Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Research Paper (National Research Council of Canada. Division of Building
Research); no. DBR-RP-463, 1970-12-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=18086360-9e63-481a-a4be-19bb4d493633 https://publications-cnrc.canada.ca/fra/voir/objet/?id=18086360-9e63-481a-a4be-19bb4d493633
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001740
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Thermal decomposition products of cellulose
Tsuchiya, Y.; Sumi, K.
JOURUAI, Oli' APPLII<D POLYMER SCII~:\CI'~ \ 01,. 1 I , PP. 2003-2013 (1970)
: ~ N & I ~ z ; B
Thermal Decomposition Products of Cellulose
YOSHIO TSUCHITA and I<II<UO SUhII, IJiuisio~l oj' Builcli~lg Reseal.ch,hTntio7ial Rescarch I'ouncil of Ca7iarla, Oiialon 7 , Ontario, Canarla
Synopsis
Untrentecl and fla~ne-re1:irtlant-t~.ented cellr~lose were thermally decomposed under vacuum and the product's were qun~rtitntively annlyzed by gas cl~romat~ography. An
unide~it~ified product a t a retcntio~l index of 2270 (bet>ween 5-metl~ylfurfural and 5-hy- drosymethylfurf~ualj, 0- niid p-11-glucose, a ~ i d a group of "dimers" that, have not been
reportecl previously were found in this study. The ide1l1,ificalion of this uiikriown prod- uct should assist ill studies on the thermal decomposil,ion of cellulose and further the un- derstanding of the mechnnism of fl:~me retardal~cy.
INTRODUCTION
Studies on the thermal decomposition of cellulose have attracted a great deal of attention over the years because this polymer is the ~ n a i n constit- uent of ~vool arid cotton. Efforts have been made to elucidate the mechanism of thermal clecomposition of cellulose for possible applications to practical problems such as t h a t of firiclirig rien- flame-retardant treat- ments. The authors are engagc.d in studies on the chemical a s ~ ~ c t s of fire and are interested in the n~echanisn~s by which flame retardants alter the thermal dccon~l~osition of polymers. I11 the present i~irrestigation or1 flame retardancjr, ccllulose treated with various inorganic salts was examined.
I<no\vleclge of the pyrolysis proclucts is necessary for understanding the mechanism of thermal decon~posit,ion of untreated and flame-retardant- treated cellulose. Recent advances that have been made on the deter- mination of these products of cellulose have been largely due to clevelop- ments in gas chroinatogral,hy. Sch~verilier arid Beclir pyrolyzed cotton by heating it to 370°C in an atmosphere of air 01. nitrogen, and identified about
30 products from methane to 3-hydroxymetl1ylf~1rf~1ral. I<ato and
I<omorita2 used pyrolysis gas chromatograpl~p to study the eft'ect of crystal- linity of cellulose on the formation of volatile compounds. They pyrolyzed microcrgstalline and tobacco celluloses at temperatures ranging from 200" t o .iOO°C in a stream of heliuln n ~ i d cluantitatively cletern~inecl the yields of acetaldehj~de, furfural, and 5 - l ~ y c l r o x y m e t l ~ y l f ~ ~ r f ~ ~ r a l . Byrne, Gardiner, and Holmes3 pyroljrzed pure and flame-retardant-treated cotton under vacuunl and analyzed the major products by estendiilg the analysis to larger conlpou~lds. They determined the amount of levoglucosan in the
2003
tar fraction : ~ t tcmpcratun>s ranging f r o n ~ 350" to .500°C' and discussed the mechanisms of pyrolytic reactions.
Experimental data on pyrolysis products of cellulose treated with flame retardants are very limited. Parlts et al.4 studied the effect of about 50 different treatments on the amounts of char, carbon monoxide, and carbon dioxide produced. Berltowit7;-JIattucl and Koguchi" decomposed APO- THPC-treated cotton and identified light gases, water vapor, and organic coml~ounds each containing three to six carbon atoms. They fol~tlcl no fundamental qualitative difference in the prrolysis mechanism for the treated and untreated cloth. Golova e t nLG decomposed cellulose treated with 17 different inorganic salts and determined the yield of levoglucosan.
Byrne et decomposed cctton treated with borax-boric acid and some
commercially available flame retardants and determined the yield of con- tlensates, tar, char, and levoglucosan.
In t,his study cellulose was pyrolyzed under vacuum at various tem- peratures ranging from 320" to 520°C and the products from light gases to the components of tar fraction were analj~zecl quantitatively. 'I'he dc- composition products of cellulose treated with various inorgn~lic com- pounds were also analyzed.
EXPERIMENTAL Materials
Whatman No. 40 filter paper nras used as the cellulose sample in this study. The ash content was 0.018%. The cellulose was treated with vari- ous compounds by dipping it into aqueous solutions of sclectecl concentra- t,ions so t h a t the desired weight of salt \vould be retained on the sample. The samples were wiped free of excess sollition prior to drying under vacuum. Specimens used in the pyrolysis expcrirnent,~ retained about
ly0
by weight of the salts when dried.Thermal Decomposition
The pyrolysis apparatus was similar to t h a t used in n previous A sample weighing about 1 . G g was placed in a I'yrex tube connected to a
liquid nitrogen trap and a vacuum pump. After the system was cvacu-
atcd to 1 X mm Hg, the vacuum line to the pump nins closed and thc
sample was heated a t one of the preselected temperatures, X20, 370, 420,
470, or 520°C, for 20 min. The Pyrex tube was positioned at about 5
degrees from the horizontal in order to allow some of thc. tar fr:~ction lo flow out of the hot zone and thus to reduce secondary decomposition. The volatile fraction in the liquid nitrogen trap was transferred to a gas- sampling bottle of known volume by rcnloving the liquid nitrogen, and the weight of thc remaining liquicl fraction in the trap \\'as determined. The residue and the t a r in the pyrolysis tube nrerc rirashed n ~ i t h water, and the solid was separated from the water cstrnct by filtering. Roth port,ions \\.ere dried and weighed.
2
2"
5 .-
4- J -n C)<
2-
I^2
k-
ce-
% W >2-
-
3-
+ C) M e , _^-
-
-
'.
.-
5
1. 2 2 2 z %-
-
I2 . s
2 %-
s - i 2 C) ?. 5 w " 2 :.$&
F.6
z Z i ? -c z - g mg,
4 2 .--
.-
.- J) -0 L " 502
2 -0TSUCI-IIYA AND SUM1
Analysis
3Iost compo~ients in the gaseous and liquid fractions were determined by the usual methods of gas chromatography. The liquid fraction from the trap was arialyzed further for carbohydrates by dehydration with anhydrous sodium sulfate followed by trimethylsilylatio~l.~ The water extract was also analyzed for carbohyclrates. The gas-chromatographic corlditions t h a t were usecl are presented in Table I.
Most of the products were identified by comparing the retention times with those of linolrrn coml)ou~ids using three or more different chromato- graphic conditions. They were cluantitatively analyzed by applying approximate substance correction factors to the areas under each peal<.9 For the trimethylsilylated products to which linown amounts of glycerin had been added as an internal standard, the areas under the peaks were compared to those of glycerin.
RESULTS AND DISCUSSION
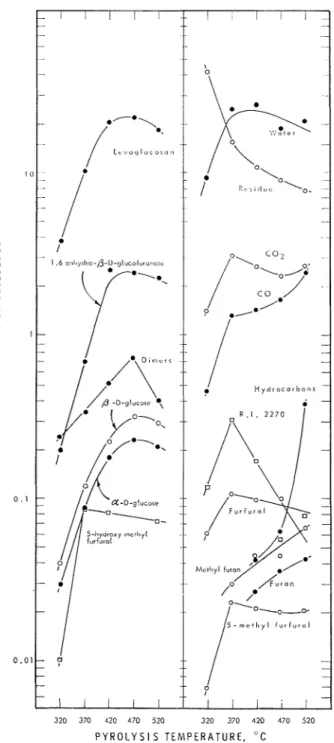
The thermal decomposition products of cellulose a t a pyrolysis tem- perature of 370°C are presented in Figure 1, where the quantitative data are represented by line charts and are plotted against retention index using ?I-alkanes as standards. The eqxrimcntal data on niatcr, carbon mo~loxidc, a i d carbon dioxide are not sho~vn.
Products c o ~ l t a i ~ l i ~ l g -OH groul)s were trimethylsilylatcd and an:~lyzed. Corresponding with the results of other investigations, levoglucosa~i was found to be by far the most abundant organic product. Other major
~)roducts included 5-11ydrosymethylfurf~ira1, 1,6-anh~idro-p-~-glucofurk~-
nose, a- :ind /3-D-glucose, ant1 an unidentified product a t a reterition index of 2270. The prcsericc of a-u-glucose is due to m u t a r o t a t i o ~ i ~ ~ ; a//3 equilibrium ~voulcl be reached in less than 10 mi11 a t pyrolysis tcmpcra-
turcs used in this study. Lcvoglucosan tind 1,6-a1ihydro-/3-i~-gluco-
furanose and the two glucoscs foulid in the products will be referred to : ~ s "monomers" i11 this pal)er. Five pcal;s were found in the 2700 region of the retelltion i~lclex scale. These peaks were l~robnbly due to either disaccharides or "dimersJJ of anhydroglucose. The :~uthors believe t h a t they were due to the latter, because the disacchs~ridcs 11:~ving pyranose rings that were forn~ed during the pyrolysis of glucose had retenti011 indices of about 2800 u~lder the same gas-chromatographic co~iclitions.
The dimers and a- and /3-1)-glucose and the unidentified cori1l)ound a t :L retention index of 2270 have not been reported previously in the literature. The qua~ititativc data on the thermal decomposition products of cellulose : ~ t various pyrolysis tcmpcraturc.~ arc prcscntcd in Table I1 a ~ l d the s:ime data are plotted in Figure 2. At 320°C, :L 1:wgc portion of the cellulose sninple rem:iined undecoml~osed, as is shoivn by the large i~mount of residue. The smaller fragments such :is the hytlrocarbons (of whicli methane and ethane were the main components) :l~lcl furan and 2-~nethyl- furan increased with increase in pyrolysis temperature. The high yield
CELLULOSE DECOMPOSITION PRODUCTS a "( " A " - T c C. 1
::
X-
U C u m , . "7 E - : : C. A'I'SUCIIIYA AND SUhll TABLE I1
Thermal Decomposition Products of Cellulosel~ Yield, wt-5, -- - --- Products 320°C 370°C 420°C -17OoC 320°C: 1,6-A11hydro-p-~-glucofura1loue" a-D-Glucose" p-~-GlucoseI' Dimers of anhydroglucosel~ Urlarialyzed tar" C:lrbori monoxide Carbon dioxide Water Char Total
Figures in c o l u m ~ ~ s :we weig111 l)crcer~lages of sample at, i~~clicatecl pyrolysis Le111per:~- t.ures.
"Tar t'ritctio~~.
of 1~ytlroc:wbons a t 520°C; suggzsts Illat greater secoiidury decoiiq)os~tio~l occurrctl a t this high t e i n l ) c r a t ~ ~ ~ * c .
'I'll(, yi~lcl of f u r f u r ~ ~ l \\.:IS O I L I ~ about, 0.1% and that of acetuldehydc \\-:is
~~cgligible in these exl)erimcnts. Iiato :111d Iiomorita2 found 1:wger :uilour~ts of' Ihc two products: 0.2% to 4.4y0 furfural and 0.01% to 4.7% acet:~ldeliyde. 7'he higher pr~ssurc~s t h a t were used in their pyrolysis gas chromatography studies prob:~bly :~ccount for the greater fragmenta- tion. The amounts of levoglucosai~ found in the present study wcrcb sm:lller than those found by Ryrtie arid co-\~orliers.~ I n their study thc rnaximunl yield of levoglucosan (44%) occurred 117hen cellulose w:ls 1,yro- lyzed a t 456°C lor G mio. I n the present study the larger sumplc size and the longer duration of pyrolysis probably accounts for the smallcr masinlunl yield (22y0).
The clunntitutive data oil tlie 1)yrolysis ~)roducts of cellulose treated ~ v i l h inorganic coiiipoaiuls :lrc pr(~sei~t(v1 111 T:~bl(s 111. 'rhe prescbnce of illorganic c.onll,ou~ids tl(xcreased the ~rield of' tar fructiou slid incre:~sed I liilt of char; tliib result correspouds with the firidirlgs of previoub wor1iei.s. The yield of organic pyrolysis products uTas lower, but that of carboll monoxide aiid dioxide and water vapor was higher. I n general, char formation iricrc~:~sc~cl with an increase in t h r effectiveness of flame retardants. Tllc decreasrx ill ihe yield of 1evoglucos:ui :uitl other 01-garlic pyrolysis
CELlJULOSE: DECOMPOSITION PHODUC'I'S
320 370 420 070 520 320 370 020 070 520
P Y R O L Y S I S T E M P E R A T U R E , " C
CEI,l,ULOSE DECOMPOSITION PRODUC'I'S
C e l l u l o s e + K 2 C 0 3
0 . 1
R E T E N T I O N I N D E X
Fig. 3. EEect of acid and alkali 011 the pyrolysis products.
though the inorganic salt is an ineffective flame retardant. The increase in carbon monoside and carbon dioxide was most pronounced ~ v i t h alkali salts.
Lipska ancl Wodleyll reported t h a t 2% treatment of I<HC03 had little efl'ect on the quality of the degradation products of cellulose having rnolecu- lar weights below about 110, although it does change their relative con- centrations. This finding is in agreement with the results obtained with all the treatments examined in the present study. Products having molecular weights greater than 110, however, differed significaritly be- tween treated arid untreated cellulose in both their quality and quantity. Typical examples showing the effect of acid and allcali on the pyrolysis products of cellulose in the retention index range of 1400 t o 2300 are shown in Figure 3. The presence of acid increased the yield of the unidentified product with the retention index of 2270 tenfold as compared to untreated cellulose, with the result that it became the most abundant organic pyroly- sis product. The presence of allrali decreased the yield of this unknown
product and formed some new products. I~iorganic coml)ounds used in
treating cellulose can be classified as acid type or allrali type from the pattern of the chromatograms. On this basis, pure cellulose follo~vs ucid- type decoml)osition.
2012 'IXIICI ILYA AND SITMI
Tlie presence of glucose in tlic pi~oducts is ilitercsting. Several investi- gators, e.g., Irvine arid O l d h a m l l ~ ~ a v e suggested Ihat glucose might be
:ill intermediate product in the formatioil of levoglucosa~i from cellulose.
This ~~ossibility was rejected by Golova et, d.L%ecause the pyrolysis of cellulose yielded 37YG levoglucosan n hile that of glucose yielded only 6YG under the same conditions. Tlie possibility that glucose might be an intermediate decomposition product of cellulose, providirig one of the routes for the formation of vo1:utile products, has, however, not been discounted.
The water extract from the tar fraction c o n t a i ~ ~ e d most of the nloliomc,rs of cellulosc. I t also coiitairiecl dimers, suggesting thnt a series of trimcrs, tctr:imers, etc., were thc main coilstituents of the tar fractio~i.
CONCLUSIONS
T l ~ e thernlal decomposition ~ ~ r o d u c t s of cellulose under vucuu~n a t temperatures between 320" and 320°C were cluantitatively analyzed by gas chromatography. An unideritified product a t a retention index of 2270
(between 5-methylfurfural :~nd 3-hydroxymethylfurfural), a- and
0-1)-
glucose and a group of dimers that were found in this study have not been reported by previous i~ivestigators. The main decompositioii prod- ucts were large organic fr:igint:~lts such :rs tllc moliolncrs (levoglucosan i ~ n d
1,G-anhydro-0-1)-glucof~~r;~iiose), cli~ners, a11 u~iideiitified product a t a re- te~itiori index of' 2270, \ \ : ~ t r r , carboll n~onoxidc, and carboll dioxide. -\lany carbo~iyl compounds, organic acids, and hgdrocarboris reported by other investigators were found in smaller amounts. Tlle authors believe thnt these minor proclucts arc. 1argc.l~ tlutb to sr~conclnry decom- position.
The uiiidentified peak a t a retention index of 2270 is probably a primary product because its yield was greatest at the lo\ver pyrolysis temperatures. The idelitificstioli of this product should assist in studies on the mechanism of thermal decoinpositioli of cellulose. Also, it should further the under- standing of the nlechanisnl of fl:une retardancy. Tllc acidity (or alka- linity) of treatment, results i l l sig::nifc:~nt cll:u1igc.s ill tlic yield of this uni-
d e n t i h d product.
Thio paper is a cont~ibutiol~ f ~ o n ~ the Division of Building liesearch, Natiollal liesearch Council of CJnlrnda, and is pl~hl~shed with the a1 p~oval of the Ili~ecto~ of the I ) i v i ~ i o ~ ~ .
The u~dhors wish to thank Mr. J. Boulallger for ass~star~ce i u the couduct of experi- ments.
References
1. It. I?. Schwenker, Jr. and L. li. Beck, Jr., J . Polym. Sci. C, No. 2, 331 (1963).
2. K. Ihlo and H. I<omorita, d g r . Biol. Chern., 32,21 (1968).
3 . G. A . Byrl~e, 1). C;;t~diner, and F. EI. Ilolrnes, J . d p p l . Chenz., 16, 81 (1966). 4. W. G. P:~rk<, ,I. (;. IClhi~r(lt, .Jr., : ~ n d TI. R . IZoberts, r l n ~ ~ r . D ~ e s l l ~ . f f Rcplr., 204
3 . J. B. Berkowitz-hIattuck and .'l' Noguchi, J . Appl. Pol!/,n. Src., 7,700 (1963).
6. 0. P. Golova, Yn. V. E p ~ t e i n , and 12. I . Dobrynina, ~ijaokoncoi. Snc,tlin., 3, Xjti
(1961).
7. Y. Tsuchiya nnd I<. Sumi, J. Polym. Sci. A-I, 7,813 (1961)).
8. C. C. Sweeley e t al., J . .lmcr. Chcnz. Sac., 85,2497 (1963).
9. 1i. Kaiser, Ch~.omrrtop.crphic in rlcr Gizsphizsc ZZZ, Bihliograph~ichei Tnst., l l a n n -
he in^, 1962, p. 136.
10. A. Rroido, Y. IIollrnl~ier, and S. Palai, J . Chct,~. Sac., B 1966, 41 1 (19001. 11. A. E. Lipska and 17'. A. Wodlejr, J. -1ppI. P o ( I / ~ ~ L . SCI., 13, 851 (1969). 12. .J. C. Irvine and J . \Tr. 11. Oldham, J. Cifot~. Soc., 119, 1744 (1!)41 )
14. 0. 1'. Golovn, E. A. Anclrievskaya, A. hI. Pnklromor, :ltld X. l r . lIel.l~s, lj1111.
. I d . Sc. GSSR, Div. CIICI~L. Sci., 1957, 399 (1057).