Publisher’s version / Version de l'éditeur:
Energy & Fuels, 32, 5, pp. 6252-6263, 2018-04-20
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/acs.energyfuels.7b04069
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Experimental and numerical investigations of soot formation in laminar
coflow ethylene flames burning in O2/N2 and O2/CO2 atmospheres at
different O2 mole fractions
Zhang, Yindi; Liu, Fengshan; Lou, Chun
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=a52d0c79-7123-43d3-ad69-41d1f305a69f https://publications-cnrc.canada.ca/fra/voir/objet/?id=a52d0c79-7123-43d3-ad69-41d1f305a69f
Experimental and Numerical Investigations of Soot Formation in
Laminar Coflow Ethylene Flames Burning in O2
/N
2
and O
2
/CO
2
Atmospheres at Different O2
Mole Fractions
Yindi Zhang,
†,‡Fengshan Liu,
*
,‡and Chun Lou
*
,§†
School of Petroleum Engineering, Yangtze University, Wuhan, Hubei 430100, People’s Republic of China
‡
Measurement Science and Standards, National Research Council Canada, 1200 Montreal Road, Building M-9, Ottawa, Ontario K1A OR6, Canada
§
State Key Laboratory of Coal Combustion, Huazhong University of Science and Technology, Wuhan, Hubei 430074, People’s Republic of China
ABSTRACT: This paper presents an experimental and numerical study of the distributions of the temperature and soot volume fraction in laminar coflow ethylene diffusion flames burning in O2/N2and O2/CO2 atmospheres with the O2 mole fraction
varying from 21 to 50% in both atmospheres. The fuel flow rate was maintained constant in all of the experiments and simulations. The two-color flame emission method based on the response spectrum of R and G bands of a three-color charge-coupled device camera was applied to measure the temperature and soot volume fraction. Numerical calculations were conducted using the C2chemistry model [Appel−Bockhorn−Frenklach (ABF), Appel, J.; Bockhorn, H.; Frenklach, M. Kinetic modeling of
soot formation with detailed chemistry and physics: Laminar premixed flames of C2hydrocarbons. Combust. Flame 2000, 121
(1−2), 122−136, DOI: 10.1016/S0010-2180(99)00135-2] with formation of polycyclic aromatic hydrocarbons (PAHs) up to pyrene and a soot model incorporating the dimerization of two pyrene molecules as the soot inception step and hydrogen-abstraction acetylene addition mechanism and PAH condensation as the surface growth processes. Numerical results are in qualitative agreement with the experimental measurements when the oxidizer stream is air. The numerical model predicts the temperature well but overpredicts the soot volume fraction in oxygen-enriched flames in both O2/N2and O2/CO2atmospheres.
With the increase of the oxygen mole fraction in the oxidizer stream, the flame becomes brighter and shorter, the peak temperature zone shifts from the flame wing to the upper part, and the peak soot volume fraction moves from the flame wing to the flame center. The soot loading grows rapidly with increasing the oxygen mole fraction in the oxidizer stream. Under the same oxygen mole fraction, the temperature and soot volume fraction in the O2/N2atmosphere are always higher than those in the
O2/CO2atmosphere as a result of the higher heat capacity of CO2and soot formation suppression by CO2. The chemical effect
of CO2may promote O and OH, which enhance the oxidation of the critical soot formation species, including H, C2H2, C6H6,
and C16H10. The primary pathway for the chemical effect of CO2is its competition for the H radical to form CO and OH,
i.e., CO2+ H ⇄ CO + OH. Soot formation in these flames is affected by two primary reactions: CO2+ H ⇄ CO + OH and
H + O2⇄O + OH.
1. INTRODUCTION
Soot and carbon dioxide emissions from combustion systems have been identified to be key contributors to global warming.1 In addition, the ultrafine soot aerosols emitted from combus-tion devices and biomass burning have harmful health and envi-ronmental effects.2Development of effective and clean combus-tion technologies to reduce CO2and soot emissions remains an
active research topic. Various technologies have been proposed and implemented for these purposes, such as the oxy-fuel combus-tion technology for CO2capture and storage (CCS), the exhaust
gas recirculation (EGR) technology for diesel and gasoline engines, and the flue gas recirculation (FGR) technology for combustors and furnaces. In oxy-combustion chambers, with the increase of the O2concentration and the addition of CO2in
the oxidizer stream, the distributions of the flame temperature and soot volume fraction (SVF) will be changed in comparison to traditional air combustion.1−3Such changes are likely to have
significant influences on combustion efficiency and CO2
emis-sion. Therefore, investigations of the flame temperature and
SVF are essential for an improved understanding of the com-bustion characteristics and maximizing the potentials of the oxy-combustion technology.
To understand the influence of CO2 addition on
oxygen-enriched diffusion flames, a number of studies of the flame tem-perature and SVF have been investigated.4−7According to Du
et al.8and Gülder and Baksh,9 CO2addition affects the flame
properties and soot formation through the following three aspects: dilution, thermal, and chemical. Liu et al.6revealed in counterflow ethylene diffusion flames that the temperature and acetylene con-centration decreased and the OH radical concon-centration increased by the addition of CO2, leading to suppressed soot inception.
Oh et al.10 experimentally identified a reduced primary soot particle number concentration in the soot inception regions of a laminar propane diffusion flame as a result of the lowered
Received: December 22, 2017
Revised: April 20, 2018
Published: April 20, 2018
pubs.acs.org/EF
Cite This:Energy Fuels 2018, 32, 6252−6263
Downloaded via NATL RESEARCH COUNCIL CANADA on July 25, 2018 at 15:39:56 (UTC).
flame temperature and enhanced OH concentration because of CO2addition to the oxidizer. Guo and Smallwood7found that
CO2addition to the fuel side of the coflow ethylene/air
diffu-sion flame suppresses inception and surface growth rates of soot through its chemical influence. Several earlier investigations9,11also speculated that addition of CO2suppresses soot formation by
chemically affecting soot inception and soot oxidation rates. The dominant pathway for the chemical effects of CO2addition
was concluded to be through CO + OH ⇄ CO2+ H.6,7
According to Haynes and Wagner12 and Fuentes et al.,13 investigation of soot formation and its impact on the local tem-perature and thermal radiation should usually focus on diffusion flames. Soot is primarily formed on the fuel side of reaction regions in diffusion flames. Soot particles are oxidized mainly by O2and OH in flames under high-temperature conditions in the
outer region of the soot layer because soot is transported down-stream mainly through convection.14−16The important effect of
the oxygen concentration in the oxidizer stream on the flame structure, soot formation, and related radiation was revealed by Merchan-Merchan et al.17The increase of the oxygen concen-tration in the oxidizer stream accelerates the fuel pyrolysis pro-cess, enhances the flame temperature, and consequently greatly promotes the soot formation reactions. In addition, the overall oxidation rates of soot and combustion in general are also signif-icantly accelerated as a result of the higher amount of oxygen available,18,19which shortens the flame heights. The total SVF is the competed result by the formation and oxidation. Hence, it is important to investigate soot formation and combustion property from both technological and fundamental points of view, which will provide a comprehensive understanding of the characteristics of oxygen-enriched combustion.
Many researchers have conducted experimental investiga-tions of the temperature and SVF from the R, G, and B bands of color cameras based on the two-color method.20−22The
two-color method uses thermal radiative emission from particulates in flames at two wavelengths in the visible spectrum to calculate the flame temperature and SVF. For laminar non-smoking diffusion flames fueled with gaseous or vaporized liquid hydrocarbon fuels, the size of soot particles is usually much smaller than the wave-lengths in the visible spectrum. As such, the radiative properties of soot particles can be described by the Rayleigh approxima-tion. Therefore, the variation of soot emissivities with the wave-length should be considered. In recent years, imaging systems combined with two-color principle and charge-coupled device (CCD) cameras have been developed to measure the soot tem-perature and volume fraction.23−27Fu et al.26 have also
con-ducted flame temperature measurement from the R, G, and B broad bands by three-color pyrometry.
The effects of oxygen enrichment on soot formation and radiative properties in diffusion flames have been experimen-tally investigated,28,29which reveal that increasing the oxygen concentration can result in a higher radiation emission rate. Also, there has been significant progress toward understanding soot formation in hydrocarbon flames.30Appel et al.31 proposed that soot surface growth is mainly through the hydrogen abstrac-tion acetylene addiabstrac-tion (HACA) mechanism, which is especially suitable for ethylene flame. Veshkini et al.32proposed an improved model to take into account the soot surface aging effect. Liu et al.33 have evaluated the dependence of the soot surface growth rate upon soot surface area (soot surface area per unit volume). They found that the square root dependence performed better in the predicted soot distribution.
Although the effects of CO2 addition on soot formation in
oxygen-enriched diffusion flames have been investigated in several previous studies,6−10,17−19there is still a lack of robust
gas-phase/soot formation models that perform equally well under different flame conditions and help gain a clear under-standing of soot formation in oxygen-enriched diffusion flames burning in O2/CO2atmospheres of different oxygen mole
frac-tions. In this paper, the soot temperature and volume fraction distributions in laminar coflow C2H4/(O2/N2) and C2H4/(O2/
CO2) diffusion flames with a varying O2mole fraction from 21
to 50% were experimentally measured using the two-color prin-ciple with the spectral band measurement technique based on a color CCD camera. Meanwhile, these flames were also numer-ically simulated using a detailed gas-phase C2chemistry
mech-anism and a soot model based on polycyclic aromatic hydro-carbon (PAH) inception and HACA mechanism for surface growth. O2/N2and O2/CO2were chosen as the oxidizer as a
result of their abundance and relevance to CCS, EGR, FGR, and oxy-combustion technologies. This study was motivated to investigate how CO2and O2influence flame and soot
forma-tion in laminar coflow C2H4diffusion flames over a range of the
O2mole fraction from 21 to 50%.
2. EXPERIMENTAL SECTION
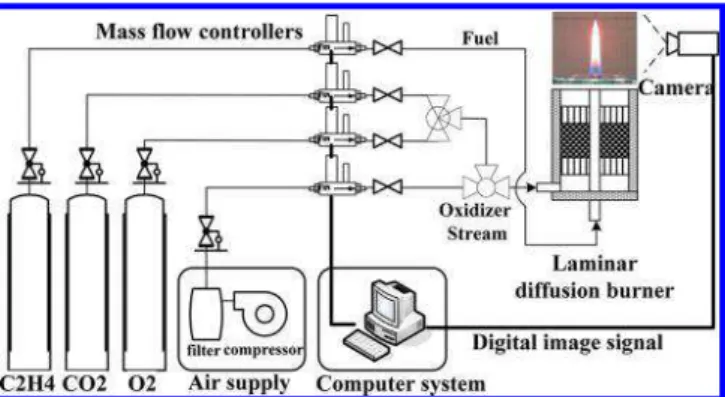
2.1. Experimental Setup.A coflow laminar diffusion flame burner studied in this paper is the same as that reported by Snelling et al.,34as
shown inFigure 1. The fuel tube is made of stainless steel and has a
10.9 mm inner diameter and a thickness of 0.9 mm. The oxidizer flows through the co-annular region between the fuel tube and an outer nozzle with an 88 mm inner diameter and 100 mm outer diameter. Glass beads and porous metal disks were used to provide a uniform flow in the oxidizer stream. Electronic mass flow controllers control the flow rates of all gases, which are delivered to the burner at room temperature and atmospheric pressure (293 K and 1 atm).Table 1
summarizes the test conditions for both O2/N2 and O2/CO2 atmo-spheres. For each set of conditions, the flow rate of fuel (ethylene) is Figure 1.Schematic diagram of the experimental setup.
Table 1. Summary of Laminar Diffusion Ethylene Flames in O2/N2and O2/CO2Atmospheres for Measurement
flame
XO2
(%)
QC2H4
(mL/min) (L/min)Qair
QO2 (L/min) QCO2 (L/min) 21% O2/N2 21 194 284 0 30% O2/N2 30 194 251 32 40% O2/N2 40 194 215 68 50% O2/N2 50 194 179 104 30% O2/CO2 30 194 85 198 40% O2/CO2 40 194 113 170 50% O2/CO2 50 194 142 142
constant at 194 mL/min, and the total flow rate of oxidizer coflow is 284 L/min. The flow rates were controlled using mass flow controllers with an accuracy of 1%. To analyze the effects of the oxygen con-centration and CO2replacement of N2 on the temperature and SVF distributions, the mole fraction of oxygen in the oxidizer stream, XO2,
was increased from 21 up to 50%.
A three-color CCD camera (type CV-M9CL), which has three CCD sensors with a size of 4.8 mm (horizontal) × 3.6 mm (vertical) and a resolution of 1024 pixels (horizontal) × 768 pixels (vertical), was used to image the flames. Using the detected radiation images of R, G, and B by the camera, the temperature and radiative property dis-tributions of soot particles in the flames can be simultaneously recon-structed. Further details of the experimental methodology have been given in refs20and21.
2.2. Two-Color Principle with Spectral Band Measurement for a Three-Color CCD Camera.The raw data obtained by the R, G, and B bands of a three-color CCD camera represent thermal radiation of the flame in the visible spectrum. According to the characteristics of the color digital camera, the spectral response curves are broad. Assuming that the spectral response curves of R, G, and B bands are described by functions ηR(λ), ηG(λ), and ηB(λ), the emissive powers ER, EG, and EB received by the R, G, and B bands of the camera can be written as
∫
∫
∫
η λ ε λ λ λ η λ ε λ λ λ η λ ε λ λ λ = = = λ λ λ λ λ λ E E T E E T E E T ( ) ( ) ( , ) d ( ) ( ) ( , ) d ( ) ( ) ( , ) d R R R b G G G b B B B b 1 2 3 4 5 6 (1) where Eb(λ, T) is the spectral emissive power of the blackbody governed by Planck’s radiation law, ε(λ) is the spectral emissivity, λ is the wavelength, andT is the absolute temperature. A blackbody furnace can be used to calibrate the relationship between the absolute emissive powers and the raw data of R, G, and B bands of the camera.For processing flame images in the visible spectrum, which are attributed to line-of-sight integrated flame emissions, the thermal radiation can be considered from soot only, because radiation from gaseous components, such as CO2 and H2O, contributes negligibly. However, in the spectral response bands of the camera from 400 to 700 nm, the chemiluminescent emission from CH at 430 nm also contributes to the B band of the camera, especially in hydrocarbon flames.35Besides, many experimental investigations have shown that the B band signal is weak and might suffer from random noise.20,36
Therefore, the emissive powers received by the R and G bands will be used to derive the soot temperature and volume fraction.
On the other hand, for a non-gray emissivity model, the empirical emissivity model of Hottel and Broughton will be substituted into
eq 1; thus, the flame temperature T and KL could be calculated from
∫
∫
η λ λ λ η λ λ λ = − = − λ λ λ λ λ λ − − α α E E T E E T ( ) ( , )(1 e ) d ( ) ( , )(1 e ) d KL KL R R b / G G b / 1 2 3 4 (2) where KL is the optical thickness of the flame along the line-of-sight under consideration and α is a constant, given by the mature soot value of 1.34.37The average flame emissivities in the response spectrum of R and G bands will then be calculated as
∫ ∫ ε λ λ λ ε λ λ λ = − − = − − λ λ λ λ λ λ − − α α (1 e ) d (1 e ) d KL KL R / 2 1 G / 4 3 1 2 3 4 (3)
The above expressions are applicable to paths of uniform temperature and SVF with negligible emission absorption along the path. In the CCD camera image processing of this study, the respective emissive powers of the R and G bands were first reconstructed to obtain radi-cally resolved local emissive powers using a standard Abel inversion procedure. Then, the local soot temperature and volume fraction were retrieved following the algorithm described by Snelling et al.34 The experimental uncertainties of the two-color spectral band measure-ments were estimated to be 5% for soot temperature and 10% for SVF.
3. NUMERICAL MODEL AND COMPUTATIONAL DETAILS
The coflow laminar diffusion flames simulated in this paper are the same as the experimental cases. The computational domain is 104.6 mm (in the streamwise direction) × 47.1 mm (in the radial direction) using 210 (z) × 88 (r) control volumes. A non-uniform mesh was used with a resolution of 0.05 mm in the
r direction and 0.066 mm in the z direction near the burner exit.
The fully elliptic governing equations for mass, momentum, energy, and species in the low-Mach number limit and axisym-metric cylindrical coordinates were solved. The transport equa-tions can be found in several previous studies.38−40 Radiative
heat transfer was calculated using the discrete ordinates method (DOM) coupled with a statistical narrow-band correlated-k (SNBCK)-based wide-band model for the absorption coef-ficients of CO, CO2, and H2O.39 The Rayleigh expression40 approximates the absorption coefficient of soot. Further details of the numerical method are given in refs38−40. The thermal and transport properties of gaseous species and the chemical reaction rates were obtained using Sandia’s CHEMKIN41and TRANSPORT42libraries and the databases associated with the Appel−Bockhorn−Frenklach (ABF) mechanism.31
The ABF gas-phase reaction mechanism containing PAHs up to pyrene (A4)31was used in this paper. The physical and chem-ical mechanisms governing the transition from large PAH molec-ules to soot particles, i.e., the soot inception processes, are currently poorly understood. The soot inception step is simpli-fied as the collision of two A4 (pyrene) molecules in the free-molecular region39in this study. The subsequent soot surface growth was simulated by the HACA mechanism and PAH (A4) condensation and oxidation by O2and OH.31,43The sectional
soot model was used to model the interactions among different sized particles. The flame code used in this study has been extensively described in previous studies,38−40 where further
details of the numerical method and soot model can be found. Numerical calculations were conducted for laminar coflow C2H4/(O2/N2) and C2H4/(O2/CO2) diffusion flames. In all of
the cases considered, the mean inlet ethylene velocity is 3.465 cm/s and the average oxidizer stream velocity is 77.0 cm/s. The boundary conditions used in the present numerical calculations were similar to those described in ref40. Parabolic and uniform velocity profiles were imposed at the fuel and oxidizer inlets, respectively. Both the fuel and oxidizer were delivered at 300 K.
Table 2provides a summary of the oxidizer compositions for all of the cases of simulation. Conditions at other boundaries can be found in ref40. All numerical simulations were conducted using 16 central processing units (CPUs).
4. RESULTS AND DISCUSSION
4.1. Visible Flame Appearance.Figure 2shows the visible flame appearances in still flame photography of the ethylene flames in O2/N2and O2/CO2atmospheres as XO
2is increased
commercial digital camera with identical settings. As displayed inFigure 2, the flame shape and brightness are strongly affected by the oxygen mole fraction. With the increase of XO2, the flame
becomes increasingly shorter and brighter, regardless of the balancing composition in the oxidizer stream (N2or CO2). In
comparison of the flame photos between O2/N2and O2/CO2
atmospheres, at a given XO2, the flames are shorter and brighter
and the flame luminosity appears closer to the burner exit in the O2/N2 atmosphere. It is obvious from Figure 2 that soot
inception is delayed and less soot is produced in the O2/CO2
atmosphere. Because pure oxygen is rarely used as an oxidizer in practical combustion systems, the case of 100% O2will not
be further considered.
4.2. Comparison of the Temperature and SVF between Measurement and Numerical Simulation in the Ethylene/Air Flame.In this section, the results of two-color R and G spectral band measurements will be compared to those of coherent anti-Stokes Raman spectroscopy (CARS) for temperature/light-of-sight attenuation (LOSA) for SVF methods34 and numerical calculation for the C
2H4/air flame.
Figure 3shows the distributions of the temperature and SVF in the ethylene/air diffusion flame by the two-color spectral band method, CARS/LOSA methods, and simulation.
FromFigure 3, it can be seen that the simulation captures the primary features of the temperature and SVF in the C2H4/air
flame, albeit the simulation fails to predict SVF in the flame centerline region. The distributions of the flame temperature obtained by all three methods are similar. However, the two-color spectral band method missed the high-temperature region
low in the flame, likely as a result of the absence of soot or very low soot concentrations. In addition, the peak temperatures in the spectral band method and simulation are about 100 K lower than those in the CARS method. The predicted peak SVF is slightly higher than those of the two measurements. Overall, the predicted flame shape characterized by the temperature and SVF distributions is closer to that based on the CARS/LOSA measurements than the two-color spectral band method.
To further verify the two-color spectral band measurements and numerical results against the CARS/LOSA data,Figure 4
compares the temperature and SVF distributions at three heights, (a) z = 20 mm, (b) z = 30 mm, and (c) z = 50 mm, in the C2H4/
air flame. FromFigure 4, the distributions of the temperature and SVF are well-predicted, except in the centerline region, where the predicted SVFs are much lower than the measure-ments. The measurements of the two-color spectral band method are also in reasonable agreement with the CARS/LOSA method for both the temperature and SVF distributions at the three heights. Figures 3 and 4 serve as validation of the two-color spectral band method and numerical model benchmarked by the CARS/LOSA results in the case of the C2H4/air flame. The
two-color spectral band measurement and simulation methods are used to investigate the effects of oxygen enrichment.
4.3. Effect of O2 Enrichment on the Diffusion Flame.
4.3.1. SVFs in C2H4/(O2/N2) Flames. Figure 5 displays the
(a) measured and (b) simulated SVF distributions in C2H4/
(O2/N2) flames for different XO2in the oxidizer stream ranging
from 21 to 50%. As demonstrated inFigure 5, the distributions of SVF are strongly affected by the oxygen content in the oxidizer stream. The sooting region or the visible flame is drastically shortened, and the soot inception starts earlier (at a location closer to the burner exit) with increasing XO2. Meanwhile, the
peak SVF increases modestly in the measurements but increases significantly by the simulation. The reason for the overprediction of SVF is likely due to neglect of the soot aging effect, as demonstrated recently by Soussi et al.16Nevertheless, the simulation captures the main effects of increasing XO2, such
as a reduced visible flame height, an earlier soot inception, and an increased peak SVF. However, the underprediction of SVFs in the centerline region persists, regardless of the level of XO2.
4.3.2. SVFs in C2H4/(O2/CO2) Flames. Figure 6 shows the
measured and predicted SVF distributions from (a) measure-ment and (b) simulation in C2H4/(O2/CO2) flames with
Table 2. Summary of Laminar Diffusion Ethylene Flames in O2/N2and O2/CO2Atmospheres for Calculation
flame X
O2(%) oxidizer stream composition (mole fraction)
21% O2/N2 21 wO2= 0.21, and wN2= 0.79 30% O2/N2 30 wO2= 0.30, and wN2= 0.70 40% O2/N2 40 wO2= 0.40, and wN2= 0.60 50% O2/N2 50 wO2= 0.50, and wN2= 0.50 21% O2/CO2 21 wO2= 0.21, and wCO2= 0.79 30% O2/CO2 30 wO2= 0.30, and wCO2= 0.70 40% O2/CO2 40 wO2= 0.40, and wCO2= 0.60 50% O2/CO2 50 wO2= 0.50, and wCO2= 0.50
increasing XO2from 30 to 50% in the oxidizer stream consisting
of O2 and CO2. As illustrated in Figure 6, the visible flame
height decreases with increasing XO2, which is similar to the
effect of XO2 in C2H4/(O2/N2) flames shown in Figure 5.
In addition, the simulations qualitatively capture the variation of peak SVF with XO2, although discrepancies still exist. For
Figure 3.Distributions of (a) temperature and (b) SVF in the ethylene/air diffusion flame.
example, the model overpredicted the peak SVF and failed to capture the significant SVFs along the flame centerline, except at XO2= 50%. In comparison ofFigures 5and6, it is evident
that, at a given level of oxygen enrichment, the soot inception location is higher, the visible flame is taller, and the peak SVF is lower in the C2H4/(O2/CO2) flame than those in the
corre-sponding C2H4/(O2/N2) flame, indicating that soot formation
in C2H4/(O2/CO2) flames is suppressed as N2is replaced with
CO2.
The results shown in Figures 3, 5, and 6 indicate that the simulation predicts reasonably well the SVF levels in the C2H4/
air flame and also qualitatively captures the effect of the oxygen content in the oxidizer stream on SVF. However, the simulation overpredicts SVF in oxygen-enriched flames in both O2/N2and
O2/CO2atmospheres.
It is also noticed fromFigures 5and6that the replacement of N2by CO2in the oxidizer leads to a significantly less delay of
soot inception in the simulation than that observed from the measurements. Although such discrepancies could be attributed to both the deficiencies in the soot model and the sensitivity issue in the present two-color method, it is believed that the
model deficiencies in the inception submodel are the main cause for the discrepancies. In the present soot inception model, A4 (pyrene) collision was assumed to lead to soot inception, instead of considering larger (such as five-ring PAHs) PAHs as the inception species. Therefore, this inception model tends to predict an earlier appearance of soot.
4.3.3. Maximum Values of the Temperature and SVF in Oxygen-Enriched Diffusion Flames. Variations of the max-imum temperature and SVF with XO2from both measurements
and simulations are compared inFigure 7. FromFigure 7a, the maximum temperature increases with increasing XO2 in both
C2H4/(O2/N2) and C2H4/(O2/CO2) flames, as expected. The
peak temperatures by measurement and simulation are in fairly good agreement over the entire range of XO2 considered, with
the maximum discrepancy between simulation and measure-ment being only about 84 K. The peak flame temperatures in the O2/CO2atmosphere are significantly lower than those in
the O2/N2 atmosphere mainly as a result of the higher heat
capacity of CO2 than N2 and the chemical and radiative
properties of CO2to a lesser degree.Figure 7b shows that the
simulation overpredicts the peak SVFs under oxygen-enriched
conditions, regardless of the compositions in the oxidizer stream, although the model predicts reasonably well peak SVF when the oxidizer is air. As already displayed inFigure 5, the simulation also
reproduces the qualitative increasing trend of the peak SVF with increasing XO2, although the simulation predicts a
signif-icantly faster increase in peak SVF than the measurements.
Figure 6.(a) Measured and (b) predicted SVF distributions in C2H4/(O2/CO2) flames.
The overprediction of peak SVF is attributed to the neglect of the soot aging effect, which was shown to be important at higher XO2but not at XO2= 0.21 (oxidizer is air).
16However,
the model predicts the correct trend of peak SVF variation with
XO2compared to the measurements.
4.3.4. Temperature Distributions at Different Flame Heights in Oxygen-Enriched Diffusion Flames. Figure 8 displays the predicted radial profiles of the temperature at three heights of z = 4, 6, and 10 mm for different XO2in both C2H4/(O2/N2)
and C2H4/(O2/CO2) flames. These results again reveal that
oxy-gen enrichment increases temperatures and replacement of O2
in the oxidizer by CO2decreases the temperature. FromFigures 7
and 8, it can be concluded that, although the flame model overpredicts SVFs with increasing XO2, it captures the main
features of the effect of the oxygen content in oxy-fuel combus-tion, such as increased temperatures, a reduced visible flame height, and an increased peak SVF.
4.4. Chemical Effect of CO2on the Temperature and
SVF. 4.4.1. Flame Shapes and Temperature Distributions.
Figure 9shows the measured (top row) and simulated (bottom row) temperature distributions in C2H4/air, C2H4/(30% O2/
70% N2), and C2H4/(30% O2/70% CO2) flames. The
measure-ments and simulations display an overall similar trend in the effect of oxidizer compositions on the flame shape; the flame becomes shorter with oxygen enrichment but it is taller in the O2/CO2atmosphere. Nevertheless, there are some differences
between the prediction and measured results. The measure-ments fail to capture the high-temperature regions near the burner exit, where soot concentrations are low. Also, the two-color measurements display high temperatures in the flame tip region (Figure 9a), while the predictions do not show this characteristic. This is likely caused by the experimental error because the soot concentrations are low in the flame tip region. The results illustrated inFigure 9indicate that the increasing O2content in the oxidizer stream increases the temperature but
the presence of CO2in the oxidizer stream decreases the
tem-perature. This is simply because increasing O2reduces the inert
concentration in the oxidizer, leading to higher flame temper-atures. When CO2is used to replace N2in the C2H4/(30% O2/
70% N2) flame, the maximum temperature declines sharply by
almost 300 K from both measurements and simulations. This is because CO2 has a strong influence on combustion reactions
and flame temperature caused mainly by its higher heat capacity and to a lesser degree its chemical and radiative effects.
4.4.2. Radial Distributions of Temperature and SVF. The radial distributions of the temperature and SVF at z = 14 mm are compared in Figure 10. From Figure 10a, the measured peak temperatures are 2058, 2125, and 1970 K for the C2H4/(21% O2/79% N2), C2H4/(30% O2/70% N2), and
C2H4/(30% O2/70% CO2) flames, respectively. The
corre-sponding simulation temperatures are 2040, 2060, and 1945 K, respectively. In comparison of the C2H4/(21% O2/79% N2)
and C2H4/(30% O2/70% N2) flames at z = 14 mm, the latter
temperature is higher and the peak value of the temperature shifts toward the flame centerline, which is associated with the
Figure 8.Calculated radial temperature distributions for the O2mole fraction increasing from 21 to 50% at the height z = 4, 6, and 10 mm.
Figure 9.Comparison of (a) measured and (b) simulated temperature distributions in C2H4/air, C2H4/(30% O2/70% N2), and C2H4/(30% O2/70% CO2) flames.
reduced flame height. When nitrogen in the oxidizer stream is replaced by CO2, the peak temperature is significantly reduced
by about 120−150 K and the flame becomes taller. The reasons for such influences of CO2 replacement of N2in the oxidizer
stream have been discussed above. FromFigures 5and6, it can also be seen that the SVF distributions are strongly affected by both the O2and CO2concentrations in the oxidizer stream.
Figure 10b shows the radial distributions of SVF at z = 14 mm in C2H4/(21% O2/79% N2), C2H4/(30% O270% N2),
and C2H4/(30% O270% CO2) flames. In comparison of the
C2H4/(21% O2/79% N2) and C2H4/(30% O2/70% N2) flames,
the latter has a higher peak SVF than the former and the peak value moves toward the flame centerline, again as a result of the reduced flame height. When N2 in the oxidizer stream of the
C2H4/(30% O2/70% N2) flame is replaced by CO2, the peak
value of SVF is significantly reduced. This is because CO2
addi-tion lowers the flame temperature and has a strong chemical effect on soot formation suppression. According to the litera-ture,6,7 CO2 suppresses soot formation mainly by its thermal
and chemical effects.
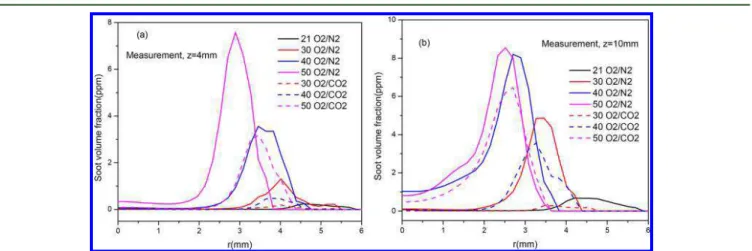
4.5. Analysis of Soot Formation in Oxygen-Enriched Diffusion Flames. 4.5.1. SVF Distributions at Different Flame Heights.The measured radial profiles of SVF at z = 4 and 10 mm are compared inFigure 11. These results again indi-cate that oxygen enrichment in general enhances soot forma-tion but replacement of N2in the oxidizer by CO2suppresses
soot formation. FromFigures 7and11, it can also be found that the maximum value of SVF (Figure 7) and the radial distribution of SVF at the height z = 4 and 10 mm (Figure 11) increase with
the oxygen concentration in the oxidizer stream of C2H4/
(O2/CO2) flames.
4.5.2. Soot Formation in the Ethylene Diffusion Flame at Different Conditions. Oxygen affects flame and soot formation both physically and chemically.10,44 Increasing the oxygen
concentration decreases the flame height, leading to a shorter residence time for soot growth. Meanwhile, it enhances the heat release rate and flame temperature, leading to higher gas-phase reaction rates as well as soot inception and surface growth rates. On the other hand, increasing oxygen will also prompt OH concentrations, which, in turn, accelerates soot oxidation.
To gain insight into the effects of oxygen enrichment and CO2replacement of N2in the oxidizer on soot inception,
sur-face growth, and oxidation processes, the calculated maximum mole fractions of H, OH, C2H2, C6H6(A1), and C16H10 (A4)
and peak SVF and temperature in the C2H4/(O2/N2) and
C2H4/(O2/CO2) flames are summarized in Table 3. From
Table 3, it can be found that the maximum mole fractions of all five species and peak SVF and temperature increase with increasing O2 in both C2H4/(O2/N2) and C2H4/(O2/CO2) flames.
In comparison of the C2H4/(O2/N2) and C2H4/(O2/CO2) flames
with the same O2 component in the oxidizer stream, there are
significant decreases for the peak mole fractions of all five species and peak SVF and temperature when N2is replaced by CO2.
The decrease of the peak mole fractions of these species can be attributed to both the thermal and chemical effects of CO2.
In this study, the particle inception is assumed to be due to the collision/dimerization of two pryene (A4) molecules. The rate of inception depends upon the temperature and pyrene
Figure 10.Comparisons of measured and simulated radial distributions of (a) temperature and (b) SVF at z = 14 mm in C2H4/air, C2H4/(30% O2/ 70% N2), and C2H4/(30% O2/70% CO2) flames.
concentration. Surface growth is caused by PAH condensation and acetylene (C2H2) addition through the HACA mechanism.
The HACA surface growth rate mainly depends upon the tem-perature, surface area, and concentrations of C2H2and radical H.
Table 3 shows that the concentration of A4 is significantly lower in C2H4/(O2/CO2) flames. The lower A4 concentration
and lower temperature are responsible for the reduced soot inception rates in C2H4/(O2/CO2) flames. The lower
concen-trations of H radical, C2H2, and A4 in C2H4/(O2/CO2) flames
imply that soot growth rates through PAH condensation and HACA mechanism are all lower in C2H4/(O2/CO2) flames,
resulting in lower SVFs in these flames.
It can be seen that, at the same oxygen concentration, using CO2to replace N2will lower the maximum value of H and OH
inTable 3. This is because CO2addition will affect the primary
pathway for the chemical effect of CO2, i.e., CO2+ H → CO +
OH. The increase in CO2prompts the forward reaction, which
lowers the H concentration. Furthermore, reaction H + O2→
O + OH also plays a vital role in combustion systems. The decreased H radical as a result of consumption by CO2lowers
OH production by H + O2→O + OH. The net change of the
OH formation rate as a result of the addition of CO2depends
upon the relative importance of the two reactions.6,7We also found that, with the increase of the oxygen concentration, the H and OH radical maximum values increase in both C2H4/(O2/N2) and
C2H4/(O2/CO2) flames inTable 3. This is because increasing the
oxygen concentration accelerates reaction H + O2⇄O + OH.
Figures 12 and 13 display the profiles of OH and H mole
fractions at two axial heights above the burner exit. These two heights cover the main soot formation region in the studied flames. It can be found that the peak values of H and OH increase with increasing the O2content in both C2H4/(O2/N2)
and C2H4/(O2/CO2) flames. FromFigure 13, we can also observe
that the H mole fraction decreases when N2is replaced by CO2.
This is due to the same reason as that discussed in the last paragraph.
As described above, we can conclude that soot formation in C2H4/(O2/CO2) flames is affected by two primary reactions,
which are CO2+ H ⇄ CO + OH and H + O2⇄O + OH.
Figures 14, 15, and 16 show the mole fractions of
C2H2, C6H6, and C16H10, respectively, at the two axial heights.
Figures 14−16 give the consistent trend that, with increasing the O2mole fraction, the peak mole fractions of C2H2, C6H6,
and C16H10 all increase. In O2/N2 flames, the distributions of
C2H2and C6H6mole fractions display a regular and consistent
trend and the peak value moves from the wing to the center of the flame. However, in O2/CO2flames, the peak values of these
two species show a weakened regularity. FromFigure16, it can be found that the peak value of A4 decreases with the increasing O2 at the flame height z = 4 mm but the peak value of A4
increases with O2at the flame height z = 10 mm.
It should be pointed out that the lower concentration of radical H in the lower region of the CO2addition flame is also a
reason for the lower pyrene concentration there. This is because pyrene is closely related to A1.Figure 15illustrates the profile of A1; they give a basic and consistent regularity. The lower Table 3. Calculated Maximum Mole Fractions of H, OH, C2H2, A1 (C6H6), and A4 (C16H10), SVF, and Temperature
oxygen mole fraction, XO2(%)
flame maximum mole fraction/temperature/SVF 21 30 40 50
O2/N2 H 0.0038 0.0056 0.00757 0.00923 OH 0.0058 0.0121 0.0193 0.0245 C2H2 0.051 0.0631 0.0704 0.0785 A1 0.00032 0.000391 0.000430 0.000498 A4 2.059 ×10−7 2.731 ×10−7 3.802 ×10−7 4.354 ×10−7 temperature (K) 2048.3 2248 2502 2688 SVF (ppm) 9.020 12.775 14.267 15.334 O2/CO2 H 0.00186 0.00225 0.00320 0.00450 OH 0.00261 0.00627 0.01100 0.0170 C2H2 0.03682 0.04842 0.05768 0.06848 A1 0.000147 0.000214 0.000367 0.000460 A4 1.4609 ×10−7 1.599 ×10−7 2.0 ×10−7 2.521 ×10−7 temperature (K) 1756 1958 2284 2450 SVF (ppm) 2.3 7.6 10.487 11.12
Figure 12.Calculated OH mole fraction distributions from 21 to 50% O2at the flame height z = 4 and 10 mm.
Figure 13.Calculated H mole fraction distributions from 21 to 50% O2at the flame height z = 4 and 10 mm.
concentrations of radical H and A1 cause the lower formation rate of pyrene (as shown inFigure16) as a result of the HACA growth mechanism of PAH. Therefore, the decreasing H decreases both the inception rate and surface growth rate in O2/CO2flames.
5. CONCLUSION
Experimental measurements and numerical calculations were conducted in laminar diffusion ethylene flames burning in O2/
N2and O2/CO2atmospheres with the O2mole fraction in the
oxidizer varied from 21 to 50% to investigate how oxygen enrich-ment and replaceenrich-ment of N2by CO2affect the temperature and
soot formation. The fuel and oxidizer flow rates are maintained constant in all of the experiments and simulations. The two-color method using the R and G spectral band measurements based on the response spectrum of R, G, and B bands of a three-color CCD camera was applied. The simulated results were obtained using the ABF mechanism and a soot model consisting of
inception by collision of two pyrene molecules and HACA and pyrene condensation for surface growth. Numerical results repro-duced the main features of the experimental results and obser-vations. However, the numerical simulation overpredicts SVF in oxygen-enriched flames. The following conclusions can be drawn on the basis of the experimental and numerical results: (1) Increasing the oxygen mole fraction in the oxidizer stream yields brighter and shorter flames and higher flame temper-atures and moves the peak temperature and SVF zones from the flame wing to the top center. (2) SVF is increased rapidly with increasing oxygen in both the O2/N2and O2/CO2
atmo-spheres. The model overpredicts the maximum SVF in oxygen-enriched flames compared to measurements. (3) CO2is
effec-tive to suppress soot formation at the O2concentration from 30
up to 50% under the present conditions mainly as a result of its thermal effect and additional chemical effect. (4) At the same oxygen mole fraction in the oxidizer, the flame height and SVF burning in the O2/N2atmosphere are always shorter and higher,
respectively, than those in the O2/CO2 atmosphere. (5) The
replacement of N2in the oxidizer by CO2lowers the
concen-trations of critical soot formation species, including H, C2H2,
C6H6, and C16H10. (6) The primary pathway for the chemical
effect of CO2is its competition for the H radical to form CO
and OH, i.e., CO2+ H ⇄ CO + OH. Soot formation in C2H4/
(O2/CO2) flames is affected by two reactions, namely, CO2+
H ⇄ CO + OH and H + O2⇄O + OH.
■
AUTHOR INFORMATIONCorresponding Authors
*Telephone: +1-613-993-9470. E-mail: fengshan.liu@nrc-cnrc.gc.ca.
*Telephone: +86-27-8754-5526. E-mail:lou_chun@sina.com.
ORCID
Yindi Zhang:0000-0003-2355-1537
Fengshan Liu:0000-0002-1790-6381
Chun Lou:0000-0003-3302-7210
Notes
The authors declare no competing financial interest.
■
ACKNOWLEDGMENTSThe authors acknowledge the financial support of the National Overseas Study Foundation of China, the National Natural Sci-ence Foundation of China (51306022, 51676078, and 51176059), the Science and Technology Innovation Foundation of Petro-China (2015D-5006-0603), and the Yangtze Youth Talents Fund (2015cqt01). The authors also acknowledge the help from the Black Carbon Metrology Research Group, Measurement Sci-ence and Standards, National Research Council Canada.
■
REFERENCES(1) Baukal, C. E., Jr. Oxygen Enhanced Combustion; CRC Press: Boca Raton, FL, 1998; pp 2−20.
(2) Buhre, B. J. P.; Elliott, L. K.; Sheng, C. D.; Gupta, R. P.; Wall, T. F. Oxy-fuel combustion technology for coal-fired power generation.
Prog. Energy Combust. Sci. 2005, 31 (4), 283−307.
(3) Andersson, K.; Johansson, R.; Johnsson, F.; Leckner, B. Radiation Intensity of Propane-Fired Oxy-Fuel Flames: Implications for Soot Formation. Energy Fuels 2008, 22, 1535−1541.
(4) Childs, P. R. N.; Greenwood, J. R.; Long, C. A. Review of temperature measurement. Rev. Sci. Instrum. 2000, 71 (8), 2959−2978. (5) Kohse-Höinghaus, K.; Barlow, R. S.; Aldén, M.; Wolfrum, J. Combustion at the focus laser: Diagnostics and control. Proc. Combust.
Inst. 2005, 30 (1), 89−123.
Figure 14.Calculated C2H2 mole fraction distributions from 21 to 50% O2at the flame height z = 4 and 10 mm.
Figure 15. Calculated A1 (C6H6) mole fraction distributions from 21 to 50% O2at the flame height z = 4 and 10 mm.
Figure 16.Calculated A4 (pyrene, C16H10) mole fraction distributions from 21 to 50% O2at the flame height z = 4 and 10 mm.
(6) Liu, F.; Guo, H.; Smallwood, G. J.; Gülder, Ö. L. The chemical effects of carbon dioxide as an additive in an ethylene diffusion flame: Implications for soot and NOx formation. Combust. Flame 2001, 125, 778−787.
(7) Guo, H.; Smallwood, G. J. A numerical study on the influence of CO2 addition on soot formation in an ethylene/air diffusion flame.
Combust. Sci. Technol. 2008, 180, 1695−1708.
(8) Du, D. X.; Axelbaum, R. L.; Law, C. K. The influence of carbon dioxide and oxygen as additives on soot formation in diffusion flames.
Symp. Combust., [Proc.] 1991, 23 (1), 1501−1507.
(9) Gülder, Ö. L.; Baksh, M. F. Influence of carbon dioxide dilution on soot formation in diffusive combustion. Proceedings of the 1993
Spring Technical Meeting of the Combustion Institute Canadian Section;
University of Laval, Quebec, Canada, May 9−12, 1993.
(10) Oh, K. C.; Shin, H. D. The effect of oxygen and carbon dioxide concentration on soot formation in non-premixed flames. Fuel 2006,
85, 615−624.
(11) Zhang, C.; Atreya, A.; Lee, K. Sooting structure of methane counterflow diffusion flames with preheated reactants and dilution by products of combustion. Symp. Combust., [Proc.] 1992, 24, 1049−1057. (12) Haynes, B. S.; Wagner, H. G. Soot formation. Prog. Energy
Combust. Sci. 1981, 7 (4), 229−273.
(13) Fuentes, A.; Henríquez, R.; Nmira, F.; Liu, F.; Consalvi, J. L. Experimental and numerical study of the effects of the oxygen index on the radiation characteristics of laminar coflow diffusion flames.
Combust. Flame 2013, 160 (4), 786−795.
(14) Kang, K. T.; Hwang, J. Y.; Chung, S. H.; Lee, W. Soot zone structure and sooting limit in diffusion flames:comparison of counterflow and co-flow flames. Combust. Flame 1997, 109 (1−2), 266−281.
(15) Hwang, J. Y.; Lee, W.; Kang, H. G.; Chung, S. H. Synergistic effect of ethylene-propane mixture on soot formation in laminar diffusion flames. Combust. Flame 1998, 114, 370−380.
(16) Soussi, J. P.; Demarco, R.; Consalvi, J. L.; Liu, F.; Fuentes, A. Influence of soot aging on soot production for laminar propane diffusion flames. Fuel 2017, 210, 472−481.
(17) Merchan-Merchan, W.; Sanmiguel, S.; Saveliev, A.; McCollam, S. Soot formation in oxygen-enhanced combustion. In
Oxygen-Enhanced Combustion, 2nd ed.; Baukal, C. E., Jr., Ed.; CRC Press:
Boca Raton, FL, 2013; pp 385−408, DOI:10.1201/b13974-16. (18) Glassman, I.; Yaccarino, P. The effect of oxygen concentration on sooting diffusion flames. Combust. Sci. Technol. 1980, 24 (3−4), 107−114.
(19) Bennett, B. A. V.; Cheng, Z.; Pitz, R. W.; Smooke, M. D. Computational and experimental study of oxygen-enhanced axisym-metric laminar methane flames. Combust. Theory Modell. 2008, 12 (3), 497−527.
(20) Zhao, H.; Ladommatos, N. Optical diagnostics for soot and temperature measurement in diesel engines. Prog. Energy Combust. Sci. 1998, 24 (3), 221−255.
(21) Zhao, H.; Feng, H.; Xu, Z.; Li, Q. Research on temperature distribution of combustion flames based on high dynamic range imaging. Opt. Laser Technol. 2007, 39, 1351−1359.
(22) Wang, F.; Wang, X. J.; Ma, Z. Y.; Yan, J. H.; Chi, Y.; Wei, C. Y.; Ni, M. J.; Cen, K. F. The research on the estimation for the NOx
emissive concentration of the pulverized coal boiler by the flame image processing technique. Fuel 2002, 81 (16), 2113−2120.
(23) Lou, C.; Chen, C.; Sun, Y.; Zhou, H. Review of soot measurement in hydrocarbon-air flames. Sci. China: Technol. Sci. 2010,
53 (8), 2129−2141.
(24) Zhang, Y.; Lou, C.; Zhou, H.; et al. Computation and measurement for distributions of temperature and soot volume fraction in diffusion flames. J. Cent. South Univ. Technol. 2011, 18 (4), 1263−1271.
(25) Musculus, M. P. B.; Singh, S.; Reitz, R. D. Gradient effects on two-color soot optical pyrometry in a heavy-duty di diesel engine.
Combust. Flame 2008, 153, 216−227.
(26) Fu, T.; Zhao, H.; Zeng, J.; Wang, Z.; Zhong, M.; Shi, C. Improvements to the three-color optical CCD-based pyrometer system. Appl. Opt. 2010, 49 (31), 5997−6005.
(27) Svensson, K. I.; Mackrory, A. J.; Richards, M. J.; Tree, D. R. Calibration of an RGB, CCD camera and interpretation of its two-color images for KL and temperature. SAE Tech. Pap. Ser. 2005,
DOI: 10.4271/2005-01-0648.
(28) Gülder, Ö. L. Effects of oxygen on soot formation in methane, propane, and n-butane diffusion flames. Combust. Flame 1995, 101, 302−310.
(29) Escudero, F.; Fuentes, A.; Consalvi, J. L.; Liu, F.; Demarco, R. Unified behavior of soot production and radiative heat transfer in ethylene, propane and butane axisymmetric laminar diffusion flames at different oxygen indices. Fuel 2016, 183, 668−679.
(30) Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41−67.
(31) Appel, J.; Bockhorn, H.; Frenklach, M. Kinetic modeling of soot formation with detailed chemistry and physics: Laminar premixed flames of C2hydrocarbons. Combust. Flame 2000, 121 (1−2), 122− 136.
(32) Veshkini, A.; Dworkin, S. B.; Thomson, M. J. A soot particle surface reactivity model applied to a wide range of laminar ethylene/air flames. Combust. Flame 2014, 161, 3191−3200.
(33) Liu, F.; Thomson, K. A.; Guo, H.; Smallwood, G. J. Numerical and experimental study of an axisymmetric coflow laminar methane-air diffusion flame at pressures between 5 and 40 atm. Combust. Flame 2006, 146, 456−471.
(34) Snelling, D. R.; Thomson, K. A.; Smallwood, G. J.; Gülder, Ö.L.; Weckman, E. J.; Fraser, R. A. Spectrally resolved measurement of flame radiation to determine soot temperature and concentration. AIAA J. 2002, 40 (9), 1789−1795.
(35) Turns, S. R. An Introduction to Combustion, 2nd ed.; McGraw-Hill: New York, 2000.
(36) Zukowski, W.; Baron, J.; Bulewicz, E. M.; Kowarska, B. An optical method of measuring the temperature in a fluidised bed combustor. Combust. Flame 2009, 156, 1445−1452.
(37) López-Yglesias, X.; Schrader, P. E.; Michelsen, H. A. Soot maturity and absorption cross sections. J. Aerosol Sci. 2014, 75, 43−64. (38) Zhang, Q.; Guo, H.; Liu, F.; Smallwood, G. J.; Thomson, M. J. Modeling of soot aggre- gate formation and size distribution in a laminar ethylene/air coflow diffusion flame with detailed PAH chemistry and an advanced sectional aerosol dynamics model. Proc.
Combust. Inst. 2009, 32, 761−768.
(39) Zhang, Q. Detailed modeling of soot formation/oxidation in laminar coflow diffusion flames. Ph.D. Thesis, University of Toronto, Toronto, Ontario, Canada, 2009.
(40) Liu, F.; He, X.; Ma, X.; Zhang, Q.; Thomson, M. J.; Guo, H.; Smallwood, G. J.; Shuai, S.; Wang, J. An experimental and numerical study of the effects of dimethyl ether addition to fuel on polycyclic aromatic hydrocarbon and soot formation in laminar coflow ethylene/ air diffusion flames. Combust. Flame 2011, 158, 547−563.
(41) Kee, R. J.; Rupley, F. M.; Miller, J. A. Chemkin-II: A Fortran
Chemical Kinetics Package for the Analysis of Gas-Phase Chemical Kinetics; Sandia National Laboratories: Livermore, CA, 1989; Sandia
Report SAND89-8009.
(42) Kee, R. J.; Dixon-Lewis, G.; Warnatz, J.; Coltrin, M. E.; Miller, J. A. A Fortran Computer Code Package for the Evaluation of Gas-Phase
Multicomponent Transport Properties; Sandia National Laboratories:
Livermore, CA, 1986; Sandia Report SAND86-8246.
(43) Frenklach, M.; Wang, H. Detailed mechanism and modeling of soot particle formation. In Soot Formation on Combustion: Mechanism
and Models; Bockhorn, H., Ed.; Springer-Verlag: Berlin, Germany,
1994; Springer Series in Chemical Physics, Vol. 59, pp 165−192, DOI:
10.1007/978-3-642-85167-4_10.
(44) Guo, H.; Gu, Z.; Thomson, K. A.; Smallwood, G. J.; Baksh, F. F. Soot formation in a laminar ethylene/air diffusion flame at pressures from 1 to 8 atm. Proc. Combust. Inst. 2013, 34, 1795−1802.