas are involved in this multisensory active integration process. As a result, the entire cortex appears as heavily multisensory. In this review, we focus on the contribution of the pulvinar to multisensory integration. This subcortical thalamic nucleus plays a central role in visual detection and selection at a fast time scale, as well as in the regulation of visual processes, at a much slower time scale. However, the pulvinar is also densely connected to cortical areas involved in multisensory integration. In spite of this, little is known about its multisensory properties and its contribution to multisensory perception. Here, we review the anatomical and functional organization of multisensory input to the pulvinar. We describe how visual, auditory, somatosensory, pain, proprioceptive and olfactory projections are differentially organized across the main subdivisions of the pulvinar and we show that topography is central to the organization of this complex nucleus. We propose that the pulvinar combines multiple sources of sensory information to enhance fast re-sponses to the environment, while also playing the role of a general regulation hub for adaptive and flexible cognition.
1. Introduction
Vision is the dominant sensory modality in both humans and nonhuman primates. Up to 50 % of identified non-human primate functional areas are involved in visual processing (20–30 % in the humans, (Van Essen and Drury, 1997; Van Essen, 2003)). As a result, vision is still to date the most studied sensory system. This contrasts with the rising view that perception and brain functions are intrinsically multisensory (Schroeder and Foxe, 2005). For example, audition, touch and proprioception play a crucial role in the sensory-motor exploration of the world. Likewise, audition, olfaction and touch are essential to social interactions and communication. These different sensory modal-ities have very similar anatomical organizational principles in the brain: incoming sensory information from the distal sensory receptors are transduced into a neuronal code and reach the cortex through special-ized primary sensory cortical areas (Fig. 1, colored cortex, Van Essen et al., 1990; Carmichael et al., 1994; Camalier et al., 2012). From there, this sensory information runs through a sequence of reciprocally con-nected cortical regions, organized along a hierarchical pattern, pro-gressively describing the incoming sensory information at higher levels of complexity (Fig. 1, associative cortices).
Though the activity of these sensory areas are dominated by one sensory modality, there is now ample evidence that they are modulated by other sensory modalities, including at the earliest processing levels (Brosch et al., 2005; Calvert et al., 1999; Cl´ery et al., 2015a; Foxe et al., 2000; Ghazanfar et al., 2005; Giard and Peronnet, 1999; Guipponi et al., 2015; Kayser et al., 2008; von Kriegstein et al., 2005; Lakatos et al., 2007; Molholm et al., 2002). For example, neuronal activity in the pri-mary visual cortex is modulated by auditory (Wang et al., 2008) as well as by tactile stimulations (Guipponi et al., 2015). At the anatomical level, direct projections between early sensory areas have been described, between the somatosensory and visual cortex (Cappe et al., 2012, 2009a; Cappe and Barone, 2005). At higher cortical levels, in the associative cortices, multisensory convergence and integration is the rule (Noesselt et al., 2007; Werner and Noppeney, 2010; Calvert, 2001; Bremmer et al., 2001; Miller and D’Esposito, 2005; Avillac et al., 2007; Guipponi et al., 2013), to the degree that the entire brain is often considered as of multisensory nature (Cl´ery et al., 2018a, 2015a; Cl´ery and Ben Hamed, 2018; Driver and Noesselt, 2008; Ghazanfar and Schroeder, 2006; Schroeder and Foxe, 2005). Most of these associative multisensory areas have direct projections to and from early sensory areas. For example, superior temporal polysensory area (STP), activated
* Corresponding authors.
E-mail addresses: Mathilda.froesel@isc.cnrs.fr (M. Froesel), benhamed@isc.cnrs.fr (S. Ben Hamed).
Contents lists available at ScienceDirect
Neuroscience and Biobehavioral Reviews
journal homepage: www.elsevier.com/locate/neubiorev
https://doi.org/10.1016/j.neubiorev.2021.02.043
by both auditory and somatosensory information, has direct projections to primary visual area V1 (Clavagnier et al., 2004). In addition, they are characterized by specific laminar and connectional patterns with cortical and subcortical structures (Foxworthy et al., 2013).
Overall, multisensory integration thus involves complex patterns of multisensory interactions i) within associative areas, ii) between distant associative areas, iii) between associative areas and early sensory cortices, iv) within early sensory cortices and v) between distant early sensory cortices. All sensory information reaches the neocortex through the thalamus and the superior colliculus. In turn, both these subcortical structures receive sensory input from the cortex and from each other. The pulvinar, the largest and most posterior thalamic nucleus, also has, as detailed below, strong feedforward and feedback connections with the cortex as well as with the superior colliculus (Benevento and Standage, 1983; Lin and Kaas, 1979; Meredith et al., 1987; Meredith and Stein, 1986; Stein and Meredith, 1993; Wallace et al., 1998, 1993). The pulvinar is thus expected to play a key role in multisensory integration. However, and quite surprisingly, very little is known about the multi-sensory organization and properties of this subcortical structure. In the following, we review current knowledge about the contribution of the pulvinar to multisensory processes and multisensory cortico-subcortical interactions, and identify current knowledge gaps thereof.
The pulvinar is a subcortical thalamic nucleus that has first been described as an early sensory relay between incoming sensory infor-mation and the cortex (to the exception of olfactory stimuli). It is the largest thalamic nucleus, located medial and dorsal to the lateral geniculate nucleus (LGN), in the most posterior aspect of the thalamus. Most relevant to the present review, most cortical regions have recip-rocal connections with the pulvinar, including early visual, somatosen-sory and auditory processing areas as well as higher order parietal, temporal, premotor and prefrontal associative areas that are known to be highly multisensory (Asanuma et al., 1985; Cappe et al., 2009a,b; Leh et al., 2008). It is actually proposed that for each direct cortico-cortical pathway (e.g. between the parietal and the prefrontal cortex), there exists an indirect cortico-pulvino-cortical pathway (e.g. parieto-pulvino-prefrontal pathway). This hypothesis, called the
repli-cation principal, thus proposes a topographical organization of the
con-nections of the pulvinar with the cortex matching cortical organization (Shipp, 2003). The pulvinar is described as a high order thalamic nu-cleus due to the multiple reciprocal pulvino-cortical pathways it is involved in (Sherman and Guillery, 2006) and its characteristic synaptic organization. Specifically, small synaptic terminals (<1 μm) are typical
of (feedback) cortico-thalamic projections, while giant synaptic termi-nals, fewer in number, are typical of (feedforward) thalamo-cortical
projections (Rouiller and Welker, 2000; Sherman, 2007). Based on these highly complex cortico-pulvino-cortical connectivity patterns, the pulvinar is proposed to play a key role as a mediator/modulator between cortical areas (Benarroch, 2015; Saalmann and Kastner, 2011).
In the following, we specifically focus onto the primate pulvinar, which is classically divided in three large distinct regions, based on their specific cytoarchitectonic properties: the lateral, the medial and the inferior pulvinar (Fig. 2, Walker, 1938; Olszewski, 1952; Gutierrez et al., 1995; Stepniewska and Kaas, 1997). The organization of the primate pulvinar complex and its connectivity with the cortex has undergone substantial changes during primate evolution (Kaas and Baldwin, 2020). Specifically, while the visual inferior and ventro-lateral pulvinar nuclei are well preserved across primates, the medial pulvinar is considered as fully identifiable only in the haplorhini primate suborder (Baldwin et al., 2017; Homman-Ludiye and Bourne, 2019), but not in the non-haplorhini primates such as the galagos or the lemurs. The rodent and carnivore homologues of the pulvinar can be defined based on the observed pattern of projections of the superficial layers of the superior colliculus or optic tectum to the primate pulvinar (Zhou et al., 2017). In spite of the fact that pulvinar lesions in these latter mammalian phylogenetic orders do not exhibit the same visual deficits as in primates, a certain functional and cortical connectivity pattern homology can be noted between the lateral-posterior pulvinar complex of rodents and carnivores and the primate inferior and ventro-lateral pulvinar nuclei (Kaas and Baldwin, 2020). No homologue for the medial primate pulvinar can be identified in rodents and carnivores as is the case for non-haplorhini primates (Kaas and Baldwin, 2020).
While the contribution of the pulvinar to visual cognition has been extensively studied including during development (Benarroch, 2015; Bourne and Morrone, 2017; Bridge et al., 2016), we will here focus onto its contribution to multisensory processes and its interactions with the multisensory cortex (Cappe et al., 2009b; Tyll et al., 2011). Indeed, and quite surprisingly, in spite of its reciprocal connections with both uni-sensory and multiuni-sensory cortical regions, the primate multiuni-sensory pulvinar functions are still by and large unknown. Here, we provide an exhaustive review of the contribution of the primate pulvinar to the processing of multisensory information, we identify the current knowl-edge gaps and we propose a novel ecologically anchored perspective onto primate pulvinar functions. We will first review the anatomical organization of the multisensory pulvinar and its connectivity pattern with the cortex. We will then revisit lesion studies beyond the commonly described visual deficits to include evidence for other categories of sensory deficits. We will next review the multisensory functions of the pulvinar, highlighting the challenge of matching these functional multisensory properties with the current anatomical knowledge of the multisensory pulvinar. We will review the sparse evidence for multi-sensory integration in the pulvinar. We will then describe the role of the pulvinar in sensory detection and selection and as a behavioral and processing regulator, i.e. in two distinct functions operating at very different time scales, an ultra-fast and a slow time scale respectively. We will conclude, proposing to generalize the role of the pulvinar in detection and selection on the one hand and emotional and attention regulation on the other hand, from the visual domain to the multisen-sory context thus shifting from a view of the pulvinar as a modulator of the visual system, to the view of the pulvinar as a general regulation hub for adaptive, flexible cognition and survival.
2. Anatomical evidence for multiple parallel cortico-pulvinar sensory pathways
Seminal and more recent studies have sought to characterize the connectivity patterns of the pulvinar with the neocortex. These con-nectivity patterns are summarized below. All connections are reciprocal, unless mentioned otherwise. Pulvino-cortical connections are discussed in relation with the major nuclei, and when possible in relation to their anatomical subdivisions. Indeed, these major pulvinar nuclei can be
Fig. 1. Organization of main sensory input to the primate brain,visual in
pink, somatosensory in purple and auditory in green. These cortices are dominated by responses to one sensory modality and are reciprocally connected to the rest of the cortex in so called-associative areas, i.e. areas associating or integrating together multiple sensory inputs.
divided in smaller anatomical units, based on their cytoarchitectonic organization. It is important to note that no direct connections between the left and right pulvinar are reported. Relevant to human pulvinar functions, a certain degree of lateralization is described.
2.1. The inferior pulvinar (PI, Fig. 2A and B, left panel)
From a cytoarchitectonic, myeloarchitectonic and chemo-architectonic point of view, PI is non-homogenous and can be sub-divided into three further distinct regions, PIc (central, 70 % of PI), PIm (medial, 20 %) and PIp (posterior, 10 %) that are encapsulated by fibers (Lin and Kaas, 1979) and characterized thanks to Nissl, myelin, cyto-chrome oxidase (CO), acetylcholinesterase (AChE), calbindin-D28 K (Cb) and monoclonal antibody Cat-301 staining. PIp is dark in Cd and expresses AChE and CO. There is a strong expression of AChE and CO and to a lesser extent Cb in PIm. Based on these staining protocols, PIc shows a complex cellular organization that leads to its further subdivi-sion as follows: PIcm, characterized by a strong Cb but light CO and AChe staining; and PIcl, characterized by a strong CO and AChE staining and only few Cb responsive neurons (Gray et al., 1999; Gutierrez et al., 2000, 1995; Stepniewska and Kaas, 1997). In terms of cortical connec-tivity, PIm and PIc are densely connected with the visual system. In particular, PIcm receives direct inputs from the retina through the thalamic lateral geniculate body (Adams et al., 2000; O’Brien et al., 2001; Warner et al., 2010). PIp is densely connected with the superior colliculus (SC) as well as PIcm (Elorette et al., 2018; Huerta and Harting, 1983; Lin and Kaas, 1979; Stepniewska et al., 2000). PI has reciprocal connections with the primary visual area V1 and the secondary visual area V2 (Benevento and Davis, 1977). PI and more so PIm, is also
connected with extrastriate dorsal visual stream areas such as MT (V5) (Adams et al., 2000; Mundinano et al., 2019; O’Brien et al., 2001; Warner et al., 2010) and MST (Baleydier and Morel, 1992; Kaas and Lyon, 2007). It is worth noting that these projections to MT are topo-graphically organized (Mundinano et al., 2019). PI, and specifically its subparts PIp and PIcm, also has dense connections with extrastriate ventral visual stream areas such as V4 (Adams et al., 2000; O’Brien et al., 2001; Warner et al., 2010), FST (Baleydier and Morel, 1992; Kaas and Lyon, 2007) and TEO (Baizer et al., 1993; Webster et al., 1993; Weller and Steele, 1992). The connectivity between PI and FST is worth high-lighting. Indeed, FST neurons are responsive to all of visual, auditory and somatosensory stimulations and is thus massively multisensory. PI is also connected with primary auditory cortex A1 (Kaas and Lyon, 2007) as well as to higher order caudal STG and to the rostral belts of the auditory cortex cAC and rAC (Gutierrez et al., 2000). To our knowledge, there is no evidence for a functional connectivity between this cortical region and primary somatosensory, proprioceptive or olfactory cortices.
2.2. The lateral pulvinar (PL, Fig. 2A and B, middle panel)
From a cytoarchitectonic point of view, PL can be distinguished by its dense fiber bundles as well as by its cellular non-homogeneity. It can be subdivided into two further distinct regions, PLdm (dorso-medial) and
PLvl (ventral) characterized respectively by Cb-dark large neurons and
PIcl-like Cb patterns (Gutierrez et al., 1995; Stepniewska and Kaas, 1997). Similarly to PI, PLvl is connected with the primary and secondary visual areas V1 and V2 (Benevento and Davis, 1977; Adams et al., 2000), as well as with the superior colliculus (SC) (Benevento and Standage, 1983; Lin and Kaas, 1979). PLdm is also connected with SC (Benevento
Fig. 2. Organization of main pulvino-cortical identified anatomical connections.A/ Left panel identifies connections between the cortex and the inferior
pulvinar, middle panel identifies connections between the cortex and the lateral pulvinar, and right panel identifies connections between the cortex and the medial pulvinar. Visual inputs are in shades of red, auditory inputs in green, somatosensory inputs in blue and multisensory inputs in purple. Smaller brain insets represent the general pulvino-cortical connectivity gradients for each of the main pulvinar nuclei. B/ Left panel identifies connections between subcortical structures and the inferior pulvinar, middle panel identifies connections between subcortical structures and the lateral pulvinar visual, and right panel identifies connections between subcortical structures and the medial pulvinar. Connections with superior colliculus (SC), lateral geniculate nuclei (LGN) and retina are in black. Connections with the amygdala in gray. C/ Global representation of pulvinar connectivity gradient with the cortex. Red indicates dominant PI and PLvl connectivity, orange, dominant PM and PLdm connectivity and yellow, dominant PM connectivity. Note that this structural general connectivity gradient matches the reported functional gradient.
and Fallon, 1975; Elorette et al., 2018; Huerta and Harting, 1983). PL is further connected with several visual ventral stream areas. Specifically,
PLvl is connected with V4 and TEO (Benevento and Rezak, 1976) while
PLdm is connected with temporal areas TEO and TE (Adams et al., 2000; Shipp, 2003; Webster et al., 1993). This connectivity pattern is very similar to the one described for PI. PL is also specifically connected with the dorsal visual stream. These connections are specific of PLdm which has dense connections with the posterior parietal and temporo-parietal cortex, including with areas MT, MST, LIP, VIP, MIP and AIP (Adams et al., 2000; Shipp, 2003; Webster et al., 1993). While these cortical regions have strong visual functions, they also play a key role in visuo-oculomotor coordination (LIP), reaching (MIP), grasping (AIP), or self-movement perception (VIP), as well as in somatosensation (VIP) and proprioception (MIP, AIP). PLdm is also densely connected with superior parietal areas PE and PEa which are involved in all of visual, somato-sensory and proprioceptive functions. This cortical region is also densely connected to the dorsolateral prefrontal cortex, as well as to the dorsal (PMd) and ventral (PMv) premotor cortices (Asanuma et al., 1985; Baizer et al., 1993; Clower et al., 2001; Gutierrez et al., 2000; Hardy and Lynch, 1992) thus defining a (or multiple) pulvino-parieto-prefrontal functional network(s). These strong parietal and prefrontal connec-tions suggest that PLdm is functionally distinct from PLvl, but quite similar to the medial pulvinar (PM) discussed next. This is further dis-cussed in the section entitled dorso-ventral functional gradient in the pulvinar below.
2.3. The medial pulvinar (PM, Fig. 2A and B, right panel)
From a structural point of view, PM lacks the fiber bundles seen in PL, and has a higher density of cells that are characterized by a rounder shape than in PL. It is also non-homogenous and can be subdivided into two further distinct regions, PMl (lateral) and PMm (medial) charac-terized by a difference in AChE staining (Gutierrez et al., 2000; Step-niewska and Kaas, 1997). PMl is strongly interconnected with the ventrolateral part of the LGN, the retina (Itaya and Van Hoesen, 1983), as well as the primary and secondary visual cortices V1 and V2 (Benevento and Davis, 1977). This region also has connections with the ipsilateral superior collicullus (SC) (Benevento and Fallon, 1975; Benevento and Standage, 1983; Elorette et al., 2018; Huerta and Hart-ing, 1983). PMl is also reciprocally connected with ventral visual stream areas V4 and TEO as well as with intraparietal area LIP, with a topo-graphical organization of its cortical projecting fibers (Romanski et al., 1997; Shipp, 2003). In contrast, PMm has reciprocal connections with the caudal and the rostral belts of the auditory cortex (cAC and rAC) (Cappe et al., 2009a; Hackett et al., 1998; de la Mothe et al., 2006, 2012; Kaas and Hackett, 1998; Hackett et al., 2007), the entire extent of the superior temporal gyrus (STG) (Gutierrez et al., 2000; Jones and Burton, 1976; Kosmal et al., 1997) as well as with the superior temporal sulcus (STS), all of which are involved in auditory processing (Romanski et al., 1997). PMm is also interconnected with somatosensory and proprio-ceptive posterior parietal areas PE and PEa, as well as with the dorsal premotor cortex (Acu˜na et al., 1990; Cappe et al., 2009a; Impieri et al., 2018; Morel et al., 2005; Romanski et al., 1997; Schmahmann and Pandya, 1990). Last but not least, PMm is characterized by strong con-nections with the parietal, temporal and prefrontal cortex (Asanuma et al., 1985; Bos and Benevento, 1975; Cappe et al., 2007; Cappe et al., 2009a; Clower et al., 2001; Hardy and Lynch, 1992). It has connections with TE, MST, LIP, VIP, and more generally with both the superior pa-rietal and the inferior papa-rietal cortices (Baizer et al., 1993; Baleydier and Morel, 1992; Webster et al., 1993). It also projects to the frontal eye field (FEF) (Trojanowski and Jacobson, 1974) and receives widespread inputs topographically organized with the dorsolateral and ventrolateral pre-frontal cortex, as well as reciprocal connections with the orbito-pre-frontal cortex (Romanski et al., 1997; Bos and Benevento, 1975). Subcorti-cally, PMm receives projections from the SC (Bender and Butter, 1987; Benevento and Standage, 1983; Elorette et al., 2018; Harting et al.,
1980). These SC projection pulvinar neurons project back to the lateral amygdala (Elorette et al., 2018) and predominantly originate from the deep/intermediate SC layers (Benevento and Standage, 1983; Elorette et al., 2018). It is to be noted that this connectivity of SC with PM is debated (Baldwin et al., 2017; Zhou et al., 2017). However, the discrepancy in the experimental observations may come from inter-species differences (Baldwin et al., 2013). Globally speaking, PM is the thalamic nucleus that presents the densest thalamocortical pro-jections to auditory and premotor cortex (Cappe et al., 2009a).
Thus, overall, although the pulvinar has globally dense connections with the striate and extrastriate visual cortex, a general multisensory pattern can be identified. Namely, the inferior pulvinar can be seen as being mainly interconnected to early visual areas and auditory circuitry, the lateral pulvinar as being mainly interconnected to extrastriate visual areas and somatosensory circuitry, and the dorsal, medial pulvinar as being interconnected to all cortical areas, in particular the fronto- parietal circuitry (hence its role in sensory-motor transformation and eye-hand coordination) –insets in Fig. 2A. This thus defines a ventro- dorsal/postero-anterior projection gradient from PI, to PL to PM (Fig. 2C) coarsely matching the functional pulvinar gradients discussed below. However, at closer inspection, more complex pulvino-cortical connectivity patterns can be identified (as described above and in Fig. 2A). This matches the complexity of the contribution of the pulvinar to multisensory cognition, as described in the lesional and functional studies described next.
3. Clinical evidence for non-visual sensory pulvinar functions
In spite of these neuro-anatomical evidence for a multisensory role of the pulvinar, from a functional point of view, primate pulvinar is clas-sically considered as a highly visual and attentional structure. This is due to the fact that subcortical lesions including the pulvinar often lead to hemineglect symptoms, that is to say an absence of conscious processing of the contralateral visual hemifield, as well as a disruption of spatial and temporal visual attention (Rafal and Posner, 1987; Ward and Arend, 2007; Zihl and von Cramon, 1979). More recent studies confirm this role. Indeed, pulvinar lesions are associated with contra-lesional visual deficits ranging from visual extinction and response competition deficits during visual attentional tasks (Danziger et al., 2004) to feature discrimination deficits in the presence of salient distracters (Snow et al., 2009). However, lesion studies in human patients often include patients with heterogeneous pulvinar lesion topographies (Snow et al., 2009). These lesions sometimes extend beyond the pulvinar. In addition, they often do not allow a precise investigation of the distinctive functions of the different pulvinar subdivisions. Monkey studies can be very infor-mative in this respect, as they allow for focal and reproducible pertur-bations of the pulvinar and its subdivisions. For example, localized inactivations or stimulations in the dorsal pulvinar in monkeys lead to similar deficits as described above (Desimone et al., 1990; Domi-nguez-Vargas et al., 2017; Petersen et al., 1987; Wilke et al., 2013) and confirm the idea of a strong functional homology between macaque and human pulvinar functional organization.
While most evaluations of pulvinar functions in lesioned patients rely on the visual modality, pulvinar lesions have also been associated with other very diverse clinical symptoms not necessarily involving visual processes. For example, lesions that cover the mediodorsal thalamus, the pulvinar and the lateral compartment, are described to lead to deficits in the detection, identification and hedonic rating of odors (Sela et al., 2009). While only the mediodorsal thalamus appears to connect to an olfactory piriform-orbitofrontal functional circuit (Price and Slotnick, 1983), sparse functional evidence suggests that the pulvinar may play a modulator role through its connectivity with layer 6 of the orbitofrontal cortex. Lesions of the pulvinar, including the anterior superior and the lateral nuclei are also described to lead to disruption in speech recog-nition and production, up to total aphasia (Van and Borke, 1969). In contrast, dorsal damage of the pulvinar leads to a slower initiation of
grasping movements while specific medio-dorsal pulvinar lesions lead to major postural deficits, thus possibly associating this region with pro-prioception (Wilke et al., 2018). A systematic multisensory evaluation of patients with pulvinar lesions is thus lacking. This systematic multi-sensory evaluation of pulvinar patients could be instructed by the functional multisensory properties of the pulvinar as described next.
4. Functional sensory maps in the pulvinar
A marked challenge in understanding the physiology of the pulvinar is the observation that its sensory functional gradients are not neces-sarily defined by the above described anatomical parcellation.
4.1. Dorso-ventral functional gradient in the pulvinar
While the pulvinar is anatomically divided into the three main nuclei described above (PI, PL and PM), functionally, a dorso-ventral gradient is often described (Arcaro et al., 2018, 2015; Dominguez-Vargas et al., 2017; Kaas and Lyon, 2007; Kinoshita et al., 2019; Komura et al., 2013; Wilke et al., 2010). The functional ventral pulvinar includes the anatomically defined inferior pulvinar as well as the lateral ventral pulvinar (PLvl). It is considered as the "visual pulvinar" (Bridge et al., 2016). The functional dorsal pulvinar corresponds to the anatomically defined medial pulvinar and dorsal lateral pulvinar (PLdm). This dorso-ventral functional gradient is thus coherent with the global anatomical pulvino-cortical connectivity organization described above (Fig. 2C). In addition, it is worth noting that, at least for the visual modality, in both the human and the non-human primate pulvinar, two independent visual field maps are described along a dorsolateral visual gradient that doesn’t coincide with neither the described anatomical subdivisions nor the dorso-medial functional gradient described above (Arcaro et al., 2015). Whether this functional gradient exists also for the other sensory modalities and how it maps onto our current knowledge of the pulvino-cortical connectivity and the well-established topographi-cally organized dorso-ventral gradient (Baizer et al., 1993; Shipp, 2003) remains to be explored. On top of these general gradients, specific functional responses have been mapped onto the anatomically defined pulvinar subdivisions. This is discussed next.
4.2. The inferior pulvinar
The neurons of inferior pulvinar respond to visual stimuli, expressing a strong orientation and movement selectivity (Mathers and Rapisardi, 1973). They also respond to eye movements in the light and in the dark (Perryman et al., 1980; Petersen et al., 1985). This is in agreement with the high connectivity of this region with the visual system including the retina. This is also in agreement with the specific connectivity pattern observed between PIm and the magnocellular pathway connecting SC to cortical area MT which has been associated with visually guided actions (Mundinano et al., 2018). PI neurons also respond to auditory stimuli during auditory discrimination (Yirmiya and Hocherman, 1987). They additionally respond to hand movement (Yirmiya and Hocherman, 1987) as well as to eye movements and saccades (Perryman et al., 1980; Robinson et al., 1991). Importantly, most neuronal responses are enhanced during active as compared to passive tasks (Yirmiya and Hocherman, 1987).
4.3. The lateral pulvinar
Neurons in the ventrolateral part of PL are involved in the processing of simple oriented as well as dynamic visual stimuli (Gattass et al., 1979; Mathers and Rapisardi, 1973) and the activity of its neurons correlates with that of temporal area TEO and extrastriate visual area V4 ( Saal-mann et al., 2012). This is further confirmed by functional connectivity MRI studies (Arcaro et al., 2015). The neurons of dorsolateral PL are responsive to more complex visual stimuli such as geometrical figures,
snakes, monkey faces and hands (Maior et al., 2010; Nguyen et al., 2013; Van Le et al., 2013). Apart from the visual modality, PL neurons are also responsive to pressure and tactile stimulations (Acu˜na et al., 1990; Gattass et al., 1979, 1978; Mathers and Rapisardi, 1973; Yirmiya and Hocherman, 1987).The ventral part of PL is involved in auditory perception (Gattass et al., 1978; Magari˜nos-Ascone et al., 1988; Yirmiya and Hocherman, 1987). Specifically, complex sounds such as vocaliza-tions and alarm screams are shown to activate the ventrolateral part of
PL (Gattass et al., 1979). Last, one study reports neuronal responses in PL to olfactory stimuli (Gattass et al., 1978). In addition to these multi-sensory properties, PL, as has been described for PI, is involved in eye movements and saccades (Perryman et al., 1980; Robinson et al., 1991). It is also involved in reaching and grasping movements (Acu˜na et al., 1990; Gattass et al., 1979, 1978; Mathers and Rapisardi, 1973; Yirmiya and Hocherman, 1987). All of these functional observations nicely correlate with the anatomical studies described above.
4.4. The medial pulvinar
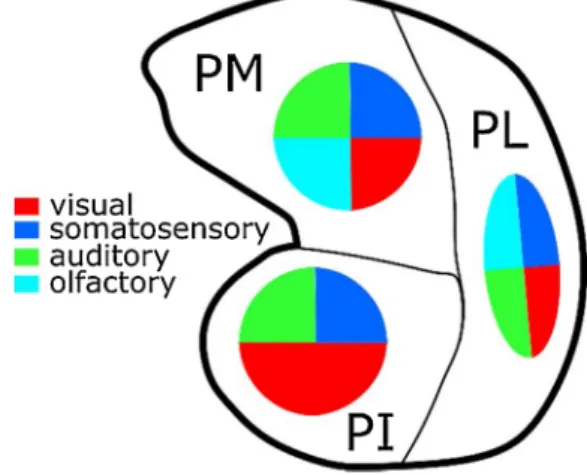
Based on the available experimental evidence, the medial pulvinar is the most multisensory subdivision of the pulvinar. The activity of its neurons correlates with the orientation, direction and velocity of visual stimuli (Magari˜nos-Ascone et al., 1988; Mathers and Rapisardi, 1973). Like has been reported for the dorsal part of PL, the medial part of PM responds to complex visual stimuli such as faces, geometrical forms, eye like patterns and snakes (Almeida et al., 2015; Maior et al., 2010; Nguyen et al., 2013). PM neurons also respond to auditory stimuli, both simple and more complex like whistles and handclaps (Gattass et al., 1978; Magari˜nos-Ascone et al., 1988; Mathers and Rapisardi, 1973; Yirmiya and Hocherman, 1987). Last, PM neurons are also active during somatosensory perception (Acu˜na et al., 1986; Gattass et al., 1978; Perryman et al., 1980) and display olfactory responses (Gattass et al., 1978). From the motor perspective, PM is responsive to both hand and eye movements (Acu˜na et al., 1986; Gattass et al., 1978; Perryman et al., 1980). These highly multisensory functional properties of the pulvinar are summarized in Fig. 3.
Touch, proprioception and pain correspond to very distinct percep-tual modalities. While touch is dominated by exteroception and the monitoring of the interaction of the outside world with the body’s margin (Cl´ery and Ben Hamed, 2018), proprioception and pain are more interoceptive senses respectively processing postural body-related in-formation and emotion-based body-related experience. The contribution of the pulvinar to these two latter modalities, namely proprioception and pain, is still hardly explored. We have thus decided to discuss these two sensory modalities independently of touch. Likewise, there are for
Fig. 3. The pulvinar is highly multisensory.Reported sensory modalities to
which the PI, PL and PM pulvinar subdivisions have been shown to respond to: visual (red), somatosensory (blue); auditory (green), olfactory (cyan).
now, no multisensory functional studies addressing the role of olfaction or gustation in primates. We have thus decided to also discuss this sensory modality independently from the other sensory modalities.
4.5. The case of proprioception and pain
The anterior part of the pulvinar (i.e. the anterior part of the dorsal medial and the lateral pulvinar, also referred to as the oral pulvinar in the older literature) has been shown to be connected with area 1, area 2 (Padberg et al., 2009), and the lateral parietal cortex (Burton, 1984) as well as area 5 (Yeterian and Pandya, 1985) and area 7b (Friedman and Murray, 1986; Pearson et al., 1978; Weber and Yin, 1984), all these regions being involved in somatosensory processing and proprioception (Delhaye et al., 2018). This is in agreement with the marked somato-sensory properties of this part of the pulvinar. It coincides with the already discussed evidence that medio-dorsal damage to the pulvinar leads to postural deficits, possibly of proprioceptive origin (Wilke et al., 2018) and that the dorsal pulvinar contributes to the maintenance of gaze during postural changes (Schneider et al., 2019).
Reinforcing the contribution of the pulvinar to somatosensation, pulvinar oscillatory power in the alpha band exerts an inhibitory in-fluence on the spike timing and firing rate in somatosensory, premotor and motor areas, as well as on the behavioural performance during visuo-tactile discrimination (Haegens et al., 2011). A similar modulatory and filtering role of the pulvinar is also observed during pain processing. For example, a PET-MRI study in humans shows that distraction during pain involves a specific functional network composed of the orbito-frontal, perigenual anterior cingulate cortex, the periaqueductal grey matter and the pulvinar. The interaction between these areas is observed only during pain stimulation in the presence of distraction but not during pain stimulation alone or (visual) distraction alone (Valet et al., 2004).
This role of the pulvinar in proprioception and posture is to be linked to its role in motor and postural control. Grieve et al. (2000) initially propose that the pulvinar plays a central role in the selection and intention to perform eye and limb movements in peripersonal space (Cl´ery and Ben Hamed, 2018). Accordingly, pulvinar inactivations disrupt the selection of movement plans (Wilke et al., 2010). Unfortu-nately, in spite of these compelling evidence, research on the contribu-tion of the pulvinar to propriocepcontribu-tion and postural control remains sparse.
Likewise, the role of the pulvinar in proprioception and posture is to be linked to its role in peripersonal space coding and motor planning in relation to the body (Grieve et al., 2000). Accordingly, in the marmoset, the dorsal pulvinar has recently been shown to co-activate during looming versus receding visual stimulation with multiple ventral (V4, FST, TE, TPO) and dorsal (MIP) visual pathway areas as well as with prefrontal areas (45b and 47) and subcortical structures (SC, putamen) (Cl´ery et al., 2020). Several of these areas are involved in the coding of near space (Cl´ery et al., 2018b) and visuo tactile impact prediction (Cl´ery et al., 2017) and are also responsive to touch (Cl´ery et al., 2017, 2015a). These areas are also expected to play a role in proprioception. As a result, the pulvinar is expected to share these same functionalities and be functionally related to this specific cortical network.
4.6. The case of olfaction and gustation
Generally speaking, there is not much evidence of anatomical con-nections between the pulvinar and gustative or olfactory areas. However
PM has reciprocal connections with the orbitofrontal cortex (Bay and Çavdar, 2013; Bos and Benevento, 1975; Romanski et al., 1997) which plays a role in the multisensory integration of visual, olfactory, gustatory and intra oral somatosensory stimuli (i.e. food, Kadohisa et al., 2005). Accordingly, olfactory related neuronal responses are reported in PL (Gattass et al., 1978) and a very recent study reveals a functional con-nectivity between the pulvinar and the amygdala during taste
perception (Roy, 2020). The contribution of this information to the over-arching functional role of the pulvinar remains to be explored.
Thus, multiple sensory modalities coexist in the pulvinar. However, multisensory processes classically imply both a multimodal sensory convergence and a multisensory integration. The following section probes whether such processes exist in the pulvinar. In spite of it spar-sity, the available reports confirm that this is the case, thus supporting the contribution of the pulvinar to multisensory processes.
5. Evidence for multisensory processing in the pulvinar
5.1. Multisensory convergence
The involvement of the pulvinar in multisensory processing has been hypothesised only quite recently (Tyll et al., 2011). Based on the anatomical and functional data presented above, both PL and PM are expected to be involved in multisensory processing and integration. Indeed, both these pulvinar subdivisions have direct connections with more than one primary sensory area, be it primary visual, auditory or somatosensory cortex, as well as direct connections with highly multi-sensory associative cortices (Asanuma et al., 1985; Leh et al., 2008). However, how these different pulvino-thalamic sensory territories organize one with respect to the other is actually unknown and very few studies have directly addressed this question. Cappe et al. (2009b) is one of the very few studies that directly tackles this question. Their work demonstrates the existence of overlapping pulvinar territories of pro-jection to the rostral and caudal auditory cortex on the one hand and to the posterior parietal somatosensory cortex on the other hand, in particular in the medial pulvinar. This is direct evidence for multisen-sory convergence.
5.2. Multisensory integration
However, multisensory processing involves, beyond multisensory convergence, i.e. the coexistence in a given area of input signals from multiple senses converging on the same neurons, an active multisensory integration process that enhances both behavioural performance and neuronal processing (for review, Cl´ery et al., 2015b). At the behavioural level, responses to multisensory stimuli are characterized by faster re-action times (Raab, 1962; Welch et al., 1986), enhanced detection rates (Grant and Seitz, 2000) as well as enhanced accuracies (Lehmann and Murray, 2005; Murray et al., 2005). At the neuronal level, multisensory processing is characterized by non-linear mechanisms whereby the response of a given neuron to a bimodal input significantly differs from the sum of its responses to each sensory input presented independently (Avillac et al., 2007).
Studies directly addressing multisensory integration in the pulvinar are scarce. Most of these studies have focussed on visuo-auditory inte-gration during speech processing. This is based on evidence of a contribution of the pulvinar to speech production and verbal memory (Hebb and Ojemann, 2013; Hugdahl and Wester, 1997). For example, the larger the left pulvinar in healthy subjects, the faster they are at understanding degraded speech and the lower are their speech percep-tion thresholds (Erb et al., 2012). Anterior superior pulvinar lesions lead to aphasia, i.e. a deficit in speech production (Van and Borke, 1969), while medial dorsal pulvinar lesions lead to anomia, i.e. a deficit in the recall of the names of everyday life objects (Ojemann et al., 1968). This is confirmed at the neuronal level, whereby medial pulvinar neurons are shown to respond to the detection of auditory statistical regularities (Barczak et al., 2018). During multisensory audio-visual speech match and mismatch tasks, a very early EEG response is observed following the presentation of audio-visual speech stimuli the source of which has been proposed to be in the pulvinar (Musacchia et al., 2006). This coincides with the description of an early cortical processing network involved in temporal audio-visual integration, composed of the insula, the posterior thalamus and including the pulvinar as well as the superior colliculus
(Bushara et al., 2001). More recent fMRI and dynamic causal modelling studies identify a specific contribution of the pulvinar to speech perception in blind people. During speech comprehension optimization, the pulvinar of blind people has an enhanced functional connectivity not only with the primary auditory cortex A1, but also with primary visual cortex V1 (Dietrich et al., 2015, 2013). This mechanism is absent in sighted subjects and suggests a recruitment of visual pathways for auditory processing following sight deprivation.
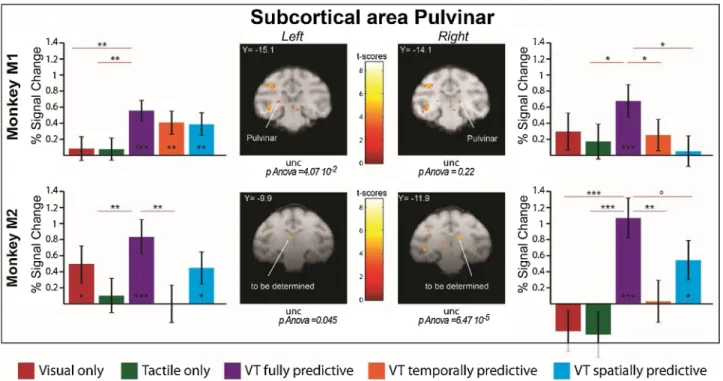
While these studies point towards a possible contribution to multi-sensory integration, direct functional evidence is missing. In a recent study (Cl´ery et al., 2017), macaque monkeys were subjected to visual stimuli looming towards the face, presented either alone, or in associ-ation with spatially or temporally congruent tactile stimuli to the face, while sitting in an MRI scanner for functional MRI acquisitions. The tactile stimuli could either predict the impact of a looming visual stimulus both spatially and temporally, only spatially or only tempo-rally. The fully predictive condition induced maximal pupil dilation, indicating integrative and anticipatory temporal prediction mecha-nisms. These physiological modifications coincided with robust activa-tions of a large functional network involving the peri-arcuate prefrontal cortex (PMv), the intraparietal cortex (VIP), the mid temporal cortex (FST), as well as the occipital visual cortex (Cl´ery et al., 2017). In in-dependent studies, this network has been described to be involved in visuo-tactile convergence (Guipponi et al., 2015) as well as in the pro-cessing visual near peripersonal space (Cl´ery et al., 2018b). Importantly, this network demonstrates both active integration of visual and tactile information, but also active integration of spatial and temporal predic-tion informapredic-tion (Cl´ery et al., 2017). Relevant to the present review, the pulvinar (PL and PM) is co-activated together with this cortical network (Fig. 4). Several criteria for multisensory integration based on fMRI activations are discussed in the literature. The mean criterium implies that the mean percentage of signal change (%SC) in either unisensory conditions is significantly different from the %SC in the multimodal condition. The max criterium implies that the %SC in the multimodal
condition is significantly higher than the %SC in the unisensory condi-tion generating the highest %SC. Last, the super-additive criterium im-plies that the %SC in the multimodal condition is significantly higher than the sum of the %SC in the two unisensory conditions. This is the most stringent condition. Four regions of interest (ROIs) can be identi-fied, based on the visuo-tactile predictive stimuli vs. fixation contrast (p < 0.001, uncorrected). In monkey M1, these ROIs are compatible with a pulvinar activation. In M2, the identified subcortical ROIs might be somewhat too anterior for a pulvinar activation. Further research is required to support these results. When the integration criteria are applied to the %SC observed in these ROIs, comparing the activations to pure visual and pure tactile stimulations to their association (i.e. reflecting multisensory integration, Fig. 4, comparing purple %SC values to red and green %SC values), the left ROI of monkey M1 fulfilled all of the multisensory integration criteria, the right ROI of monkey 1 fulfilled one of them, the left ROI of monkey 2 fulfilled one of them, and the right ROI of monkey 2 fulfilled the three of them. When the inte-gration criteria are applied to the %SC observed in these ROIs, comparing the activations to visual stimulations spatially, temporally or spatio-temporally predictive of the tactile stimulation (i.e. reflecting spatio-temporal predictive mechanisms, Fig. 4, comparing purple %SC values to orange and blue %SC values), the left ROI of monkey M1 didn’t fulfil any of the multisensory integration criteria, the right ROI of monkey 1 fulfilled two of them, the left ROI of monkey 2 fulfilled one of them, and the right ROI of monkey 2 fulfilled two of them. This is a strong indicator that the observed activations reflect active integrative processes rather than mere convergence of information. Quite surpris-ingly, to our knowledge, very few electrophysiological recordings in the pulvinar have directly addressed this issue. A very recent study by Cappe et al. (2020) demonstrates audio-visual multisensory integration in the local field potential of the medial pulvinar.
Thus, although sparse, converging evidence suggest that the pulvinar implements multisensory convergence and integration. This raises the question of whether and how these processes contribute to the cognitive
Fig. 4. Temporal and spatial prediction enhances thalamic pulvinar activations in both monkeys. Histograms represent the percent signal change for Visual
(Red), Tactile (Green), VT fully predictive (Purple), VT temporally predictive (Orange) and VT spatially predictive (Blue) conditions for monkey 1 and 2, for selected ROIs in the pulvinar. The contrast used to extract percent signal change is the VT predictive versus fixation contrast (P < 0.001, uncorrected level (unc). For each ROI, block effect is assessed by a repeated measure one-way ANOVA; significance of PSCs difference with respect to baseline and amongst themselves is assessed using paired t-tests, *P < 0.05; **P < 0.01; ***P < 0.001, ◦0.05 < P < 0.07). The M1 ROIs are located in the pulvinar. The M2 ROIs are somewhat too anterior for a pulvinar
role of the pulvinar, that has predominantly been investigated from the perspective of vision. Indeed, very much like is described in the asso-ciative cortical areas to which it projects, pulvinar sensory responses are often associated with complex cognitive processes. In a recent meta- analysis of human functional neuroimaging studies by Barron et al. (2015), the authors show that all of pulvinar subdivisions are involved in the processing of perception, attention, emotions, actions and other higher cognitive functions. This has been thoroughly and repeatedly reviewed elsewhere. One of the functions that has been associated with the pulvinar is its role in speeded responses to high saliency external events as well as in emotional regulation. This is discussed next.
6. The pulvinar in sensory detection and selection
6.1. Ultra-fast emotional processing and the innate alarm system
A first emotion processing pathway involving the superior colliculus, the dorsolateral pulvinar and the lateral amygdala has also been demonstrated with probabilistic tractography in both humans and ma-caques (Rafal et al., 2015). These results have recently been supported by a tracer study that shows overlapping SC projections and lateral amygdala projections to the pulvinar. These projections are localized in PM as well as in PI (Elorette et al., 2018). Visual cortex lesions result in an increase in the strength of the fiber tracts between PL and the amygdala ipsilaterally to the V1 lesion, at the same time that the pulvino-collicular fibers are decreased (Tamietto et al., 2012). The au-thors suggest the existence of two different emotion processing path-ways, one involved in conscious emotion perception (including the pulvinar, the amygdala and the orbitofrontal cortex) and one involved in unconscious (including the superior colliculus, the pulvinar and the amydgala, see Elorette et al., 2018) emotion perception (Tamietto et al., 2012; Soares et al., 2017). This hypothesis is supported by an MRI study presenting perceived and non-perceived fear-conditioned faces. When the faces are processed but not perceived, an increased connectivity is observed between the right amygdala, the pulvinar and the superior colliculus while the connectivity between the amygdala, the fusiform and the orbitofrontal cortices decreases (Morris et al., 1999). It is pro-posed that this unconscious emotional pathway plays a crucial role in survival in complex social groups, contributing to a very rapid conscious or unconscious perception of emotion and as fast behavioural reactions as possible.
Based on a recent human fMRI study, it is proposed that during the processing of threatening visual stimuli, information directly reaches the superior colliculus, without going through the visual cortex (Liddell et al., 2005), and is then rapidly transmitted to the amygdala and the locus coeruleus. This results in a noradrenergic cortical neuro-modulation, notably in the anterior cingulate cortex (ACC) and in fronto-parieto-temporal attentional network. This innate alarm system (IAS) is proposed to allow for ultra-fast behavioural responses to conscious or unconscious threatening stimuli (Lanius et al., 2017). This IAS is proposed to interact with both the orienting attentional network (via the inferior parietal cortex, the frontal eye fields, the superior col-liculus, and the pulvinar, and acetylcholine as neuromodulator) and the
alerting network (via the locus coeruleus and NA as neuromodulator) for
slower sensory processing (Petersen and Posner, 2012; Posner et al., 1997).
6.2. Attention, sensory selection, distractor filtering and perception
The pulvinar also contributes to conscious sensory processing. The visual response of pulvinar neurons reflects perceptual awareness (Wilke et al., 2009) and confidence during visual categorization (Komura et al., 2013) rather than overt behavioural report. The pulvinar functions are proposed to be implemented in interaction with early visual extrastriate processing in area V4 (Wilke et al., 2006). In addition to its contribution to conscious perception, the pulvinar is also associated with attention
and visual selection (Saalmann and Kastner, 2009). Lesions to the pul-vinar in humans result in an impairment in spatial and temporal atten-tion (Michael et al., 2001; Ward et al., 2007; Rafal and Posner, 1987; Zihl and von Cramon, 1979; Arend et al., 2008). A slowed down visual search and abnormal prolonged fixations are also observed (Ungerleider and Christensen, 1979), as well in most extreme cases a hemineglect syndrome whereby patients do not have awareness of contralesional visual stimuli (Karnath et al., 2002). Supporting these lesion observa-tions, spatial attention enhances visual responses in the pulvinar (Zhou et al., 2016) and the enhanced response of the pulvinar to behaviourally relevant stimuli precedes that observed in either the parietal and the temporal cortices (Benevento and Port, 1995). This modulation of visual responses by attention is stronger in the presence of distractors, sug-gesting a contribution of the pulvinar to sensory filtering, i.e. to the selection of input stimuli of major salience or behavioural relevance, at the expanse of non-relevant stimuli (Fischer and Whitney, 2012; Rob-inson and Petersen, 1992). This applies to the visual responses of all of
PI, PLvl and Pldm. Supporting this point, the reversible inaction of
ventral PL during spatial attention orientation for object recognition in the presence of distractors, result in a strong decrease in correct trials to visual items presented in the contralateral field (Zhou et al., 2016). This behavioral effect is less pronounced in the absence of distractors. Importantly, PL inactivation effects coincide with a reduction of sensory evoked responses in extrastriate visual area V4. Last, the reversible inactivation of the pulvinar results in depressed visual responses in V1 while a stimulation of the pulvinar results in enhanced responses in V1 at the corresponding visual receptive field sites, together with a sup-pression of the activity of neurons representing other spatial locations (Purushothaman et al., 2012). The contribution of the pulvinar to sen-sory selection and filtering is further supported by the clinical obser-vation that ventral pulvinar lesions lead to contralesional deficits in response competition (Danziger et al., 2004) and in the discrimination of target features, specifically in the presence of salient distracters (Snow et al., 2009). Importantly, while both the ventral and the dorsal pulvinar are associated with distractors filtering, they are not activated by task-switching (Strumpf et al., 2013).
7. The pulvinar as a processing regulator
7.1. Emotional regulation
From an anatomic perspective, medial pulvinar PM is densely con-nected with the limbic system. Specifically, PM has reciprocal connec-tions with the posterior limbic neocortex, the posterior cingulate gyrus (Baleydier and Mauguiere, 1985) as well as the paralimbic and para-hippocampal regions (Baleydier and Mauguiere, 1985; Yeterian and Pandya, 1997). The medial part of PM is reciprocally connected with the rostral part of the superior temporal gyrus, the cingulate cortex, the posterior insula as well as the lateral amygdala (Mufson and Mesulam, 1984). The lateral part of PM is reciprocally connected with the insula, the posterior cingulate cortex and the superior temporal cortex (Jones and Burton, 1976; Romanski et al., 1997). Last, orbital frontal, medial prefrontal and temporal polar proiscortices project to both dorsal and ventral PM whereas the anterior cingulate proisocortex projects only to dorsal PM (Yeterian and Pandya, 1988). From a functional perspective, cortical evoked responses have been found in the temporal, temporo-parietal junction, the insula, the frontal parietal opercular cortex and in mesial temporal regions following PM stimulations in humans, and vice versa (Rosenberg et al., 2009). All this taken together indicates a strong functional link between the medial pulvinar and the limbic system.
Confirming this, during an emotional face processing fMRI task, it has been determined that patient suffering of a general social anxiety disorder (gSAD) have stronger functional interactions within the frontal emotion regulation regions, i.e. between the pulvinar and the middle occipital gyrus, the orbitofrontal cortex and the superior frontal gyrus
(Tadayonnejad et al., 2016). Independently, the amygdala and PM are proposed to coordinate cortical networks to evaluate the biological significance of affective visual stimuli (Pessoa and Adolphs, 2010), amplifying the responses of cortico-pulvino-cortical circuits to weak emotional visual stimulus (Padmala et al., 2010).
Overall, the pulvinar is thus proposed to act as a modulator of the limbic system. As a result, the pulvinar is proposed to play a key role in emotional regulation.
7.2. Attentional regulation
Several recent studies demonstrate that spatial attention orientation correlates with enhanced functional LFP coupling between the pulvinar and the cortex. PM displays an enhanced functional coupling with both the dorsal visual pathways (LIP, Fiebelkorn et al., 2019), and the pre-frontal cortex (Fiebelkorn et al., 2019). Specifically, Fiebelkorn et al. (2019) show a significant coupling between PM spikes and the phase of both FEF (8–19 Hz) and LIP (15–20 Hz) alpha/low-beta activity at recording sites with coincident visual response fields. Importantly, they show that this synchronization is unidirectional, the spikes of the pul-vinar impacting the phase of the LFP in both the FEF and LIP (note that LIP spikes also impacted the pulvinar LFPs, but did so independently from the effects of the pulvinar onto LIP). Likewise, PL (mostly PLvl) displays a marked enhanced coupling with both the ventral (V4, TEO, Saalmann et al., 2018, 2012) and the dorsal visual pathways (LIP, Saalmann et al., 2018). Specifically, both Saalmann et al. (2012) and Zhou et al. (2016) show, using Granger causality statistics (a method that determines whether one time series is useful to predict another one, without however inferring causation or causality, in contrast with inactivation studies), that the pulvinar synchronizes neuronal activity between interconnected visual areas, in the alpha/low beta range (10− 20 Hz) during attention orientation. Zhou et al. (2016) additionally show that attention increases the influence of extrastriate visual cortex (V4) on pulvinar while decreasing the reverse influence of the pulvinar onto V4 in higher gamma band frequencies. It is thus proposed that PL regulates the transmission of information across cortical areas by syn-chronizing cortical activity at alpha frequencies (Kastner et al., 2020). The reversible inactivation of PL increases alpha frequency power con-tent in the visual cortical areas (Zhou et al., 2016). More alpha is often associated with inattention (Bollimunta et al., 2011; Schmid et al., 2012). Accordingly, this increased visual cortex alpha is concomitant with decreased attentional performance and decreased neuronal re-sponses in V4. These lesion observations (more visual cortical alpha and less attention-related spikes) contrast with control observations (pulvi-nar alpha power causally synchronizing attention-related spiking ac-tivity in visual cortical areas). However, if one considers the complex recurrent cortico-pulvino-cortical connectivity (Lakatos et al., 2016), this apparent contradiction actually suggests that the pulvinar might play a crucial role in organizing cortical intra- and inter-areal activity. Confirming this hypothesis, a recent computational modeling study (Jaramillo et al., 2019) shows that a pulvino-cortical circuit model, composed of the pulvinar and two cortical areas, reproduces the exact control and lesion behavioral and visual cortex neurophysiological sig-natures described above. In this model, effective connections between the two cortical areas are directly gated by the pulvinar. As a result, Jaramillo et al. (2019) suggest that the feedforward and feedback pulvino-cortical pathways modify the relative hierarchical positions of cortical areas by a direct control over the frequency-dependent inter--areal interactions (see also Halassa and Kastner, 2017). This putative role of the pulvinar is expected to generalize beyond the visual function. Supporting this regulatory role of the pulvinar in cognition beyond attentional regulation, functional correlations between the pulvinar and the ipsilateral superior frontal gyrus (SFG), the contralateral tempora-l–parietal junction (TPJ) and the visual cortex increase when the per-formance in a working memory task increase (Rotshtein et al., 2011). In addition, while noradrenergic alpha2 agonist (dexmedotomidine)
induces sedation and interferes with behavioral success in attentional detection task, the introduction of white noise (making the task more complex perceptually speaking) recovers behavioral performance and this effect coincides with an increased activity in the left pulvinar. This argues in favor of a contribution of the pulvinar, beyond the attentional function, to phasic arousal or alerting process and the noradrenergic neuromodulation system (Coull et al., 2004).
It is worth noting that sensory detection and selection, as well as emotional and attentional regulation involve a rapid adjustment of behavior to both the environment and the subject’s current covert goals and states. Such flexible behavior is crucial for optimal behavior. This includes the ability of rapidly shifting from a state of intense focus on the ongoing behavior (e.g. foraging) to a high alertness state (e.g. identi-fying a competitor or a predator), or the ability of shifting from peaceful social interactions to a state of high alertness to negative emotions. The pulvinar thus appears as a major modulator of behavioral and cognitive flexibility.
8. Generalizing the role of the pulvinar in sensory detection, selection and regulation
Converging evidence thus suggest a very strong contribution of the pulvinar to two independent general cognitive functions operating at different time scales, mostly based on studies performed in the visual modality: 1) the selection of sensory input based on its emotional valence, intrinsic salience as well as extrinsic behavioural relevance, a process requiring fast and flexible adjustments to the environment and 2) the regulation of cognitive states and arousal, a much slower process possibly fluctuating with a variety of internal state modulators ranging from circadian influences, to emotional and anxiety states, to energetic status etc. This suggests that this structure is part of multiple functional loops, transmitting bottom up and top down information to the many cortical sensory, and associative areas it is related too, as well as to other key subcortical structures such as the amygdala. We will argue that the same two independent general cognitive functions reported in the visual domain are at play for such sensory modalities as the auditory and so-matosensory modality, involving the same type of interactions between the pulvinar and the cortex, and relying on the same type of neuro-modulator system. That is to say that the pulvinar is proposed to contribute to sensory processing and notably to sensory detection and selection at a fast time scale, as well as in sensory regulation, at a much slower time scale, along all sensory modalities, including olfaction, proprioception and pain. Whether this is achieved through common or multiple functional networks and whether some of the sensory modal-ities such as pain and olfaction are processed through specific distinct functional networks remains to be explored. This exploration would entail a more systematic multisensory approach to the anatomical, functional and neuropsychological evaluation of the role of the pulvinar. Overall, the time seems ripe to move from a view of the pulvinar as a modulator of the visual system, to the view of the pulvinar as a general regulation hub for adaptive, flexible cognition and survival.
Fundings
M.F., C.C. and S.B.H. were funded by the French National Research Agency (ANR)ANR-16-CE37-0009-01 grant and the LABEX CORTEX funding (ANR-11-LABX-0042) from the Universit´e de Lyon, within the program Investissements d’Avenir (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
CRediT authorship contribution statement
Mathilda Froesel: Conceptualization, Writing - original draft,
Writing - review & editing. C´eline Cappe: Conceptualization, Writing - review & editing, Funding acquisition. Suliann Ben Hamed: Concep-tualization, Writing - original draft, Writing - review & editing, Funding