HAL Id: hal-03255870
https://hal.archives-ouvertes.fr/hal-03255870
Submitted on 9 Jun 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
lithosphere
Veronique Le Roux, Benjamin Urann, Daniele Brunelli, Enrico Bonatti, Anna
Cipriani, Sylvie Demouchy, Brian Monteleone
To cite this version:
Veronique Le Roux, Benjamin Urann, Daniele Brunelli, Enrico Bonatti, Anna Cipriani, et al..
Post-melting hydrogen enrichment in the oceanic lithosphere. Sci.Adv., 2021. �hal-03255870�
G E O C H E M I S T R Y
Postmelting hydrogen enrichment in the
oceanic lithosphere
Veronique Le Roux1*, Benjamin M. Urann1,2, Daniele Brunelli3,4, Enrico Bonatti4,5,
Anna Cipriani3,5, Sylvie Demouchy6, Brian D. Monteleone1
The large range of H2O contents recorded in minerals from exhumed mantle rocks has been challenging to
inter-pret, as it often records a combination of melting, metasomatism, and diffusional processes in spatially isolated samples. Here, we determine the temporal variations of H2O contents in pyroxenes from a 24-Ma time series of
abyssal peridotites exposed along the Vema fracture zone (Atlantic Ocean). The H2O contents of pyroxenes
cor-relate with both crustal ages and pyroxene chemistry and increase toward younger and more refractory peridotites. These variations are inconsistent with residual values after melting and opposite to trends often observed in mantle xenoliths. Postmelting hydrogen enrichment occurred by ionic diffusion during cryptic metasomatism of peridotite residues by low-degree, volatile-rich melts and was particularly effective in the most depleted peridotites. The presence of hydrous melts under ridges leads to widespread hydrogen incorporation in the oceanic lithosphere, likely lowering mantle viscosity compared to dry models.
INTRODUCTION
The incorporation of crystallographically bonded hydrogen (also referred to as H2O or water) as atomic impurity in mantle minerals
plays a key role in mantle dynamics (1–4). However, the mechanisms that control H2O distribution in upper mantle rocks remain poorly
understood. Most of the current constraints on H2O distribution in
exhumed mantle rocks come from xenoliths. These rocks typically sample a cold, variably oxidized, fluid-rich lithospheric mantle (5) and may not be representative of asthenospheric conditions. Previous studies have shown that pyroxenes hold most of the H2O budget of
nominally anhydrous peridotites, but significant variations are ob-served (6–8). For example, xenolithic orthopyroxene (opx) contain from <10 to 400 g g−1 H
2O, while H2O in xenolithic clinopyroxene
(cpx) range from <10 to 900 g g−1 H
2O (6, 7). Deciphering the
processes that control these variations remains a challenge because well-defined correlations between H2O and major or trace elements
are rarely found (9, 10). In some xenoliths, H2O in pyroxene
cor-relates with major elements (e.g., Al), as experimentally predicted (11), but abundances are often too high to represent a partial melting residue, complicating the reconstruction of mantle H2O budgets (6).
Abyssal peridotites are mantle residues left after mid-ocean ridge basalt extraction from the asthenospheric mantle (12), now emplaced in the lithosphere and uplifted tectonically to the seafloor. Their constituent minerals (olivine, opx, and cpx) have only been analyzed for H2O and other volatiles in a limited number of studies (13–16),
likely because their significant alteration represents a technical chal-lenge. Earlier studies on abyssal peridotites have reported variations in the H2O contents of pyroxenes, interpreted to reflect residual
H2O contents of the oceanic upper mantle (15) and/or H2O addition
after partial melting (14, 15). It was also argued that the H2O content
of pyroxene minerals from the Southwest Indian Ridge and Hess Deep, East Pacific Rise, may have been modified after initial melt extraction (13, 17). Although these studies provide information on H2O variability in abyssal peridotites, most of the samples are spatially
isolated and sometimes lack a broader tectonic context. Therefore, this study focuses on a time series of genetically related abyssal peridotites to untangle the processes that control H2O distribution
over time and determine how they compare to xenoliths.
In particular, we use secondary ion mass spectrometry (SIMS) to measure the in situ volatile contents (in particular H2O and F) of
opx and cpx in abyssal peridotites from the Vema fracture zone (11°N; Equatorial Atlantic). The Vema lithospheric section (VLS) exposes a >300-km-long, ~1-km-thick segment of lithospheric mantle overlain by oceanic crust made of gabbros, dykes, and basalts (18). The peridotite samples used in this study were collected every 10 to 20 km along the VLS and span crustal ages from 1.5 to 24 million years (Ma) (18, 19). Crustal ages were estimated from geomagnetic time scales (20) and U-Pb ages in crustal gabbros (21). The time at which peridotite was emplaced into the lithosphere is estimated using the crustal age and the spreading rate, considering the delay of emplacement between basalts and its parent peridotite (18, 19). The degree of melting in peridotites along the VLS increases toward younger crustal ages, from 8 to 14% on average (18, 19, 22). Lower degrees of melting recorded in the older peridotites (>15 Ma) may reflect undercooling of the peridotite mantle due to the presence of pyroxenite in the source (23). Crustal thickness increased over time from 4.8 ± 0.2 km to 5.4 ± 0.2 km (18, 19), concomitant with a de-crease in full spreading rates from ~35 to 27 mm year−1 (18). The
peridotite time series used in this study have porphyroclastic, protogranular, and/or occasionally mylonitic textures and were all equilibrated in the spinel stability field. Amphibole is sometimes present in peridotites along the VLS (24), but none of the samples investigated here contain amphibole. The samples are typically 70 to 80% altered, with variably large (2-mm- to >1-cm-diameter) relic pyroxene, and small (<500-m-diameter) relic cores of olivine in some of the samples (19, 22). This study combines volatile measure-ments with selected trace and major element analyses in pyroxenes to elucidate the processes that control H2O variations in peridotite 1Department of Geology and Geophysics, Woods Hole Oceanographic Institution,
Woods Hole, MA 02543, USA. 2MIT-WHOI Joint Program, Marine Geology and
Geo-physics, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA.
3Dipartimento di Scienze Chimiche e Geologiche, Università di Modena e Reggio
Emilia, Modena, Italy. 4Istituto di Scienze Marine, CNR, Bologna, Italy. 5Lamont
Doherty Earth Observatory, Columbia University, New York, NY 10027, USA. 6
Géo-sciences Montpellier, Université Montpellier & CNRS, Montpellier, France. *Corresponding author. Email: vleroux@whoi.edu
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC). on June 9, 2021 http://advances.sciencemag.org/ Downloaded from
from ridge settings. Last, we discuss the implications of hydrogen incorporation in residual abyssal peridotites for the viscosity of the oceanic lithosphere.
RESULTS
Volatile distribution in VLS pyroxenes
SIMS analyses were performed in 31 VLS cpx and 31 VLS opx (including 28 samples with both opx and cpx data), resulting in a total of 235 analyses after filtering for data quality (see the Supple-mentary Materials for details). Average H2O and fluorine contents
in opx vary from 81 to 362 g g−1 and 0.15 to 21 g g−1, respectively,
while average H2O and fluorine contents in cpx vary from 189 to
724 g g−1 and 0.25 to 51 g g−1, respectively (table S1). No systematic
hydrogen loss or gain from cores to rims is observed as a function of crustal ages, but cpx H2O abundances are slightly more scattered
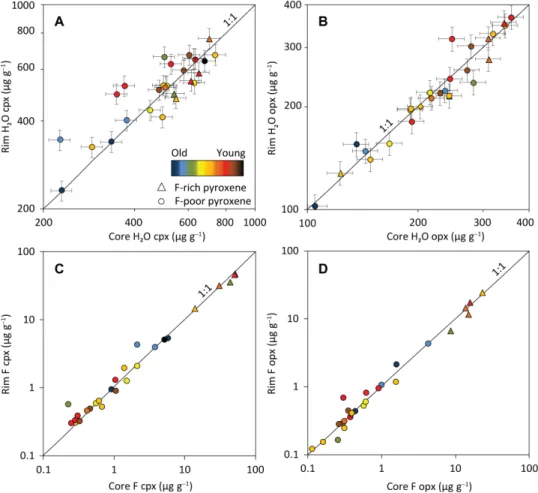
from the 1:1 line compared to the opx (Fig. 1). Fluorine core-to-rim concentrations show tight correlations in both opx and cpx, with limited deviation from the 1:1 line.
The distribution of H2O between cpx and opx ( D H 2 Ocpx/opx ) has been
reported in a large number of studies on natural peridotites (7, 13) and experimental samples (25). Data from this study are reported in Fig. 2 along with data from the literature. H2O partitions preferentially
into cpx over opx. Natural samples (mantle xenoliths and oceanic peridotites) show more H2O enrichment in cpx over opx ( D H 2 Ocpx/opx =
2.6 ± 0.9) compared to experimental samples ( D H 2 Ocpx/opx = 1.3 ± 0.3)
(13). The VLS pyroxene data also exhibit this difference to experimental data, with a D H 2 Ocpx/opx of 2.3 ± 0.3 (1SD, N = 28) consistent with
D H 2 Ocpx/opx of 2.4 ± 0.3 previously reported for oceanic peridotites only (13). A preliminary Fourier transform infrared spectroscopy (FTIR) study of VLS pyroxenes from 10 to 19 Ma (14) yielded an average
D H 2 Ocpx/opx of 3.1 ± 0.6, higher than most natural peridotites. Data for
fluorine partitioning are significantly scarcer for both natural and experimental samples. In the VLS pyroxenes, we find an average
D Fcpx/opx of 2.2 ± 1, similar to what has been found in a limited set of natural and experimental samples ( D Fcpx/opx of 2.4 ± 1.3) (26). No sys-tematic variation in D H 2 Ocpx/opx and D
F
cpx/opx is observed with crustal ages.
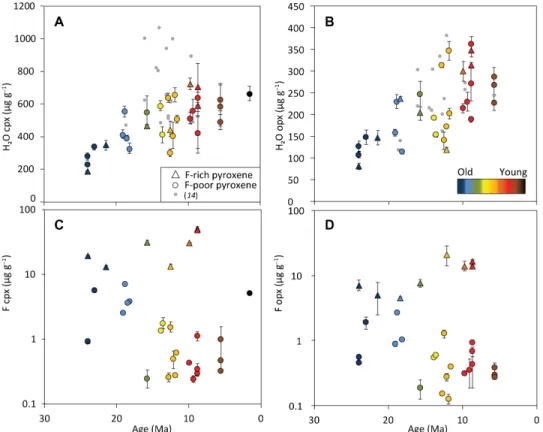
Volatile variations with crustal ages and degree of depletion Crustal ages and H2O contents of opx and cpx are negatively
correlated (Fig. 3). The H2O contents of cpx and opx increase
toward younger crustal ages and are about two times higher in the <10-Ma interval compared to the 20- to 24-Ma interval. A previous VLS FTIR study (14) reported weak to no correlation (R2 = 0.06 to 0.23), possibly due to limited sample availability. On the other hand, fluorine shows two distinct trends. Opposite to H2O, fluorine
Fig. 1. Core-to-rim variations of H2O and F (g g−1) in pyroxenes. H2O in (A) cpx rims versus cores (R2 = 0.66) and (B) opx rims versus cores (R2 = 0.88), and F in (C) cpx
rims versus cores (R2 = 0.99) and (D) opx rims versus cores (R2 = 0.97). Color code refers to associated crustal ages. Each point represents one analysis. Error bars are typical
propagated 2 SE of individual analyses (internal error and propagated error from calibration curve; see the Supplementary Materials) and smaller than symbols when not apparent. F-rich cpx (>10 g g−1) and F-rich opx (>4 g g−1) appear as triangles.
on June 9, 2021
http://advances.sciencemag.org/
concentrations in both pyroxenes generally decrease toward younger crustal ages, except in the few fluorine-rich samples (triangle symbols) where F concentrations are high (>10 g g−1 for cpx and >4 g g−1 for opx) and follow a reverse trend. High abundances of F do not reflect analytical artifacts or the presence of mineral inclusions, as
high F concentrations are found throughout each grain in both opx and cpx from the same samples.
Broad correlations are also found between the degree of melting recorded by the peridotites and pyroxene volatile contents (Fig. 4). The degree of melting in VLS peridotites increases toward younger
Fig. 2. Covariations of H2O and F (g g−1) in pyroxenes. (A) H2O in opx versus cpx from this study (R2 = 0.80) compared to experimental studies (11, 25, 39), xenoliths (7),
and other oceanic peridotites (13, 14). (B) F in opx versus cpx from this study (R2 = 0.95) compared to experimental studies (11, 69) and peridotitic pyroxenes from various
tectonic environments (26). Error bars are omitted for clarity. Color code refers to associated crustal ages. Each point represents the average of all grains in one sample. F-rich cpx (>10 g g−1) and F-rich opx (>4 g g−1) appear as triangles.
Fig. 3. H2O and F (g g−1) in pyroxenes over 23-Ma time series. (A) H2O in cpx and (B) in opx versus crustal ages (R2 = 0.52 and 0.43, respectively). Note that the x axis
is reversed to reflect older rocks in the West compared to the East. Error bars are omitted for clarity for a previous FTIR study (14). (C) F in cpx and (D) in opx versus crustal ages. F-rich cpx (>10 g g−1) and F-rich opx (>4 g g−1) appear as triangles. In (C), R2 values for F-poor and F-rich samples are 0.1 and 0.51, respectively. In (D), R2 values for
F-poor and F-rich samples are 0.2 and 0.61, respectively. Error bars for volatiles are 2 SE of sample averages and smaller than symbols when not apparent; each point represents the average of all grains in one sample. Color code refers to associated crustal ages.
on June 9, 2021
http://advances.sciencemag.org/
crustal ages, as shown by previous studies (18, 19, 22). For consistency between previous studies, the degree of melting was recalculated using spinel compositions only (27). The degree of melting in the source correlates broadly with the H2O contents of opx and cpx,
whereby the more depleted pyroxenes contain the highest H2O
con-tents. On the other hand, fluorine decreases with increasing degrees of melting in fluorine-poor pyroxenes, while data are too limited in fluorine-rich pyroxenes to assess the effect of melting.
Volatile variations with pyroxene chemistry
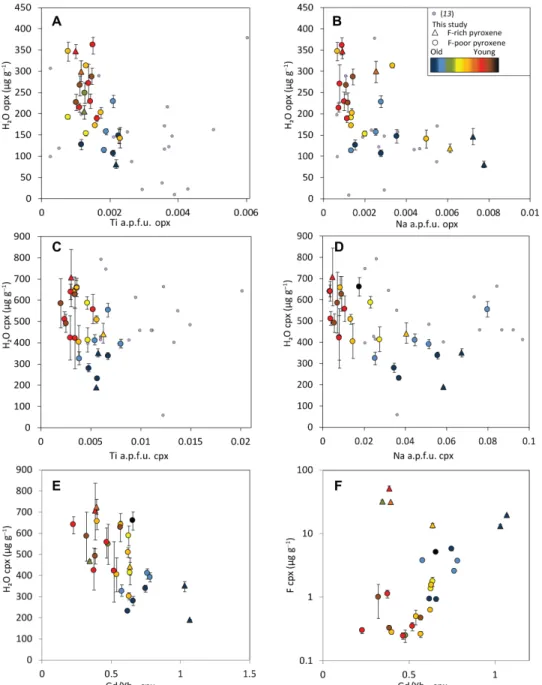
As observed in a previous study of abyssal peridotites (13), H2O
in pyroxene does not correlate with tetrahedrally or octahedrally coordinated Al3+ (fig. S1). This behavior is opposite to experimental
predictions after partial melting (11) and to elemental trends observed in some xenoliths (28). Further, H2O negatively correlates with Ti
and Na in both pyroxenes (Fig. 5). Opposite to that trend, fluorine positively correlates with Ti and Na in both cpx and opx in fluorine- poor samples (fig. S2). The few fluorine-rich samples, however, show negative correlations between F, Ti, and Na.
In addition to major elements, H2O and fluorine covary with other
incompatible trace elements (Figs. 5 and 6). In particular, H2O
negatively correlates with rare earth elements (REE) Sm, Gd, and Yb and, despite a partitioning behavior close to Ce (29), shows the strongest negative correlation with mid-REE Gd. H2O concentrations
in cpx increase with decreasing Gd/Yb normalized to chondrite (30) in cpx. Similarly, in fluorine-rich samples, fluorine displays negative
correlations with REE and positive correlations with H2O (Fig. 6).
In contrast, fluorine abundances in fluorine-poor samples display broad positive correlations with REE and a broad negative correlation with H2O.
DISCUSSION
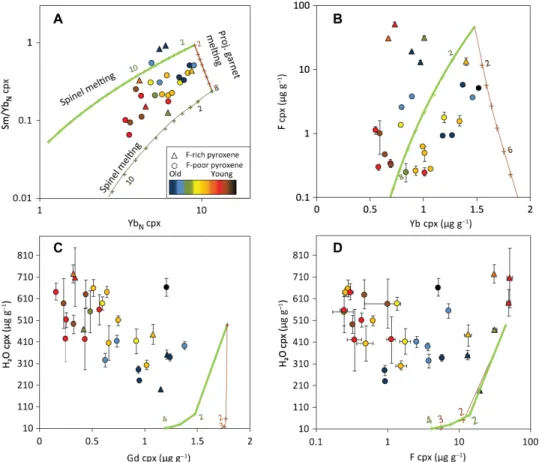
Tracers of partial melting and their relationship to H2O and fluorine
Incompatible trace elements, such as normalized (30) Sm/Yb and Yb in VLS cpx, follow residual trends after partial melting. Figure 6 illustrates that REE variability in VLS cpx can be explained by frac-tional melting of a depleted MORB (mid-ocean ridge basalt) mantle (DMM) in the garnet stability field, followed by fractional melting of DMM in the spinel stability field. Melting parameters (11, 25, 26, 31–34) are reported in table S2 and follow the method described in previous studies (22, 35). A mantle source that contains ~10 g g−1 F (26) and
melted less than 20% would yield residual fluorine and REE abun-dances within the range observed in this study.
On the other hand, H2O displays negative correlations with major
and trace elements predicted to behave similarly to H2O during partial
melting. The H2O variations in VLS pyroxenes cannot be explained
solely by partial melting, whether the source is typical DMM (~100 to 200 g g−1 H2O) (32) or more H2O-rich. Similarly, some pyroxenes
contain too much fluorine to represent residues after partial melting. Stochastic F enrichments are unlikely to have been produced during
Fig. 4. H2O and F (g g−1) in pyroxenes versus degree of melting of peridotites. (A) H2O in cpx and (B) in opx versus melting degree. R2 values are 0.19 and 0.21,
respectively. (C) F in cpx and (D) in opx versus melting degree. In (C), R2 values for F-poor and F-rich samples are 0.32 and 0.27, respectively. In (D), R2 values for F-poor and
F-rich samples are 0.26 and 0.42, respectively. Error bars for volatiles are 2 SE of sample averages and smaller than symbols when not apparent; each point represents the average of all grains in one sample. Color code refers to associated crustal ages. F-rich cpx (>10 g g−1) and F-rich opx (>4 g g−1) appear as triangles.
on June 9, 2021
http://advances.sciencemag.org/
modal metasomatism because elements that are typically enriched during melt metasomatism (e.g., REE, Na, and Ti) negatively correlate with fluorine. Instead, the positive correlation between fluorine and hydrogen in F-rich samples (Fig. 6) could indicate that F-H incorporation occurred as a complex defect similar to mecha-nism observed in olivine (36) and that F enrichment resulted from localized cryptic metasomatism by a F-rich fluid. This fluid could have originated in a subridge magma chamber and sporadically per-colated overlying peridotites during their emplacement at shallow levels. Although the reason for high fluorine abundances in selected pyroxenes remains to be fully understood, these observations cast
doubt on the ability to use fluorine as a direct tracer of H2O in
peridotite minerals (13).
Effects of serpentinization and subsolidus re-equilibration on pyroxene H2O contents
H2O variations are about two times higher in depleted pyroxenes
compared to fertile ones, yet petrographic observations do not indi-cate significant differences in H2O contents linked to degree of
alteration or changes in microstructure such as grain size. FTIR spectra obtained on a subset of serpentinite-free grains (14) are broad-ly consistent with SIMS results, and negative correlations between
Fig. 5. H2O and F (g g−1) versus minor and trace elements in pyroxenes. H2O versus (A) Ti in atoms per formula unit (a.p.f.u.) (R2 = 0.36) and (B) Na a.p.f.u. in opx
(R2 = 0.39), (C) H
2O versus Ti a.p.f.u. (R2 = 0.25), and (D) Na a.p.f.u. in cpx (R2 = 0.52). (E) H2O versus Gd/YbN (30) in cpx (R2 = 0.37) and (F) F versus Gd/YbN in cpx (R2 values
for F-poor and F-rich samples are 0.50 and 0.62, respectively). Error bars for volatiles are 2 SE of sample averages and smaller than symbols when not apparent; each point represents the average of all grains in one sample. Color code refers to associated crustal ages. F-rich cpx (>10 g g−1) and F-rich opx (>4 g g−1) appear as triangles.
on June 9, 2021
http://advances.sciencemag.org/
H2O and other incompatible elements are not expected during
serpentinization. In addition, no large core to rim variations are ob-served, inconsistent with transient serpentinization. Last, hydrogen diffusivities at serpentinization temperatures may be too low to affect primary H2O abundances in pyroxenes, although this is still
debated (37).
Previous studies have reviewed the effects of depth and pressure on H partition coefficients between olivine and opx for peridotites equilibrated in the spinel and garnet stability field. Natural pyroxenes and bulk peridotites from global datasets display order of magnitude variations in H2O contents at similar pressures of equilibration in
the spinel stability field (7), casting doubt that pressure is the main controller of hydrogen distribution in the oceanic lithospheric mantle. In addition, a broad increase in D H 2 Ool/opx can be observed in cratonic peridotites equilibrated at depths of >150 km, but no sys-tematic variations are observed in the spinel stability field (7). Thus, the potential effects of pressure of melting and/or equilibration are likely overprinted by other processes in the shallow oceanic lithospheric mantle. Further, hydrogen redistribution during cooling has been inferred to explain the discrepancy (38) in D H 2 Ocpx/opx be-tween natural samples (average of 2.4; samples typically equilibrated
at <1000°C) (13) and experiments (average of 1.3; samples typically equilibrated at >1200°C) (25, 38, 39). Temperatures of equilibration of VLS peridotites were calculated using major element composition of opx and cpx (40, 41) for an average pressure of 1.5 GPa, i.e., in the spinel stability field (23). Neither H2O concentrations nor D H 2 Ocpx/opx
display correlations with temperatures of re-equilibration at subsolidus conditions (fig. S3). This observation comes with the caveat that H could have further diffused after closure of the major element sys-tems. However, if H enrichments in VLS pyroxenes were primarily controlled by variable extents of subsolidus cooling, core-to-rim profiles may show disequilibrium distribution, and D H 2 Ocpx/opx should
vary systematically with crustal ages, with samples re-equilibrated at higher temperatures displaying a lower D H 2 Ocpx/opx . This is not observed
in the VLS samples. Therefore, although subsolidus redistribution of hydrogen likely occurred before exhumation on the seafloor (be-cause D H 2 Ocpx/opx = 2.3), it cannot be the main mechanism behind the 24-Ma H2O enrichment trend and had to be preceded by H enrichment
in the bulk system. Subsolidus redistribution cannot provide enough H2O to double concentrations over time in both pyroxenes, as this
would not satisfy mass balance requirements. Therefore, an external reservoir of hydrogen (e.g., melt/fluid) is needed.
Fig. 6. Volatile and trace element variations compared to fractional melting model of DMM (31). (A) Sm/YbN in cpx versus YbN in cpx (30) compared to spinel-field
melting (thick light green) and projected garnet-field melting (22) (brown trend) followed by spinel-field melting (dark green). Small colored numbers next to cross symbols indicate the degree of melting. The source is assumed to have 16 g g−1 F and 142 g g−1 H2O (32). (B) F in cpx (g g−1) versus Yb in cpx (g g−1), compared to the melting
models. R2 values for F-poor and F-rich samples are 0.16 and 0.50, respectively. (C) H2O versus Gd (g g−1) in cpx (R2 = 0.34) compared to the melting models. Mid-REEs
display the best inverse correlations with H2O. (D) H2O versus F (g g−1) in cpx compared to the melting models. R2 values for F-poor and F-rich samples are 0.02 and 0.51,
respectively. Error bars for volatiles are 2 SE of sample averages and smaller than symbols when not apparent; each point represents the average of all grains in one sample. Color code refers to associated crustal ages. F-rich cpx (>10 g g−1) and F-rich opx (>4 g g−1) appear as triangles.
on June 9, 2021
http://advances.sciencemag.org/
Hydrogen enrichment during melt metasomatism
Limited metasomatism has been reported in VLS peridotites, where 0.1 to 1% of residual melt could be trapped (22). Modal metasoma-tism leads to precipitation of new minerals from a migrating melt and produces concomitant enrichments of incompatible elements in newly crystallized pyroxenes (42). In that scenario, H2O should
positively correlate with REEs (e.g., La and Ce), Al, Na, and Ti, but the opposite is observed in the VLS pyroxenes. Therefore, hydrogen variations in the VLS time series do not primarily reflect modal metasomatism.
However, hydrogen alone could have diffused from these small melt fractions trapped at grain boundaries. Hydrogen diffuses at a faster rate than any other trace, minor, and major elements (6, 7). Diffusivities of hydrogen in pyroxene (43–45) at temperatures of 1000 to 1200°C are in the range of ~10−12 to 10−10 m2 s−1. These diffusivities yield a minimal characteristic distance of <10 m/Ma compared to <0.6 mm/Ma for REE (46), assuming a diffusion co-efficient of 10−21 m2 s−1. Considering that the peridotites were
con-tinuously percolated by basalts, while they ascended under the ridge from ~70- to 100-km depth (23) at a minimum rate of 1 m year−1
for 70 to 100 ka, hydrogen could have diffused at least ~1 m in all peridotite samples, well above the observed grain size in these rocks. Therefore, while the trace and major element abundances of VLS pyroxenes primarily reflect partial melting (22), hydrogen concen-trations record a superimposed gain of hydrogen that occurred by ionic diffusion during cryptic metasomatism.
Hydrogen can be incorporated in pyroxene both by coupled substitution mechanisms and by occupying metal vacancies, mean-ing that partition coefficients between pyroxene and melt are, in part, dictated by crystal chemistry and point defect populations (25, 34, 44, 47–49). Point defect theory predicts that hydrogen incorporation is favored by increasing concentration of intrinsic as well as extrinsic (minor and trace elements) point defects (9, 50, 51). Vacancies are the most common type of point defects that can be hydrogenated, and their concentrations typically increase with in-creasing temperature, oxygen fugacity (ƒo2), and concentrations of trace elements (52), particularly trivalent impurities. Pyroxenes that contain high H2O abundances can record more reducing conditions
than H2O-poor pyroxenes (53, 54). This has been linked to the
in-corporation of hydrogen via the proton-polaron exchange reaction (55) or variations of that reaction (56), resulting in reduction of Fe3+ to Fe2+. Seafloor abyssal peridotites record some of the lowest mantle
ƒo2 observed for spinel peridotites (57), which could favor the large uptake of hydrogen observed in the VLS time series. Although accurate ƒo2 calculations would require olivine and corrected Fe3+ spinel data (58), these reactions are favored under reducing conditions and the presence of lattice vacancies related to Fe3+. As the mantle composition at the VLS changed from a (potentially oxidized) pyroxenite-bearing source to a (potentially more reduced) peridotite (23), it is possible that the source composition influenced hydrogen uptake. It could also be speculated that the loss of incompatible trace elements during melting could create transient vacancies and opportunities for elemental substitutions with H, favored by a short time lapse between melting and subsequent metasomatism under ridges. This increase in vacancy concentrations could favor additional H incorporation (59). Associated substitutions may include replace-ment of Na+ (lost during melting) by similarly charged H+, as suggested for olivine (60) and supported by negative correlations between Na and H2O in the VLS pyroxenes (Fig. 5).
To our knowledge, the reverse correlations between H2O
abun-dances in pyroxenes and the degree of depletion in the residual mantle at the VLS have not been observed in mantle xenoliths. Depending on the mechanism of hydrogen incorporation, xenoliths can show positive (56) or negative (54) correlations between H2O
and ƒo2 so that oxidizing conditions under which xenoliths evolve are not a prediction for their water contents. Although mantle xenoliths are prone to melt-rock reactions (5), some retain broad positive correlations between H2O and other incompatible elements
(28, 61), opposite to the VLS time series. It is possible that, in xenoliths, a loss of Na+ during melting would not necessarily be followed by H+ addition from a percolating melt, as samples may re-equilibrate
in the lithospheric mantle for millions of years before metasomatism and/or eruption occurs.
Hydrous melts under ridges
Only a fluid or melt occurring on a large scale could create the trends seen in the 24-Ma time series. Therefore, volatile-rich melts must occur under ridges, at least in the vicinity of fracture zones and potentially elsewhere. The H2O contents of equilibrium melts are
calculated using experimentally determined pyroxene-melt partition coefficients [ D H 2 Ocpx/melt of 0.016; (11, 25)] and cpx H
2O abundances.
Lacking olivine measurements, we apply a first-order correction factor of 1.4 to the measured H2O abundances in cpx, which corresponds
to the average correction factor reported for samples equilibrated at ~900° to 1100°C (38, 41) and similar to equilibrium temperatures reported here (table S1). For example, a cpx that contains 545 g g−1 H2O after subsolidus re-equilibration would be assumed to contain
only 389 g g−1 H2O at mantle conditions. The equilibrium hydrous
melt contains ~1.6 weight % (wt %) H2O in the older portion of the
VLS (>15 Ma) and ~2.4 wt % H2O in the younger portion of the
VLS (<15 Ma). This is significantly higher than the average MORB H2O contents [0.2 wt % (62); MgO > 8 wt % only] and H2O averages
of VLS basalts (<0.3 wt % H2O) (62). Although hydrous melts may
be diluted by more abundant dry melts under ridges, hydrous melts (>1 wt % H2O) have been observed in the vicinity of fracture zones
(63). Numerical simulations also predict that low-degree, volatile- rich melts could be trapped in the lithospheric mantle under ridges (64). Hydrogen enrichment observed in large swaths of VLS perid-otites support this hypothesis. In addition, crystallization of small melt fractions has been observed in most abyssal peridotites (35, 65), and 0.1 to 1% of trapped melt may be present in the VLS peridotite residue (22). These melts, potentially trapped at grain boundaries (66), are ideal candidates to carry H and other incompatible elements, but only H may have enough time to re-equilibrate in the system. This is consistent with the observation that most abyssal peridotites are enriched in light REE (LREE) compared to their corresponding cpx (67), which can be explained by a late incompatible element enrichment at grain boundaries.
Water budget of abyssal peridotites and lithosphere viscosity
On the basis of our results, the bulk H2O budget of VLS abyssal
peridotites (unaltered primary assemblage) should be largely controlled by pyroxenes (>75%). Using a range of well-established partition coefficients between opx, cpx, and olivine (ol) at subsolidus and mantle conditions ( D H 2 Ool/opx = 0.04 to 0.11; D H 2 Ool/cpx = 0.02 to 0.07)
(11, 13, 25, 39), a representative range of olivine and bulk abundances can be calculated. Modal compositions of typical abyssal peridotite (27)
on June 9, 2021
http://advances.sciencemag.org/
(ol, 73%; opx, 22%; cpx, 4%; spinel, 1%) and depleted MORB mantle (31) (ol, 57%; opx, 28%; cpx, 13%; spinel, 2%) are combined with cpx and opx H2O contents of 545 to 371 and 240 to 163 g g−1 H2O,
respectively. This range corresponds to average H2O values in
pyroxenes for <15-Ma and >15-Ma VLS peridotites. Bulk (unaltered) primary assemblages of residual peridotites at the VLS should therefore contain 55 to 160 g g−1 H2O (table S3). In theory, residual
oceanic peridotites should be dehydrated after melting (4). However, this first-order estimate indicates that the water contents of abyssal peridotites are systematically higher than predicted for a residual lithosphere, and high H2O abundances do not just reflect local
anomalies. In addition, olivine is the primary phase that controls the rheological properties of the mantle lithosphere. Residual olivine water contents equilibrated with H2O-rich pyroxenes would range
from 8 to 50 g g−1, assuming the full range of cpx H2O reported for
VLS peridotites. The presence of low-degree, volatile-rich melts in ridge settings should therefore affect the rheological properties of the residual oceanic lithosphere. These hydrous melts may rarely erupt but instead metasomatize the residual lithosphere under oceanic ridges (64) and reduce mantle rock viscosity compared to models of dry lithosphere (4). These observations point to a critical need to understand H2O distribution in the oceanic lithospheric
mantle from the perspective of abyssal peridotites, in particular in large swaths of genetically related samples.
MATERIALS AND METHODS Secondary ion mass spectrometry
SIMS analyses were conducted at the Northeast National Ion Micro-probe facility at the Woods Hole Oceanographic Institution on a Cameca IMS 1280. Opx and cpx separates, ranging from 3 mm to <500 m, were mounted in crystal bound and polished down to 1-m grit using a diamond solution. After extraction from the crystal bound, the polished separates were thoroughly cleaned with acetone and re-mounted in indium. The indium mounts were re-polished with 1-m diamond solution. Indium mounts were cleaned with ethanol and deionized water to remove surface contamination, and then the mounts were placed in a vacuum oven to dry. Samples were gold-coated to ~160-nm thickness and placed into a vacuum chamber for storage until analysis. Samples were loaded into the instrument sample chamber at least 12 hours before analysis to allow adequate pump down time and achieve chamber pressures of no more than ~5 × 10−9 torr. At least three spots per grain were analyzed, typically combined with core-to-rim profiles on most grains when possible. Spot locations were chosen to avoid any visible crack, inclusion, or anomalous surface appearance. A primary 133Cs+ beam
of 5.0 to 7.5 nA was sputtered through the sample surface with a 30 m by 30 m raster and a 400-m field aperture, allowing only transmission of ions from the innermost 5 m by 5 m of the beam crater. Secondary magnet mass calibration was done before each measurement with mass resolving power of >6000 (m/m at 10% peak height). We measured 12C/30Si, 16O1H/30Si, 19F/30Si, (±31P/30Si), 32S/30Si, and 35Cl/30Si ratios in glass reference materials D51-3, D52-5,
ALV519-4-1, 46D, 1649-3, 1654-3 6001, and AII107-D20 to produce a calibration slope for each 1-week analytical session (example cali-bration curves in fig. S4). Calicali-bration slopes (m) were obtained by plotting measured isotope ratios (x) against known reference material concentrations (y) of the form y = mx for each element of interest. Sample unknowns were then calculated by multiplying measured
ratios by m. Calibration slope uncertainties were assessed using a bootstrapping technique (5000 iterations) to derive 95% confidence intervals, with the intercept set to zero. The bootstrapping code per-forms a nonlinear maximum likelihood inversion of a straight line to x and y data, where x and y are considered random variables with known errors. SD for glasses is based on multiple sessions over the years and is conservatively estimated to be 2 = 10%; SDs for indi-vidual pyroxene reference materials were reported in a previous study (68). Points that constitute the calibration curve are randomly sampled 5000 times to generate confidence limits of parameter estimates. The glass calibration slope was used to reduce data for C, P, S, and Cl. Analytical uncertainties over five counting cycles were combined with calibration slope uncertainties to yield typical total uncertainty (2 SE, 95% confidence intervals) of <12, <12, <8, and <9% for C, P, S, and Cl, respectively. For reference, total uncer-tainties for OH and F in glass reference materials are <4 and <3%, respectively. Because volatile measurements on the SIMS are often affected by matrix effects, we also measured the same volatile elements on opx-cpx reference materials requested from the De-partment of Minerals Sciences, Smithsonian Museum (USA) and calibrated using mount NMNH 118331. Opx reference materials that were used for the calibration curve included NMNH 116610-29, 117322-245, 116610-5, and 109426-1. Cpx reference materials that were used for the calibration curve included NMNH 117213-5, 11610-18, 116610-15, 118317-1 (KH03-27), and 118318 (SC-J1). These reference materials have been calibrated for SIMS in a previ-ous study using reference materials from Carnegie Institution (68). The reference materials from Carnegie Institution had been cross-calibrated by multiple independent absolute techniques such as FTIR, elastic recoil detection analysis, and vacuum manometry; see complete list of references in (68). Representative calibration curves are presented in fig. S5 and were used to calculate the water and fluorine contents of pyroxenes. Measurements where the in-ternal precision of 16O1H/30Si and/or 19F/30Si was greater than
10% were excluded from the dataset. The vast majority of internal 2 SE are much lower than 10%, with an average of 0.4% for OH and 0.8% for F in cpx, and 0.2% for OH and 0.8% for F in opx. These low-precision measurements were typically also associated with elevated C, S, and/or Cl concentrations and are likely the result of analyses conducted on slightly uneven surfaces or nonvisible microfractures. Analytical uncertainties over five counting cycles were combined with calibration slope uncertainties to yield typical total uncertainty (2 SE, 95% confidence intervals) of <5 and <3% for OH and F in cpx, respectively, and <6 and <3% for OH and F in opx, respectively. Continuous measurements of ALV519-4-1 were used throughout the session every couple of hours to monitor in-strument drift, which was found to be negligible. The volatile con-centrations of Suprasil 102 and Herasil 102 (optical-quality glasses), as well as synthetic forsterite, were measured regularly throughout the session to quantify volatile background abundances. There are no published values for the OH, F, and Cl content of Suprasil 102, Herasil 102, and synthetic forsterite, but those samples are believed to have very low OH, F, and Cl concentrations, respectively (less than a few g g−1). To quantify our maximum backgrounds, we assume
that Suprasil 102 contains no OH, Herasil contains no F, and synthetic forsterite contains no Cl. Background F and Cl values were less than 0.15 and 0.09 g g−1, respectively, for all sessions. Background OH varied by mount, from ~5 to ~80 g g−1, in a few samples analyzed
at the very beginning of the session (typically 5 to 10 g g−1 when
on June 9, 2021
http://advances.sciencemag.org/
properly corrected for Si content of glasses; see below). Although most samples were prepared in crystal bound and then mounted in indium specifically for this study, a small subset of samples that we remounted in indium had been previously mounted in epoxy. Not all tiny pieces of epoxy could be removed from all fragile grains, leading to higher backgrounds than when using our usual preparation procedure (26, 33). To ensure proper correction of these few high background measurements, the high background analyses were reanalyzed at a later time when the background was three to four times lower. Several tests have been done to ensure proper correction of the background values, as synthetic glasses and pyroxenes do not have the same composition (e.g., glasses have ~100% SiO2, while
pyroxenes have SiO2 < 60 wt %). The potential effects of differences
in transmission of Si (counts) and composition (Si contents) have been evaluated. Results from the recommended correction method are presented in fig. S6. We recommend that the measured OH con-tents of Suprasil 102 be conservatively multiplied by 1.85 for opx background and multiplied by 1.95 for cpx background, reflecting the average difference in Si content between the pyroxene and glasses. For example, if the Suprasil 102 background is ~5 g g−1, it means that
the real background is ~9 g g−1 for an opx and ~10 g g−1 for a cpx. By applying this conservative multiplier, we found that volatile values in samples that were initially associated with 60 to 80 g g−1 OH background, and then remeasured with a <20 g g−1 OH background
matched the best (closest to a 1:1 line). Without this correction, background values could be slightly underestimated, although it would not change the conclusions of this study. Nevertheless, we recommend that Si-corrected background values should be system-atically reported in further volatile studies of minerals by SIMS. Indi-vidual mineral analyses were not corrected for background when volatile background values were less than propagated 2 SE uncertainty.
Rare earth elements Sm, Gd, and Yb in selected pyroxenes were analyzed by SIMS with a Cameca IMS 4f ion microprobe at the Istituto di Geoscienze e Georisorse (Pavia) following the method described in a previous study (22).
Electron Probe Micro-Analyses
Major element concentrations of pyroxenes were measured with a Cameca SX100 electron probe housed at the American Museum of Natural History (NY) using a 15-kV acceleration voltage, a 20-nA beam, a 10-m-diameter beam, and 30-s counting times. Sodium and potassium were run under different conditions to attain a higher precision and monitor their mobility, with a 5-nA beam and counting times of 80 s. For details of the method, readers are referred to previous studies (19, 22).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/ content/full/7/24/eabf6071/DC1
REFERENCES AND NOTES
1. S.-I. Karato, M. S. Paterson, J. D. Fitzgerald, Rheology of synthetic olivine aggregates: Influence of grain size and water. J. Geophys. Res. B Solid Earth 91, 8151–8176 (1986). 2. S. J. Mackwell, D. L. Kohlstedt, M. S. Paterson, The role of water in the deformation
of olivine single crystals. J. Geophys. Res. B Solid Earth 90, 11319–11333 (1985). 3. S. D. Jacobsen, Effect of water on the equation of state of nominally anhydrous minerals.
Rev. Mineral. Geochem. 62, 321–342 (2006).
4. G. Hirth, D. L. Kohlstedt, Water in the oceanic upper mantle: Implications for rheology, melt extraction, and the evolution of the lithosphere. Earth Planet. Sci. Lett. 144, 93–108 (1996). 5. D. G. Pearson, D. Canil, S. B. Shirey, Treatise on Geochemistry, D. H. Heinrich, K. T. Karl, Eds.
(Pergamon, 2003), pp. 171–275.
6. A. H. Peslier, A review of water contents of nominally anhydrous natural minerals in the mantles of Earth, Mars and the Moon. J. Volcanol. Geotherm. Res. 197, 239–258 (2010). 7. S. Demouchy, N. Bolfan-Casanova, Distribution and transport of hydrogen
in the lithospheric mantle: A review. Lithos 240-243, 402–425 (2016).
8. A. H. Peslier, M. Schönbächler, H. Busemann, S.-I. Karato, Water in the Earth’s interior: Distribution and origin. Space Sci. Rev. 212, 743–810 (2017).
9. H. Skogby, Water in natural mantle minerals I: Pyroxenes. Rev. Mineral. Geochem. 62, 155–167 (2006).
10. S. Demouchy, S. D. Jacobsen, F. Gaillard, C. R. Stern, Rapid magma ascent recorded by water diffusion profiles in mantle olivine. Geology 34, 429–432 (2006).
11. J. A. O'Leary, G. A. Gaetani, E. H. Hauri, The effect of tetrahedral Al3+ on the partitioning
of water between clinopyroxene and silicate melt. Earth Planet. Sci. Lett. 297, 111–120 (2010).
12. H. J. B. Dick, R. L. Fisher, W. B. Bryan, Mineralogic variability of the uppermost mantle along mid-ocean ridges. Earth Planet. Sci. Lett. 69, 88–106 (1984).
13. J. M. Warren, E. H. Hauri, Pyroxenes as tracers of mantle water variations. J. Geophys. Res. Solid Earth 119, 1851–1881 (2014).
14. P. Li, Q. K. Xia, L. Dallai, E. Bonatti, D. Brunelli, A. Cipriani, M. Ligi, High H2O content
in pyroxenes of residual mantle peridotites at a Mid Atlantic Ridge segment. Sci. Rep. 10, 579 (2020).
15. E. Schmädicke, J. Gose, R. Stalder, Water in abyssal peridotite: Why are melt-depleted rocks so water-rich? Geochem. Geophys. Geosyst. 19, 1824–1843 (2018).
16. J. Gose, E. Schmädicke, A. Beran, Water in enstatite from Mid-Atlantic Ridge peridotite: Evidence for the water content of suboceanic mantle? Geology 37, 543–546 (2009). 17. K. T. Hesse, J. Gose, R. Stalder, E. Schmädicke, Water in orthopyroxene from abyssal spinel
peridotites of the East Pacific Rise (ODP Leg 147: Hess Deep). Lithos 232, 23–34 (2015). 18. E. Bonatti, M. Ligi, D. Brunelli, A. Cipriani, P. Fabretti, V. Ferrante, L. Gasperini, L. Ottolini,
Mantle thermal pulses below the Mid-Atlantic Ridge and temporal variations in the formation of oceanic lithosphere. Nature 423, 499–505 (2003).
19. A. Cipriani, E. Bonatti, D. Brunelli, M. Ligi, 26 million years of mantle upwelling below a segment of the Mid Atlantic Ridge: The Vema Lithospheric Section revisited. Earth Planet. Sci. Lett. 285, 87–95 (2009).
20. S. C. Cande, D. V. Kent, Revised calibration of the geomagnetic polarity timescale for the Late Cretaceous and Cenozoic. J. Geophys. Res. B Solid Earth 100, 6093–6095 (1995). 21. C. J. Lissenberg, M. Rioux, N. Shimizu, S. A. Bowring, C. Mével, Zircon dating of oceanic
crustal accretion. Science 323, 1048–1050 (2009).
22. D. Brunelli, M. Seyler, A. Cipriani, L. Ottolini, E. Bonatti, Discontinuous melt extraction and weak refertilization of mantle peridotites at the vema lithospheric section (Mid-Atlantic Ridge). J. Petrol. 47, 745–771 (2006).
23. D. Brunelli, A. Cipriani, E. Bonatti, Thermal effects of pyroxenites on mantle melting below mid-ocean ridges. Nat. Geosci. 11, 520–525 (2018).
24. A. Cipriani, E. Bonatti, M. Seyler, H. K. Brueckner, D. Brunelli, L. Dallai, S. R. Hemming, M. Ligi, L. Ottolini, B. D. Turrin, A 19 to 17 Ma amagmatic extension event at the Mid-Atlantic Ridge: Ultramafic mylonites from the Vema Lithospheric Section. Geochem. Geophys. Geosyst. 10, (2009).
25. C. Aubaud, E. H. Hauri, M. M. Hirschmann, Hydrogen partition coefficients between nominally anhydrous minerals and basaltic melts. Geophys. Res. Lett. 31, L20611 (2004). 26. B. M. Urann, V. Le Roux, K. Hammond, H. R. Marschall, C.-T. A. Lee, B. D. Monteleone,
Fluorine and chlorine in mantle minerals and the halogen budget of the Earth’s mantle. Contrib. Mineral. Petrol. 172, 51 (2017).
27. J. M. Warren, Global variations in abyssal peridotite compositions. Lithos 248–251, 193–219 (2016).
28. A. W. Ashley, M. Bizimis, A. H. Peslier, M. Jackson, J. Konter, Metasomatism and hydration of the oceanic lithosphere: A case study of peridotite xenoliths from Samoa. J. Petrol. 61, egaa028 (2020).
29. P. J. Michael, The concentration, behavior, and storage of H2O in the suboceanic upper
mantle: Implications for mantle metasomatism. Geochim. Cosmochim. Acta 52, 555–566 (1988).
30. E. Anders, N. Grevesse, Abundances of the elements—Meteoritic and solar. Geochim. Cosmochim. Acta 53, 197–214 (1989).
31. R. K. Workman, S. R. Hart, Major and trace element composition of the depleted MORB mantle (DMM). Earth Planet. Sci. Lett. 231, 53–72 (2005).
32. A. E. Saal, E. H. Hauri, C. H. Langmuir, M. R. Perfit, Vapour undersaturation in primitive mid-ocean-ridge basalt and the volatile content of Earth's upper mantle. Nature 419, 451–455 (2002).
33. B. M. Urann, V. Le Roux, T. John, B. G. M, J. D. Barnes, The distribution and abundance of halogens in eclogites: An in situ SIMS perspective of the Raspas Complex (Ecuador). Am. Mineral. 105, 307–318 (2020).
34. E. H. Hauri, G. A. Gaetani, T. H. Green, Partitioning of water during melting of the Earth's upper mantle at H2O-undersaturated conditions. Earth Planet. Sci. Lett. 248, 715–734
(2006).
on June 9, 2021
http://advances.sciencemag.org/
35. E. Hellebrand, J. E. Snow, P. Hoppe, A. W. Hofmann, Garnet-field melting and late-stage refertilization in ‘residual’ abyssal peridotites from the Central Indian Ridge. J. Petrol. 43, 2305–2338 (2002).
36. C. Crépisson, M. Blanchard, H. Bureau, C. Sanloup, A. C. Withers, H. Khodja, S. Surblé, C. Raepsaet, K. Béneut, C. Leroy, P. Giura, E. Balan, Clumped fluoride-hydroxyl defects in forsterite: Implications for the upper mantle. Earth Planet. Sci. Lett. 390, 287–295 (2014). 37. K. J. Lynn, J. M. Warren, The potential for aqueous fluid-rock and silicate melt-rock
interactions to re-equilibrate hydrogen in peridotite nominally anhydrous minerals. Am. Mineral. 106, 701–714 (2020).
38. L. A. Schaffer, A. H. Peslier, A. D. Brandon, M. Bizimis, R. Gibler, M. Norman, J. Harvey, Effects of melting, subduction-related metasomatism, and sub-solidus equilibration on the distribution of water contents in the mantle beneath the Rio Grande Rift. Geochim. Cosmochim. Acta 266, 351–381 (2019).
39. C. Aubaud, M. M. Hirschmann, A. C. Withers, R. L. Hervig, Hydrogen partitioning between melt, clinopyroxene, and garnet at 3 GPa in a hydrous MORB with 6 wt.% H2O. Contrib.
Mineral. Petrol. 156, 607–625 (2008).
40. C. G. Sun, Y. Liang, Distribution of REE between clinopyroxene and basaltic melt along a mantle adiabat: Effects of major element composition, water, and temperature. Contrib. Mineral. Petrol. 163, 807–823 (2012).
41. G. P. Brey, T. Köhler, Geothermobarometry in four-phase lherzolites II. New
thermobarometers, and practical assessment of existing thermobarometers. J. Petrol. 31, 1353–1378 (1990).
42. V. Le Roux, J.-L. Bodinier, A. Tommasi, O. Alard, J.-M. Dautria, A. Vauchez, A. J. V. Riches, The Lherz spinel lherzolite: Refertilized rather than pristine mantle. Earth Planet. Sci. Lett.
259, 599–612 (2007).
43. E. Ferriss, T. Plank, D. Walker, Site-specific hydrogen diffusion rates during clinopyroxene dehydration. Contrib. Mineral. Petrol. 171, 55 (2016).
44. R. Sundvall, H. Skogby, Hydrogen defect saturation in natural pyroxene. Phys. Chem. Miner. 38, 335–344 (2011).
45. Y. Xu, W. Tang, H. Hui, R. L. Rudnick, S. Shang, Z. Zhang, Reconciling the discrepancy between the dehydration rates in mantle olivine and pyroxene during xenolith emplacement. Geochim. Cosmochim. Acta 267, 179–195 (2019).
46. J. A. Van Orman, T. L. Grove, N. Shimizu, Rare earth element diffusion in diopside: Influence of temperature, pressure, and ionic radius, and an elastic model for diffusion in silicates. Contrib. Mineral. Petrol. 141, 687–703 (2001).
47. R. Stalder, H. Skogby, Hydrogen diffusion in natural and synthetic orthopyroxene. Phys. Chem. Miner. 30, 12–19 (2003).
48. A. J. Berry, H. S. C. O'Neill, J. Hermann, D. R. Scott, The infrared signature of water associated with trivalent cations in olivine. Earth Planet. Sci. Lett. 261, 134–142 (2007). 49. Q. Bai, D. L. Kohlstedt, Effects of chemical environment on the solubility and
incorporation mechanism for hydrogen in olivine. Phys. Chem. Miner. 19, 460–471 (1993). 50. A. Nakamura, H. Schmalzried, On the nonstoichiometry and point defects of olivine.
Phys. Chem. Miner. 10, 27–37 (1983).
51. D. R. Bell, G. R. Rossman, R. O. Moore, Abundance and partitioning of OH in a high-pressure magmatic system: Megacrysts from the Monastery Kimberlite, South Africa. J. Petrol. 45, 1539–1564 (2004).
52. J. R. Holloway, B. J. Wood, Simulating the Earth: Experimental Geochemistry, J. R. Holloway, B. J. Wood, Eds. (Springer Netherlands, 1988), pp. 143–168.
53. H. Skogby, OH incorporation in synthetic clinopyroxene. Am. Mineral. 79, 240–249 (1994). 54. A. H. Peslier, J. F. Luhr, J. Post, Low water contents in pyroxenes from spinel-peridotites
of the oxidized, sub-arc mantle wedge. Earth Planet. Sci. Lett. 201, 69–86 (2002). 55. S. J. Mackwell, Q. Bai, D. L. Kohlstedt, Rheology of olivine and the strength
of the lithosphere. Geophys. Res. Lett. 17, 9–12 (1990).
56. P. Tollan, J. Hermann, Arc magmas oxidized by water dissociation and hydrogen incorporation in orthopyroxene. Nat. Geosci. 12, 667–671 (2019).
57. D. J. Frost, C. A. McCammon, The redox state of Earth’s mantle. Annu. Rev. Earth Planet. Sci.
36, 389–420 (2008).
58. F. A. Davis, E. Cottrell, S. K. Birner, J. M. Warren, O. G. Lopez, Revisiting the electron microprobe method of spinel-olivine-orthopyroxene oxybarometry applied to spinel peridotites. Am. Mineral. 102, 421–435 (2017).
59. J. R. Smyth, D. R. Bell, G. R. Rossman, Incorporation of hydroxyl in upper-mantle clinopyroxenes. Nature 351, 732–735 (1991).
60. P. M. E. Tollan, H. S. C. O’Neill, J. Hermann, The role of trace elements in controlling H incorporation in San Carlos olivine. Contrib. Mineral. Petrol. 173, 89 (2018). 61. A. H. Peslier, M. Bizimis, Water in Hawaiian peridotite minerals: A case for a dry
metasomatized oceanic mantle lithosphere. Geochem. Geophys. Geosyst. 16, 1211–1232 (2015).
62. M. Le Voyer, E. H. Hauri, E. Cottrell, K. A. Kelley, V. J. M. Salters, C. H. Langmiur, D. R. Hilton, P. H. Barry, E. Furi, Carbon fluxes and primary magma CO2 contents along the global
mid-ocean ridge system. Geochem. Geophys. Geosyst. 20, 1387–1424 (2019).
63. M. Ligi, E. Bonatti, A. Cipriani, L. Ottolini, Water-rich basalts at mid-ocean-ridge cold spots. Nature 434, 66–69 (2005).
64. T. Keller, R. F. Katz, M. M. Hirschmann, Volatiles beneath mid-ocean ridges: Deep melting, channelised transport, focusing, and metasomatism. Earth Planet. Sci. Lett. 464, 55–68 (2017).
65. M. Seyler, M. Toplis, J. P. Lorand, A. Luguet, M. Cannat, Clinopyroxene microtextures reveal incompletely extracted melts in abyssal peridotites. Geology 29, 155–158 (2001). 66. T. Hiraga, I. M. Anderson, D. L. Kohlstedt, Grain boundaries as reservoirs of incompatible
elements in the Earth’s mantle. Nature 427, 699–703 (2004).
67. Y. L. Niu, Bulk-rock major and trace element compositions of abyssal peridotites: Implications for mantle melting, melt extraction and post-melting processes beneath mid-ocean ridges. J. Petrol. 45, 2423–2458 (2004).
68. K. M. Kumamoto, J. M. Warren, E. H. Hauri, New SIMS reference materials for measuring water in upper mantle minerals. Am. Mineral. 102, 537–547 (2017).
69. C. Beyer, S. Klemme, M. Wiedenbeck, A. Stracke, C. Vollmer, Fluorine in nominally fluorine-free mantle minerals: Experimental partitioning of F between olivine, orthopyroxene and silicate melts with implications for magmatic processes. Earth Planet. Sci. Lett. 337–338, 1–9 (2012).
Acknowledgments: We thank an anonymous reviewer, L. Doucet, C. Langmuir, and P. Tollan
for thoughtful reviews that improved this manuscript. We thank B. Schoene for editorial handling of the manuscript. Funding: Funding for this study was supported by NSF EAR-P&G 1524311 and 1839128 to V.L.R. and the Andrew W. Mellon Foundation Award for Innovative Research to V.L.R. A.C. and D.B. were funded by the Italian Programma di Rilevante Interesse Nazionale PRIN 20178LPCPW and PRIN2017KY5ZX8, respectively. Revisions were performed within the duration of a “Visiting Scholar at SCIENCE 2020” award to V.L.R. (University of Copenhagen, Denmark), with support from the Department of Geosciences and Natural Resource Management, Section for Geology. Author contributions: V.L.R. designed the study and wrote the manuscript. V.L.R. and B.M.U. performed the volatile analyses by SIMS at WHOI. E.B., D.B., and A.C. provided the samples and performed major and trace element analyses. S.D. contributed to the discussion on diffusional processes. B.D.M. assisted with technical developments of SIMS analyses at WHOI. All coauthors commented on the manuscript. Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are
present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Submitted 6 November 2020 Accepted 21 April 2021 Published 9 June 2021 10.1126/sciadv.abf6071
Citation: V. Le Roux, B. M. Urann, D. Brunelli, E. Bonatti, A. Cipriani, S. Demouchy, B. D. Monteleone, Postmelting hydrogen enrichment in the oceanic lithosphere. Sci. Adv. 7, eabf6071 (2021).
on June 9, 2021
http://advances.sciencemag.org/
DOI: 10.1126/sciadv.abf6071 (24), eabf6071.
7 Sci Adv
ARTICLE TOOLS http://advances.sciencemag.org/content/7/24/eabf6071
MATERIALS
SUPPLEMENTARY http://advances.sciencemag.org/content/suppl/2021/06/07/7.24.eabf6071.DC1
REFERENCES
http://advances.sciencemag.org/content/7/24/eabf6071#BIBL
This article cites 66 articles, 9 of which you can access for free
PERMISSIONS http://www.sciencemag.org/help/reprints-and-permissions
Terms of Service
Use of this article is subject to the
is a registered trademark of AAAS.
Science Advances
York Avenue NW, Washington, DC 20005. The title
(ISSN 2375-2548) is published by the American Association for the Advancement of Science, 1200 New
Science Advances
License 4.0 (CC BY-NC).
Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of
on June 9, 2021
http://advances.sciencemag.org/