HAL Id: tel-02003464
https://tel.archives-ouvertes.fr/tel-02003464
Submitted on 1 Feb 2019
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de
Drug Delivery : detection of Netrin-1 in Breast Cancer
& Immunomodulation in Hepatocellular Carcinoma
Jennifer Wischhusen
To cite this version:
Jennifer Wischhusen. Ultrasound Microbubbles for Molecular Imaging and Drug Delivery : detection of Netrin-1 in Breast Cancer & Immunomodulation in Hepatocellular Carcinoma. Biotechnology. Université de Lyon, 2017. English. �NNT : 2017LYSE1317�. �tel-02003464�
N° d’ordre NNT : 2017LYSE1317
THESE de DOCTORAT DE L’UNIVERSITE DE LYON
opérée au sein del’Université Claude Bernard Lyon 1
Ecole Doctorale
N° 205Ecole Doctorale Interdisciplinaire Sciences-Santé (EDISS)
Spécialité de doctorat
: Biologie Moléculaire et BiotechnologieDiscipline
: SantéSoutenue publiquement le 19/12/2017, par :
Jennifer WISCHHUSEN
Ultrasound Microbubbles for
Molecular Imaging and Drug Delivery:
Detection of Netrin-1 in Breast Cancer &
Immunomodulation in Hepatocellular Carcinoma
Devant le jury composé de :
DUMONTET, Charles Professeur des Universités - Praticien Hospitalier Université de Lyon 1, CRCL-INSERM U1052 Président FERRARA, Katherine Directrice de Recherche University of California Davis Rapporteure
KIESSLING, Fabian Directeur de Recherche RWTH Aachen University Rapporteur LENTACKER, Ine Directrice de Recherche Ghent University Rapporteure MEHLEN, Patrick Directeur de Recherche/ INSERM Université de Lyon 1, CRCL-INSERM U1052 Examinateur BRIDAL, Lori Directrice de Recherche Sorbonne Universités, LIB - CNRS UMR 7371 Examinatrice GRUELL, Holger Directeur de Recherche University of Cologne Examinateur PADILLA, Frédéric Directeur de Recherche/ CNRS LabTAU- INSERM U1032 Université de Lyon 1, Directeur de thèse WILLMANN, Juergen K. Directeur de Recherche Stanford University Invité
Abstract
Ultrasound plays an important role in cancer diagnosis. B-mode imaging and contrast-enhanced ultrasound are used to detect cancerous lesions in breast and liver. Ultrasound contrast agents such as microbubbles can be functionalized with ligands recognizing molecular targets thereby enabling ultrasound molecular imaging. Ultrasound molecular imaging was shown to provide high sensitivity (10-12 M) and resolution (~100 μm). Netrin-1 is upregulated in a large fraction of human cancers and in 70% of metastatic breast cancers. Netrin-1 promotes tumor progression by enhancing cancer cell survival. A newly developed netrin-1 interference therapy requires the identification of patients who overexpress the target protein and, could benefit from anti-netrin-1 therapy. The first major aim of the present thesis was the development of ultrasound molecular imaging of netrin-1 as a companion diagnostic in breast cancer. In the first phase, a novel method, called MB-ELISA and based on Enzyme-Linked ImmunoSorbent Assay (MB-ELISA), was developed for the assessment of targeted microbubble binding in in vitro static binding assays. To validate MB-ELISA, microbubble binding analyses were performed with both MB-ELISA and mosaic microscopy; the latter serving as a reference method. Specificity and sensitivity of MB-ELISA were equivalent or better than that of mosaic microscopy in the two microbubble assay formats in 24- and 96-well plates. While the minimum concentration of coated target protein, required for significant microbubble attachment and detection, was of 500 ng/mL for microscopy, it was of 250 ng/mL for MB-ELISA. MB-ELISA enabled the reliable quantification of microbubble binding in a rapid, high-throughput, and whole-well analysis, facilitating the characterization of new targeted contrast agents. In the second phase, netrin-1 was assessed as a new target for ultrasound molecular imaging: netrin-netrin-1-targeted microbubbles were prepared, validated in in vitro binding assays and feasibility and specificity of netrin-1 imaging were tested in multiple netrin-1-positive and negative murine breast tumor models in vivo. In vivo netrin-1 labeling and ex vivo immunofluorescence analysis confirmed the expression and co-localization of netrin-1 with the vascular marker CD31 in human SKBR7, murine 4T1, and murine MMTV-PyMT breast tumors. In vitro flow chamber assays showed specific binding of anti-netrin-1-MBs to netrin-1-overexpressing breast cancer cell lines. In vivo imaging of netrin-1 showed significantly increased imaging signal with anti-netrin-1-microbubbles in netrin-1-positive breast tumors compared to signals from isotype control microbubbles or after blocking of netrin-1. In netrin-1-negative breast tumors, as well as in wild type normal mammary glands, no molecular imaging signal was observed with anti-netrin-1-microbubbles or isotype control microbubbles. These results suggest that ultrasound molecular imaging allows accurate detection of netrin-1 on the vascular endothelium of netrin-1-positive tumors and has the potential to become a companion diagnostic for netrin-1 interference therapy in breast cancer patients.
Therapeutic ultrasound is intensively studied for treatment of different types of cancer. In addition to thermal ablation mediated by high-intensity focused ultrasound,
ultrasound-targeted microbubble destruction triggers cavitation and sonoporation thereby permeabilizing the tissue and facilitating the penetration of drugs from the blood stream
into the tumor tissue. This type of mechanical ultrasound further induces immune cell infiltration and tumor antigen release, thereby triggering anti-tumor immune system activation. Hepatocellular carcinoma is the most common cause of cancer-related deaths as patients are frequently diagnosed at intermediate or late stage and show bad response to the currently available therapies, creating the need for novel treatments. The immune microenvironment plays a crucial role in hepatocellular carcinoma development. Tumor-infiltrating lymphocytes promote tumor cell growth and suppress anti-tumoral immune responses. Thus, the modulation of tumor immunology might re-activate anti-tumoral activities and improve hepatocellular carcinoma management. The second major aim of the present thesis was the study of the immunomodulatory effects of an ultrasound-targeted microbubble destruction approach for the delivery of nanoparticles loaded with microRNA-122 and anti-microRNA-21, with respectively anti-cancer, chemo-sensitizing, and immuno-regulating activities. In the first phase, a novel immuno-competent mouse model bearing subcutaneous hepatocellular carcinoma was developed and its response to the previously reported combination treatment, composed of ultrasound delivery of microRNAs followed by intraperitoneal doxorubicin administration, was assessed. In this thesis, ultrasound-targeted microbubble destruction-mediated microRNA-122 and anti-microRNA-21 delivery followed by doxorubicin injections was shown to reduce tumor growth in the immuno-competent mouse model; thus confirming therapeutic efficacy. In the second phase, potential immunomodulatory effects of mechanical ultrasound or microRNA-122 and anti-microRNA-21 delivery were assessed. To do so, different cytokine screening assays were set up to assess expression profiles in hepatocellular carcinoma samples, including tumor tissue, lymph nodes, and serum, after treatment with either mechanical ultrasound alone or after ultrasound-mediated microRNA delivery. Cytokine screenings in tumors, lymph nodes, and serum within the first 24 h after mechanical ultrasound treatment indicated heterogeneous pro- and anti-tumoral activities. Lymph nodes adjacent to tumors treated with ultrasound-mediated microRNA delivery showed pro-tumor cytokine downregulation and anti-tumor cytokine upregulation, suggesting an overall positive therapy response with regard to tumor immunology. Some of the immunomodulatory effects were observed both in the treated tumor, but also in the contralateral control tumor, suggesting systemic immune system activation. Though, further immune cell characterization studies are required to understand whether a phenotypic shift can be achieved. The results identified ultrasound-targeted microbubble destruction-mediated microRNA delivery as a potential immunomodulatory therapeutic approach.
In conclusion, ultrasound molecular imaging and ultrasound-targeted microbubble destruction turned out to be powerful techniques that have the potential to improve diagnosis and therapy of different types of carcinomas.
Keywords: Targeted microbubbles, in vitro static binding assays, molecular imaging, ultrasound molecular imaging in vivo, netrin-1, breast cancer, personalized medicine,
Microbulles Ultrasonores pour l’Imagerie Moléculaire et
la Délivrance de Médicaments:
Détection de la Nétrine-1 dans le Cancer du Sein & Modulation
de la Réponse Immunitaire dans le Carcinome Hépatocellulaire
Les ultrasons ont un rôle important dans le diagnostic du cancer. L’imagerie B-mode et l’imagerie de contraste ultrasonore permettent de visualiser des lésions cancéreuses dans le sein ou le foie. Plus récemment, avec l’utilisation de microbulles ou agents de contraste ultrasonores, fonctionnalisées avec un ligand ciblant un marqueur moléculaire, l’imagerie moléculaire ultrasonore a été introduite. L’imagerie moléculaire ultrasonore montre une excellente sensibilité (10-12 M) et résolution (~100 μm). Nétrine-1 est une protéine surexprimée dans différents type de cancer humain, incluant 70% des cancers du sein métastatiques. Nétrine-1 contribue à la progression tumorale en supprimant l’apoptose et en induisant la survie et la prolifération cellulaire. Une thérapie moléculaire récemment développée, interférant avec l’activité de la nétrine-1, nécessite maintenant l’identification des patientes qui surexpriment la nétrine-1 et peuvent bénéficier de ce traitement. Le premier objectif de la thèse était le développement de l’imagerie moléculaire par ultrasons de la nétrine-1 en tant que test d’accompagnement pour le cancer du sein. Dans un premier temps, une nouvelle méthode, appelée MB-ELISA et basée sur l’Enzyme-Linked ImmunoSorbent Assay (ELISA), a été développé pour la validation de microbulles ciblées lors de tests d’accrochement in vitro. Afin de valider le test MB-ELISA, l’accrochement des microbulles a été analysé par MB-ELISA et par microcopie mosaïque ; la dernière servant de technique de référence. Pour les deux formats de test d’accrochement en plaque à 24 puits et à 96 puits, la spécificité et sensibilité de MB-ELISA se sont révélées équivalentes ou meilleures que celles de la microscopie mosaïque. Alors que la concentration minimale de la protéine cible requise pour un accrochement et une détection significative était de 500 ng/mL pour la microscopie, elle n’était que de 250 ng/mL pour MB-ELISA. La spécificité et la sensibilité de MB-ELISA permettent de quantifier l’accrochement de microbulles de manière fiable et rapide sur un grand nombre d’échantillons, ce qui facilite la caractérisation de nouveaux agents de contraste ciblés. Dans un deuxième temps, nétrine-1 a été étudiée comme cible pour l’imagerie moléculaire par ultrasons. Des microbulles anti-nétrine-1 ont été préparées, validées lors de tests d’accrochements in vitro, et la faisabilité et la spécificité de l’imagerie de la nétrine-1 ont été testées dans plusieurs modèles in vivo de tumeurs du sein, positif ou négatif pour la nétrine-1. Le marquage de la nétrine-1 in vivo et sa révélation ex vivo par immunofluorescence ont confirmé l’expression et la co-localisation de la nétrine-1 avec le marqueur vasculaire CD3nétrine-1 dans les tumeurs du sein humain SKBR7 et de souris 4Tnétrine-1 et MMTV-PyMT. Des essais in vitro en chambre à flux ont démontré l’accrochement spécifique des microbulles anti-nétrine-1 aux cellules de lignées de cancer du sein qui surexpriment la nétrine-1. L’imagerie in vivo de la nétrine-1 a démontré un signal plus élevé avec des microbulles anti-nétrine-1 dans les tumeurs positives pour la nétrine-1, comparé au
signal des microbulles de contrôle isotypique ou après avoir bloqué la nétrine-1. Dans les tumeurs négatives pour la nétrine-1 ainsi que dans les glandes mammaires normales, aucun signal d’imagerie moléculaire n’a été observé avec les microbulles anti-nétrine-1 et de contrôle isotypique contrôle. Ces résultats suggèrent que l’imagerie moléculaire par ultrasons permet la détection spécifique et fiable de la nétrine-1 présentée sur l’endothélium des tumeurs positives pour la nétrine-1, et qu’elle a le potentiel de devenir un test d’accompagnement pour la thérapie d’interférence de la nétrine-1 chez les patientes atteintes de cancer du sein.
Les ultrasons thérapeutiques sont également utilisés pour le traitement de différents types du cancer. En addition de la technique d’ablation thermique par les ultrasons focalisés à haute intensité, la destruction ciblée des microbulles par ultrasons, qui induit cavitation et sonoporation, est utilisée pour perméabiliser le tissu et faciliter la délivrance de médicaments. Ce type d’action mécanique des ultrasons a également le potentiel d’induire l’infiltration de cellules immunitaires et la libération d’antigènes tumoraux, déclenchant une réponse anti-tumorale du système immunitaire. Le carcinome hépatocellulaire est la cause de mort par cancer la plus fréquente, car les patients sont seulement diagnostiqués à un stade intermédiaire ou avancé, et la réponse aux traitements actuellement disponible est insuffisante. Il y donc un besoin pour de nouvelles approches thérapeutiques. Le microenvironnement immunitaire joue un rôle important dans le développement du carcinome hépatocellulaire. Les lymphocytes infiltrant la tumeur promeuvent la croissance tumorale et suppriment les réponses immunitaires anti-tumorales. Par conséquent, la modulation de l’immunologie tumorale pourrait réactiver les réponses anti-tumorales et améliorer la gestion du carcinome hépatocellulaire. Le deuxième objectif de la thèse est l’étude des effets immuno-modulatoires d’une approche de destruction ciblée des microbulles par ultrasons pour la délivrance de nanoparticules chargées en microARN-122 et anti-microARN-21, ayant des activités anti-cancéreuses, chimio-sensibilisantes, et immuno-régulatrices. Dans un premier temps, un nouveau modèle de souris immuno-compétent avec des tumeurs du carcinome hépatocellulaire sous-cutanées a été développé et la réponse de la tumeur au traitement combiné décrit dans des études préalables, composé de la délivrance de microARNs par ultrasons suivi par l’administration intra-péritonéale de la doxorubicine, a été analysée. Dans cette thèse, la destruction ciblée des microbulles par ultrasons pour la délivrance de nanoparticules chargées en microARN-122 et anti-microARN-21 suivi par des injections de doxorubicine a induit une diminution de la croissance tumorale dans le modèle murin immuno-compétent ; confirmant ainsi son efficacité thérapeutique. Dans un deuxième temps, les effets immuno-modulatoires potentiels des ultrasons mécaniques ou de la délivrance de microARN-122 et anti-microARN-21 ont été analysés. Pour cela, différents tests de quantification de cytokines ont été établis afin d’analyser les profils d’expression de cytokines dans différents échantillons, tels que le tissue du carcinome hépatocellulaire, les nœuds lymphocytaires et le sérum, après un traitement soit avec les
au cours des premières 24 h après traitement avec les ultrasons mécaniques seuls a indiqué des activités hétérogènes à la fois pro- et anti-tumorales. Les nœuds lymphocytaires adjacents aux tumeurs traitées avec la délivrance de microARNs par ultrasons ont démontré une baisse d’expression des cytokines pro-tumorales et une augmentation d’expression des cytokines anti-tumorales, suggérant une réponse thérapeutique globale positive par rapport à l’immunologie tumorale. Des effets immuno-modulatoires ont été observés dans la tumeur traitée ainsi que dans la tumeur contrôle contra-latérale, indiquant une activation systémique du système immunitaire. Cependant, des études de caractérisation des populations de cellules immunitaires seront nécessaires afin de comprendre si des changements phénotypiques sont déclenchés. Les résultats ont identifiés l’approche de la destruction ciblée des microbulles par ultrasons pour la délivrance de micro-ARN comme un outil thérapeutique et immuno-modulatoire puissant.
En conclusion, l’imagerie moléculaire par ultrasons et la destruction ciblée des microbulles par ultrasons s’avèrent être des techniques puissantes qui ont le potentiel d’améliorer le diagnostic et la thérapie de différents types de carcinomes.
Mots-clés : Microbulles ciblées, test d’accrochement statique in vitro, imagerie moléculaire, imagerie moléculaire ultrasonore in vivo , nétrine-1, cancer du sein, médecine personnalisée, destruction ciblée des microbulles par ultrasons, délivrance de médicament, cavitation, microARN-122, microARN-21, carcinome hépatocellulaire, réponses immunitaire
L’intitulé et l’adresse du laboratoire où la thèse a été préparée: LabTAU INSERM U1032
151 cours Albert Thomas 69424 cedex 03 Lyon
Acknowledgements
The work in this thesis was supported by the LabEx DEVweCAN (ANR-10-LABX-0061) of the University of Lyon, within the program "Investissements d'Avenir" (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR), the French Ligue nationale contre le cancer, and by NIH R01CA155289 (JKW). The ultrasound molecular imaging study was performed at the Stanford Center for Innovation in In-Vivo Imaging (SCI3), supported by NIH S10OD010344 for the VisualSonics Vevo2100. Confocal microscopy was performed at the Stanford Neuroscience Microscopy Service, supported by NIH NS069375.
My PhD was supported by the LabEx DEVweCAN (ANR-10-LABX-0061) of the University of Lyon, within the program "Investissements d'Avenir" (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR), by the German-American Fulbright Commission, by the France-Stanford Center for Interdisciplinary Studies, and by NIH R01CA155289.
With regard to the different experiments in the ultrasound molecular imaging study, I wish to thank Annabelle Bouchardon from the Fédération de Recherche Santé Lyon-Est for assistance with mosaic microscopy, the Laboratoire d’Automatique et de Génie des Procédés (LAGEP) for access to the Zetasizer Nano ZS, Malvern Instruments, and Dr. Thierry Bettinger from Bracco for assistance with the preparation of targeted microbubbles.
I would like to thank Frederic Padilla for being a great mentor and a friend during this adventure, called PhD. I appreciated your guidance through the different projects, your answers to all my questions, and your support with scientific but also administrative hurdles. I am proud of our achievements and I believe we surpassed ourselves. Thank you for everything!
I would like to thank the whole team of the LabTAU for a great time. I enjoyed our lunch break discussions, coffee breaks, lab days, Christmas festivities, and end-of-the-day beers. The LabTAU provided me with a second family while I was away from mine! I would like to say a special thank you to Dr. Cyril Lafon who co-supervised me. I would like to say a big thank you to Rodolfo Molina-Peña who was an excellent internship student. Another big thank you goes to Jacqueline Ngo who was always available to help me out with preparations, assist me with experiments, but also to listen and give advice whenever needed! My coup de cœur at the LabTAU is Dr. Charlène Bouyer. Charlène, you made many of my days since your return from Stanford in 2015. I thank you for being who you are! All the best to you!
Furthermore, I would like to thank the team of Prof. Patrick Mehlen at the CRCL. You were great collaborators. I learned a lot from you and I appreciated your numerous efforts to help me progress with my projects. Special thank goes to Dr. Jean-Guy Delcros who helped on a
nearly daily basis planning, performing, analyzing, and interpreting the experiments for the molecular and cellular biology work. It was a pleasure to work with you!
Also the team at Stanford University deserves my thanks. I am happy that I was given the opportunity to spend one year of my PhD in the laboratory of Prof. Juergen K. Willmann. I learnt a lot from Prof. Willmann and his lab. Thank you very much, Juergen!
Dear Juergen, I will always be incredibly grateful that I had the chance to meet you and work with you. I was very impressed when I first met you a few years ago, and even more when I saw you working at Stanford, in research and the clinic, and on so many different and always highly relevant projects. You were so determined, motivated and invested, and you still found the time to listen to and support young researchers. This tragedy is a big loss for the scientific community, but an even bigger one for your family and friends. My sympathy goes out to your wife, Amelie, and your children, Juliana and Alexander… (January 30, 2018)
I would also like to thank Dr. Ramasamy Paulmurugan for lots of discussions and guidance. A special thank you goes to Rayhaneh Afjei for welcoming me so kindly and accompanying me as a friend during my time at Stanford. My coup de cœur at Stanford was Dr. Katheryne Wilson. Katie, I am infinitely glad we met and had the opportunity to spend lots of beautiful moments together in California, but also on our trips to Seattle/Chehalis, New York, and Philadelphia. You, Nora and Igor became my surrogate family on the other side of the world and it broke my heart when I had to leave you. I am happy we managed to stay in contact ever since!
Ich möchte außerdem meiner Familie und meinen Freunden danken für ihren unermüdlichen guten Zuspruch über Jahre hinweg und trotz der großen Distanz. Vielen Dank Papa und Mama für das Vertrauen, die Unterstützung und eure Besuche in Lyon und in Kalifornien! Vielen Dank Benni und Vanessa, dass ihr an mich geglaubt und mich unterstützt habt. Lieben Dank, Oma, für ein offenes Ohr und ein bisschen Ablenkung hier und da! Ich sende auch einen stillen Gedanken an Opa. Vielen lieben Dank auch an Alicia und Daniel! Und vielen Dank an Franzi, für viele liebe Worte! Ihr seid die Besten!
Un grand merci aussi à mes proches en France: Denise, Jean-Claude, Romain et Géraldine de Marseille, Dany, Karine, Luce, Anouck et Jules de Bron, Allan et Laurène de Paris, Christelle, Anne-Gaëlle, Lucie et Ali de Lyon. Merci à tous ! Vous êtes au top !
Ce dernier mot est pour mon amour, Pierre-Julien. Mille mercis parce que tu as toujours été à mes côtés ! Tu m’as soutenue dans mon désir de faire une thèse et de partir aux Etats-Unis. Tu as partagé mes réussites et moments de bonheur, mais aussi mes moments de doutes et de peine. Nous avons surmonté un an de relation à longue distance. Pour moi, cette thèse était notre bataille et nous avons réussi !
“Wahrlich es ist nicht das Wissen, sondern das Lernen, nicht das Besitzen, sondern das Erwerben, nicht das Da-Seyn, sondern das Hinkommen, was den grössten Genuss gewährt.“
Table of Contents
Chapter I – General Introduction ... 15
1 Cancer ... 16
1.1 Cancer Development ... 16
1.2 Breast Cancer ... 17
1.3 Liver Cancer ... 19
2 Biomedical Ultrasound in Cancer ... 22
2.1 Diagnostic Ultrasound ... 22
2.2 Therapeutic Ultrasound ... 24
3 Objective of the Thesis ... 26
Chapter II – Ultrasound Molecular Imaging of Netrin-1 in Breast Cancer ... 28
1 Molecular Imaging ... 29
1.1 Positron Emission Tomography ... 30
1.2 Single Photon Emission Computed Tomography ... 30
1.3 Molecular Computed Tomography ... 30
1.4 Molecular Magnetic Resonance Imaging ... 31
1.5 Optical Molecular Imaging ... 31
1.6 Photoacoustic Molecular Imaging ... 31
1.7 Ultrasound Molecular Imaging ... 32
2 Netrin-1 ... 38
2.1 Physiological Functions ... 38
2.2 Dependence Receptors ... 38
2.3 Pathological Functions ... 39
2.4 Endothelial Expression and Function ... 39
2.5 Netrin-1 as a Therapeutic Target ... 40
2.6 Can Netrin-1 be a Diagnostic Target for USMI with Targeted MBs? ... 40
3 Specific Aims ... 42
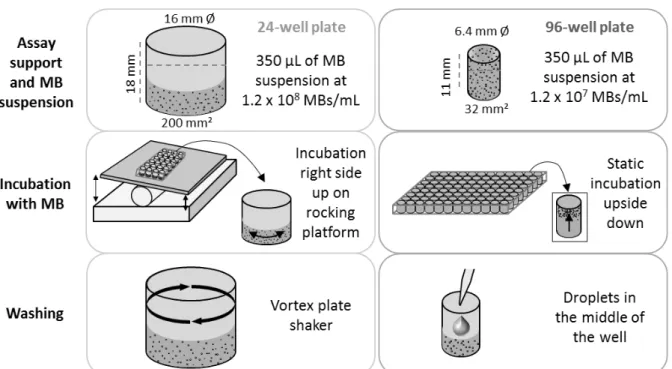
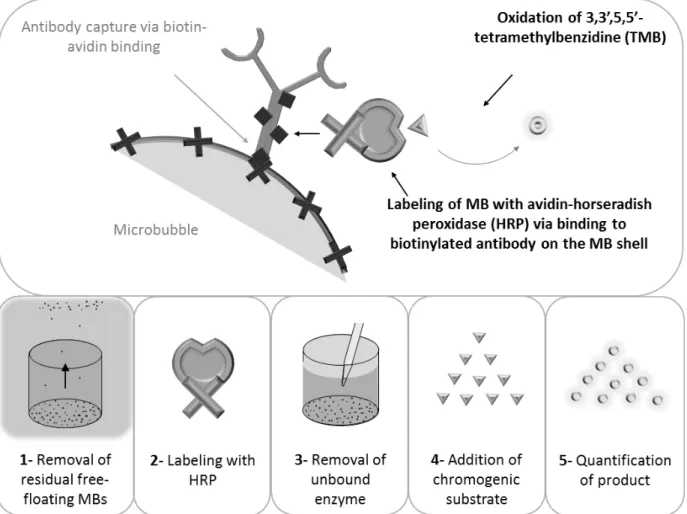
4 Study – Microbubble Enzyme-Linked ImmunoSorbent Assay (MB-ELISA) for the Detection of Targeted Microbubbles in In Vitro Static Binding Assays... 43
4.1 Abstract ... 43
4.3 Material and Methods ... 45
4.4 Results ... 52
4.5 Discussion ... 59
4.6 Conclusion ... 62
4.7 Supplement ... 63
5 Study – Ultrasound Molecular Imaging as a Non-Invasive Companion Diagnostic for Netrin-1 Interference Therapy in Breast Cancer ... 64
5.1 Abstract ... 64
5.2 Introduction ... 65
5.3 Material and Methods ... 68
5.4 Results ... 69
5.5 Discussion ... 79
5.6 Conclusion ... 82
5.7 Supplement ... 83
6 Conclusions on Ultrasound Molecular Imaging ... 95
Chapter III – Ultrasound Drug Delivery & Immunomodulation in Hepatocellular Carcinoma 99 1 Localized Drug Delivery in Cancer ... 100
1.1 Drug Carriers ... 100
1.2 Implanted Depot Systems ... 100
1.3 Passive Accumulation ... 101
1.4 Endogenous Stimuli-Responsive Drug Carriers: pH, Enzyme, Redox ... 101
1.5 Exogenous Stimuli-Responsive Drug Carriers: Temperature, Magnetic Field, Electric Pulses, Light ... 103
1.6 Ultrasound-Targeted Microbubble Destruction ... 105
2 Cancer Immunology ... 112
2.1 Anti- and Pro-Tumoral Immune Responses and Immunotherapy... 112
2.2 Immune System and Immunotherapy in Hepatocellular Carcinoma ... 114
2.3 Ultrasound Cancer Immunotherapy ... 115
2.4 Roles of microRNA-122 and -21 in Hepatocellular Carcinoma ... 117
2.5 Can Ultrasound-Mediated microRNA Delivery Reactivate Anti-Tumoral Immune Responses in Hepatocellular Carcinoma? ... 117
4 Study – Ultrasound-Mediated Delivery of miRNA-Loaded Nanoparticles Modulates Immune Responses in Subcutaneous Murine Hepa1-6 Hepatocellular Carcinoma in
Syngeneic C57BL/6J Mice ... 120
4.1 Abstract ... 120
4.2 Introduction ... 121
4.3 Material and Methods ... 122
4.4 Results ... 128
4.5 Discussion ... 142
4.6 Conclusion ... 153
4.7 Supplement ... 154
5 Conclusions on Ultrasound Drug Delivery & Immunomodulation ... 171
Chapter IV - General Discussion ... 174
References ... 179
Chapter I
– General Introduction
General Introduction
This chapter provides a general overview on cancer development, with special focus on breast cancer and hepatocellular carcinoma, and on the role of ultrasound in cancer management.
1 Cancer
1.1 Cancer Development
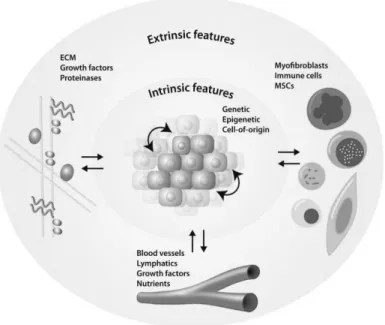
Cancer is a worldwide health issue with 14.1 million new cases and 8.2 million deaths in 2012 (1). Incidence is highest for lung, breast, and colorectal cancer, and mortality is highest for lung, liver, and stomach cancer (1). Cancer is a highly diverse group of diseases, and carcinomas, which derive from epithelial cells of the body, present the highest number of clinical cases (2). More than 100 different types of cancer are described, and subtypes of cancer can develop in the same organ (3). Cancer is a genetic and highly dynamic disease. Genetic predisposition, ageing, and exposure to environmental factors cause genetic alterations which in turn lead to genetic instability. Oncogenes get activated causing gain of function, and tumor suppressor genes are inactivated causing loss of function (3). Today, ten common hallmarks of cancer are identified including resisting cell death, sustained proliferative signaling, evading growth suppressors, enabling replicative immortality, activating invasion and metastasis, inducing angiogenesis, deregulating cellular energetics, avoiding immune destruction, tumor-promoting inflammation, and genome instability and mutation (4). Cancer development is characterized by a multistep process starting with the transformation of a normal cell and leading to pre-cancerous lesions, carcinoma in situ, and metastasis (5). Tumors are composed of genetically and epigenetically distinct neoplastic cell populations, with different tumorigenic potentials, causing intra-tumor and inter-tumoral heterogeneity (2). The clonal evolution theory was the first attempt to describe the complex process of cancer development. Accordingly, stepwise accumulation of somatic-cell mutations followed by subclonal selection, on the principle of Darwinian selection, drives the survival and expansion of tumors in the tissue ecosystem (6). Second, the cancer stem cell hypothesis was proposed in which a normal tissue stem cell acquires transforming mutations and initiate cancer and metastasis (7). Alternatively, cells of origin in cancer development were described as transit-amplifying cells with mutations that trigger dedifferentiation towards stem cell state suggesting that tumor development is caused by plasticity (5). Here, cancer stem cells produce proliferating progeny acquiring novel mutations and converting them into novel cancer stem cells promoting tumor growth and metastasis. The different theories explain different findings in cancer research and it is expected that all three processes are involved in human tumorigenesis (2). In addition to the genetic and epigenetic variability and the cancer cell of origin, which are cell intrinsic factors, extrinsic factors were described to drive cancer development. The microenvironment with the extracellular matrix, blood vessels and lymphatics, stromal cells, and the respective associated bioactive components, such as growth factors and nutrients, are important determinants for progression of carcinoma (Fig.I.1.1) (8,9). Epithelial-to-mesenchymal transition (EMT) plays a central role in cancer progression and metastasis. EMT transcription factors provide cancer cells with migratory and invasive capacities, tumor-initiating potential, and drug resistance (10). There are three types of mutations that can occur in tumor cells: founder mutation
tumor growth, and passenger mutations which do not provide a selective advantage for cancer cells (11). Founder and driver mutations are considered crucial for tumor survival and progression, but the identification of these key mutations is again challenged by phenotypic heterogeneity.
The different contributors and processes involved in cancer initiation, progression, and metastasis provide the current basis for identification of cancer biomarkers for detection, staging, and therapy response prediction, but also for the development of anti-cancer therapies for personalized anti-cancer medicine. As of today, our knowledge about anti-cancer development, despite phenomenal advances in the last decades, is still incomplete, and mechanism-based targeted therapies show variable and transitory responses (12). The complexity and heterogeneity of tumor parenchyma and stroma creates the need to find innovative, comprehensive, multidisciplinary approaches to control cancer. In this thesis, highly frequent breast cancer and highly lethal liver cancer were selected for the study of novel diagnostic and therapeutic strategies.
1.2 Breast Cancer
Breast cancer is the second most frequent cancer and the fifth cause of cancer death worldwide (13). In women, breast cancer is the most frequent cancer, the second cause of cancer death in more developed regions, and the first cause of cancer death in less developed regions in the world (13). In 2012, 1.67 million new breast cancer cases were diagnosed and presented 25% of all cancers (13). Breast mainly consists of mammary glands, including several lobes and ducts, and blood vessels. Among breast carcinomas, 55% are invasive ductal carcinoma, 13% ductal carcinoma in situ, and 5% are invasive lobular carcinoma (14). Invasive carcinomas have a 85% chance of 5-year survival (14). Age, reproductive factors, endogenous and exogenous hormone exposures, genetic predisposition, and environmental factors, such as alcohol consumption, physical activity, obesity, and radiation, are associated with breast cancer development (15).
Figure I.1.1: Intrinsic and extrinsic factors contributing to tumor development. Image reproduced from (2) with permission.
Screening has largely decreased breast cancer mortality as cancer early detection presents the best chances for positive outcome and survival (15). Breast cancer screening includes breast self-examination by palpation, and imaging by mammography with X-rays, by magnetic resonance imaging (MRI) which has higher sensitivity but less specificity than X-ray, and by ultrasound especially in patients with dense breast tissue (15). The diagnostic workflow is summarized in Tab.I.1.1. Patient history and physical examination serve to determine breast cancer symptoms. Diagnostic imaging protocols and image-guided needle biopsies are performed according to the patient’s age and lesion characteristics. Mammography is the gold standard for breast cancer detection, image analysis is standardized by the breast imaging reporting and database system (BI-RADS), and carcinomas are principally described by mass, asymmetry, and calcifications (15). Ultrasound and MRI are also integral parts of the breast cancer diagnosis guidelines for selected patients with inconclusive or suspicious mammography findings or patients at high risk (16,17). Biopsies are used to determine estrogen receptor, progesterone receptor, and HER2 expression to predict response to endocrine or anti-HER2 therapies (15). In addition to immunohistochemistry (IHC) analysis, mRNA screening assays have been developed for breast cancer and verify the expression of up to 70 genes for prognosis (Oncotype DX, Mammaprint) (18). Indeed, breast cancer is a pioneer for the concept of personalized medicine in which target expression is analyzed prior to therapy decision.
Breast cancer patients are staged according to carcinoma type and size, regional lymph node infiltration, and metastasis (19). Breast-conserving surgery is preferred for women with early stage breast cancer. This does not apply to patients with multicentric disease, diffuse and malignant calcifications, inflammatory breast, persistent positive margins after surgery, and those who cannot receive radiation. A negative surgical margin decreases the risk for local recurrence, though the notion of a negative margin is not
generally defined (15). Neo-adjuvant chemotherapy helps to reduce tumor sizes and enable breast-conserving surgery, but also to eradicate local or distant residual disease. To further decrease the risk of local recurrence, surgery is accompanied by radiation therapy. Adjuvant systemic endocrine and targeted therapies reduce recurrence due to micro-metastatic disease (15). Depending on the HER and hormone receptor status, patients are divided into four different subtypes: luminal A (positive for hormone receptors, negative for HER2, low Ki-67 proliferation marker), luminal B (same as luminal A, but with high Ki-67), HER2-enriched, and basal-like (HER2 and hormone receptor negative) (20). According to subtype, patients are treated with trastuzumab in combination with chemotherapy, or endocrine therapy (19). If breast-conserving surgery is not feasible or sufficient, e.g. in the case of axillary lymph node involvement, radiation, chemotherapy, total mastectomy with or without breast reconstruction are applied. After therapy, women undergo surveillance and follow-up with an, at least, bi-annual interval for five years.
The heterogeneity in breast cancer challenges the successful development of molecularly targeted agents. Innovative approaches are required to improve clinical outcome for patients with heterogeneous disease (21). The dynamic character of breast cancer further underlines the need for molecular monitoring to react to disease evolution with therapy adjustments (18).
1.3 Liver Cancer
Hepatocellular carcinoma (HCC) is the second most common cause of death from cancer worldwide (22). It has been estimated that HCC caused 746,000 deaths in 2012, which represents 9.1% of all cancer-related deaths. In men, HCC is the fifth most common cancer and in women the ninth most common cancer. In 2012, 782,000 new HCC cases were registered. Despite a predominance of liver cancer in Asian countries (50% of new cases in China in 2012), trends in incidence indicate an increase in the USA and Europe (3.8% and 6.6%, respectively, in 2012). Liver cancer is associated with bad prognosis with an overall ratio of mortality to incidence of 0.95. Primary hepatic cancers include HCC, intrahepatic bile duct cancer (cholangiocarcinoma), angiosarcoma and hemangiosarcoma, and hepatoblastoma, which are histologically distinct diseases. HCC is the most frequent and represents 80% of all cases (23). HCC develops in 70-90% of all cases in the context of chronic liver disease (24). Common risk factors are hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, alcohol abuse, and aflatoxin B1 exposure (a toxin produced by fungi Aspergillus flavus and A. parasiticus) (24,25). Like other cancers, HCC develops in a multi-step process (26). The mentioned etiological factors induce hepatic injury which leads to necrosis of hepatocytes, and necrosis induces hepatocyte proliferation. Continuous destruction-proliferation cycles finally lead to chronic liver disease. Oxidative stresses generated during injury activate stellate cells that produce fibroblastic cells without hepatic functionality, and extracellular matrix components such as collagen that promote liver fibrosis and cirrhosis. Cirrhosis describes the replacement of functional liver tissue by abnormal liver nodules surrounded by scar tissue. Further mutational insults from mutagenic aflatoxin, genome-integrating HBV, chronic HCV infection, and chronic alcohol-mediated
inflammation continue to deregulate cell proliferation. Hyperplastic nodules progress to dysplastic nodules, and finally to HCC, which dedifferentiates with progression and becomes more malignant. The first part of pathogenesis leading to liver cirrhosis is accompanied by telomere shortening, while the second part of pathogenesis resulting in HCC is characterized by telomerase activation, p53 loss or mutation, and genomic instability (26).
Screening for HCC is widely applied for high risk patients and reduces HCC-related mortality (27,28). Ultrasound is the recommended surveillance test as it is well tolerated, widely available, and has good sensitivity of 60-80% and specificity higher than 90% (24). The optimal screening interval is 6 months independent of the individual patient risk (27). The diagnostic algorithm starts with the detection of a nodule during ultrasound examination. If the mass is <1 cm, ultrasound is repeated every three months. For diagnosis of masses of sizes >1 cm, multi-detector computed tomography (MDCT) and/or dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) are applied. If the lesion shows arterial hypervascularization and a washout in the venous or delayed phase, it is considered HCC. Otherwise a complementary imaging modality is used to confirm or disapprove the result. In case of inconclusive results, a biopsy is performed to further analyze the liver mass. If the biopsy is negative for HCC, ultrasonography surveillance at 3 months interval is conducted until the nodule either disappears or displays diagnostic characteristics of (27).
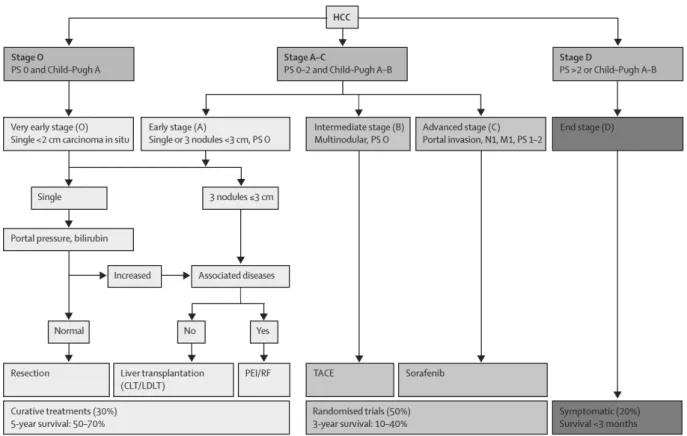
The current approach for treatment decision is based on the patient stratification strategy of the Barcelona Clinic Liver Cancer (BCLC) and has been tested and confirmed since 2003 (Fig.I.1.2) (24,27,29–35). Patients are stratified according to tumor size and numbers, Child-Pugh score, which describes liver function (A=best to C=worst), performance status (PS, 0=best to 2=worst), which quantifies the cancer patients’ general well-being, and the impact of treatment on life expectancy. Early-stage patients (single HCC <5 cm or up to three HCC lesions <3 cm) are subjected to potentially curative treatments like partial liver resection, complete liver resection in combination with liver transplantation or ablation using percutaneous ethanol infusion or radiofrequency. These patients have a good 5-year survival rate of 50-70%. Intermediate stage patients who are excluded from surgery due to large/multifocal HCC without vascular invasion or extrahepatic spread undergo palliative therapy called transarterial chemoembolization (TACE). TACE occludes the hepatic artery and thereby reduces the major blood supply to the tumor. Simultaneously, infusion of chemotherapy into the hepatic artery increases drug exposure to the tumor. Advanced stage patients with preserved liver function are treated with the targeted therapy drug sorafenib, a small molecule kinase inhibitor. Intermediate and advanced patients have a 3-year survival rate of 10-40%. End stage HCC patients receive symptomatic treatment for pain relief and survival prolongation, but survival is limited to less than three months. Liver cancer management is challenging due to resistance to current anti-cancer drugs, lack of biomarkers to detect very early disease and underlying liver disease that limits the use of chemotherapeutics (26). Locally limited chemotherapy delivered in form of TACE shows
and the recently approved Regorafenib, a multi-kinase inhibitor, are indicated for advanced HCC patients and increases overall survival by three months (36–38). As response rates are low and side effects are frequent (36), there is still an urgent need to improve HCC therapy.
Figure I.1.2: Barcelona Clinic Liver Cancer staging and treatment approach. Figure reproduced from (32) with permission. Abbreviations: PS= performance status, N1=lymph node involvement, M1=metastasis, CLT=cadaveric liver transplantation, LDLT=live-donor liver transplantation, PEI=percutaneous ethanol injection, RF=radiofrequency, TACE=transarterial chemoembolization.
2 Biomedical Ultrasound in Cancer
2.1 Diagnostic Ultrasound
Ultrasound is widely used for biomedical imaging. It is safe, cost-effective, non-invasive, can be used at the bed side, provides images in real-time, and does not produce ionizing radiation (39). Limitations in the use of ultrasound for diagnostic purposes are tissues containing gases or bones as ultrasound cannot pass (39). Ultrasound allows imaging up to 15 cm deep into the body at the classical frequency range of 1-20 MHz (40). Depending on the ultrasound transducer and the generated pulse, spatial resolution varies from 0.1 to 1.5 mm (40). Ultrasound imaging is based on the principle that longitudinal sound waves are sent out into the tissue and get reflected by structures with differences in impedance. This creates an echo that can be detected and resolved to depict tissue anatomy in so-called brightness mode (B-mode) (40). As tumors develop into abnormal masses of tissue, B-mode imaging early on served for detection of neoplastic lesions (40). In addition to B-mode, elastography is an ultrasound imaging approach that detects tissue stiffness based on the propagation of shear waves (40). Elastography is an interesting diagnostic approach in cancer as tumor tissue stiffness was shown to differ from surrounding normal tissue (40). Besides anatomical imaging, ultrasound can provide functional imaging, such as blood flow, based on Doppler, a change in frequency of longitudinal acoustic waves due to motion (41). The shift in frequency can therefore be used to extract information about blood flow and the technique is called Doppler imaging. Blood flow information is particularly interesting in tumors as tumoral blood vessels differ from normal blood vessels. Tumor angiogenesis induces a partly immature, disorganized vasculature in which the blood flow is disturbed (42). In case of slowed blood flow, contrast-enhanced ultrasound (CEUS) can be applied to improve cancer detection and tumor characterization (43).
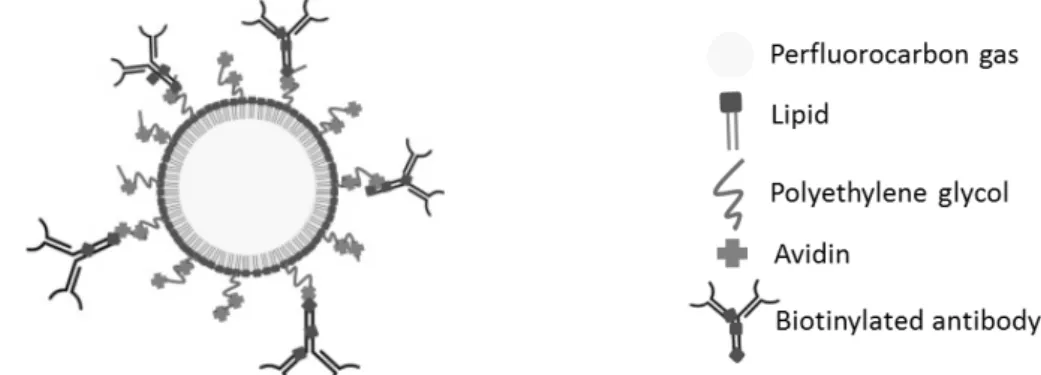
CEUS requires the use of ultrasound contrast agents (UCAs). The first FDA-approved UCA was an albumin-coated and air-filled microsphere (44). Since then, UCAs have been largely improved with regard to stability and safety. Today, UCAs are mostly microbubbles (MBs), which have a diameter in the micrometer range and consist of a gas core most often stabilized by a lipid shell containing polyethylene glycol (PEG) molecules for stearic protection (Fig.I.2.1) (45). Due to their size, MBs are limited to the blood circulation upon intravenous injection. Thus, MBs are intravascular contrast agents which are used to assess functional perfusion parameters such as vessel sizes, density, and flow rate (43). To distinguish MBs from tissue, specific ultrasound imaging sequences were developed exploiting the fact that tissue shows a linear response to ultrasound while MBs have a non-linear response (46). MBs were developed further and conjugated with targeted ligands to enable ultrasound molecular imaging (USMI) (47). The ligand can be coupled to the MB shell e.g. via a non-covalent avidin-biotin bridge if MBs carry avidin molecules on PEG and/or lipids and get incubated with biotinylated antibodies (48). An example of a clinical-grade targeted UCA is BR55, which is targeted towards the Vascular Endothelial Growth Factor
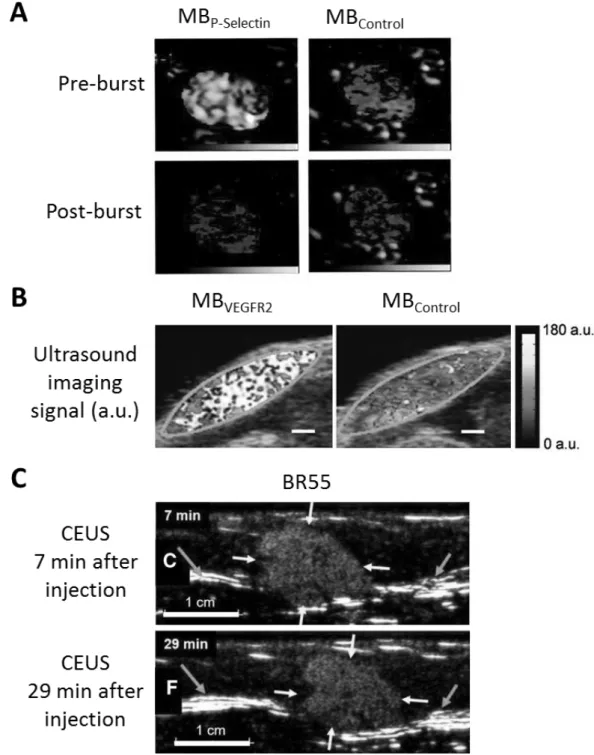
(49). Targeted MBs have the capacity to recognize and bind molecular markers that are expressed on the vascular endothelium (Fig. I.2.2). USMI enables diagnosis and monitoring of pathological processes involving the endothelium (48). In order to discriminate free-circulating and attached MBs from each other, different imaging and data treatment approaches have been developed: e.g. MBs can be destroyed to detect residual free-circulating MBs and calculate the targeted MB signal by signal subtraction (destruction-replenishment method), or, alternatively, images are acquired during the MB wash-out phase so that only specifically bound MBs are left in the image (50,51).
Recent advances in computer technology and software enabled the implementation of three-dimensional real-time ultrasound imaging in the clinic (52). Three-dimensional ultrasound provides a more complete anatomic assessment for better reliability in diagnosis and disease monitoring. This imaging approach was also combined with CEUS and USMI and proved useful for early and precise therapy response assessment upon anti-tumor therapy (53–55). Another revolutionizing advancement in ultrasound diagnostics is the introduction of ultrafast imaging with plane-wave transducers driven by novel graphical processing units (56). Ultrafast processing was applied for shear wave elastography, Doppler, and CEUS (56,57).
Figure I.2.1: Microbubble (MB) functionalized with an antibody ligand for molecular targeting.
Figure I.2.2: Ultrasound molecular imaging (USMI) with microbubbles (MBs) targeting a biomarker of the tumor endothelium.
Ultrasound and CEUS are integral components of clinical disease management for different cancers, including breast and liver (58,59), which distinguish cancer from normal tissues as abnormal masses with uncoordinated vasculature. As the causes of cancer are aberrantly expressed proteins that dysregulate cellular proliferation and survival, molecularly targeted therapies were developed to specifically attack the tumor cells while preserving normal tissue. Information about the location, size, and perfusion of the tumor are not sufficient to predict the patient’s response to a targeted therapy. USMI has the potential to visualize the expression level and distribution of tumor-related vascular markers and allow for treatment decision and monitoring of the therapeutic response.
2.2 Therapeutic Ultrasound
Ultrasound is not limited to diagnostic purposes, but can also be used for therapeutic applications. While diagnostic ultrasound is defined by low energy levels of 0.1-100 mW/cm², therapeutic ultrasound requires high energy levels of 5-5000 W/cm² (60,61). Ultrasound therapy is achieved through thermal and mechanical effects. Thermal therapies include physical therapy to warm tissue, improve blood flow, and accelerate blood flow, hyperthermia at 42 °C for cancer therapy, and high-intensity focused ultrasound (HIFU) to ablate tumor tissue by a local but very high rise in temperature (62). Alternatively, HIFU can also be used to trigger local hyperthermia for drug release from temperature-sensitive liposomes into tumor tissue (63). Since recently, HIFU ablation in combination with immunotherapy is studied for the enhancement of tumor therapy efficacy (64,65) Mechanical ultrasound therapy includes HIFU-induced histotripsy for tissue disintegration in tumors, shock wave lithotripsy for kidney stone destruction, but also low-intensity pulsed ultrasound for bone fracture healing (62,66,67). A relatively novel mean of ultrasound therapy is the combination of low frequency, moderate power ultrasound with MBs or nanodroplets or the use of very high power, pulsed ultrasound (62). These ultrasound regimens trigger cavitation and sonoporation, which are exploited for sonothrombolysis or tissue permeabilization (68). Ultrasound was also explored for cancer immunotherapy. HIFU is expected to expose immune cells to tumor debris thereby stimulating anti-tumor immune responses, and ultrasound in combination with MBs are used to deliver antigen-encoding genes or antigens (69).
MBs under ultrasound exposure compress and expand correspondingly to the compression and expansion phases of the ultrasound pulse: a process called stable cavitation (70). With ultrasound pulses of higher pressure, MBs oscillate non-linearly leading to a larger degree of expansion than compression which ultimately results in MB collapse: a process called inertial cavitation. The physical effects of stable and inertial cavitation bring along diverse biological effects. MBs are pushed in the direction of the wave propagation due to the radiation force of ultrasound pulses so that MBs are brought in contact with the endothelial cell layer of the vasculature (71). Stable cavitation of MBs induces microstreaming effects around the bubble. Microstreaming describes the flow of fluid that
MB collapse due to inertial cavitation, strong mechanical forces are generated in form of shock waves, microstreaming, microjets, and free radical production, which can perforate cell membranes as well as blood vessels (i.e. endothelial cell layer disruption) (72).
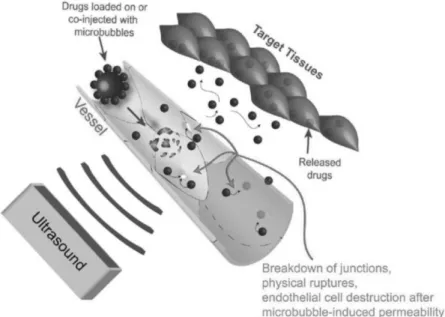
The different bio-effects acting together on cell membrane and vessel wall permeability facilitate drug delivery across these natural barriers. As with today’s ultrasound devices the ultrasound beam can be focalized, high energy ultrasound can be applied in a highly localized manner; thus paving the way for local treatment with minimized damage to the surrounding healthy tissue (73). Focal ultrasound in combination with free-circulating MBs provides a drug delivery approach called ultrasound-targeted MB destruction (UTMD). In this delivery approach, the drug is either co-injected with the cavitation nuclei, which are the MBs, or loaded on or into the MBs. Upon inertial cavitation and MB collapse, drug extravasation is increased (Fig.I.2.3).
In vitro studies with cell layers and suspensions provided proof of principle of ultrasound gene delivery, and in vivo studies for cancer gene therapy achieved tumor growth reduction (74,75). The permeabilizing effects of stable and inertial cavitation can be applied for different kinds of molecules and target tissues. Indeed, in vitro and in vivo studies of ultrasound-mediated delivery of chemotherapeutics have shown drug accumulation at the target site and tumor volume reduction in different models (76). Thus, the ultrasound-mediated local gene and drug delivery approach presents an interesting tool that can permeabilize cells in different set-ups and that can be translated into applications requiring the delivery of genes such as in developmental studies, and the delivery of therapeutic agents such as in preclinical cancer therapy studies.
Figure I.2.3: Ultrasound drug delivery via cavitation of MBs at the target site. Figure reproduced
3 Objective of the Thesis
Cancer is a highly diverse group of diseases. Heterogeneity was observed between different tumors, but also within the same tumor. Thus, clinical challenges are different for different cancer types and individual and innovative approaches are required to address the complexity of the disease.
Breast cancer has high incidence and mortality despite screening programs for cancer early detection. The treatment algorithm includes surgery, chemotherapy, hormone therapy and molecularly targeted therapies. It has been shown that the identification of breast cancer subtypes facilitates the selection of appropriate therapies. Imaging is already an integral part of the screening and diagnosis workflow. Though, patients need to undergo invasive biopsies to determine their breast cancer subtype, and biopsy results depend on where the sample was collected and how it was processed ex vivo. With the development of molecular imaging technologies and clinical-grade targeted contrast agents, it is imaginable to use non-invasive molecular imaging as companion diagnostics for specific targeted therapies.
USMI is a novel powerful technique which allows intravascular molecular imaging. Identification of novel breast cancer biomarkers for imaging and therapy, development of corresponding targeted contrast agents, and establishment of robust, reproducible, and reliable imaging protocols can significantly improve tumor characterization and provide physicians with crucial information for personalized medicine.
HCC is a highly lethal disease due to diagnosis at advanced stage, and bad therapy response. HCC usually develops in underlying liver disease such as fibrosis and cirrhosis, which means that the patient’s health is significantly impaired. The immune system also has an important role in HCC development, and has to be taken into consideration in clinical management. Novel more efficient therapies are required and should come along with reduced off-target delivery and associated side effects to improve drug tolerance by the patient, and on-site delivery should be enhanced to achieve efficient anti-tumor effects.
UTMD is emerging as a powerful tool for locally-targeted drug delivery. Combination of UTMD with molecular drugs which have therapeutic effects in cancer cells, but not in normal cells could significantly improve on-site delivery and reduce side effects so that drug doses could be increased and better responses achieved. Immuno-modulatory effects of ultrasound can also have an important role in therapy. The identification of innovative drugs, the development of stable and safe drug carriers, and the establishment of efficient, locally controlled, and image-guided delivery approaches, and the understanding of therapeutic bio-effects can significantly improve tumor treatment and the life quality of cancer patients.
The work of my thesis drives research towards the application of USMI for personalized medicine and UTMD for immunotherapy. Novel techniques and protocols were developed and validated for both the detection of a novel cancer biomarker and therapy target in breast cancer using USMI, and the delivery of molecular drugs with
immuno-In Chapter II, USMI of the novel cancer biomarker and therapy target netrin-1 is presented including an introduction into the state of the art of molecular imaging, USMI, and the role of netrin-1 in cancer. The development of a novel technique for in vitro validation of targeted MBs is presented in form of a published research article. The development of USMI as a companion diagnostic for netrin-1 interference therapy is also presented in form of a submitted research article. The chapter is completed with the discussion of future perspectives of this work.
In Chapter III, the effect of UTMD-mediated microRNA delivery on the immune system in HCC is presented. First, the state of the art in local drug delivery, more specifically UTMD drug delivery, and a novel UTMD microRNA delivery approach, which was recently developed by our collaborators, are described, followed by an introduction into HCC immune responses. The study of immune-modulatory effects of the UTMD microRNA delivery approach in HCC is presented in form of a prepared research article. Finally, the chapter is complemented with the next steps for the development of an anti-HCC immunotherapy.
In Chapter IV, further reaching conclusions are drawn of all results taken together in the context of current and future challenges and promises for cancer management.
Chapter II
– Ultrasound Molecular Imaging of Netrin-1 in
Breast Cancer
Ultrasound Molecular Imaging of
Netrin-1 in Breast Cancer
In this chapter, ultrasound molecular imaging of a novel protein, which is secreted by breast cancer cells and involved in cancer cell survival, is developed.
1 Molecular Imaging
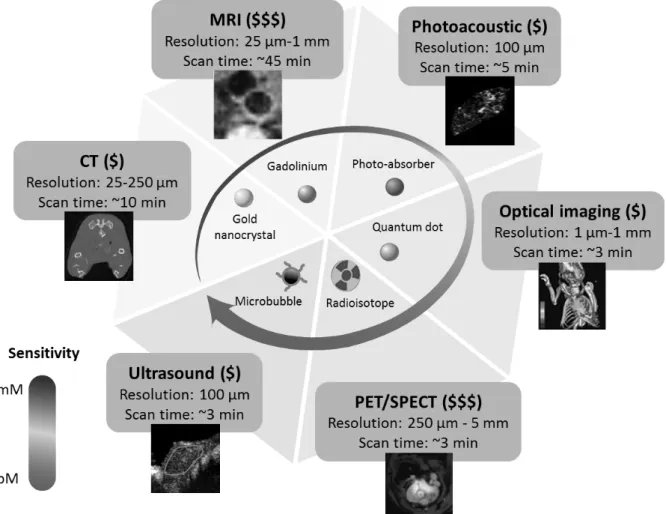
While classical imaging approaches provide information about anatomy and physiology, molecular imaging enables non-invasive visualization of cellular and subcellular phenomena (77). This is particularly interesting for cancer diagnostics as cancer cells can be studied in their normal environment within living subjects revealing molecular complexity and heterogeneity. Positron emission tomography (PET) was the first clinical molecular imaging technique. Today, it is complemented by novel innovative approaches, which enter clinical phases, including molecular magnetic resonance imaging (MRI), molecular CT, molecular optical imaging, molecular photoacoustic imaging, and ultrasound molecular imaging (USMI) (Figure II.1.1) (77). Molecular imaging generally requires the use of molecularly targeted contrast agents with imaging modality-specific characteristics that interact with tissues at the molecular level and whose detection reflects molecular processes. Molecular imaging is expected to significantly improve cancer early detection, disease progression monitoring, therapy response assessment, and prognostic evaluations for disease recurrence (78). We will now discuss these imaging techniques and the position of USMI among them.
Figure II.1.1: Comparison of different molecular imaging modalities. Techniques differ in sensitivity for contrast agents, resolution, scan time, and pricing. CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; SPECT: Single Photon Emission Computed Tomography. Figure modified and reproduced with permission from (79).
1.1 Positron Emission Tomography
PET is a nuclear imaging technique that requires the injection of radiolabeled tracers at non-pharmacological doses. In combination with CT, three-dimensional images are reconstructed and indicate the localization and amount of tracer (80). The tracer can be a biological molecule conjugated with an isotope capable of producing two gamma-rays upon positron emission. For whole-body image acquisition, the high-energy gamma-rays are collected within a few seconds to several minutes by a camera. The gamma-rays emitted by positron emission travel in opposite directions from each other (i.e. in a 180° angle), which is exploited to determine the precise localization of the isotope in the body. Isotopes are produced in cyclotrons or generators and have a half-life in the range of minutes. Thus, isotope preparation, injection, and imaging have to occur quickly (80). Spatial resolution in PET imaging is in the millimeter range and sensitivity is in the range of 10-11-10-12 molar requiring that several hundred million cells in close proximity accumulate the isotype to reach sufficient quantity to discriminate a specific signal from background noise (80). The best known PET tracer is fluoro-deoxy-glucose (FDG), which is taken up by tumors with high metabolic activity and glucose consumption (81). Alternative tracers of metabolism, proliferation, perfusion and hypoxia were shown to be useful for neoplasm detection, but they are non-specific for tumors and ineffective in slowly growing tumors. Thus, tumor-targeting tracers have to be developed to improve clinical PET (80).
1.2 Single Photon Emission Computed Tomography
Single Photon Emission Computed Tomography (SPECT) is used for whole-body quantitative molecular imaging. In SPECT, radioisotopes are used which capture electrons and emit gamma-rays upon decay as opposed to positron emissions in PET (82). SPECT radioisotopes are more stable than PET isotopes, facilitating their use. Gamma photons are collected by collimators which collect only photons with parallel trajectories to extrapolate their localization. SPECT sensitivity, quantification, and spatial resolution are inferior to PET (82,83). Though, SPECT scanners are more widely available and more cost-effective, and thus more often used than PET (82). Different tumor-specific molecularly targeted contrast agents are available for SPECT (83). Hybrid SPECT/CT allows combining molecular and anatomical imaging and is indicated for neoplasm and lymph node detection and staging (84).
1.3 Molecular Computed Tomography
CT is the three-dimensional reconstruction of anatomy relying on differences in X-ray attenuation (85). It is a rapid imaging technique for whole-body imaging at high spatial resolution (77). Though, CT has a low sensitivity for contrast agents (101-10-2 molar) and shows limited soft-tissue contrast (77). CT contrast agents are iodinated molecules which effectively absorb x-rays. The non-specific accumulation and short half-life of these agents limits their use (77). The development of alternative CT contrast agents is in the pre-clinical
their detection (77,86,87). These agents were used in vivo in mice for imaging of blood vessels, lymph nodes, and liver (88).
1.4 Molecular Magnetic Resonance Imaging
MRI is based on the measurement of magnetic relaxation times of diploes such as hydrogen atoms in a magnetic field (85). High spatial resolution and excellent anatomical detail is achieved. Functional MRI such as diffusion-weighted MRI is performed using specific imaging sequences depicting the decelerated diffusion of water molecules in cancer. MRI contrast agents are composed of iron oxide nanoparticles which have different T2 relaxation times than tissue, or of paramagnetic gadolinium chelates with different T1 relaxation characteristics than tissue (85). Superparamagnetic iron oxide nanoparticles or paramagnetic gadolinium chelates can be coupled to targeting ligands and used as molecular probes. A limitation in molecular MRI is its low sensitivity for molecular probes requiring micro-molar concentrations (10-3-10-5 molar) (85,86). Cancer-specific receptors, enzymes, and signaling molecules, lymph nodes, angiogenic activities, and drug distribution were detected in pre-clinical studies (89).
1.5 Optical Molecular Imaging
Optical imaging is based on the interaction of light with tissue resulting in fluorescence, absorption, reflectance, or bioluminescence (90). Accordingly, optical imaging approaches include confocal and two-photon microscopy, fluorescence imaging, diffuse optical tomography, fluorescence molecular tomography and bioluminescence imaging. The latter achieves the deepest light penetration of 2-3 cm (90). Either fluorescent contrast agents are used and excited by an external light source, or, for bioluminescence imaging, the photo-protein luciferase catalyzes oxidation of the bioluminescent substrate luciferin leading to emission of visible photons (90). These in vivo imaging techniques are used to study human diseases and development of novel therapeutics. The use of optical imaging in the clinics is limited due to the low tissue penetration, but optical imaging is used in endoscopy and provides high spatial resolution (83).
1.6 Photoacoustic Molecular Imaging
In photoacoustic (PA) imaging, pulsed laser irradiation is used to induce thermos-elastic expansion generating acoustic waves that can be detected with traditional ultrasound transducers (91). For PA imaging, either intrinsic tissue chromophores or exogenous contrast agents can be used and their optical absorption properties serves for image construction (91). When the endogenous tissue absorption is imaged, a multi-spectroscopic approach is applied to isolate information about hemoglobin concentration, oxygen saturation, and blood flow (92). Using a molecularly targeted contrast agent, molecular markers can be visualized. PA contrast agents need a high extinction coefficient and a narrow absorption spectrum to facilitate the identification of the contrast agent among the endogenous optical absorbers (93). Small molecule dyes and metallic nanoparticles are coupled to targeting moieties, and upon injection they can interact with intravascular and extravascular molecular target (93). PA imaging is a real-time, non-invasive technique which visualizes the
optical absorption properties of tissues up to 5 cm deep into the tissue and with a resolution of 100 μm (91,93). So far, molecular PA imaging was studied in pre-clinical models for the detection of tumor angiogenesis and specific cancer cells (91).
1.7 Ultrasound Molecular Imaging
Ultrasound molecular imaging (USMI) was first introduced by Klibanov and colleagues 20 years ago (47). USMI is based on the use of ultrasound contrast agents (UCAs) which are functionalized with targeting ligands. To distinguish specifically bound MBs from free-circulating MBs, specific ultrasound imaging sequences were developed. Today, a first clinical UCA targeting Vascular Endothelial Growth Factor Receptor-2 (VEGFR2) is available for intravascular imaging of tumor angiogenesis. In this thesis USMI is studied as a companion diagnostic for personalized medicine in breast cancer. Thus, this section describes the state of the art of UCAs, targeting ligands, molecular targets, imaging strategies, and applications of USMI in pre-clinical and clinical studies.
Ultrasound contrast agents
Microbubbles (MBs) are the most commonly used UCAs. Their size ranges from 1-4 μm in diameter and they are composed of a gas core stabilized by a shell made of phospholipids, polymers or proteins (51). The echogenic gas can be room air, nitrogen, or inert gases such as perfluorocarbons, which increase the half-life of MBs (94). Protein- and lipid-shelled MBs are prepared through gas dispersion into protein or lipid suspension under high-shear mixing (95). In the case of polymeric MBs, an organic phase containing the polymer is emulsified in an aqueous phase, and the solvent is evaporated (96). Usually polyethylene glycol (PEG) is added to the shell formulation to increase stability and reduce uptake by the reticuloendothelial system (RES) (43,97). Several types of MBs are today approved for clinical use including lipid-shelled Definity/Luminity, Lumason/SonoVue, Sonazoid, and albumin-shelled Optison (43). There are used for diagnosis of breast, kidney, and liver lesions (98). MBs are provided as lyophilized cakes, which have to be re-suspended prior to use. Upon injection, MBs have a relatively short half-life ranging from a few minutes up to 30 min for the different MB types (98). MBs are retained in lung, liver and spleen and they degrade in small vessels, although a partial redistribution after lung passage was reported (98,99). The gas of disintegrated MBs is exhaled through the lungs, shell components are eliminated by kidneys or phagocytosed by macrophages (98). Due to their size, MBs are restricted to the vascular compartment upon intravenous injection, and considered intravascular contrast agents.
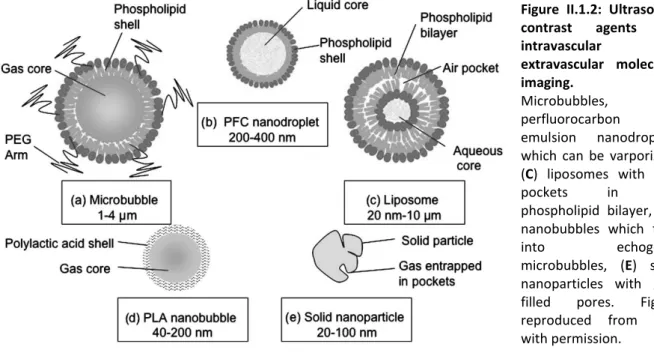
Alternatively to the described intravascular UCAs, nanometer-sized UCAs were more recently proposed, that could passively extravasate into the tumor tissue through the enhanced permeability and retention (EPR) effect (Fig.II.1.2) (91,98). Echogenic liposomes composed of a phospholipid bilayer in which air or other gases are entrapped were developed (100). As these liposomes can be loaded with drugs, a theranostic application of these agents is possible (98). Other forms of nanometer-sized UCAs are nanobubbles,
compared with MBs, to avoid coalescence and preserve small sizes (98). Further, nanobubbles synthesis is associated with low purity, stability and toxicity, which renders the use of these agents less attractive for clinical applications (98). Initially, size reduction rendered the first generation of these novel UCAs instable and less echogenic than MBs (101). Today, novel formulations of echogenic liposomes and nanobubbles were reported with similar echogenicity than MBs (102,103). A last class of nanoscale UCAs are phase-change contrast agents (PCCAs) which are droplets with a liquid perfluorocarbon core stabilized by lipid, protein or polymer shells (91). Perfluorocarbons are used that have boiling points close to or below body temperature. They are kept in liquid state due to high pressure in the small stabilized droplet (104). Upon ultrasound exposure or temperature rise, perfluorocarbons switch into gas state, which results in a 4-10 times expansion in size, and strong echogenic response (104). PCCA can be prepared by a technique called MB condensation (105). The conversion of nanodroplets into MBs causes cavitation and sonoporation, suggesting the use of PCCAs in drug delivery applications (98).
MBs are the best studied UCAs so far, clinical-grade FDA-approved MBs are available, USMI protocols for targeted MBs were developed, and multiple pre-clinical and the first clinical studies of USMI were reported. Thus, the development of USMI for a novel breast cancer target in this thesis was based on the use of MBs.
Figure II.1.2: Ultrasound contrast agents for intravascular and extravascular molecular imaging. (A) Microbubbles, (B) perfluorocarbon emulsion nanodroplets which can be varporized, (C) liposomes with auir pockets in the phospholipid bilayer, (D) nanobubbles which fuse
into echogenic microbubbles, (E) solid
nanoparticles with gas-filled pores. Figure reproduced from (50) with permission.