Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
The Journal of Chemical Physics, 134, 12, pp. 121104-1-121104-3, 2011-03-29
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=bafe5b26-a0e0-4c0c-8e2d-e301e14a4f66 https://publications-cnrc.canada.ca/fra/voir/objet/?id=bafe5b26-a0e0-4c0c-8e2d-e301e14a4f66
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1063/1.3574393
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Communication: Single crystal x-ray diffraction observation of hydrogen bonding between 1-propanol and water in a structure II clathrate hydrate
Single Crystal X-ray Crystallographic Observation of Hydrogen
Bonding Between 1-propanol and Water in a Binary Structure II
Clathrate Hydrates
Konstantin Udachin, Saman Alavi, and John A. Ripmeester
Steacie Institute for Molecular Sciences, National Research Council of Canada 100 Sussex Dr., Ottawa, Ontario K1A 0R6 Canada
Abstract
Single crystal X-ray crystallography is used to detect guest-host hydrogen bonding in structure II (sII) binary clathrate hydrate of 1-propanol and methane. X-ray structural analysis shows that the 1-propanol oxygen atom is a distance of 2.749 and 2.788 Å from the closest clathrate hydrate water oxygen atoms from the hexagonal face of the large sII cage. The 1-propanol hydroxyl hydrogen atom is disordered and at distances of 1.956 and 2.035 Å from the closest cage water oxygen atoms. These distances are compatible with guest-water hydrogen bonding. The C-C-C-O torsional angle in 1-propanol in the cage is 91.47° which corresponds to a staggered conformation for the guest. Molecular dynamics studies of this system demonstrated guest-water hydrogen bonding in this hydrate. The molecular dynamics simulations predict most probable distances for the 1-propanol – water oxygen atoms to be 2.725 Å, and the average C-C-C-O torsional angle to be ~59° consistent with a gauche conformation. The individual cage distortions resulting from guest – host hydrogen bonding from the simulations are rather large, but due to the random nature of the hydrogen bonding of the guest with the 24 water molecules making up the hexagonal faces of the large structure II cages, these distortions are not observed in the X-ray structure.
Clathrate hydrates are traditionally considered to form when hydrophobic molecules or molecules with large hydrophobic moieties are reacted with water or ice at low temperatures.[ref]The molecular driving force of the hydrate formation is considered to be
minimization of the water-hydrophobic interactions by forming water cages that isolate the guests by encapsulation. However, it has long been known that this picture is not complete and guest molecules miscible in water also form clathrates if reacted under appropriate thermodynamic conditions. These water-miscible guest molecules have hydrophilic functional groups in addition to the hydrophobic moieties of the molecule and have been predicted to form hydrogen bonds with the water molecules in the solid clathrate structures. Recent evidence from direct single crystal X-ray crystallographic observation of the hydrogen bonding in the hydrate structure in the sH pinacole and sII tert-butylamine binary hydrates,[2] differences between NMR relaxation times[3] and
pressure – temperature phase boundaries[4] of similarly structured guest molecules with
and without hydrogen bonding functional groups, IR vibrational frequencies, and observations from molecular dynamics simulations[2-4,6] shown direct evidence of
hydrogen bonding of guest molecules with hydrophilic functional groups.
. The binary structure II clathrate hydrate phase of the water-miscible 1-propanol guest with methane help gas was recently characterized by a number of groups. Also, a recent molecular dynamics simulation study predicted that significant hydrogen bonding should be expected between 1-propanol guests and host water molecules. In this letter, we present the results of single crystal X-ray structural analysis and further molecular dynamics simulations of hydrogen bonding and guest conformation in this hydrate. Understanding gained from studying factors that affect formation of alcohol clathrate
hydrates can help in understanding the action hydrate formation inhibitors and lead to the design of other hydrate structures with polar and water soluble guests species that may be suitable for gas storage or separation by clathrate technology. .
A single crystal of n-propanol and methane hydrate was grown in a sealed tube from a 1 : 16 n-propanol to water molecular ratio solution with methane pressure of 6 atm at -40 °C over a period of several months. Crystals suitable for diffraction were selected using a microscope mounted in a cold box. Subsequent handling and transfer of the crystals were always done under cold conditions where the crystals were stable.
Single crystal X-ray diffraction data were measured on a Bruker Apex 2 Kappa diffractometer at 100 K, using graphite monochromatized Mo Kradiation ( = 0.71073
Å). The unit cell was determined from randomly selected reflections obtained using the Bruker Apex2 automatic search, center, index, and least squares routines. Integration was carried out using the program SAINT, and an absorption correction was performed using SADABS.[7] The crystal structures were solved by direct methods and the structure was
refined by full-matrix least-squares routines using the SHELXTL program suite.[8] All
atoms were refined anisotropically. Hydrogen atoms on guest molecules were placed in calculated positions and allowed to ride on the parent atoms.
The details of the X-ray structural determination are as follows: chemical formula C4.57H48.30O18[1.57(CH4)·C3H7OH·17H2O], M = 366.37, Z = 8, crystal size 0.4×0.3×0.15
mm3, cubic space group Fd 3 , a = 17.1507(1) Å, V = 5044.8(2) Åm 3, T = 100.0(1) K, ρ
= 1.031 g/cm3, 17531 reflections measured, 461 unique 392 [I>2(I)] , final R indices
differential peak and hole 0.30 and -0.15 e·Å-3, respectively. The water O-H distances
range between 0.816 – 0.872 Å and the 1-propanol O-H distances are measured to be 0.842 Å. The CIF file for the clathrate hydrate is available from the CCDC.[9]
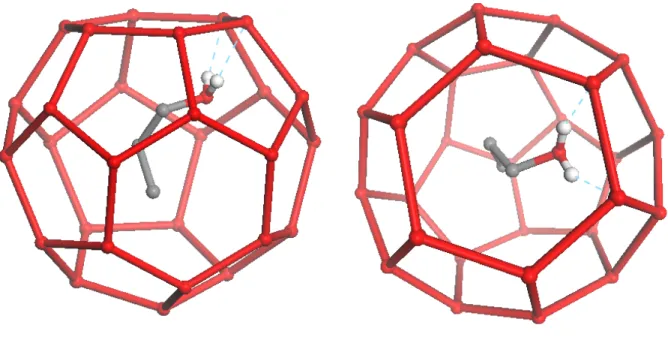
The 1-propanol molecules occupy 24-fold disordered positions in the large cages. One of the 24-fold degenerate position of the 1-propanol molecule in the large sII cage from the X-ray crystal structure is shown in Figure 1. The OH group of the 1-propanol points towards water molecules of the hexagonal faces of the large cage. The measured distances of the propanol hydroxyl oxygen (OH) to the closest cage water oxygens (OW)
are 2.749 and 2.788 Å, and the distance of the hydroxyl hydrogen (HO) to two closest OW atoms are 1.956 and 2.035 Å. These distances are consistent with hydrogen bonding between the 1-propanol guest and the cage water. The 1-propanol proton donating hydrogen bond is seen in the X-ray crystal structure, but the proton accepting hydrogen bond (proton from water) is not directly observed. The reason for this is the proton disorder at the water sites in the cage. The O-C-C-C dihedral angle of the 1-propanol molecule is determined to be 91.446° which corresponds to a staggered conformation.
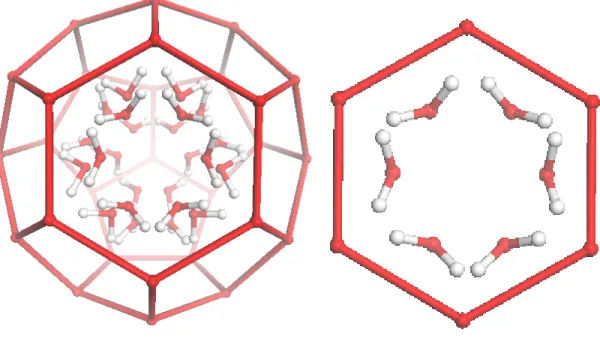
The large sII cage with the 24-fold disordered OH groups of the propanol molecules are shown in Figure 2. The OH groups of the 1-propanol molecules are aligned towards the four hexagonal faces of the cage which are tetrahedrally arranged. The arrangements of the OH groups of the 1-propanol guests are symmetric with respect to all six water molecules of the hexagonal face (shown in Figure 2). The twelve disordered position adjacent to each face are shown more clearly in Figure 2b.
The details of the molecular dynamics simulations and force fields used are similar to those of our previous work.[4,6(b)] and are given in the Supporting
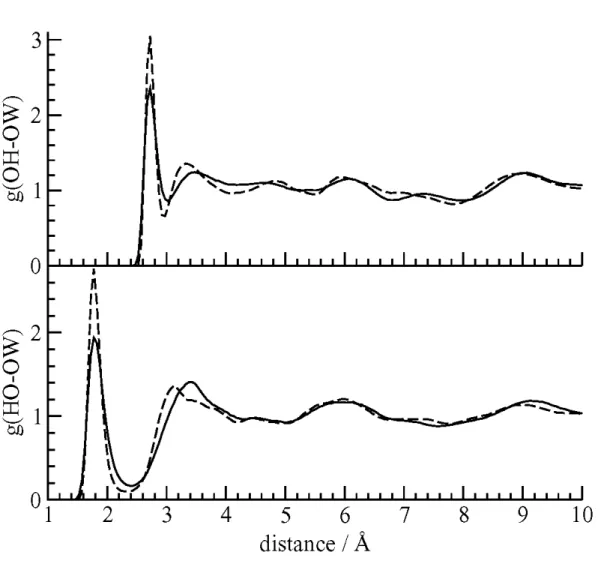
Information.[10] The computed radial distribution functions (RDF) for the O
H-OW and
HO-OW pairs are shown in Figure 3. The peaks in the computed OH-OW and HO-OW
RDFs are at 2.725. and 1.8 Å, respectively which show excellent agreement with the crystallographic results.
The average computed O-C-C-C dihedral angle of 1-propanol from the simulations at 100 K is 58.8° which indicates a gauche conformation for the molecule. The average dihedral angle is constant within 1° at higher temperatures.
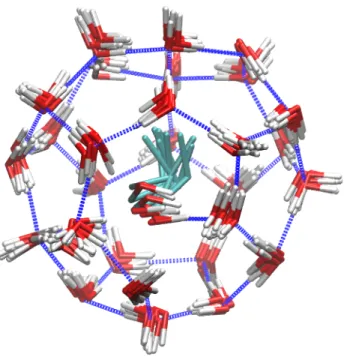
Overlaid snapshots of the motion of a 1-propanol guest in a large cage over a 200 ps period from a 100 K simulation are showed in Figure 4. The ranges of motions of the guest and water molecules are seen in this figure. An animation showing this cage from different perspectives is given in the Supporting Information. Due to hydrogen bonding with the guests, the hexagonal faces of the cage become distorted, but due to the disorder in the 1-propanol distribution in all cages in the simulation cell and the real sample, on average, the water oxygen atoms will be found at the ideal clathrate hydrate positions in the X-ray crystal structure determination. (anything in the thermal ellipsoids of the waters?)
We note that the resolution required to discern the hydrogen bonding of the guest 1-propanol molecule to the cage water molecules is only available from single crystal X-ray analysis and powder X-X-ray diffraction (PXRD) do not provide sufficient resolution. (I would suggest omitting the previosu sentence; “resolution” has different meanings in SC vs
powder diffraction; according to Gary, the H-bonding couldn’t be derived from fitting the powder pattern, but something might be said about the shape and size of the diffraction spots – so a rather more elaborate analysis).
References
1) General clathrate references
2) S. Alavi, K. Udachin, and J. A. Ripmeester, Chem. Eur. J. 16, 1017 (2010). 3) R. Susilo, S. Alavi, I. Moudrakovski, P. Englezos, and J. A. Ripmeester,
ChemPhysChem 10, 824 (2009).
4) T. Makiya, T. Murakami, S. Takeya, A. K. Sum, S. Alavi, and R. Ohmura, Phys. Chem. Chem. Phys. 12, 9927 (2010).
5) (a) V. Buch, J. P. Devlin, I. A. Monreal, B. Jagoda-Cwiklik, N. Aytemiz-Uras, and L. Cwiklik, Phys. Chem. Chem. Phys. 11, 10245 (2009). (b) I. A. Monreal, L. Cwiklik, B. Jagoda-Cwiklik, and J. P. Devlin, J. Phys. Chem. Lett. 1, 290 (2010).
6) (a) S. Alavi, R. Susilo, and J. A. Ripmeester, J. Chem. Phys. 130, 174501 (2009). (b) S. Alavi, S. Takeya, R. Ohmura, T. K. Woo, and J. A. Ripmeester J. Chem. Phys. 133, 074505 (2010).
7) G. M. Sheldrick, 2002, SADABS Ver. 2.03, University of Gottingen, Germany. 8) G. M. Sheldrick, SHELXTL, Version 6.10; Bruker AXS Inc., Madison, Wisconsin,
USA, 2000.
9) CCDC under number ...
10) See Supplementary Material Document No._________ for Tables of the force
field parameters and Cartesian coordinates of optimized water – guest hydrogen bonded clusters. Figures showing the OH-OW radial distribution function, hydrogen bond dynamics are also give. Cartesian coordinates of four sample structure I hydrate cages with ethanol guests along the simulation trajectory at 90 K are also provided. These can be used in viewing animations of the 1-propanol and cage water motions.
Figure 2. The 24 disordered configurations of the hydroxyl groups of the 1-propanol
molecule in the large sII cage as determined by X-ray crystallography (left). The six OH configurations adjacent to a single hexagonal face are shown on the right. The OH groups point towards oxygen atoms of water molecules.
Figure 3. The RDFs for the oxygen atom (top) and hydrogen atom (bottom) of the
1-propanol hydroxyl group with the water oxygen atoms. The sharp peaks at short distances indicate the formation of guest – host hydrogen bonds.
Figure 4. Overlaid snapshots of a 1-propanol molecule in a sII large cage during a 250 ps
simulation from MD simulations at 100 K. The distortion of the hexagonal face of the cage and the gauche conformation of the guest are seen.