HAL Id: hal-03085571
https://hal.archives-ouvertes.fr/hal-03085571
Submitted on 21 Dec 2020
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
The multiscale and multiphase organization of the
transcriptome
Danielle Adekunle, Arnaud Hubstenberger
To cite this version:
Danielle Adekunle, Arnaud Hubstenberger. The multiscale and multiphase organization of the tran-scriptome. Emerging Topics in Life Sciences, 2020, 4 (3), pp.265-280. �10.1042/ETLS20190187�. �hal-03085571�
The multiscale and multiphase
organization of the transcriptome
Danielle A. Adekunle
1,2; Arnaud Hubstenberger
31. Department of Biology, Massachusetts Institute of Technology, Cambridge, MA
02139, U.S.A.
2. Department of Molecular Genetics and Microbiology, UF Genetics Institute, Center
for Neurogenetics, University of Florida, Gainesville, FL, U.S.A
3. Université Côte D'Azur, CNRS, Inserm, iBV, Nice, France
Correspondence: Arnaud Hubstenberger (
Arnaud.HUBSTENBERGER@univ-cotedazur.fr
)
Abstract. Gene expression must be co-ordinated to cellular activity. From transcription to
decay, the expression of millions of RNA molecules is highly synchronized. RNAs are covered
by proteins that regulate every aspect of their cellular life: expression, storage, translational
status, localization, and decay. Many RNAs and their associated regulatory proteins can
coassemble to condense into liquid droplets, viscoelastic hydrogels, freeze into disorganized
glass-like aggregates, or harden into quasi-crystalline solids. Phase separations provide a
framework for transcriptome organization where the single functional unit is no longer a
transcript but instead an RNA regulon. Here, we will analyze the interaction networks that
underlie RNA super-assemblies, assess the complex multiscale, multiphase architecture of the
transcriptome, and explore how the biophysical state of an RNA molecule can define its fate.
Phase separations are emerging as critical routes for the epitranscriptomic control of gene
expression.
Keywords: post-transcriptional regulation, ribonucleoproteins, RNA condensates, RNA phase
separation and transition, stress granules, transcriptomics
Introduction.
There are hundreds of thousand mRNA molecules [1] and millions of total RNA molecules in a
single mammalian cell [2]. Transcriptome organization must, therefore, be highly co-ordinated
from transcription to decay. In the nucleus, RNAs are co-transcriptionally bound by regulatory
RNA binding proteins (RBPs) that regulate RNA fate. Ribonucleoproteins (RNPs), RNAs
complexed with RBPs, are the functional units upon which the mechanisms organizing the
transcriptome act. These RNPs can coassemble into super-assemblies called RNA condensates
(
Figure 1
) [3,4], that demix from the cytosol or nucleoplasm to provide a means to orchestrate
the transcriptome that would not be possible by simple Brownian diffusion.
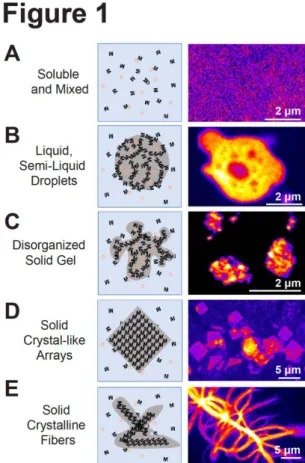
Figure 1. RNP coassembly can induce phase
separation.
Left panel, schematic representations of different states of RNP organization. Right panel, confocal image of the, respective, fluorescently labelled RNP superassembly. (A) Soluble RNPs, ‘M’, and small molecules ‘O’, can be homogenously mixed within the cytoplasm or nucleoplasm. Right panel, repressed mRNPs homogenously mixed in the cytosol of a C. elegans oocyte (B) Dynamic, multivalent RNP interactions can induce demixing of RNPs into semi-liquid or liquid droplets. Right panel, repressed mRNPs condensed into a viscoelastic droplet within the cytosol of a quiescent C. elegans oocyte. (C) Stronger interactions can promote RNP coassembly into amorphous hydrogels. Right panel, heat shock-induced stress granules in C. elegans oocytes. (D) RNPs can polymerize into regular arrays. Right panel, square sheet granules induced by the loss of function of CGH-1 helicase in C. elegans oocyte are toxic to the cell but reversible. (E) RNPs can stably polymerize into crystalline fibres. Right panel, mutations in the prion-like domain of FUS can induce irreversible fibrous aggregation that are pathological. In (B) and (C), small molecules can freely diffuse into and out of these porous condensates. Image (A–D) are adapted from Hubstenberger et al. [15], Image (E) from Patel et al. [20].Phase-separated RNA condensates can contain thousands of transcripts and hundreds of
proteins, providing a higher scale of cellular compartmentalization [5–13]. Nucleoli, Cajal
bodies (CBs), nuclear histones bodies, P-bodies, stress granules (SGs), translation factories,
and cell-type-specific condensates such as myo-granules, neuro-granules, and germ granules
are all well-characterized RNA super-assemblies [3,4]. These granules coassemble, at least in
part, through phase separation, a distinct mode of RNA spatial organization [3,4]. The
architecture of RNA condensates and their regulation by phase transitions provides a
mechanism for synchronizing RNA expression to cellular activity, development, and the
environment, allowing groups of RNAs to function co-ordinately.
This review will examine the various models of molecular interactions that drive RNP phase
separation with a focus on the multiscale and multiphase nature of RNA condensates. It will
also explore how phase separations co-ordinate the organization and fate of large RNA
regulons.
The physical nature of RNA phase transitions and the emergent material
properties of RNA condensates.
Transcripts are compartmentalized within condensates whose material properties control
RNA exchange, dynamics, and biochemical microenvironment. Upon coassembly, RNPs can
condense into liquid droplets, semi-liquid hydrogels, solid glass state, or crystal-like solids
(
Figure 1
). In a seminal study, live-imaging demonstrated the liquid nature of
micrometer-sized germ granules [14]. These droplets dock, fuse, mix, and relax toward a spherical shape
under surface tension (
Figure 2A
). While some droplets are liquid others are viscoelastic
(
Figure 2A,B
) [15]. Some RNP gels do not coalesce and can further solidify, limiting
intracondensate, or cytosolic exchange [16–20]. All of these low-density super-assemblies are
porous and permeable structures, largely comprised of water, that allow the passive diffusion
of small molecules [21]. The scaffolding components of liquid droplets are disordered [21,22];
but some can polymerize into geometrical shapes [15], or crystalline fibers (
Figure 1
) [16,18–
20,23–26]. Although crystalline aggregates are often associated with degenerative diseases,
diffractive assemblies have also been found in myo-granules [27]. We have only begun to
unravel the influence of these material properties on transcriptome organization.
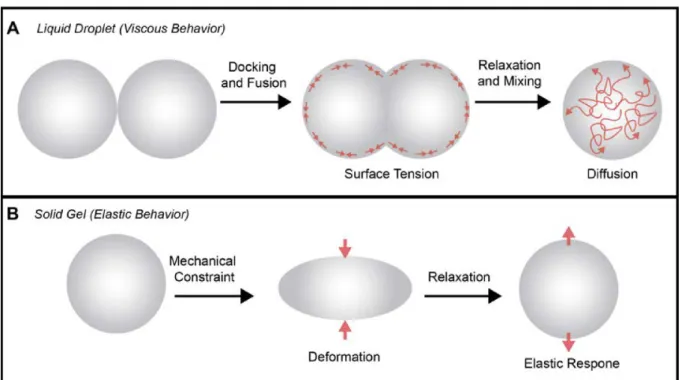
Figure 2. Characteristic properties of liquid droplet and solid gel RNP super-assemblies.
(A) Liquid droplets are typified by their ability to dock, fuse, and relax into spheres under surface tension [14,15]. Molecules can freely diffuse within liquid condensates and exchange at the interface of the condensate and the cytoplasm or nucleoplasm. (B) Solid gel condensates are deformed under mechanical stress, but characteristically recover their initial shape in an elastic response [15].Four types of interactions may trigger RNP phase transition to organize the
transcriptome.
Characterizing the molecular mechanisms that drive condensate coassembly is critical to
elucidating how condensates organize the transcriptome. As demonstrated in simplified
reconstituted systems in vitro and overexpression and ectopic expression studies in cellulo,
protein–protein, RNA–protein, and RNA–RNA interactions are individually sufficient to drive
RNP phase separations (
Figure 3
). What we have learned from these reconstituted systems is
that RNA can either drive condensation, as scaffolding components, or can be recruited into
super-assemblies by RBPs.
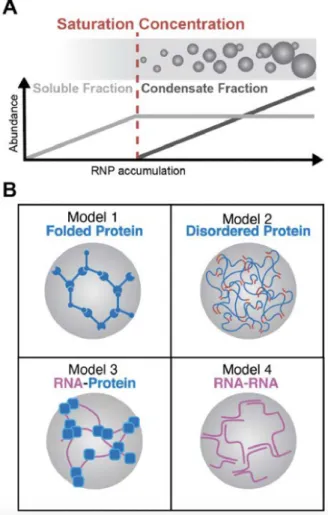
Figure 3. Mechanisms of phase separation.
(A) Phase separations are concentration dependent. Above a specific concentration threshold, RNPs will condense and the size of the condensates will increase according to the amount of RNPs in excess of this saturation concentration. While RNPs accumulate within condensates, cytosolic RNP concentration remains constant. (B) Alternative reconstituted models that induce phase separation in vitro: Model 1. Multivalent interactions of folded protein domains into highly connected networks can induce phase separation. Increased protein multivalency leads to a lowering of the saturation concentration required for condensate formation. Model 2. Unfolded disordered protein regions trigger phase separation through weak and promiscuous but highly multivalent interactions. Model 3. RNA scaffolds can bridge RBPs into clusters, lowering their local concentration and triggering phase separation. Model 4. Multivalent RNA–RNA interactions promote demixing independent of proteins. In Model 1 and Model 2, RNAs can be passively recruited as clients of RBPs.Model 1. Protein–protein interactions through folded domains.
As demonstrated in synthetic systems, when proteins coassemble into branched networks
through stereospecific interactions, the super-assemblies can lead to supramolecular
condensates that can reach microns in size [28]. The more interactions a protein engages in,
the lower the concentration required for the protein's superassembly to phase separate
(
Figure 3
). Phase separation depends on two main parameters: the concentration and the
multivalency of the scaffolding components. Condensates nucleated by protein–protein
interactions can assemble with RNAs through RNA binding domains (RBDs).
Model 2. Protein–protein interactions through intrinsically disordered regions (IDRs).
RBPs are highly enriched in IDRs [16,29]: typically low complexity sequences that do not fold
in solution and consequently do not participate to stable stereospecific interactions. To
promote self-assembly, some IDRs amino acids may work as ‘stickers’ promoting weak,
promiscuous and multivalent IDR–IDR interactions, while the rest of the disordered sequences
function as spacers within the interaction network (
Figure 3
). Various classes of IDRs, including
RGG, G/S-F/Y-G/S and polyQ repeats are soluble at low concentration and self-assemble into
viscoelastic droplets that phase separate above a saturation concentration [16,19,20,30–36].
Highly transient interactions between poorly folded IDR domains promote the liquidity of the
system, whose material properties are influenced by IDR composition [37,38]. Strikingly,
artificial droplets mimic the fluidity of endogenous ones and are also sufficient to create ∼5
nm polymer meshworks that exclude molecules larger than 50 kDa. This is similar to what has
been seen in germ granules and nucleoli [21]. Many IDRs carry prion-like sequences, through
which disorganized, weak, and transient interactions can develop into strong, irreversible
cross-β-strands with crystallin-like order as observed by X-ray diffraction [16,18–
20,25,31,33,36,39–42]. Therefore, the material properties of IDR-rich RBP granules range
from liquid to solid.
Model 3. RNA–protein interactions.
RNAs can play an important scaffolding role in condensate assembly. RNAs recruit and link
RBPs and, accordingly increase local RBP concentration leading to phase separation (
Figure
3
). In reconstituted systems, it has been observed that increased RNA repeats that recruit
RBPs lower the concentration at which RNA–protein complexes demix [28]. In vitro, IDRs can
condense on their own without RNA but at very high concentrations [16,26]. To condense at
physiological concentrations RBP–RNA interactions are required [18,19,25,36,43,44]. RBPs
must be in excess of RNAs for multiple RBPs to associate with the same RNA molecule and
maintain the multivalency required for phase separation. When RNAs are in excess and
proteins are limiting, condensate formation is restricted [25,43,45]. RNAs also contribute to
the material properties of condensates; short and long RNAs limit and increase the viscosity
of RNP droplets, respectively [21,32,36].
Model 4. RNA–RNA interactions.
Even in the absence of protein, RNA–RNA interactions are sufficient to drive phase separation,
including at physiological concentrations (
Figure 3
) [46–49]. RNAs tend to separate into
homotypic clusters. These clusters can either dock without merging or coalesce without
mixing, meaning smaller droplets can become embedded in larger ones. One phase can also
surround the other as a surfactant [50]. RNA secondary structures modulated by RBPs can add
increased specificity to RNA–RNA interactions [48].
The four aforementioned interaction models combine in vivo to drive the coassembly
of endogenous condensates. Even in the simplest phase separation model where a
single component can condense on its own, many factors control nucleation and
modulate saturation concentration above which scaffolding components demix. For
many endogenous condensates, multiple components can independently cause
demixing, meaning there are many alternative and redundant pathways that can trigger
condensate formation. One must distinguish the contribution of the nucleators that
seed phase separation, the scaffolding components that drive condensate growth, and
the ‘hitchhikers’ that are passive clients.
The diversity and complex composition of endogenous condensates.
Analyzing condensate composition is central to understanding how condensates organize the
transcriptome. Various condensate purifications approaches have been utilized, including
differential centrifugation coupled to immunoprecipitation [5,7,8,51,52], and a recently
developed technology, fluorescence-activated particle sorting (FAPS) [9]. These approaches
revealed that condensates typically consist of numerous protein species; more than 50
proteins coassemble in neuronal granules [7] and hundreds coassemble in nucleoli [10],
P-bodies [9], and SGs [5,11,12]. Additionally, they demonstrated that IDRs such as RGG, polyQ,
or F/Y-G/S repeats, are extremely enriched in condensates, suggesting that these domains
dictate biophysical condensate properties. At the proteomic level, there are many protein–
protein interactions that create dense stereospecific networks, as revealed by studies
employing proximity labelling of proteins in these networks [11,12]. The culmination of these
studies has made clear that stereospecific protein–protein and IDR–IDR interactions drive
coassembly.
Condensate transcriptomes are even more complex than proteomes. P-bodies, SGs, neuronal
granules, and germ granules contain hundreds to thousands of mRNA species [6,8,9,13].
Although no method exists currently to simultaneously purify distinct cellular condensate
subtypes and directly characterize their transcriptome, ATLAS-Seq, a novel fractionation
method coupled to RNA-sequencing identified hundreds of clusters of RNAs that co-segregate
with their regulatory proteins [53], supporting a model where RNAs coassemble into
supramolecular assemblies that have not yet been described. Small noncoding RNA (ncRNA)
content has not been characterized on a transcriptome-wide scale, but miRNAs are stably
anchored and numerous proteins from the Argonaute family collect in condensates [9,54–57].
Although there are various condensate types, condensates can be classified into two main
classes: translation factories and those accumulating translationally repressed RNAs. In fungi,
translationally repressed cytosolic mRNAs accrue in SGs, P-bodies, and Whi3 condensates
[6,48,52], while actively translated glycolytic and translation factors encoding mRNAs
condense into translation factories [58,59]. In metazoans, repressed mRNAs accumulate in
P-bodies [9], SGs [6], germ granules [8], neuronal granules [13,60,61], myo-granules [27], while
translated mRNAs can coassemble into translation factories [62].
RNAs are nucleating factors and condensate organizers.
RNAs have emerged as condensate nucleators and structuring factors. The lncRNA NEAT-1 is
perhaps the most well-characterized illustration of this. NEAT-1 bridges together RBPs [63,64]
to promote Paraspeckle phase separation. NEAT-1 defines Paraspeckle architecture by
creating condensate polarity through the positioning of its 5ʹ and 3ʹ ends at the nucleoplasmic
interface and the interring of its core within Paraspeckles [65,66]. This organizes RBPs along a
radial axis around NEAT-1 lncRNAs. The structural role of RNAs in recruiting RBPs to trigger
condensation is also evident in histone locus bodies, CBs, nuclear speckles, nuclear stress
bodies [64], and nucleoli [67–69].
However, no master scaffolding RNA components have been identified in cytosolic
condensates. While a collective of RNAs scaffold P-bodies and SGs [70,71], the most abundant
RNAs within P-bodies or SGs comprise less than 1% of their transcriptome [6,9], suggesting a
more decentralized organization for cytosolic condensates compared with nuclear
condensates. Still, RNAs determine condensate structure and composition in the cytosol. This
was demonstrated by clustering RNA targets of Pumilio protein by tethering the RBD of
Pumilio to an artificial scaffold. RNA clustering was sufficient to trigger P-body-like assembly
formation, despite the fact that Pumilio is a non-essential P-body protein [72]. Similarly, SG
assembly is triggered by RNA release from polysomes which leads to the recruitment of RBPs
that assemble SGs [73–75]. Unfolded mRNAs trigger a conformational switch and the
condensation of the master organizer of SG assembly, G3BP [76,77]. G3BP dimers directly
interact with mRNAs, and by recruiting numerous RBPs, acts as a central node within the
interaction network whose high degree of multivalency relies on RNAs to phase separate
[76,78]. Like RNA–protein and protein–protein interactions, RNA–RNA interactions contribute
to SG assembly through sense–antisense RNA hybrids [49]. In protein-free extracts RNA
precipitation partially recapitulates the SG transcriptome, suggesting that RNA–RNA
interactions are important for SG formation [49]. The most elegant demonstration to date of
mRNAs as condensate organizers is Whi3 RBP condensates in fungi. The secondary structure
of mRNAs, modulated by Whi3, control intermolecular RNA–RNA pairing, to dictate whether
two mRNA molecules will co-segregate in the same droplet, or separate into distinct droplets
[48]. Taken together, RNAs are critical to condensate formation: they nucleate RBP–RBP
interactions and regulate RNA–RNA liquid–liquid demixing specificity.
Redundant, alternative protein scaffolds create robust super-assemblies.
Multiple components can independently cause demixing, meaning various alternative and
redundant pathways can trigger condensate formation. Tethering any of the abundant
proteins of CBs, including those non-essential to endogenous CB assembly, to a repetitive DNA
array is sufficient to nucleate de novo condensates [79]. Similarly, the artificial clustering of
Pumilio, a non-essential component of P-bodies, described above, demonstrated that an RBD
is sufficient to trigger P-body-like assembly formation [72]. SGs are perhaps the most striking
example of robust and redundant assembly. Even in the absence of stress, overexpression of
any of the following components is sufficient to induce SG assembly: G3BP [80], the prion-like
domain of TIA-1 [81], CPEB1 [82], Caprin-1 [83], DYRK3 [84], hnRNPA2, and FUS [19]. All of
these examples, fit a phase separation model where concentration and multivalency are
critical determinants of condensation [28,70,76,78].
Condensate coassemblies require two types of protein–protein interactions: folded–folded
and IDR–IDR. Several proteins that participate to folded–folded interactions are required for
CB, SG, and PB formation [11,12,75–79,85]. In addition, prion-like IDR–IDR polyQ/N polymers
work synergistically with folded domains to assemble P-bodies [39,41,86]. Folded–folded
interactions bring specificity and stability to the system while IDR–IDR interactions may
provide droplet fluidity [87,88]. Essential protein components in certain environmental
contexts were found to be dispensable in others [75,85]. This signifies that alternative
assembly mechanisms may underlie the plasticity of RNA condensates and clarifies the lack of
a unique master scaffolding protein. Master organizers appear to be interchangeable central
nodes with the highest degree of multivalent interactions within the condensate network
[28,70,76,78].
RNA partitioning in RNA condensates: a sorting mechanism.
RNA sorting to condensates is central to understanding how phase separations organize the
transcriptome. Granules can be distinguished by their RNA composition [6,9,89]. Although all
mRNAs condense in SGs to some degree, the condensed fraction of mRNAs varies from <1%
to >95% [6]. Any mRNA can transiently dock on condensates, including translated ones, but
only repressed mRNAs can be stably anchored, leading to stronger enrichment [56,90].
Similarly, mRNAs condense to various degree in P-bodies [9], and single-molecule live imaging
confirmed that the fraction and dynamics of transcripts that localize to SGs and PBs is different
for each mRNA [56,91]. The wide variations in RNA inclusion suggest that RNA sorting to
condensates is fine-tuned. Whether bound in 3ʹUTRs or 5ʹUTRs Argonaute promotes
translation repression, but only 3ʹUTR bound RNAs are enriched in P-bodies: translational
repression alone is not enough to stabilize RNAs in P-bodies [9,56]. From global CLIP analyses,
we know that RNA–protein interactions are predictors of RNA enrichment in P-bodies [9] and
live single mRNA imaging demonstrated that these interactions anchor mRNAs and lncRNAs
to condensates [56,91]. From these studies, we can conclude that low specificity and
low-affinity binding allows transient RNA docking, while high specificity and high-low-affinity docking
promotes RNA anchoring. Overall, RNA condensation and solubilization within cytosolic
condensates, whose diversity reaches far beyond P-bodies and SGs to include a wide array of
cell-type-specific condensates, as well as an increasing list of nuclear bodies, provides a
powerful mechanism to sort and organize RNA transcriptome-wide (
Figure 4
). Novel
sequencing approaches such as ATLAS-Seq, suggest that hundreds of RNA supramolecular
assemblies remain to be elucidated [53].
The complex control of RNA organization through phase separation has been
evolutionarily selected.
Cells must adapt RNA expression to environmental variations. Changes in temperature, ionic
strength, pH, or Redox state may reorganize the transcriptome by passively triggering phase
transitions, where RBPs switch from diffuse to condensed states [30,32,34,77,87,92–95].
Illustrating the diversity of the responses, PAB1 condensation is triggered by heat [87],
whereas DDX4 is triggered by cold [34]; while FUS and DDX4/LAF-1 condense at high and low
salt concentrations, respectively [30,32,34]. Similarly, the pH dependent protonation of
G3BP's IDR induces a conformational switch for this SG master protein, promoting its ability
to interact and phase separate with unfolded RNAs that are released from polysomes upon
stress, which in turn may be critical to limit RNA entanglement [77]. To promote cellular
fitness, IDRs seem to have been evolutionarily selected to control the temperature or pH
levels at which proteins phase separate [87,93–95]. As an example, the stress-induced
condensation which inactivates Ded1p protein translation initiation triggers a translational
switch from housekeeping to stress protein production. The translation of mRNAs carrying
complex 5ʹUTRs is inhibited while the translation of shorter and less structured 5ʹUTRs is
promoted [95]. Taken together, phase separations have a transcriptome-wide but selective
impact on RNA expression.
In addition to these passive regulations, and the active regulations by helicases or chaperones,
many protein post-translational modifications (PTM) control phase separations depending on
cellular activity, environment, or developmental stage [96]. Similarly, the number and
distribution of direct RNA m6A methylations regulate and influence the composition of the
phase-separated transcriptome [97]. These PTMs and RNA modifications can either work as
switches that trigger major transcriptome reorganization from a well-mixed state to a
condensed state, or fine-tune condensate dynamics and composition.
The dynamics, composition, and organization of condensates are stress specific [75,87,98–
101]. Twenty percent of SG proteome diversity is stress-dependent [11]. The transcriptome of
P-bodies and SGs is similarly organized in a stress-specific manner; stress associated RNA
motifs were found to be enriched in these granules [6,52]. Taken together, the simultaneous
condensation or dissolution of RNA droplets in response to the environment provides a
mechanism for the synchronization of large pools of RNAs that share the same fate (
Figure 4
).
Architecture of condensates: channelling RNA through subcompartments.
Despite their liquid nature at the macroscopic level [14], RNA condensates are not
homogenously mixed. Nucleoli and germline P-bodies are subcompartmented by liquid–liquid
phase separations (LLPS) [15,102,103]. The biogenesis of ribosomes in nucleoli involves
condensate organization around newly synthesized rRNAs in an assembly-line fashion from
the core to periphery subcompartments [104]. However, some components may not
coassemble through phase separations: 4 out of 6 nucleoli proteins mechanistically analyzed
fit a phase separation model, the other proteins were recruited in an active manner [105]. In
SGs, super-resolution imaging showed that multiple solid cores with different composition
reversibly cluster together sharing the same liquid shell, further illustrating the
subcompartmentalization of condensates [5,106–109].
The remodelling of germ granules during development is well-characterized. During early
meiosis, liquid germ droplets wet the nuclear pore (
Figure 5
) [14]. Electron microscopy
analysis has revealed droplet asymmetry along the axis of RNA efflux [110]. Droplets organize
around PGL-1/3 proteins that are sufficient to condense [44,111]. On the nuclear surface, GLH
proteins promote germ droplet docking on nuclear pores through hydrophobic FGF repeats
[112]. At the cytosolic interface, the FBF-2 translation repressor loads onto exiting mRNAs
[113]. These polarized germ condensates further dock with two other condensates, Z granules
and Mutator foci [114]. As transcription is arrested and oogenesis progresses, highly fluid
germ droplets are released from the pore to the cytosol. They are subsequently engulfed by
semi-liquid germline P-bodies, and without mixing become subcompartments of larger
condensates (
Figure 5
) [15]. Upon fertilization, condensates dissolve. This allows maternal
mRNPs to be sequestered and ‘frozen’ within droplet gels during oogenesis arrest and quickly
mobilized upon embryonic program activation [15]. At each mitotic cycle, germ droplets
condense posteriorly and somatic P-bodies condense anteriorly to be asymmetrically
inherited [8,14,15,115]. At this developmental stage, RNAs cluster MEG-3 gel cores around
liquid PGL-3 droplets [8,116,117]. The ability of multiple proteins: PGL-1/3 [44,111], MEG-3
[116–118], Ddx4/ LAF-1 [32,34] to phase separate can mechanistically explain LLPS
subcompartmentalization. Compartmentalized granules have also been observed in
Drosophila germlines [119–121]. From transcription to RNA decay, phase separations channel
RNP remodelling through an organized path. The functional unit is no longer an isolated RNA,
but RNA regulons: RNAs that are co-segregated through phase separation to be co-regulated
(
Figure 4
).
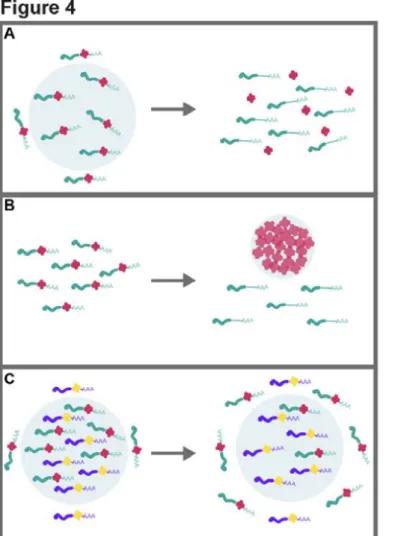
Figure 4. The impact of phase separations on transcriptome organization.
(A) RNP droplet dissolution can work as a developmental or environmental switch and synchronize the simultaneous release within the cytosol of large RNA regulons. For example, numerous nuclear condensates, as well as cytosolic P-bodies dissolve upon mitosis [130,147]. (B) The condensation of RBPs can alternatively inhibit their interaction with mRNA targets, as observed upon Pab1, Ded1p, or Whi3 condensation upon stress [87,95,135]. (C) Some RNAs can be released from droplets into the cytosol, while others are retained in a condensed state, as exemplified by maternal mRNAs during early embryonic development whose release from germ granules correlatess with the different temporal waves of translation activation [8,148]. Granule material properties can fine-tune RNA exchange. For example, highly viscous droplets can sequester mRNAs, whereas fluid ones can promote dynamic exchange [15].Figure 5. Condensate architecture is remodelled during germline development.
In early oogenesis, germ droplets wet nuclear pores [14], which are sites of RNA export. These liquid droplets are subsequently released to the cytosol where they can be engulfed into semi-liquid P-bodies upon oogenesis arrest [15]. Fertilization triggers the dissolution of maternal RNPs that were solidified in highly viscous germline P-bodies during the arrest. Embryogenesis promotes asymmetric distribution of maternal RNPs. At the anterior pole, RNPs phase separate into somatic P-bodies [15,115]. At the embryo's posterior pole, RNPs condense into germ granules that contain a liquid core and gel shell [8,14,44,116]. Scale bars 2 μm.Although RNA granules have been historically classified into discrete types according to
specific markers, recent work suggests RNA granules exist in a continuum of phases that guide
RNA exchange, and whose liquid–liquid demixing is tightly modulated. SGs are considered
compositionally and functionally distinct from P-bodies. In SGs, repressed mRNAs are loaded
with translation initiation complexes and the 40S ribosomal subunit, whereas P-body
repressors inhibit the loading of initiation factors [73,122]. However, SGs and P-bodies share
numerous interactors [5,9,11,12,85], and thus compete for these exchanging factors. Shared
factors promote docking between P-bodies and SGs, while competition for these factors and
interaction stoichiometry within networks control the degree of mixing between coexisting
phases [78]. Exchange of RNA between SGs and P-bodies is bidirectional, as illustrated by live
imaging [90]. However, as described above for nucleoli, competition between weak
multivalent interactions and strong stereotypic interactions can also asymmetrically drive RNA
flux from one compartment to another [104]. From nuclear to cytosolic condensates,
transcripts can be localized through phase separations to distinct compartments, providing a
mechanism to organize the transcriptome spatially and temporally and to control associated
RNA biochemical reactions and fate.
Condensation is actively limited, and micro-condensates may be
underestimated.
Hyper-aggregation has long been known to be induced by overexpressing or mutating
prion-like IDRs. RNAi screens demonstrated that multiple genes function to limit condensation and
solidification into crystal-like arrays, suggesting that RNP polymerization is the default state
[86]. In an energy-depleted context condensates grow and solidify, signifying that the cell
actively restricts condensation [15,102]. Helicases ensure transcriptome liquidity. They
comprise 10% of the condensate proteome and their depletion causes RNP solidification
[9,15]. By participating in protein–RNA and IDR–IDR interactions, helicases promote
condensate assembly, and by disrupting intermolecular RNA–RNA interactions maintain
liquidity [43,50,123]. Similarly, chaperones that prevent the accumulation of misfolded
proteins regulate granule dynamics [5,17,24,124–126]. Super-resolution microscopy
uncovered that chaperones limit condensate growth to subresolution sizes and further
suggested that many biomolecules are supersaturated, forming condensates whose growth is
actively limited [127,128]. Mild stresses induce condensates below imaging resolution [87]
and proximity labelling revealed pre-assembled sub-microscopic SGs under non-stress
conditions that may serve as ‘seeds’ to promote rapid assembly upon stress [11,12].
Illustrating the diversity of mechanisms that limit growth, endoplasmic reticulum tubules
promote condensate fission [129]. A decade of research has revealed that RNP's ability to
phase separate is the rule rather than the exception, and that condensation is the default
state and must be actively limited.
To co-ordinate condensate dissolution to cellular activity, the cell can control multiple
parameters that are critical to phase separation: the concentration, interaction strength, and
the multivalency of scaffolding components. Nuclear envelope breakdown upon mitosis
dilutes nuclear component concentrations that drop below saturation concentrations, and
thus induce nuclear condensates dissolution [130]. Signalling pathways induce PTMs that limit
interaction strength between scaffolding components to trigger condensate dissolution [96].
Similarly, helicases and chaperones can disrupt scaffolding interactions leading to condensate
collapse. Cells can also express competing ligands that disrupt the multivalency of the central
nodes of condensate networks, leading to the dissolution of condensates [78]. For example,
RNA multivalency is critical to germ granule assembly, and soluble RBP can compete with
condensates for mRNAs and thus trigger their dissolution [44]. Thus, the dissolution of
condensate can be finely tuned by cellular activity, providing a mechanism to synchronize the
release and cytosolic access of large RNA regulons.
Condensation function: sequestration of RNAs vs. catalyzing RNA processing.
RNA condensates organize transcriptional and post-transcriptional regulations. Translation
repression associated with mRNA condensation uncouples protein production from mRNA
expression on a transcriptome-wide scale [9]. Condensation could limit translation through
two alternative mechanisms: passively sequestering mRNAs away from the translation
machinery or catalyzing mRNA processing events that prevent translation. The two alternative
models function in vitro, sequestration through FMRP condensation is sufficient to inhibit
translation [35], while condensates catalyze deadenylation that limits translation [57,131]. In
a third model, non-functional miRISCs are trapped within P-bodies where they can scan
potential mRNA targets that traverse granules more transiently [56]. In this model,
condensates favour the loading of miRNAs onto mRNAs to promote mRNA repression. Of
note, very few copies of some transcripts are expressed per cell [1] and storing them in
dedicated structures like P-bodies could allow them to be more easily ‘found’. Condensates
also provide a new solvent environment that can passively melt and remodel RNA structures
even in the absence of active helicases or chaperones [132], and thus may limit RNA
entanglement [77], or promote new interactions such as RNA–RNA interactions that are
unstable in the cytosolic environment [50]. Translation factories could promote the
co-translational assembly of protein complexes or ribosome recycling [58,59,62]. Taken together
these recent studies suggest that RNA condensates are more than sorting centers that
sequester RNA molecules, but may catalyze transcriptome remodelling through specific
catalytic activities.
Buffering RNA expression variation through condensation.
Homeostasis maintenance is critical to cellular survival. To do so, cells need to buffer large
variations in RNA expression. In a saturated system, when molecules accumulate above the
saturation concentration, molecules in excess are buffered by condensates, keeping cytosolic
concentrations constant (
Figure 3
). The same may apply to the overexpression of numerous
RBPs that phase separate through self-interactions [133]. However, for complex heterotypic
super-assemblies, the saturation concentration depends on scaffolding partners
[28,70,76,78,104,134]. Condensates can also absorb large variations in interaction
stoichiometry [70,104,134], providing a potential protective mechanism to the cell. For
example, upon stress hyper-reactive mRNAs are released from polysomes, drastically
changing the interacting ratio between mRNAs and RBPs, which leads to the coassembly of
protective SGs [73–78]. This property distinguishes condensates from stereospecific
complexes, for which variation in stoichiometry between partners often leads to cellular
toxicity as monomers tend to interact non-specifically when they accumulate in excess. RNA
exchange and partitioning can also be finely tuned within condensates. Interaction strength,
client multivalency, or scaffold stoichiometry are predicted to modulate the partitioning of
RNAs and control condensate composition [70,76,78,104,134]. Most biological systems
require energy-dependent feed-back loops, but the thermodynamics of phase transitions
provides a robust energy-free buffering system to the cell.
Condensate epitranscriptomics control gene expression.
The epitranscriptome consists of RNP structural organizations and PTMs that control gene
expression and are transgenerationally inherited. The ability of the PolyQ-rich RBP, Whi3
super-assemblies in yeast to be asymmetrically inherited illustrates that phase separations
regulate translation to mediate epigenetic memory [135]. Some translation factories can also
be asymmetrically inherited [59]. In metazoans, germ granules are asymmetrically inherited
in the germline [14,44,117]. Consistent with the transmission of an epigenetic signal, germ
granule mutants become RNAi defective, express somatic transcripts, and become sterile over
generations [114,136–139]. These condensates accumulate small ncRNAs and their regulatory
proteins are essential for ensuring transgenerational epigenetic inheritance [114,140–146].
Conclusion.
Phase transitions have emerged as an essential compartmentalization mechanism to provide
a higher scale of transcriptome organization. From bacteria to eukaryotes, this mode of
organization is conserved and may have provided one of the first organization mechanisms in
the RNA world that preceded current forms of life. Great progress has been made in
mechanistically dissecting phase separations. It is clear, we are only beginning to uncover their
biological function.
Summary.
•
Thousands of transcripts and hundreds of proteins coassemble into RNA
condensates.
•
Phase separations provide a higher scale of transcriptome organization.
•Phase transitions synchronize the transcriptome with cellular activity.
•Diffuse, liquid, semi-liquid, and solid RNP states influence RNA fate.
Competing Interests.
The authors declare that there are no competing interests associated with the manuscript.
Funding.
Core funding for this work was provided by CNRS and Inserm, and grants from the ATIP-Avenir
program to AH. This work has also been supported by the French government, through the
UCAJEDI Investments in the Future project managed by the National Research Agency (ANR)
with the reference number ANR-15-IDEX-01.
Author contributions.
D.A. and A.H. contributed equally to the manuscript.
Abbreviations.
CBs : Cajal bodies
IDRs : intrinsically disordered regions
LLPS : liquid–liquid phase separations
ncRNA : noncoding RNA
PTM : post-translational modifications
RBDs : RNA binding domains
RBPs : RNA binding proteins
RNPs : ribonucleoproteins
SGs : stress granules
References:
1 Marinov, G.K., Williams, B.A., McCue, K., Schroth, G.P., Gertz, J., Myers, R.M. et al (2014) From single-cell to cell-pool transcriptomes: stochasticity in gene expression and RNA splicing. Genome Res. 24, 496–510 https://doi-org.insb.bib.cnrs.fr/10.1101/gr.161034.113
2 Palazzo, A.F. and Lee, E.S. (2015) Non-coding RNA: what is functional and what is junk? Front. Genet. 6, 2 https://doi-org.insb.bib.cnrs.fr/10.3389/fgene.2015.00002
3 Banani, S.F., Lee, H.O., Hyman, A.A. and Rosen, M.K. (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 https://doi-org.insb.bib.cnrs.fr/10.1038/nrm.2017.7
4 Shin, Y. and Brangwynne, C.P. (2017) Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 https://doi-org.insb.bib.cnrs.fr/10.1126/science.aaf4382
5 Jain, S., Wheeler, J.R., Walters, R.W., Agrawal, A., Barsic, A. and Parker, R. (2016) ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498
https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2015.12.038
6 Khong, A., Matheny, T., Jain, S., Mitchell, S.F., Wheeler, J.R. and Parker, R. (2017) The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol. Cell
7 Fritzsche, R., Karra, D., Bennett, K.L., Ang, F.Y., Heraud-Farlow, J.E., Tolino, M., et al (2013) Interactome of two diverse RNA granules links mRNA localization to translational repression in neurons. Cell Rep. 5, 1749– 1762 https://doi-org.insb.bib.cnrs.fr/10.1016/j.celrep.2013.11.023
8 Lee, C.-Y.S., Putnam, A., Lu, T., He, S., Ouyang, J.P.T. and Seydoux, G. (2020) Recruitment of mRNAs to P granules by condensation with intrinsically-disordered proteins. eLife 9, e52896
https://doi-org.insb.bib.cnrs.fr/10.7554/eLife.52896
9 Hubstenberger, A., Courel, M., Bénard, M., Souquere, S., Ernoult-Lange, M., Chouaib, R., et al (2017) P-body purification reveals the condensation of repressed mRNA regulons. Mol. Cell 68, 144–157.e5 https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2017.09.003
10 Andersen, J.S., Lam, Y.W., Leung, A.K.L., Ong, S.-E., Lyon, C.E., Lamond, A.I. et al (2005) Nucleolar proteome dynamics. Nature 433, 77–83 https://doi-org.insb.bib.cnrs.fr/10.1038/nature03207
11 Markmiller, S., Soltanieh, S., Server, K.L., Mak, R., Jin, W., Fang, M.Y., et al (2018) Context-dependent and disease-specific diversity in protein interactions within stress granules. Cell 172, 590–604.e13 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2017.12.032
12 Youn, J.-Y., Dunham, W.H., Hong, S.J., Knight, J.D.R., Bashkurov, M., Chen, G.I., et al (2018) High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell 69, 517–532.e11 https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2017.12.020
13 Heraud-Farlow, J.E., Sharangdhar, T., Li, X., Pfeifer, P., Tauber, S., Orozco, D., et al (2013) Staufen2 regulates neuronal target RNAs. Cell Rep. 5, 1511–1518 https://doi-org.insb.bib.cnrs.fr/10.1016/j.celrep.2013.11.039 14 Brangwynne, C.P., Eckmann, C.R., Courson, D.S., Rybarska, A., Hoege, C., Gharakhani, J. et al (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 https://doi-org.insb.bib.cnrs.fr/10.1126/science.1172046
15 Hubstenberger, A., Noble, S.L., Cameron, C. and Evans, T.C. (2013) Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell 27, 161–173 https://doi-org.insb.bib.cnrs.fr/10.1016/j.devcel.2013.09.024
16 Kato, M., Han, T.W., Xie, S., Shi, K., Du, X., Wu, L.C., et al (2012) Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767
https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2012.04.017
17 Kroschwald, S., Maharana, S., Mateju, D., Malinovska, L., Nüske, E., Poser, I. et al (2015) Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife 4, e06807 https://doi-org.insb.bib.cnrs.fr/10.7554/eLife.06807
18 Lin, Y., Protter, D.S.W., Rosen, M.K. and Parker, R. (2015) Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219
19 Molliex, A., Temirov, J., Lee, J., Coughlin, M., Kanagaraj, A.P., Kim, H.J. et al (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2015.09.015
20 Patel, A., Lee, H.O., Jawerth, L., Maharana, S., Jahnel, M., Hein, M.Y., et al (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2015.07.047
21 Wei, M.-T., Elbaum-Garfinkle, S., Holehouse, A.S., Chen, C.C.-H., Feric, M., Arnold, C.B. et al (2017) Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 9, 1118–1125 https://doi-org.insb.bib.cnrs.fr/10.1038/nchem.2803
22 Brady, J.P., Farber, P.J., Sekhar, A., Lin, Y.-H., Huang, R., Bah, A., et al (2017) Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc. Natl. Acad. Sci. U.S.A. 114, E8194–E8203 https://doi-org.insb.bib.cnrs.fr/10.1073/pnas.1706197114
23 Kim, H.J., Kim, N.C., Wang, Y.-D., Scarborough, E.A., Moore, J., Diaz, Z., et al (2013) Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 https://doi-org.insb.bib.cnrs.fr/10.1038/nature11922
24 Mateju, D., Franzmann, T.M., Patel, A., Kopach, A., Boczek, E.E., Maharana, S. et al (2017) An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 36, 1669–1687 https://doi-org.insb.bib.cnrs.fr/10.15252/embj.201695957
25 Schwartz, J.C., Wang, X., Podell, E.R. and Cech, T.R. (2013) RNA seeds higher-order assembly of FUS protein. Cell Rep. 5, 918–925 https://doi-org.insb.bib.cnrs.fr/10.1016/j.celrep.2013.11.017
26 Sun, Z., Diaz, Z., Fang, X., Hart, M.P., Chesi, A., Shorter, J. et al (2011) Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLOS Biol. 9, e1000614 https://doi-org.insb.bib.cnrs.fr/10.1371/journal.pbio.1000614
27 Vogler, T.O., Wheeler, J.R., Nguyen, E.D., Hughes, M.P., Britson, K.A., Lester, E., et al (2018) TDP-43 and RNA form amyloid-like myo-granules in regenerating muscle. Nature 563, 508–513
https://doi-org.insb.bib.cnrs.fr/10.1038/s41586-018-0665-2
28 Li, P., Banjade, S., Cheng, H.-C., Kim, S., Chen, B., Guo, L., et al (2012) Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 https://doi-org.insb.bib.cnrs.fr/10.1038/nature10879 29 Castello, A., Fischer, B., Eichelbaum, K., Horos, R., Beckmann, B.M., Strein, C., et al (2012) Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149, 1393–1406
https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2012.04.031
30 Burke, K.A., Janke, A.M., Rhine, C.L. and Fawzi, N.L. (2015) Residue-by-Residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol. Cell 60, 231–241
https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2015.09.006
31 Conicella, A.E., Zerze, G.H., Mittal, J. and Fawzi, N.L. (2016) ALS mutations disrupt phase separation mediated by α-helical structure in the TDP-43 low-complexity C-terminal domain. Structure 24, 1537–1549 https://doi-org.insb.bib.cnrs.fr/10.1016/j.str.2016.07.007
32 Elbaum-Garfinkle, S., Kim, Y., Szczepaniak, K., Chen, C.C.-H., Eckmann, C.R., Myong, S. et al (2015) The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. U.S.A. 112, 7189–7194 https://doi-org.insb.bib.cnrs.fr/10.1073/pnas.1504822112 33 Murakami, T., Qamar, S., Lin, J.Q., Schierle, G.S.K., Rees, E., Miyashita, A., et al (2015) ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88, 678–690 https://doi-org.insb.bib.cnrs.fr/10.1016/j.neuron.2015.10.030
34 Nott, T.J., Petsalaki, E., Farber, P., Jervis, D., Fussner, E., Plochowietz, A., et al (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936– 947 https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2015.01.013
35 Tsang, B., Arsenault, J., Vernon, R.M., Lin, H., Sonenberg, N., Wang, L.-Y. et al (2019) Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proc. Natl. Acad. Sci. U.S.A. 116, 4218–4227
https://doi-org.insb.bib.cnrs.fr/10.1073/pnas.1814385116
36 Zhang, H., Elbaum-Garfinkle, S., Langdon, E.M., Taylor, N., Occhipinti, P., Bridges, A.A. et al (2015) RNA controls PolyQ protein phase transitions. Mol. Cell 60, 220–230
https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2015.09.017
37 Quiroz, F.G. and Chilkoti, A. (2015) Sequence heuristics to encode phase behaviour in intrinsically disordered protein polymers. Nat. Mater. 14, 1164–1171 https://doi-org.insb.bib.cnrs.fr/10.1038/nmat4418
38 Wang, J., Choi, J.-M., Holehouse, A.S., Lee, H.O., Zhang, X., Jahnel, M., et al (2018) A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699.e16 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2018.06.006
39 Decker, C.J., Teixeira, D. and Parker, R. (2007) Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J. Cell Biol. 179, 437–449 https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.200704147
40 Han, T.W., Kato, M., Xie, S., Wu, L.C., Mirzaei, H., Pei, J., et al (2012) Cell-free formation of RNA granules: bound RNAs identify features and components of cellular assemblies. Cell 149, 768–779 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2012.04.016
41 Protter, D.S.W., Rao, B.S., Van Treeck, B., Lin, Y., Mizoue, L., Rosen, M.K. et al (2018) Intrinsically disordered regions can contribute promiscuous interactions to RNP granule assembly. Cell Rep. 22, 1401–1412 https://doi-org.insb.bib.cnrs.fr/10.1016/j.celrep.2018.01.036
42 Xiang, S., Kato, M., Wu, L.C., Lin, Y., Ding, M., Zhang, Y. et al (2015) The LC domain of hnRNPA2 adopts similar conformations in hydrogel polymers, liquid-like droplets, and nuclei. Cell 163, 829–839 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2015.10.040
43 Hondele, M., Sachdev, R., Heinrich, S., Wang, J., Vallotton, P., Fontoura, B.M.A. et al (2019) DEAD-box ATPases are global regulators of phase-separated organelles. Nature 573, 144–148
https://doi-org.insb.bib.cnrs.fr/10.1038/s41586-019-1502-y
44 Saha, S., Weber, C.A., Nousch, M., Adame-Arana, O., Hoege, C., Hein, M.Y., et al (2016) Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166, 1572– 1584.e16 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2016.08.006
45 Maharana, S., Wang, J., Papadopoulos, D.K., Richter, D., Pozniakovsky, A., Poser, I., et al (2018) RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 360, 918–921
https://doi-org.insb.bib.cnrs.fr/10.1126/science.aar7366
46 Aumiller, W.M., Pir Cakmak, F., Davis, B.W. and Keating, C.D. (2016) RNA-based coacervates as a model for membraneless organelles: formation, properties, and interfacial liposome assembly. Langmuir 32, 10042– 10053 https://doi-org.insb.bib.cnrs.fr/10.1021/acs.langmuir.6b02499
47 Jain, A. and Vale, R.D. (2017) RNA phase transitions in repeat expansion disorders. Nature 546, 243–247 https://doi-org.insb.bib.cnrs.fr/10.1038/nature22386
48 Langdon, E.M., Qiu, Y., Ghanbari Niaki, A., McLaughlin, G.A., Weidmann, C.A., Gerbich, T.M., et al (2018) mRNA structure determines specificity of a polyQ-driven phase separation. Science 360, 922–927 https://doi-org.insb.bib.cnrs.fr/10.1126/science.aar7432
49 Van Treeck, B., Protter, D.S.W., Matheny, T., Khong, A., Link, C.D. and Parker, R. (2018) RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome. Proc. Natl. Acad. Sci. U.S.A. 115, 2734–2739 https://doi-org.insb.bib.cnrs.fr/10.1073/pnas.1800038115
50 Tauber, D., Tauber, G., Khong, A., Treeck, B.V., Pelletier, J. and Parker, R. (2020) Modulation of RNA condensation by the DEAD-box protein eIF4A. Cell 180, 411–426.e16
https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2019.12.031
51 Namkoong, S., Ho, A., Woo, Y.M., Kwak, H. and Lee, J.H. (2018) Systematic characterization of stress-induced RNA granulation. Mol. Cell 70, 175–187.e8
https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2018.02.025
52 Wang, C., Schmich, F., Srivatsa, S., Weidner, J., Beerenwinkel, N. and Spang, A. (2018) Context-dependent deposition and regulation of mRNAs in P-bodies. eLife 7, e29815
https://doi-org.insb.bib.cnrs.fr/10.7554/eLife.29815
53 Adekunle, D.A. and Wang, E.T. (2020) Transcriptome-wide organization of subcellular microenvironments revealed by ATLAS-Seq. Nucleic Acids Res. gkaa334 https://doi-org.insb.bib.cnrs.fr/10.1093/nar/gkaa334 54 Liu, J., Valencia-Sanchez, M.A., Hannon, G.J. and Parker, R. (2005) MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 7, 719–723
https://doi-org.insb.bib.cnrs.fr/10.1038/ncb1274
55 Pillai, R.S. (2005) Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science 309, 1573– 1576 https://doi-org.insb.bib.cnrs.fr/10.1126/science.1115079
56 Pitchiaya, S., Mourao, M.D.A., Jalihal, A.P., Xiao, L., Jiang, X., Chinnaiyan, A.M. et al (2019) Dynamic recruitment of single RNAs to processing bodies depends on RNA functionality. Mol. Cell 74, 521–533.e6 https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2019.03.001
57 Sheu-Gruttadauria, J. and MacRae, I.J. (2018) Phase transitions in the assembly and function of human miRISC. Cell 173, 946–957.e16 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2018.02.051
58 Lui, J., Castelli, L.M., Pizzinga, M., Simpson, C.E., Hoyle, N.P., Bailey, K.L. et al (2014) Granules harboring translationally active mRNAs provide a platform for P-body formation following stress. Cell Rep. 9, 944–954 https://doi-org.insb.bib.cnrs.fr/10.1016/j.celrep.2014.09.040
59 Pizzinga, M., Bates, C., Lui, J., Forte, G., Morales-Polanco, F., Linney, E., et al (2019) Translation factor mRNA granules direct protein synthetic capacity to regions of polarized growth. J. Cell Biol. 218, 1564–1581
https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.201704019
60 De Graeve, F. and Besse, F. (2018) Neuronal RNP granules: from physiological to pathological assemblies. Biol. Chem. 399, 623–635 https://doi-org.insb.bib.cnrs.fr/10.1515/hsz-2018-0141
61 Formicola, N., Vijayakumar, J. and Besse, F. (2019) Neuronal ribonucleoprotein granules: dynamic sensors of localized signals. Traffic 20, 639–649 https://doi-org.insb.bib.cnrs.fr/10.1111/tra.12672
62 Pichon, X., Bastide, A., Safieddine, A., Chouaib, R., Samacoits, A., Basyuk, E. et al (2016) Visualization of single endogenous polysomes reveals the dynamics of translation in live human cells. J. Cell Biol. 214, 769–781 https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.201605024
63 Mao, Y.S., Sunwoo, H., Zhang, B. and Spector, D.L. (2011) Direct visualization of the co-transcriptional assembly of a nuclear body by noncoding RNAs. Nat. Cell Biol. 13, 95–101
64 Shevtsov, S.P. and Dundr, M. (2011) Nucleation of nuclear bodies by RNA. Nat. Cell Biol. 13, 167–173 https://doi-org.insb.bib.cnrs.fr/10.1038/ncb2157
65 Lin, Y., Schmidt, B.F., Bruchez, M.P. and McManus, C.J. (2018) Structural analyses of NEAT1 lncRNAs suggest long-range RNA interactions that may contribute to paraspeckle architecture. Nucleic Acids Res. 46, 3742–3752 https://doi-org.insb.bib.cnrs.fr/10.1093/nar/gky046
66 Souquere, S., Beauclair, G., Harper, F., Fox, A. and Pierron, G. (2010) Highly ordered spatial organization of the structural long noncoding NEAT1 RNAs within paraspeckle nuclear bodies. Mol. Biol. Cell 21, 4020–4027 https://doi-org.insb.bib.cnrs.fr/10.1091/mbc.e10-08-0690
67 Berry, J., Weber, S.C., Vaidya, N., Haataja, M. and Brangwynne, C.P. (2015) RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl. Acad. Sci. U.S.A. 112, E5237–E5245 https://doi-org.insb.bib.cnrs.fr/10.1073/pnas.1509317112
68 Falahati, H., Pelham-Webb, B., Blythe, S. and Wieschaus, E. (2016) Nucleation by rRNA dictates the precision of nucleolus assembly. Curr. Biol. 26, 277–285 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cub.2015.11.065 69 Mitrea, D.M., Cika, J.A., Guy, C.S., Ban, D., Banerjee, P.R., Stanley, C.B. et al (2016) Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. eLife 5, e13571 https://doi-org.insb.bib.cnrs.fr/10.7554/eLife.13571
70 Banani, S.F., Rice, A.M., Peeples, W.B., Lin, Y., Jain, S., Parker, R. et al (2016) Compositional control of phase-separated cellular bodies. Cell 166, 651–663 https://doi-
71 Teixeira, D., Sheth, U., Valencia-Sanchez, M.A., Brengues, M. and Parker, R. (2005) Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11, 371–382
https://doi-org.insb.bib.cnrs.fr/10.1261/rna.7258505
72 Garcia-Jove Navarro, M., Kashida, S., Chouaib, R., Souquere, S., Pierron, G., Weil, D. et al (2019) RNA is a critical element for the sizing and the composition of phase-separated RNA–protein condensates. Nat. Commun. 10, 3230 https://doi-org.insb.bib.cnrs.fr/10.1038/s41467-019-11241-6
73 Kedersha, N. (2002) Evidence that ternary complex (eIF2-GTP-tRNAiMet)-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13, 195–210
https://doi-org.insb.bib.cnrs.fr/10.1091/mbc.01-05-0221
74 Kedersha, N., Cho, M.R., Li, W., Yacono, P.W., Chen, S., Gilks, N. et al (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151, 1257–1268 https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.151.6.1257
75 Kedersha, N., Panas, M.D., Achorn, C.A., Lyons, S., Tisdale, S., Hickman, T., et al (2016) G3BP–Caprin1–USP10 complexes mediate stress granule condensation and associate with 40S subunits. J. Cell Biol. 212, 845–860 https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.201508028
76 Yang, P., Mathieu, C., Kolaitis, R.-M., Zhang, P., Messing, J., Yurtsever, U., et al (2020) G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345.e28 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2020.03.046
77 Guillén-Boixet, J., Kopach, A., Holehouse, A.S., Wittmann, S., Jahnel, M., Schlüßler, R., et al (2020) RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181, 346–361.e17 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2020.03.049
78 Sanders, D.W., Kedersha, N., Lee, D.S.W., Strom, A.R., Drake, V., Riback, J.A., et al (2020) Competing protein–RNA interaction networks control multiphase intracellular organization. Cell 181, 306–324.e28 https://doi-org.insb.bib.cnrs.fr/0.1016/j.cell.2020.03.050
79 Kaiser, T.E., Intine, R.V. and Dundr, M. (2008) De novo formation of a subnuclear body. Science 322, 1713– 1717 https://doi-org.insb.bib.cnrs.fr/10.1126/science.1165216
80 Tourrière, H., Chebli, K., Zekri, L., Courselaud, B., Blanchard, J.M., Bertrand, E. et al (2003) The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831 https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.200212128
81 Gilks, N., Kedersha, N., Ayodele, M., Shen, L., Stoecklin, G., Dember, L.M. et al (2004) Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398 https://doi-org.insb.bib.cnrs.fr/10.1091/mbc.e04-08-0715
82 Wilczynska, A., Aigueperse, C., Kress, M., Dautry, F. and Weil, D. (2005) The translational regulator CPEB1 provides a link between dcp1 bodies and stress granules. J. Cell Sci. 118, 981–992
https://doi-org.insb.bib.cnrs.fr/10.1242/jcs.01692
83 Solomon, S., Xu, Y., Wang, B., David, M.D., Schubert, P., Kennedy, D. et al (2007) Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs. Mol. Cell. Biol. 27, 2324–2342 https://doi-org.insb.bib.cnrs.fr/10.1128/MCB.02300-06
84 Wippich, F., Bodenmiller, B., Trajkovska, M.G., Wanka, S., Aebersold, R. and Pelkmans, L. (2013) Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152, 791– 805 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2013.01.033
85 Ayache, J., Benard, M., Ernoult-Lange, M., Minshall, N., Standart, N., Kress, M. et al (2015) P-body assembly requires DDX6 repression complexes rather than decay or Ataxin2/2L complexes. Mol. Biol. Cell 26, 2579–2595 https://doi-org.insb.bib.cnrs.fr/10.1091/mbc.E15-03-0136
86 Hubstenberger, A., Cameron, C., Noble, S.L., Keenan, S. and Evans, T.C. (2015) Modifiers of solid RNP granules control normal RNP dynamics and mRNA activity in early development. J. Cell Biol. 211, 703–716 https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.201504044
Google ScholarCrossref PubMed
87 Riback, J.A., Katanski, C.D., Kear-Scott, J.L., Pilipenko, E.V., Rojek, A.E., Sosnick, T.R. et al (2017) Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168, 1028–1040.e19 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2017.02.027
88 Vijayakumar, J., Perrois, C., Heim, M., Bousset, L., Alberti, S. and Besse, F. (2019) The prion-like domain of Drosophila Imp promotes axonal transport of RNP granules in vivo. Nat. Commun. 10, 2593 https://doi-org.insb.bib.cnrs.fr/10.1038/s41467-019-10554-w
89 Courel, M., Clément, Y., Bossevain, C., Foretek, D., Vidal Cruchez, O., Yi, Z. et al (2019) GC content shapes mRNA storage and decay in human cells. eLife 8, e49708 https://doi-org.insb.bib.cnrs.fr/10.7554/eLife.49708 90 Moon, S.L., Morisaki, T., Khong, A., Lyon, K., Parker, R. and Stasevich, T.J. (2019) Multicolour single-molecule tracking of mRNA interactions with RNP granules. Nat. Cell Biol. 21, 162–168
https://doi-org.insb.bib.cnrs.fr/10.1038/s41556-018-0263-4
91 Wilbertz, J.H., Voigt, F., Horvathova, I., Roth, G., Zhan, Y. and Chao, J.A. (2019) Single-molecule imaging of mRNA localization and regulation during the integrated stress response. Mol. Cell 73, 946–958.e7 https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2018.12.006
92 Kato, M., Yang, Y.-S., Sutter, B.M., Wang, Y., McKnight, S.L. and Tu, B.P. (2019) Redox state controls phase separation of the yeast Ataxin-2 protein via reversible oxidation of its methionine-rich low-complexity domain. Cell 177, 711–721.e8 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2019.02.044
93 Franzmann, T.M., Jahnel, M., Pozniakovsky, A., Mahamid, J., Holehouse, A.S., Nüske, E., et al (2018) Phase separation of a yeast prion protein promotes cellular fitness. Science 359, eaao5654
https://doi-org.insb.bib.cnrs.fr/10.1126/science.aao5654
94 Kroschwald, S., Munder, M.C., Maharana, S., Franzmann, T.M., Richter, D., Ruer, M. et al (2018) Different material states of Pub1 condensates define distinct modes of stress adaptation and recovery. Cell Rep. 23, 3327–3339 https://doi-org.insb.bib.cnrs.fr/10.1016/j.celrep.2018.05.041
95 Iserman, C., Altamirano, C.D., Jegers, C., Friedrich, U., Zarin, T., Fritsch, A.W., et al (2020) Condensation of Ded1p promotes a translational switch from housekeeping to stress protein production. Cell 181, 818–831.e19 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2020.04.009
96 Snead, W.T. and Gladfelter, A.S. (2019) The control centers of biomolecular phase separation: how membrane surfaces, PTMs, and active processes regulate condensation. Mol. Cell 76, 295–305 https://doi-org.insb.bib.cnrs.fr/10.1016/j.molcel.2019.09.016
97 Ries, R.J., Zaccara, S., Klein, P., Olarerin-George, A., Namkoong, S., Pickering, B.F. et al (2019) M6a enhances the phase separation potential of mRNA. Nature 571, 424–428 https://doi-org.insb.bib.cnrs.fr/10.1038/s41586-019-1374-1
98 Aulas, A., Fay, M.M., Lyons, S.M., Achorn, C.A., Kedersha, N., Anderson, P. et al (2017) Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci. 130, 927–937 https://doi-org.insb.bib.cnrs.fr/10.1242/jcs.199240
99 Buchan, J.R., Yoon, J.-H. and Parker, R. (2011) Stress-specific composition, assembly and kinetics of stress granules in Saccharomyces cerevisiae. J. Cell Sci. 124, 228–239
https://doi-org.insb.bib.cnrs.fr/10.1242/jcs.078444
100 Hoyle, N.P., Castelli, L.M., Campbell, S.G., Holmes, L.E.A. and Ashe, M.P. (2007) Stress-dependent
relocalization of translationally primed mRNPs to cytoplasmic granules that are kinetically and spatially distinct from P-bodies. J. Cell Biol. 179, 65–74 https://doi-org.insb.bib.cnrs.fr/10.1083/jcb.200707010
101 Wallace, E.W.J., Kear-Scott, J.L., Pilipenko, E.V., Schwartz, M.H., Laskowski, P.R., Rojek, A.E., et al (2015) Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162, 1286–1298 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2015.08.041
102 Brangwynne, C.P., Mitchison, T.J. and Hyman, A.A. (2011) Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 4334–4339
https://doi-org.insb.bib.cnrs.fr/10.1073/pnas.1017150108
103 Feric, M., Vaidya, N., Harmon, T.S., Mitrea, D.M., Zhu, L., Richardson, T.M. et al (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697
https://doi-org.insb.bib.cnrs.fr/10.1016/j.cell.2016.04.047
104 Riback, J.A., Zhu, L., Ferrolino, M.C., Tolbert, M., Mitrea, D.M., Sanders, D.W. et al (2020) Composition-dependent thermodynamics of intracellular phase separation. Nature, 581, 209–214
https://doi-org.insb.bib.cnrs.fr/10.1038/s41586-020-2256-2
105 Falahati, H. and Wieschaus, E. (2017) Independent active and thermodynamic processes govern the nucleolus assembly in vivo. Proc. Natl. Acad. Sci. U.S.A. 114, 1335–1340
https://doi-org.insb.bib.cnrs.fr/10.1073/pnas.1615395114
106 Cirillo, L., Cieren, A., Barbieri, S., Khong, A., Schwager, F., Parker, R. et al (2020) UBAP2L forms distinct cores that act in nucleating stress granules upstream of G3BP1. Curr. Biol. 30, 698–707.e6 https://doi-org.insb.bib.cnrs.fr/10.1016/j.cub.2019.12.020
![Figure 5. Condensate architecture is remodelled during germline development. In early oogenesis, germ droplets wet nuclear pores [14], which are sites of RNA export](https://thumb-eu.123doks.com/thumbv2/123doknet/13534452.418113/12.892.113.780.154.364/figure-condensate-architecture-remodelled-germline-development-oogenesis-droplets.webp)