Whole Body Protein Breakdown Is Less Inhibited by
Insulin, But Still Responsive to Amino Acid, in
Nondiabetic Elderly Subjects
CHRISTELLE GUILLET, AUDE ZANGARELLI, PIERRE GACHON, BE´ ATRICE MORIO,
CHRISTOPHE GIRAUDET, PAULETTE ROUSSET, AND YVES BOIRIE
Unite´ du Me´tabolisme Prote´ino-Energe´tique, Unite´ Mixte de Recherche, Universite´ d’Auvergne/Institut National de la Recherche Agronomique, Centre de Recherches en Nutrition Humaine, Centre Hospitalier de l’Universite´, 63009 Clermont-Ferrand, France
Responses of whole body glucose disposal (GDR) and protein breakdown (PB) to physiological insulin levels are altered in nondiabetic elderly subjects. Amino acids enhance inhibition of PB by insulin in young subjects. We hypothesized that ad-dition of amino acid to insulin may improve the defect in PB regulation by insulin in elderly people. Therefore, we inves-tigated the effect of hyperinsulinemia combined to either eua-minoacidemia (EuAA) or hyperaeua-minoacidemia (HyperAA) on GDR and PB, using isotopic dilution ofD-[6,6-2H
2]glucose and
L-[1-13C]leucine, in young (meanⴞSEM, 24.4ⴞ 0.8 yr) and
el-derly (70.2ⴞ 0.7 yr) subjects. GDR was lower in elderly than in young subjects in all situations (P < 0.05). Despite a greater
inhibition with HyperAA, PB was less inhibited in elderly than in young subjects during both clamps (ratio between change over basal PB and change over basal insulinemia,ⴚ0.014 ⴞ 0.002 vs.ⴚ0.024 ⴞ 0.003 in EuAA and ⴚ0.022 ⴞ 0.002 vs. ⴚ0.036 ⴞ 0.003mol/ml䡠U/kg fat-free mass䡠min in HyperAA; elderly vs. young, P < 0.05). In conclusion, in nondiabetic elderly sub-jects, PB is less inhibited by insulin with either basal or high amino acid concentrations. Addition of amino acid potenti-ates insulin-induced suppression of PB in both groups to the same extent, suggesting a specific dysregulation of PB by in-sulin with age. (J Clin Endocrinol Metab 89: 6017– 6024, 2004)
A
PHYSIOLOGICAL DECLINE in lean body mass occurs with age (1, 2). Numerous mechanisms are implicated in this alteration, but a defect in protein metabolism regu-lation, precisely a decreased response of protein turnover to anabolic factors, has been proposed (3). Insulin and amino acids exert different, but complementary, effects leading to-gether to an increase in net protein anabolism. The main invivo effect of insulin on protein metabolism is an inhibition
of protein breakdown (PB) (4, 5), whereas its effect on protein synthesis is still debated (5– 8). Inversely, amino acids have a stimulating effect on protein synthesis as well as enhanced whole body PB suppression by insulin (8 –12).
During aging, a progressive impairment of in vivo insulin action on glucose metabolism has been described (13, 14). Indeed, it is usually reported that peripheral glucose utili-zation mediated by insulin is reduced in elderly humans (13, 15, 16), with normal suppression of hepatic glucose produc-tion (13, 14, 17). Nevertheless, age-related changes in insulin action on body protein homeostasis have been less well doc-umented. A previous study determined whether the age-related defect in insulin action on glucose metabolism ex-tends to amino acid metabolism (18). The researchers concluded that there was actually a lower glucose disposal
rate in elderly subjects, but a normal reduction of whole body PB under different insulin infusion rates. Noticeably, plasma insulin levels were higher in the elderly than in the young group during the clamps. Thus, the effect of insulin on PB observed in elderly subjects was obtained with higher insulin concentrations than in young subjects. Moreover, plasma amino acid concentrations were not maintained at their basal levels during insulin infusion. A later study (7) examined the effect of age on the response of protein metabolism to phys-iological increases in insulin, in healthy young and elderly subjects, with maintenance of basal plasma amino acid con-centrations. A lesser insulin dose-dependent reduction of PB was documented in the aging population compared with that in the young group. Thus, the response of whole body PB to a physiological increase in insulin levels appeared to be affected by aging. Because amino acids can potentiate insu-lin-mediated inhibition of PB (9, 10), amino acid concentra-tions also have to be considered in the action of insulin on protein metabolism. For instance, during the fed state, a situation where insulin and amino acid are increased, a lesser inhibition of PB has been reported in elderly subjects despite a normal increase in the amino acid concentration (19, 20). Therefore, the combined effects of insulin and amino acids at postprandial concentrations on protein metabolism should be investigated in elderly subjects.
Hence, in the present study the response of whole body PB to insulin was investigated with either basal or high plasma amino acid concentrations in healthy young and elderly sub-jects. For this purpose, we used two euglycemic hyperinsu-linemic clamps differing by amino acid infusion levels: euaminoacidemic (EuAA) and hyperaminoacidemic
(Hy-Abbreviations: BMI, Body mass index; EuAA, euaminoacidemia; FFM, fat-free mass; GDR, whole body glucose disposal; HyperAA, hy-peraminoacidemia; IS-E, insulin-sensitive elderly; IS-R, insulin-resistant elderly; KIC, ketoisocaproate; MPE, molar percent excess; NS, not sig-nificant; PB, protein breakdown; Ra, rate of appearance.
JCEM is published monthly by The Endocrine Society (http://www. endo-society.org), the foremost professional society serving the en-docrine community.
doi: 10.1210/jc.2003-031323
6017
perAA). The data indicate that in elderly subjects, whole body PB is resistant to insulin action, but amino acids are still able to inhibit PB in this population.
Subjects and Methods
Subjects
The study group consisted of 14 young (mean⫾ sem, 24.4 ⫾ 0.8 yr; body mass index, 22.1⫾ 0.6 kg/m2) and 24 healthy elderly (70.2⫾ 0.7 yr; 25.4⫾ 0.5 kg/m2) male subjects. All volunteers had normal physical examinations without any medical history of digestive, renal, cardio-vascular, endocrine, or chronic diseases. The physical characteristics of the subjects are indicated in Table 1. Body composition was assessed by dual energy x-ray absorptiometry (QDR-4500A, Hologic, Inc., Waltham, MA).
The purpose and potential risks of the study were explained to all subjects, and their voluntary written consents were obtained before their participation. The ethical committee of the Auvergne region approved the experimental protocol.
Materials d-[6,6-2H
2]Glucose [96 molar percent excess (MPE)], l-[1-13C]leucine (99 MPE), and sodium [13C]bicarbonate (99 MPE) were obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). The isotopic and chemical purities of glucose and leucine were checked by gas chroma-tography-mass spectrometry. Solutions of tracers were tested for ste-rility and pyrogenicity before use and were prepared in sterile apyrogen water. Throughout each experiment, tracers were membrane-filtered through 0.22-m pore size filters. Human insulin (Actrapid, Novo-Nordisk Pharmaceutique, Copenhagen, Denmark) was diluted in sterile saline just before the infusion. A 20% glucose solution (Braun Medical, Boulogne, France) was used to maintain blood glucose at the baseline
level as previously described (7). Amino acid mixtures (5% and 10% Primene) were purchased from Clintec Parenteral (Montargis, France). Experimental protocol
All subjects were studied in the postabsorptive state after a 10-h overnight fast. On the day of the experiment, two venous tracts were laid on the arms. One catheter was retrogradely inserted into a dorsal vein of the left arm and was used for blood sampling. The hand of the subject was introduced into a ventilated box heated to 60 C to obtain arterialized blood. A second catheter was inserted into the contralateral arm and was used for tracer, insulin, and amino acid infusions. A third catheter was inserted into the same arm for the administration of a 20% glucose solution at rates adjusted for periodic plasma glucose measurements. After a prime dose of [13C]bicarbonate (6 mg/5 ml within 1 min), a primed [EuAA, 5.9 mol/kg fat-free mass (FFM); HyperAA, 8.4 mol/kg FFM] continuous (EuAA, 0.098 mol/kg FFM䡠min; HyperAA, 0.14mol/kg FFM䡠min) infusion of l-[1-13C]leucine in combination with a primed (EuAA, 1.8 mg/kg; HyperAA, 3 mg/kg) continuous (EuAA, 0.03 mg/kg䡠min; HyperAA, 0.05 mg/kg䡠min) infusion of
d-[6,6-2H
2]glucose were performed for 8 h. The l-[1-13C]leucine infusion rate was increased (0.21mol/kg FFM䡠min) during the HyperAA insuline-mic clamp to account for the higher dilution rate of the tracer by ex-ogenous amino acids. After a 4-h basal period, the EuAA or HyperAA, euglycemic, hyperinsulinemic clamps were performed in young (EuAA, n⫽ 6; HyperAA, n ⫽ 8) and elderly (EuAA, n ⫽ 12; HyperAA, n ⫽ 12; Fig. 1) male subjects. Subjects undergoing the EuAA clamp were from a previous study (7). However, because a gender effect was observed, only data from men were considered in the present study. For the two clamps, a continuous infusion of insulin was administered at a rate of 0.7 mIU/kg FFM䡠min. The plasma glucose concentration was deter-mined every 5 min using a glucose oxidase method (glucose analyzer 2, Beckman Coulter, Fullerton, CA) and was maintained constant by a
TABLE 1. Physical characteristics of young and elderly subjects during EuAA and HyperAA clamps Young EuAA
(n⫽ 6) Young HyperAA(n⫽ 8) Elderly EuAA(n⫽ 12) Elderly HyperAA(n⫽ 12)
Age (yr) 22.8⫾ 1.3 26.1⫾ 1.5 69.4⫾ 0.7a 71.0⫾ 1.2a Body weight (kg) 66.8⫾ 3.2 78.7⫾ 2.4 65.4⫾ 3.6 75.9⫾ 2.7 BMI (kg/m2) 20.5⫾ 0.5 24.6⫾ 0.9 24.4⫾ 0.9a 26.2⫾ 0.6a FFM (kg) 56.8⫾ 2.2 64.9⫾ 1.7 47.5⫾ 3.1a 59.2⫾ 2.3 Fat mass (%) 15.0⫾ 0.7 17.3⫾ 2.4 27.4⫾ 2.2a 22.1⫾ 1.5a Fasting glucose (mg/dl) 82.8⫾ 1.6 88.2⫾ 0.9 84.6⫾ 2.3 88.6⫾ 1.2
Values are the mean⫾SEM. Fat mass and FFM were evaluated from dual-energy x-ray absorptiometry.
aP⬍ 0.05, elderly vs. young subjects.
FIG. 1. Protocol design for the measure-ments of whole body glucose and protein metabolism in young and elderly sub-jects during hyperinsulinemic, euglyce-mic clamps with normal (EuAA) and high (HyperAA) amino acid concentrations.
periodic adjustment of the 20% glucose infusion according to the neg-ative feedback principle.
During the first clamp, an amino acid solution (5% Primene) com-bined with insulin and glucose was infused at the rate of 8.2⫻ 10⫺3 ml/kg FFM䡠min1to maintain plasma amino acid concentrations constant at the postabsorptive level, as previously described (7). The second clamp was performed as described above, but the rate of amino acid infusion (10% Primene) was increased to 28⫻ 10⫺3ml/kg FFM䡠min to achieve plasma amino acid concentrations 2- to 3-fold above baseline. Blood and breath samples were taken before any infusions, then at 20-min intervals during the last hour of each period. From the whole blood, the plasma supernatant was separated, an internal standard was added, and the sample was kept at⫺20 C until additional analysis. Breath samples were kept in 10-ml Vacutainers (BD Biosciences, Grenoble, France). Total carbon dioxide production rates were measured during the last hour of the two periods by open circuit indirect calo-rimetry (Deltatrac, Datex, Geneva, Switzerland) to determine leucine oxidation.
Insulin sensitivity assessment
Insulin resistance was not considered for glucose and protein me-tabolism together in all previous studies. Because the response of PB to insulin seemed to be altered in insulin-resistant elderly subjects (7), the question was raised of how to know whether this response was related to various degrees of insulin sensitivity for glucose metabolism. The influence of insulin resistance on protein metabolism regulation by insulin and amino acid was thus explored in elderly subjects, who were divided a posteriori into two groups: insulin sensitive (IS-E) or insulin resistant (IR-E).
The differentiation between IS-E (n⫽ 12) and IR-E (n ⫽ 12) elderly subjects, with six IS-E and six IR-E subjects per clamp, was determined on the basis of both insulin clamp data [whole body glucose disposal (GDR)/insulinemia] (21) and a classical index of clamp-derived insulin sensitivity [glucose infusion rate/(glycemia ⫻ (insulinemia during clamp⫺ insulinemia during basal state)] (22). Both indexes indicated the same value to separate the two populations (first index mean (21): IS-E, 0.18; IR-E, 0.11; second index mean (22): IS-E, 0.17; IR-E, 0.09). Mean indexes for IR-E and IS-E were significantly different (P⬍ 0.05). Elderly subjects were considered to be insulin resistant when the insulin sen-sitivity index value was lower than the insulin sensen-sitivity index (0.15) determined at similar insulin infusion rate by Bergman et al. (23). Analytical methods
Plasma [2H
2]glucose, [13C]leucine, and ketoisocaproate (KIC) enrich-ments and concentrations were measured by gas chromatography-mass spectrometry (5971A, Hewlett-Packard, Palo Alto, CA) as previously described (7, 20).13CO
2isotopic enrichments were measured with a gas isotope ratio mass spectrometer (Gas System, Fisons Instruments, VG Isotech, Middlewich, UK). Plasma insulin concentrations were mea-sured by ELISA (BioSource Europe, Nivelles, Belgium).
Calculations
Endogenous glucose production and glucose disposal rates were calculated from the dilution of labeled glucose in plasma using a mono-compartment model and Steele’s equations (24). The glucose disposal rate was obtained by calculating total glucose flux considering the time changes in concentrations and enrichment of plasma glucose. Glucose production was estimated by subtraction of the unlabeled glucose in-fusion rate from the total glucose rate of appearance.
Leucine kinetics were calculated according to the reciprocal pool model, using KIC as an indicator of intracellular leucine enrichment (25). Those parameters were normalized for FFM to consider the differences in body composition between young and elderly subjects. The total leucine rate of appearance (Ra; micromoles per kilogram of FFM per minute) was calculated from plasma isotopic dilution of [13C]KIC. This flux includes the tracer infusion and unlabeled leucine infused with other amino acids to maintain amino acid concentrations at the basal level (EuAA) or to elevate those concentrations 2- to 3-fold above the basal level (HyperAA). From this equation, whole body PB (Endo Leu Ra; micromoles per kilogram of FFM per minute) was calculated by subtracting from the total leucine Ra, the infused labeled leucine and the leucine administered with the amino acid solution. Leucine oxidation (micromoles per kilogram of FFM per minute) was then calculated by measuring13CO
2production as the product of CO2production and
13CO
2enrichment divided by [13C]KIC enrichment, because KIC is the immediate precursor of irreversible leucine decarboxylation in cells.
13CO
2enrichment was corrected for incomplete recovery by a factor of 0.70 in the basal state and 0.84 during the clamp according to previous clamp studies (26). The contribution of natural enrichment of the infused glucose and amino acid solutions to13CO
2production was determined in three additional subjects receiving these solutions during 4 h at rates similar to those used previously. This infusion increased the 13CO
2 enrichment in the expired air (⫹2 delta per thousand). This contribution has been corrected for the subsequent calculation of leucine oxidation
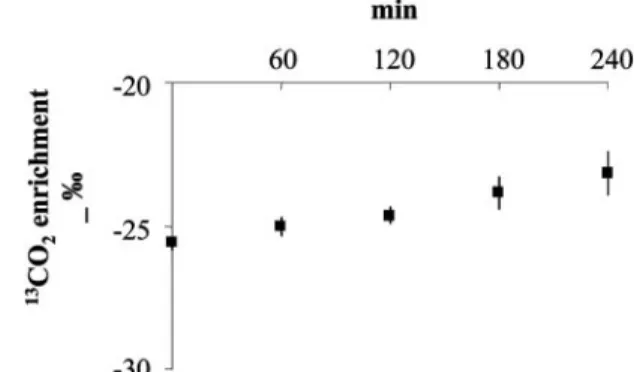
FIG. 2. Influence of 4-h glucose and amino acid solutions infusion on
13CO
2enrichment in the expired air determined in three subjects.
TABLE 2. Physical characteristics, plasma insulin concentrations, whole body glucose, and leucine changes induced by insulin in IS-E and IR-E elderly subjects during EuAA and HyperAA clamps
IS-E EuAA
(n⫽ 12) IS-E HyperAA(n⫽ 12) IR-E EuAA(n⫽ 12) IR-E HyperAA(n⫽ 12)
Age (yr) 69.4⫾ 1.4 72.5⫾ 1.7 69.8⫾ 0.8 69.5⫾ 1.9 Body weight (kg) 67.2⫾ 5.7 71.8⫾ 2.1 61.4⫾ 5.9 80.0⫾ 4.7 BMI (kg/m2) 24.5⫾ 0.9 25.0⫾ 0.9 24.4⫾ 2.1 27.4⫾ 0.7a FFM (kg) 50.3⫾ 6.4 57.4⫾ 2.0 43.1⫾ 2.5 61.0⫾ 4.5 Fat mass (%) 26.0⫾ 3.9 20.2⫾ 1.0 28.8⫾ 3.9 24.0⫾ 2.9 Fasting glucose (mg/dl) 85.8⫾ 4.3 91.1⫾ 3.3 83.2⫾ 3.8 86.9⫾ 3.6
Plasma insulin (U/ml) 5.8⫾ 0.7 5.3⫾ 0.6 6.6⫾ 0.4b 10.5⫾ 3.1a
Changes over basal GDR/changes over basal insulin (mg/ml䡠U/kg FFM䡠min)
0.13⫾ 0.02 0.13⫾ 0.04 0.06⫾ 0.02b 0.04⫾ 0.01a
Changes over basal PB/changes over basal insulin
(mg/ml䡠U/kg FFM䡠min) ⫺0.012 ⫾ 0.003 ⫺0.026 ⫾ 0.003
c ⫺0.016 ⫾ 0.002 ⫺0.018 ⫾ 0.003
aP⬍ 0.05, IR-E HyperAA vs. IS-E HyperAA subjects. bP⬍ 0.05, IR-E EuAA vs. IS-E EuAA subjects. cP⬍ 0.05, IS-E HyperAA vs. IS-E EuAA subjects.
(Fig. 2). Nonoxidative leucine disposal (micromoles per kilogram of FFM per minute), an index of whole body protein synthesis, is the difference between total leucine Ra and leucine oxidation. Finally, leucine balance is the difference between total leucine intake (from tracer and amino acid infusions) and leucine oxidation.
To consider the various insulin concentrations between subjects, the ratio between the change over the basal whole body glucose disposal rate or PB, and the change over the basal plasma insulin concentration were calculated: (clamp glucose disposal rate⫺ basal glucose disposal rate)/(clamp plasma insulin concentration⫺ basal plasma insulin con-centration) or (clamp PB⫺ basal PB)/(clamp plasma insulin concen-tration⫺ basal plasma insulin concentration).
Statistical analysis
Results are expressed as the mean ⫾ sem. Body composition and biological data between the groups were compared using a one-way ANOVA (StatView 5.0, Abacus Concepts, Inc., Berkeley, CA). Glucose and leucine parameters were compared between the groups by two-way ANOVA for repeated measurements, with age and insulin sensitivity as the classifying factors. For all parameters in which significant differences were detected in the basal state ([13C]leucine, [13C]KIC, and [2H
2]glucose enrichments; [13C]leucine infusion rate; plasma branched chain, essen-tial, nonessenessen-tial, and total amino acid concentrations; plasma insulin concentrations; endogenous leucine Ra; leucine oxidation; nonoxidative leucine disposal; and leucine balance), an analysis of covariance model was applied using the basal values as covariates. However, the statistical outcomes were not affected by such analysis. The ratios between the change over basal in the whole body glucose disposal rate or PB and the change over basal in the plasma insulin concentration were also ana-lyzed by two-way factorial ANOVA. When a significant effect was detected, differences among individual means were assessed with Fish-er’s protected least significant difference post hoc test to determine pair-wise differences. The level of significant difference was set at P⬍ 0.05 for all statistical tests.
Results
Body composition
Body mass index (BMI) was higher in elderly subjects than in young subjects (P⬍ 0.05; Table 1). FFM was not statisti-cally different between young and elderly individuals [P⫽ not significant (NS)] in absolute value, but was significantly lower in elderly subjects in proportion to body weight (young, 80.5⫾ 1.4%; elderly, 74.7 ⫾ 1.5%; P ⬍ 0.05, elderly
vs. young). The contribution of fat mass to body weight was
greater in elderly subjects than in young subjects (P⬍ 0.05), whereas there was no difference in body weight in either groups. Fasting glucose was slightly higher, but not signif-icantly so, in elderly compared with young individuals, but none had fasting hyperglycemia.
BMI, body weight, FFM, fat mass, and fasting glucose were not statistically different between IS-E and IR-E subjects (P⫽ NS), except for IR-E, who had higher BMIs than IS-E subjects during the HyperAA clamp (Table 2).
Plasma leucine, KIC, and glucose enrichments and expired
13CO
2production
[13C]Leucine and [13C]KIC enrichments (Table 3) remained constant when amino acid infusion enabled maintenance of the basal plasma leucine concentration and were diminished during the infusion of high amount of leucine with amino acid infusion (P⬍ 0.05). The [13C]leucine enrichments were higher during the clamp in elderly subjects than in young subjects regardless of the plasma amino acid level (Table 3).
[2H
2]Glucose enrichment decreased during the clamp re- TABLE
3. Plasma leucine, KIC and glucose enrichments, expired 13 CO 2 production and [ 13 C]leucine and [ 2H 2 ]glucose infusion rates during the basal state and the two insulin clamps in young and elderly subjects EuAA HyperAA Young (n ⫽ 6) Elderly (n ⫽ 12) Young (n ⫽ 8) Elderly (n ⫽ 12) Basal Clamp Basal Clamp Basal Clamp Basal Clamp [ 13 C]Leucine enrichment (MPE) 5.04 ⫾ 0.12 5.25 ⫾ 0.14 5.66 ⫾ 0.25 5.78 ⫾ 0.25 a 7.36 ⫾ 0.35 5.66 ⫾ 0.37 b 8.43 ⫾ 0.21 6.31 ⫾ 0.28 a,b,c [ 13 C]KIC enrichment (MPE) 4.06 ⫾ 0.09 4.32 ⫾ 0.10 4.55 ⫾ 0.19 4.54 ⫾ 0.20 5.85 ⫾ 0.21 5.24 ⫾ 0.32 b,c 6.67 ⫾ 0.15 5.64 ⫾ 0.25 b,c [ 2H 2 ]Glucose enrichment (MPE) 1.13 ⫾ 0.03 0.68 ⫾ 0.08 b 1.22 ⫾ 0.05 0.78 ⫾ 0.07 b 1.78 ⫾ 0.11 0.81 ⫾ 0.03 b 2.06 ⫾ 0.04 1.16 ⫾ 0.12 a,b,c 13 CO 2 excretion ( mol/kg FFM 䡠min) 0.014 ⫾ 0.001 0.023 ⫾ 0.001 b 0.016 ⫾ 0.001 0.028 ⫾ 0.001 b 0.019 ⫾ 0.001 0.054 ⫾ 0.003 b,c 0.017 ⫾ 0.001 0.054 ⫾ 0.004 b,c [ 13 C]Leucine infusion rate ( mol/kg FFM 䡠min) 0.093 ⫾ 0.001 0.093 ⫾ 0.001 0.102 ⫾ 0.004 0.102 ⫾ 0.004 0.134 ⫾ 0.005 0.185 ⫾ 0.014 0.133 ⫾ 0.002 0.193 ⫾ 0.011 [ 2H 2 ]Glucose infusion rate (mg/kg FFM 䡠min) 0.036 ⫾ 0.001 0.036 ⫾ 0.001 0.039 ⫾ 0.002 0.039 ⫾ 0.002 0.051 ⫾ 0.001 0.051 ⫾ 0.001 0.057 ⫾ 0.001 0.057 ⫾ 0.001 Data are the mean ⫾ SEM . a P ⬍ 0.05, elderly vs. young. b P ⬍ 0.05, clamp vs. basal. cP ⬍ 0.05, HyperAA vs. EuAA.
gardless of the amino acid concentration (Table 3; P⬍ 0.05). During insulin infusion, these enrichments were higher dur-ing the HyperAA clamp in elderly than in young subjects. The insulin infusion induced an increase in expired13CO
2 production in both conditions (P⬍ 0.05; Table 3). However, this parameter was higher during the HyperAA clamp (P⬍ 0.05).
Plasma insulin concentration
Plasma insulin concentrations (see Table 5) were system-atically higher in the elderly compared with the young sub-jects (P⬍ 0.05). The basal plasma insulin level was not dif-ferent between groups, except for IR-E subjects in HyperAA conditions, in whom it was significantly higher than in other groups (Table 2). During the HyperAA clamp, IR-E subjects had higher plasma insulin concentrations than during the EuAA clamp (Table 2).
Plasma amino acid concentrations
Plasma amino acid concentrations (Table 4) were not dif-ferent during the EuAA clamp in young and elderly subjects. During the HyperAA clamp, plasma amino acid concentra-tions were increased in both groups, but in elderly subjects, branched chain, essential, and total amino acid levels were higher than those in young subjects (P⬍ 0.05).
Glucose metabolism
As indicated in Table 5, the basal glucose disposal rate was not different between young and elderly subjects during the two clamps. However, the insulin-mediated increase in glu-cose disposal, represented by the ratio between the change over the basal glucose disposal rate and the change over the basal plasma insulin concentration (Fig. 3A), was lower in elderly than in young subjects during both the EuAA clamp (elderly vs. young, 0.10⫾ 0.02 vs. 0.20 ⫾ 0.03 mg/ml䡠U/kg FFM䡠min; P ⬍ 0.05) and the HyperAA clamp (elderly vs. young, 0.05⫾ 0.01 vs. 0.13 ⫾ 0.02 mg/ml䡠U/kg FFM䡠min;
P ⬍ 0.05). The utilization of glucose was lower after
Hy-perAA clamp than after EuAA clamp only in young subjects (P⬍ 0.05). Endogenous glucose production was not different between groups in the basal state. This parameter was re-duced by insulin similarly in young and elderly subjects in both conditions (P⫽ NS).
Regarding the insulin sensitivity of elderly subjects, the insulin-mediated increase in glucose disposal was greater in
IS-E subjects compared with IR-E subjects in both conditions (Table 2).
Leucine metabolism
As indicated in Table 5, basal total leucine flux normalized for FFM was similar in young and elderly subjects during both clamps (P ⫽ NS). During the HyperAA clamp, total leucine Ra was stimulated in both young and elderly subjects (P⬍ 0.0001). In the postabsorptive state, PB was not different between groups. After insulin infusion, rates of PB, in ab-solute values (Table 5), were lower in HyperAA than in EuAA groups (P ⬍ 0.05). When the differences in insulin concentration between the groups were considered (Fig. 3B), the ratio between the change over basal PB and the change over basal insulin concentration was statistically lower in elderly subjects than in young subjects in both conditions [EuAA,⫺0.014 ⫾ 0.002 vs. ⫺0.024 ⫾ 0.003mol/ml䡠U/kg FFM䡠min (elderly vs. young, P ⬍ 0.05); HyperAA, ⫺0.022 ⫾ 0.002 vs.⫺0.036 ⫾ 0.003mol/ml䡠U/kg FFM䡠min (elderly
vs. young, P⬍ 0.05)]. The insulin-mediated PB inhibition was
greater after HyperAA clamp than after EuAA clamp (P⬍ 0.05) regardless of the age of the subjects.
When the insulin sensitivity of elderly subjects was con-sidered, the inhibition of PB was similar in IS-E and IR-E individuals (Table 2). Nevertheless, addition of amino acids to the insulin infusion increased the inhibition of PB only in IS-E subjects (P⬍ 0.05, HyperAA vs. EuAA).
Leucine oxidation (Table 5) remained stable during the EuAA clamp and was increased during the HyperAA clamp (P⬍ 0.05) regardless of the age of the subjects. Nonoxidative leucine disposal (Table 5), an index of protein synthesis, increased during the HyperAA clamp (P⬍ 0.05), but was not changed compared with the basal value during the EuAA clamp. Leucine balance (Table 5) was less negative after the EuAA (P⬍ 0.05) clamp and became strongly positive during the HyperAA clamp (P⬍ 0.05), similarly in young and el-derly subjects.
Discussion
In the present study we demonstrated that elderly subjects were resistant to insulin for both glucose and protein me-tabolism, particularly for PB regulation by insulin. Never-theless, the ability of amino acids to enhance the insulin-mediated inhibition of PB is preserved in this population. In addition, the response of PB to insulin was similarly affected TABLE 4. Plasma branched chain, essential, nonessential, and total amino acid concentrations in the basal state and during the two insulin clamps in young and elderly subjects
EuAA HyperAA
Young (n⫽ 6) Elderly (n⫽ 12) Young (n⫽ 8) Elderly (n⫽ 12)
Basal Clamp Basal Clamp Basal Clamp Basal Clamp
BCAA (mol/liter) 345⫾ 47 314⫾ 46 356⫾ 38 377⫾ 49 503⫾ 59 882⫾ 75a,b 395⫾ 24 1024⫾ 60a,b,c
EAA (mol/liter) 798⫾ 109 772⫾ 126 774⫾ 117 871⫾ 124 992⫾ 96 1679⫾ 114a,b 804⫾ 96 1934⫾ 102a,b,c
NEAA (mol/liter) 1642⫾ 144 1533 ⫾ 130 1541 ⫾ 136 1642 ⫾ 137 1797 ⫾ 156 2235 ⫾ 175a,b 1581⫾ 156 2256 ⫾ 147a,b
Total AA (mol/liter) 2440 ⫾ 118 2305 ⫾ 108 2314 ⫾ 112 2513 ⫾ 114 2789 ⫾ 180 3914 ⫾ 235a,b 2385⫾ 59 4190⫾ 108a,b,c
Data are the mean⫾SEM. BCAA, Branched chain amino acid; EAA, essential amino acid; NEAA, nonessential amino acid.
aP⬍ 0.05, clamp vs. basal. bP⬍ 0.05, HyperAA vs. EuAA. cP⬍ 0.05, elderly vs. young.
in elderly subjects regardless of their degree of insulin sen-sitivity for glucose metabolism.
As previously described, we noticed a reduced insulin action not only on glucose utilization (7, 13–18), but also on PB in the aged population (7). Only a few studies have shown that the insulin sensitivities of glucose and protein metabo-lism may be differently affected during aging (9, 18, 27). This is also the case in other circumstances, such as diabetes (28) or obesity (29). In all of these studies, proteolysis inhibition was calculated without consideration of the plasma insulin levels of the subjects. Indeed, plasma insulin concentrations were significantly increased in obese (29) and noninsulin-dependent diabetes mellitus (28) subjects in the fasted state and during the insulin clamp. Insulin levels were higher during the clamp in elderly subjects than in young subjects
TABLE 5. Whole body glucose and leucine kinetics during the basal state and the insulin clamps in young and elderly subjects EuAA HyperAA Young (n ⫽ 6) Elderly (n ⫽ 12) Young (n ⫽ 8) Elderly (n ⫽ 12) Basal Clamp Basal Clamp Basal Clamp Basal Clamp Plasma insulin ( U/ml) 5.7 ⫾ 0.4 28.1 ⫾ 1.5 a 6.0 ⫾ 0.4 35.2 ⫾ 1.3 a,b 9.8 ⫾ 1.4 45.8 ⫾ 5.5 a,c 7.9 ⫾ 1.7 62.0 ⫾ 8.0 a,b,c Glucose disposal (mg/kg FFM 䡠min) 2.89 ⫾ 0.25 7.25 ⫾ 0.80 a 3.34 ⫾ 0.29 6.07 ⫾ 0.33 a 2.98 ⫾ 0.16 7.12 ⫾ 0.31 a 2.70 ⫾ 0.07 6.32 ⫾ 0.76 a Glucose production (mg/kg FFM 䡠min) 2.78 ⫾ 0.24 1.37 ⫾ 0.32 a 3.26 ⫾ 0.24 1.14 ⫾ 0.35 a 2.93 ⫾ 0.16 0.52 ⫾ 0.29 a 2.68 ⫾ 0.06 0.88 ⫾ 0.38 a Total leucine Ra ( mol/kg FFM 䡠min) 2.29 ⫾ 0.06 2.16 ⫾ 0.05 2.24 ⫾ 0.07 2.23 ⫾ 0.08 2.30 ⫾ 0.08 3.51 ⫾ 0.10 a,c 1.89 ⫾ 0.10 3.23 ⫾ 0.17 a,b,c Endo Leu Ra ( mol/kg FFM 䡠min) 2.20 ⫾ 0.06 1.71 ⫾ 0.05 a 2.14 ⫾ 0.07 1.73 ⫾ 0.07 a 2.16 ⫾ 0.07 0.95 ⫾ 0.05 a,c 1.87 ⫾ 0.03 0.79 ⫾ 0.07 a,b,c Leucine oxidation ( mol/kg FFM 䡠min) 0.49 ⫾ 0.03 0.52 ⫾ 0.02 0.52 ⫾ 0.02 0.61 ⫾ 0.02 0.47 ⫾ 0.02 1.27 ⫾ 0.07 a,c 0.36 ⫾ 0.02 1.16 ⫾ 0.07 a,b,c Nonoxidative leucine disposal ( mol/kg FFM 䡠min) 1.81 ⫾ 0.05 1.65 ⫾ 0.04 1.77 ⫾ 0.05 1.63 ⫾ 0.07 1.83 ⫾ 0.07 2.25 ⫾ 0.14 a,c 1.64 ⫾ 0.03 2.24 ⫾ 0.10 a,c Leucine balance ( mol/kg FFM 䡠min) ⫺ 0.40 ⫾ 0.03 ⫺ 0.07 ⫾ 0.03 a ⫺ 0.41 ⫾ 0.02 ⫺ 0.10 ⫾ 0.03 a ⫺ 0.33 ⫾ 0.02 1.18 ⫾ 0.16 a ⫺ 0.22 ⫾ 0.02 1.46 ⫾ 0.07 a Data are the mean ⫾ SEM . a P ⬍ 0.05, clamp vs. basal. b P ⬍ 0.05, elderly vs. young. cP ⬍ 0.05, HyperAA vs. EuAA.
FIG. 3. Effect of insulin and amino acid on glucose disposal increase (A) and PB inhibition induced by insulin (B) in young (䡺) and elderly (f) subjects during EuAA and HyperAA, euglycemic, hyperinsuline-mic clamps. *, P⬍ 0.05 for age effect; ‡, P ⬍ 0.05 for amino acid effect.
(18), suggesting reduced insulin clearance with age, as pre-viously reported (30). Because higher plasma insulin levels were required in the elderly to obtain the same result as in young, the insulin action on PB may be interpreted as de-fective in elderly individuals. This aspect was demonstrated previously (7) and in our present study. Actually, during both clamps, a lesser inhibition of PB mediated by insulin was found in elderly subjects despite a greater insulinemia than in young subjects. Therefore, the inhibition of PB should be evaluated according to the individual insulinemia to com-pare protein metabolism in individuals differing in plasma insulin levels. The effect of amino acid infusion on protein metabolism was also investigated in our experiment. Indeed, proteolysis may be inhibited by amino acids alone (8, 11) or in conjunction with insulin (9, 10). Amino acids induced a greater inhibition of PB in both young and elderly subjects, but PB inhibition was still less in the elderly. Thus, amino acid infusion could not compensate for the decreased re-sponse of proteolysis to the inhibitory effect of insulin in elderly subjects. This observation is consistent with the con-cept of a relative resistance to the anabolic action of insulin and amino acids during aging, because it has been shown previously for protein synthesis (3, 31, 32). It seems that in elderly population, the alteration in the anabolic process involves resistance not only to the stimulatory action of amino acids and nutrients on protein synthesis, but also to the inhibitory effect of insulin on PB. This defective response to nutritional factors could contribute to the age-related loss of protein mass or to a redistribution of protein metabolism from the muscle to the splanchnic tissues (20, 33). Therefore, leucine balance may not reflect what is happening at the muscle level. Indeed, metabolic alterations can reduce pro-tein deposition in the postprandial state, which may not compensate for protein lost during the postabsorptive phase. Nevertheless, recent studies have shown an improvement of protein retention in elderly subjects by increasing amino acid availability (34, 35). One possibility would be to modulate the distribution of daily protein intake; when 80% of protein intake was consumed at one meal (a pulse pattern feeding), the increase in nitrogen balance was higher than when the same intake was distributed over four meals (a spread pat-tern) in elderly women (34). The inverse tendency was found in young women (a better nitrogen balance with the spread pattern) (36). The protein digestion rate of proteins, i.e. the slow and fast protein concept (37) could also increase amino acid availability. A fast absorbed protein included in a mixed meal induced a better protein balance than a slow one in elderly subjects, contrary to what was observed in young subjects (35). In so far as the pulse feeding condition could be assimilated to the administration of a fast protein, these studies clearly demonstrated the major relevance of amino acid availability in combination with a postprandial increase in insulin to improve protein retention and thus to prevent sarcopenia in the aged population.
In the present study the influence of hyperaminoacidemia on glucose metabolism was also investigated in young and elderly individuals. The modulation of insulin action on glu-cose disposal by amino acids has been previously docu-mented in young subjects (38 – 42), but not in the elderly. Infusion of a mixture of amino acids decreased the rate of
infusion of exogenous glucose required to maintain eugly-cemia during a hyperinsulinemic clamp (38, 42), suggesting a reduction in whole body glucose utilization (40). The mech-anism involved in skeletal muscle insulin resistance induced by plasma amino acids may be an inhibition of glucose trans-port/phosphorylation, resulting in a marked reduction of glycogen synthesis (43). In addition, amino acids could de-crease glucose oxidation (44, 45) by substrate competition at the mitochondrial level, such as for glucose/lipid competi-tion, or by interaction with early steps of insulin signaling (46). The whole body glucose disposal rate, in this study, is reduced after the infusion of high amino acid levels in young subjects only. Actually, the resistance to insulin action on glucose metabolism already existed in elderly subjects. Thus, the effect of amino acid administration on the glucose me-tabolism response to insulin may not be effective in the elderly. Therefore, considering previous observations in young subjects, we could hypothesize that insulin resistance in the elderly occurred at the glucose transport or phosphor-ylation level.
To a lesser extent, the sensitivity of insulin for glucose and protein metabolism has been evaluated in the elderly pop-ulation under euglycemic hyperinsulinemic conditions. Two groups were distinguished on the basis of glucose disposal rate: IR-E and IS-E. Because insulin resistance and glucose intolerance develop with aging, previous studies have been designed to determine the impact of diet or physical exercise on improvement in insulin sensitivity in aged individuals (47– 49). However, the relationship between insulin sensi-tivity for glucose and protein metabolism was poorly doc-umented. In our study the increase in glucose disposal me-diated by insulin was greater in the IS-E group than in the IR-E group, but insulin action was similarly reduced on PB in both elderly groups. Despite a better response of glucose metabolism to insulin in the IS-E group, it seemed to have more difficulty acting on PB.
In conclusion, this study showed that 1) elderly subjects developed resistance to insulin action for both glucose uti-lization and PB; 2) hyperaminoacidemia enhanced insulin-mediated PB inhibition; and 3) the sensitivity of glucose metabolism is dissociated from the sensitivity of protein me-tabolism to insulin. The results of this study support the concept that aminoacidemia appears to be as important as insulinemia in the regulation of protein metabolism in young and elderly individuals.
Acknowledgments
We thank Liliane Morin for monitoring the subjects throughout the clamp studies, Marion Brandolini for dietary recall analysis, and Sandrine Corny for tracer preparations.
Received August 14, 2003. Accepted September 2, 2004.
Address all correspondence and requests for reprints to: Dr. Yves Boirie, Unite´ du Me´tabolisme Prote´ino-E´nerge´tique, Laboratoire de Nu-trition Humaine, BP 321, 58 rue Montalembert, 63009 Clermont-Ferrand Cedex 1, France. E-mail: boirie@clermont.inra.fr.
This study was presented in part at the 25th Congress of the European Society of Parenteral and Enteral Nutrition, September 2003, Cannes, France [Clin Nutr S1:14(052)].
References
1. Flynn MA, Nolph GB, Baker AS, Martin WM, Krause G 1989 Total body potassium in aging humans: a longitudinal study. Am J Clin Nutr 50:713–717 2. Forbes GB 1999 Longitudinal changes in adult fat-free mass: influence of body
weight. Am J Clin Nutr 70:1025–1031
3. Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR 2000 The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab 85:4481– 4490
4. Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier
DM, Young VR1985 Insulin-mediated reduction of whole body protein
break-down. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest 76:2306 –2311
5. Tessari P, Trevisan R, Inchiostro S, Biolo G, Nosadini R, De Kreutzenberg
SV, Duner E, Tiengo A, Crepaldi G1986 Dose-response curves of effects of
insulin on leucine kinetics in humans. Am J Physiol 251:E334 –E342 6. Biolo G, Declan Fleming RY, Wolfe RR 1995 Physiologic hyperinsulinemia
stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 95:811– 819
7. Boirie Y, Gachon P, Cordat N, Ritz P, Beaufrere B 2001 Differential insulin sensitivities of glucose, amino acid, and albumin metabolism in elderly men and women. J Clin Endocrinol Metab 86:638 – 644
8. Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA 1987 Effect of insulin and plasma amino acid concentrations on leucine metabolism in man. Role of substrate availability on estimates of whole body protein syn-thesis. J Clin Invest 80:1784 –1793
9. Fukagawa NK, Minaker KL, Young VR, Matthews DE, Bier DM, Rowe JW 1989 Leucine metabolism in aging humans: effect of insulin and substrate availability. Am J Physiol 256:E288 –E294
10. Flakoll PJ, Kulaylat M, Frexes-Steed M, Hourani H, Brown LL, Hill JO,
Abumrad NN1989 Amino acids augment insulin’s suppression of whole body
proteolysis. Am J Physiol 257:E839 –E847
11. Giordano M, Castellino P, DeFronzo RA 1996 Differential responsiveness of protein synthesis and degradation to amino acid availability in humans. Di-abetes 45:393–399
12. Tessari P, Inchiostro S, Biolo G, Trevisan R, Fantin G, Marescotti MC, Iori
E, Tiengo A, Crepaldi G1987 Differential effects of hyperinsulinemia and
hyperaminoacidemia on leucine-carbon metabolism in vivo. Evidence for dis-tinct mechanisms in regulation of net amino acid deposition. J Clin Invest 79:1062–1069
13. Defronzo RA 1979 Glucose intolerance and aging: evidence for tissue insen-sitivity to insulin. Diabetes 28:1095–1101
14. Robert JJ, Cummins JC, Wolfe RR, Durkot M, Matthews DE, Zhao XH, Bier
DM, Young VR1982 Quantitative aspects of glucose production and
metab-olism in healthy elderly subjects. Diabetes 31:203–211
15. Gumbiner B, Thorburn AW, Ditzler TM, Bulacan F, Henry RR 1992 Role of impaired intracellular glucose metabolism in the insulin resistance of aging. Metabolism 41:1115–1121
16. Pagano G, Marena S, Scaglione L, Bodoni P, Montegrosso G, Bruno A,
Cassader M, Bonetti G, Cavallo Perin P1996 Insulin resistance shows selective
metabolic and hormonal targets in the elderly. Eur J Clin Invest 26:650 – 656 17. Broughton DL, James OW, Alberti KG, Taylor R 1991 Peripheral and hepatic insulin sensitivity in healthy elderly human subjects. Eur J Clin Invest 21:13–21 18. Fukagawa NK, Minaker KL, Rowe JW, Matthews DE, Bier DM, Young VR 1988 Glucose and amino acid metabolism in aging man: differential effects of insulin. Metabolism 37:371–377
19. Arnal MA, Mosoni L, Boirie Y, Gachon P, Genest M, Bayle G, Grizard J,
Arnal M, Antoine JM, Beaufrere B, Mirand PP2000 Protein turnover
mod-ifications induced by the protein feeding pattern still persist after the end of the diets. Am J Physiol Endocrinol Metab 278:E902–E909
20. Boirie Y, Gachon P, Beaufrere B 1997 Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 65:489 – 495
21. Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
22. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ2000 Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
23. Bergman RN, Prager R, Volund A, Olefsky JM 1987 Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 7:790 – 800
24. Jacquez JA 1992 Theory of production rate calculations in steady and non-steady states and its application to glucose metabolism. Am J Physiol 262: E779 –E790
25. Schwenk WF, Beaufrere B, Haymond MW 1985 Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol 249:E646 –E650 26. Reaich D, Graham KA, Cooper BG, Scrimgeour CM, Goodship THJ 1994 Recovery of 13C in breath from infused NaH13CO3 increases during eugly-caemic hyperinsulinemia. Clin Sci (Lond) 87:415– 419
27. Marchesini G, Cassarani S, Checchia GA, Bianchi G, Bua V, Zoli M, Pisi E 1987 Insulin resistance in aged man: relationship between impaired glucose tolerance and decreased insulin activity on branched-chain amino acids. Me-tabolism 36:1096 –1100
28. Luzi L, Petrides AS, De Fronzo RA 1993 Different sensitivity of glucose and amino acid metabolism to insulin in NIDDM. Diabetes 42:1868 –1877 29. Luzi L, Castellino P, DeFronzo RA 1996 Insulin and hyperaminoacidemia
regulate by a different mechanism leucine turnover and oxidation in obesity. Am J Physiol 270:E273–E281
30. Fink RI, Revers RR, Kolterman OG, Olefsky JM 1985 The metabolic clearance of insulin and the feedback inhibition of insulin secretion are altered with aging. Diabetes 34:275–280
31. Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY,
Patureau Mirand P1995 Altered response of protein synthesis to nutritional
state and endurance training in old rats. Am J Physiol 268:E328 –E235 32. Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L,
Grizard J, Boirie Y2004 Impaired anabolic response of muscle protein
syn-thesis is associated with S6K1 dysregulation in elderly humans. FASEB J 18:1586 –1587
33. Uauy R, Winterer JC, Bilmazes C, Haverberg LN, Scrimshaw NS, Munro HN,
Young VR1978 The changing pattern of whole body protein metabolism in
aging humans. J Gerontol 33:663– 671
34. Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P,
Antoine JM, Prugnaud J, Beaufrere B, Mirand PP1999 Protein pulse feeding
improves protein retention in elderly women. Am J Clin Nutr 69:1202–1208 35. Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C,
Reiffers-Magnani K, Fauquant J, Ballevre O, Beaufrere B2003 The rate of
protein digestion affects protein gain differently during aging in humans. J Physiol 549:635– 644
36. Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P,
Antoine JM, Prugnaud J, Beaufrere B, Mirand PP2000 Protein feeding pattern
does not affect protein retention in young women. J Nutr 130:1700 –1704 37. Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B 1997
Slow and fast dietary proteins differently modulate postprandial protein ac-cretion. Proc Natl Acad Sci USA 94:14930 –14935
38. Boden G, Tappy L 1990 Effects of amino acids on glucose disposal. Diabetes 39:1079 –1084
39. Flakoll PJ, Wentzel LS, Rice DE, Hill JO, Abumrad NN 1992 Short-term regulation of insulin-mediated glucose utilization in four-day fasted human volunteers: role of amino acid availability. Diabetologia 35:357–366 40. Pisters PW, Restifo NP, Cersosimo E, Brennan MF 1991 The effects of
eu-glycemic hyperinsulinemia and amino acid infusion on regional and whole body glucose disposal in man. Metabolism 40:59 – 65
41. Tappy L, Acheson K, Normand S, Schneeberger D, Thelin A, Pachiaudi C,
Riou JP, Jequier E1992 Effects of infused amino acids on glucose production
and utilization in healthy human subjects. Am J Physiol 262:E826 –E833 42. Tessari P, Inchiostro S, Biolo G, Duner E, Nosadini R, Tiengo A, Crepaldi
G1985 Hyperaminoacidaemia reduces insulin-mediated glucose disposal in healthy man. Diabetologia 28:870 – 872
43. Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M,
Nowotny P, Roth E, Waldhausl W, Roden M2002 Mechanism of amino
acid-induced skeletal muscle insulin resistance in humans. Diabetes 51: 599 – 605
44. Walajtys-Rode E, Williamson JR 1980 Effects of branched chain␣-ketoacids on the metabolism of isolated rat liver cells. III. Interactions with pyruvate dehydrogenase. J Biol Chem 255:413– 418
45. Chang TW, Goldberg AL 1978 Leucine inhibits oxidation of glucose and pyruvate in skeletal muscles during fasting. J Biol Chem 253:3696 –3701 46. Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR 1998 Bidirectional
modulation of insulin action by amino acids. J Clin Invest 101:1519 –1529 47. Cox JH, Cortright RN, Dohm GL, Houmard JA 1999 Effect of aging on
response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol 86:2019 –2025
48. Fukagawa NK, Anderson JW, Hageman G, Young VR, Minaker KL 1990 High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr 52:524 –528
49. Houmard JA, Egan PC, Neufer PD, Friedman JE, Wheeler WS, Israel RG,
Dohm GL1991 Elevated skeletal muscle glucose transporter levels in
exercise-trained middle-aged men. Am J Physiol 261:E437–E443
JCEM is published monthly by The Endocrine Society (http://www.endo-society.org), the foremost professional society serving the endocrine community.