See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/321482195
A new species of Calonectria causing rot on ripe
strawberry fruit in Brazil
Article in Australasian Plant Pathology · December 2017 DOI: 10.1007/s13313-017-0532-x CITATIONS0
READS181
6 authors, including: Some of the authors of this publication are also working on these related projects: The rise of Pyricularia graminis-tritici sp. nov. as the wheat blast pathogen in Brazil: sympatric speciation inferred from multilocus gene phylogeny, pathogenicity spectra and avirulence genes evolutionView project Levantamento das principais doenças bióticas e abióticas que incidem nas plantações florestais do estado de Mato Grosso View project Ueder Pedro Lopes Universidade Federal Rural de Pernambuco 30 PUBLICATIONS 141 CITATIONS SEE PROFILE Rafael Alfenas Universidade Federal de Mato Grosso (UFMT) 30 PUBLICATIONS 235 CITATIONS SEE PROFILE Laércio Zambolim Universidade Federal de Viçosa (UFV) 138 PUBLICATIONS 571 CITATIONS SEE PROFILE Pedro W Crous Westerdijk Fungal Biodiversity Institute 1,995 PUBLICATIONS 34,381 CITATIONS SEE PROFILE All content following this page was uploaded by Ueder Pedro Lopes on 17 January 2018. The user has requested enhancement of the downloaded file.
ORIGINAL PAPER
A new species of
Calonectria causing rot on ripe strawberry
fruit in Brazil
Ueder P. Lopes1&Rafael F. Alfenas2&Laércio Zambolim3&Pedro W. Crous4&Hélcio Costa5&Olinto L. Pereira3
Received: 20 March 2017 / Accepted: 28 November 2017 # Australasian Plant Pathology Society Inc. 2017
Abstract
A major problem in the strawberry production chain is the occurrence of fruit rot caused by fungi, thus, accurate identification of the pathogens associated with these rots is important for suitable management of diseases. During an assessment of postharvest diseases of strawberry plants, a rot caused by Calonectria was found. The surface of the infected fruits showed a slight leakage of liquid and the presence of mycelium with clearly defined hyphae containing a mass of conidiophores and conidia. Morphological examination was performed on isolates derived from single germinated conidium cultures, and phylogenetic analyses of four DNA loci (β-tubulin, histone H3, elongation factor and calmodulin) was undertaken. In order to confirm the pathogenicity, strawberry fruits and plants were dipped or sprayed with a conidial suspension. Strawberry fruits showed rot symptoms similar to those initially observed, and the plants developed leaf spot symptoms. The fungus was successfully reisolated from the infected tissues. Based on morphological and phylogenetic analysis, the fungus was shown to be distinct, and is described here as Calonectria fragariae sp. nov. This finding provides additional information for the better understanding of strawberry diseases in Brazil, enabling the development of more effective strategies of management of these diseases.
Keywords Cylindrocladium . Eucalyptus . Fragaria x ananassa . Strawberry disease
Introduction
Strawberry (Fragaria x ananassa) is produced in different regions in Brazil, which is considered a major producer of this fruit in South America (Antunes and Peres2013). The occur-rence of fruit rot is the main cause of losses in the strawberry production chain, especially during the postharvest period (Costa et al.2003; Ruaro et al.2014). Losses of 98% of the
fruits have been recorded after five days of storage, due to infection of pathogens (Henz et al.2008).
Pathogenic fungi commonly causing postharvest rot in strawberry fruits in Brazil are Botrytis cinerea, Colletotrichum s pp . i n cl u d i n g C . s i a m e n s e , P i l i d i um c o nc a v u m, Neofusicoccum kwambonambiense and N. parvum, and Rhizopus stolonifer (Costa et al. 2003; Tanaka et al. 2005; Lopes et al.2010,2014; Ruaro et al.2014; Capobiango et al.
2016). Other fungal pathogens that have been reported less frequently include Alternaria spp., Gnomoniopsis comari, Mucor spp., Pestalotiopsis longisetula, Phytophthora spp., Rhizoctonia solani, and Sclerotinia sclerotiorum (Costa et al.
2003; Tanaka et al.2005; Ruaro et al.2014). Among the path-ogens affecting fruits at postharvest, the most commonly found are B. cinerea, and R. stolonifer which are present in over 30% of fruit (Lopes2011).
Although the rot caused by Calonectria is less common in fruits when compared to leaf spot and root rot, Calonectria species have been associated with fruit rots on barbados cherry (Silva et al.2001), rambutan (Serrato-Diaz et al. 2013), and durian (Sivapalan et al. 1998). During a survey of strawberry fruit diseases on twenty Brazilian farms, a rot which developed after harvest
* Ueder P. Lopes ueder.lopes@ufrpe.br 1
Unidade Acadêmica de Garanhuns, Universidade Federal Rural de Pernambuco, Garanhuns, PE 55292-270, Brazil
2 Universidade Federal de Mato Grosso, Sinop, MT 78557-267, Brazil 3
Departamento de Fitopatologia, Universidade Federal de Viçosa, Viçosa, MG 36570-900, Brazil
4
CBS-KNAW Fungal Biodiversity Centre, Uppsalalaan 8, 3584, CT Utrecht, The Netherlands
5 Centro Regional Centro Serrano, Instituto Capixaba de Pesquisa Assistência Técnica e Extensão Rural, Venda Nova do Imigrante, ES 29375-000, Brazil
caused by Calonectria sp. (= Cylindrocladium) species was found in one of them (Fig.1) (Lopes2011).
Species of Calonectria affect a broad range of hosts, in-cluding approximately 335 plant species (Crous 2002). Among the important forestry species infected by Calonectria species are those within the families Fabaceae (Acacia spp.), Myrtaceae (Eucalyptus spp.) and Pinaceae (Pinus spp.) (Crous2002). Furthermore, Calonectria and their anamorph Cylindrocladium causes leaf blight and stem rot diseases on several other economically important crops such as peanut, soybean, potato, rice, alfalfa and strawberry (Crous
2002; Hirooka et al.2009; Lombard et al.2010a). Although Hirooka et al. (2009) have reported the occurrence of Calonectria canadiana (as Cylindrocladium candense), grouped in a Ca. kyotensis species complex, causing damping-off and leaf blight in strawberry, there are no studies showing the occurrence of postharvest rot caused by species of Calonectria, especially species belonging to the Ca. candelabra complex. The complex Ca. candelabra is one of the most common in the world consisting of 27 species of which 18 species are found in South America (Alfenas et al.
2013b, 2015; Crous 2002; Lombard et al. 2010b, 2015; Schoch et al.1999).
Then the aim of this study was describe a new species of Calonectria causing strawberry fruit rot in Brazil, using mor-phological and phylogenetic analyses.
Materials and methods
Fungal isolates
Strawberry fruits were collected from one commercial straw-berry farm (cv.‘Oso Grande’) in Santa Maria Jetibá, Espírito Santo State, Brazil, in 2010. The fruits were maintained in moist chambers at 25 °C and viewed daily, for seven days, for sporulating fungi using a magnifying glass or dissecting microscope. Fungal isolates were collected from fruits show-ing symptoms of rot and superficial mycelial growth. To ob-tain single conidial cultures, pathogen structures observed un-der a stereoscopic microscope (45 x) were deposited on Petri dishes containing water agar medium [WA, 1.5% (w/v) agar].
Subsequently 2 mL of sterile distilled water were added to each WA dish, and the dish was gently tilted in each direction to evenly spread the water and fungal structures. Excess water was removed by inverting the Petri dish, and under a stereo-microscope (45 x) a single conidia was transferred to a Petri dish containing malt extract agar (MEA), and maintained at 25 °C for 5 days. One single conidial isolate obtained was stored in sterile water (Castellani 1939) and maintained in the culture collection of the Laboratory of Plant Protection (LPP), Department of Phytopathology, Universidade Federal de Viçosa, Brazil, as LPP040, and in the CBS Fungal Biodiversity Institute in the Netherlands (CBS), as CBS 133607. Two additional isolates were maintained as LPF141.1 and LPF141.2 in the culture collection of the Laboratory of Forest Pathology (LPF). Diseased tissue sam-ples were mounted on a sheet of paper, dried, and deposited in the herbarium of the Universidade Federal de Viçosa (VIC).
Morphological characterization
Cultures were grown on synthetic nutrient agar (SNA) medi-um (1 g KH2PO4, 1 g KNO3, 0.5 g MgSO47H2O, 0.5 g KCl, 0.2 g glucose, 0.2 g sucrose, 0.6 ml NaOH (1 M) and 18 g agar/l distilled water) at 26 °C, following the protocols de-scribed for Calonectria by Lombard et al. (2009). After 7 days of incubation, the morphological characteristics were deter-mined by mounting the fungal structures in clear lactic acid at 85% v/v. Thirty measurements of the conidiogenous appa-ratus, stipe, vesicle and conidia at ×1000 magnification were determined using a Zeiss Axioscope 2 microscope with inter-ference contrast (DIC) illumination. The 95% confidence levels of conidia measurements were determined and the ex-tremes are given in parentheses. For other structures, only extremes are presented.
DNA extraction, amplification and sequencing
Genomic DNA was extracted from 7-day-old fungal colonies grown on MEA (malt extract agar) medium, following the protocol of the Wizard® Genomic DNA purification kit (Promega Corporation, WI, USA). The partial gene sequences of β-tubulin (TUB2), histone H3 (HIS3), elongation factor
Fig. 1 Strawberry fruits collected from a farm on Santa Maria Jetibá, Espírito Santo State, Brazil, in 2010, and stored at 25 °C. Symptoms of rot caused by Calonectria fragariae, three days after harvest
(TEF1α) and calmodulin (CAL) were amplified by PCR using the primer pairs T1 (O’Donnell & Cigelnik 1997) and C Y LT U B 1 R ( C r o u s e t a l . 2 0 0 6) , C Y L H 3 F a n d CYLH3R (Crous et al. 2006), EF1-728F (Carbone and Kohn 1999) and EF-2 (O’Donnell et al. 1998), CAL-228F and CAL-737R (Carbone and Kohn1999), respec-tively, using the DreamTaq™ Master Mix (MBI Fermentas, Vilnius, Lithuania) according to the manufac-turer’s protocol. The PCR conditions were as described in Alfenas et al. (2013b).
Phylogenetic analysis
Bidirectional sequencing was done with primers used for ampli-fications. Electropherograms were visualized with the Sequence Scanner Software v. 1.0 (Applied Biosystems) and PHPH (http://www.biomol.unb.br/phph/). Consensus sequences were determined using MEGA v. 6 (Tamura et al.2013).
The generated sequences aligned with other sequences of closely related Calonectria spp. obtained from GenBank (GenBank (http://www.ncbi.nlm.nih.gov) (Table 1), using MAFFT v. 7.0 (Katoh and Toh2013). Alignments were man-ually corrected using MEGA v. 6 (Tamura et al.2013). Gaps (insertions / deletions) were treated as absent. The best evolu-tionary model of nucleotide substitution for each gene region was selected according to the Akaike Information Criterion (AIC) using MrModeltest v. 2.3 (Posada and Crandall1998) and incorporated into the analyses.
Bayesian Inference (BI) phylogenetic analysis with MrBayes v. 3.2.1 (Ronquist and Heulsenbeck 2003) was performed using the Markov chain Monte Carlo (MCMC) algorithm and employed two sets of four chains started in parallel from a random tree topology with the heating parameter set at 0.3. The MCMC analysis ran until the average standard deviation of split frequencies came be-low 0.01, with trees saved every 1000 generations. The first 25% of saved trees were discarded as the Bburn-in^ phase and posterior probabilities (PP) were determined from the remaining trees.
The partition homogeneity test and the 70% reciprocal bootstrap method were applied to determine whether the data sets were consistent and combinable using PAUP (Phylogenetic Analysis Using Parsimony, v. 4.0b10) (Swofford2002).
Phylogenetic relationships were estimated by heuristic searches based on 1000 random addition sequences and by tree bisection-reconnection, with the branch swapping option set on‘best trees’ only. All characters were equally weighted, and alignment gaps were treated as missing data. Measures calculated for parsimony included the tree length (TL), con-sistency index (CI), retention-index (RI) and rescaled consis-tence index (RC).
Evolution models GTR + I for TEF1α, HKY + I for TUB2, HKY + I + G for CAL and HKY + G for HIS3 were selected and incorporated into the Bayesian Inference (BI) analysis.
Bootstrap analyses were based on 1000 replications. Calonectria colombiensis and Calonectria chinensis was used as the outgroup taxa in the BI analysis (Lombard et al.2009). The tree was deposited in TreeBase (ID20749).
Pathogenicity tests
A conidial suspension (1.0 × 104conidia.mL−1) was prepared from isolate LPP040 (CBS 133607), rinsing sporulating col-onies (ca. 15–day-old) with distilled water using a glass cane. This suspension was used to inoculate strawberry fruits and strawberry and eucalyptus plants.
Twenty-five strawberry fruits (cv. ‘Oso grande’) were dipped for 10 s into the conidial suspension, and 25 fruits were dipped in distilled water (control group). All fruits (inoculated and control) were distributed on trays, placed in plastic bags, and maintained at 25 °C. The fruits were evaluated daily for the presence of rot symptoms.
Two strawberry plants of each cultivar (‘Aromas’, ‘Tudla’ and‘Oso Grande’) were inoculated and two were not inocu-lated (control plants). One plant of the hybrid clone Eucalyptus grandis x E. urophylla, susceptible to Calonectria Blight (CLB), was inoculated and another was used in a negative control test. Strawberry and eucalyptus plants were approximately 120 days old. Plants were inocu-lated on both sides of the leaves by spraying the conidial suspension to the point of runoff using a plastic hand sprayer. The control plants were sprayed with distilled water. All plants (inoculated and control) were placed in a moist chamber for 48 h and subsequently transferred to a greenhouse at 25 °C and high humidity. The plants were evaluated daily for the appearance of symptoms.
Four days after inoculation of the strawberry fruits, the fungus was re-isolated by removing tissue at the edge of le-sions and plating it onto potato dextrose agar (PDA) medium. Two weeks after inoculation of strawberry and eucalyptus plants, infected leaves were collected and placed in germina-tion boxes containing moistened foam for 48 h. The fungus was isolated by removing the white conidial mass from spor-ulating conidiophores with the aid of a scalpel. The colony obtained from re-isolation was compared with the growth of isolates LPP040, LPF141.1 and LPF141.2.
Results
Morphological characterization
Morphological characteristics of the isolate CBS 133607 (=LPP040).
Ta bl e 1 Access ion numbers of se quenced gen e reg ions of Cal onect ria spp. us ed in the phylogenetic analys is S p eci es 1 Isolates nb Gen B ank acces sion nr . 2 Subst rat e L oca lity Ref er enc e β -tubulin Elo ngation fa ctor Calmodulin His tone (TU B2) (TE F1 α )( CAL )( HI S3 ) Ca . brass iana CBS 134857 KM395971 KM395884 KM396058 KM396141 Soil (E. br assiana plantation) T eres ina, P ia uí , B ra zil A lf ena s et al . ( 2015 ) Ca . brass iana CBS 134855 KM395969 KM395882 KM396056 KM396139 Soil (E. br assiana plantation) T eres ina, P ia uí , B ra zil A lf ena s et al . ( 2015 ) Ca . brass iana CBS 134856 KM395970 KM395883 KM396057 KM396140 Soil (E. br assiana plantation) T eres ina, P ia uí , B ra zil A lf ena s et al . ( 2015 ) Ca . can d elabr a CMW 31000 FJ972426 FJ972525 GQ26 7367 FJ972476 Eucalyptus sp. B razil L ombard et al. ( 2010b ) Ca . can d elabr a CMW 31001 GQ42 1779 GQ26 7298 GQ26 7368 GQ2 67246 Eucalyptus sp. B razil L ombard et al. ( 2010b ) Ca . chi n ensi s CBS 112744 A Y 725618 A Y 725709 A Y 725746 A Y 725660 Soil China C rous et al. ( 2004 ) Ca . co lom b iana CBS 1 15127 FJ972423 FJ972492 GQ26 7455 FJ972442 Soil Colombia Lombard et al. ( 2010c ) Ca . co lom b iana CBS 1 15638 FJ972422 FJ972491 GQ26 7456 FJ972441 Soil Colombia Lombard et al. ( 2010c ) Ca . co lom b ie ns is CBS 112220 GQ26 7207 A Y 72571 1 A Y725748 A Y 725662 Eucalyptus grandis C o lom b ia C rous et al . ( 2004 ) Ca . euc a ly ptic ola CBS 134846 KM395963 KM395876 KM396050 KM396133 Eucalyptus sp. (l ea f) E unápol is, B ah ia, B ra zil A lf ena s et al . ( 2015 ) Ca . euc a ly ptic ola CBS 134847 KM395964 KM395877 KM396051 KM396134 Eucalyptus sp. (s eedi ng) Sa nta B ár bar a, M ina s G era is, B razi l A lf ena s et al . ( 2015 ) Ca . euc a ly ptic ola CBS 134848 KM395965 KM395878 KM396052 KM396135 Soil (Eucalyptus plantation) Monte D ourado, Pa rá, B raz il Alf ena s et al . ( 2015 ) Ca . fra gariae CBS 133607 KM998965 KM998963 KM998966 KM998964 Fraga ria x a nanassa Brazil This st udy Ca . fra gariae LPF 141.1 K X50 0195 KX50 0197 KX50 0191 KX5 00194 Fraga ria x a nanassa Brazil This st udy Ca . fra gariae LPF 141.2 K X50 0196 KX50 0198 KX50 0192 KX5 00193 Fraga ria x a nanassa Brazil This st udy Ca . glaebicola CBS 134852 KM395966 KM395879 KM396053 KM396136 Soil (Eucalyptus plantation) Martinho Campos, M inas G era is, B ra zil Alf ena s et al . ( 2015 ) Ca . glaebicola CBS 134853 KM395967 KM395880 KM396054 KM396137 Eucalyptus sp . (le af ) B ic o d o P apa g ai o, T o ca nti n s, B raz il A lfe nas et al. ( 2015 ) Ca . glaebicola CBS 134854 KM395968 KM395881 KM396055 KM396138 Eucalyptus sp . (le af ) B ic o d o P apa g ai o, T o ca nti n s, B raz il A lfe nas et al. ( 2015 ) Ca . gracilipes CBS 1 1 1 141 DQ19 0566 GQ26 731 1 G Q26 7385 DQ1 90644 Colombia Crous et al. ( 2006 ) Ca . me tr oside ri CBS 133603 KC29431 3 K C294310 KC294304 KC29430 7 Metr osider os polymorpha Braz il Alfena s et al . ( 2013a ) Ca . me tr oside ri CBS 133604 KC29431 4 K C29431 1 K C294305 KC29430 8 Metr osider os polymorpha Braz il Alfena s et al . ( 2013a ) Ca . me tr oside ri CBS 133605 KC29431 5 K C294312 KC294306 KC29430 9 Metr osider os polymorpha Braz il Alfena s et al . ( 2013a ) Ca . nemuricola CBS 134837 KM395979 KM395892 KM396066 KM396149 Soil (tr opical rainforest) Arap onga, M inas Gerais, B razil A lfenas et al. ( 2015 ) Ca . nemuricola CBS 134838 KM395980 KM395893 KM396067 KM396150 Soil (tr opical rainforest) Arap onga, M inas Gerais, B razil A lfenas et al. ( 2015 ) Ca . nemuricola CBS 134839 KM395981 KM395894 KM396068 KM396151 Soil (tr opical rainforest) Arap onga, M inas Gerais, B razil A lfenas et al. ( 2015 ) Ca . pauciramo sa CMW 5683 FJ918514 FJ918565 GQ26 7405 FJ918531 Eucalyptus grandis So uth A frica L ombard et al. ( 2010b ) Ca . pauciramo sa CMW 30823 FJ918515 FJ918566 GQ26 7404 FJ918532 Eucalyptus sp. B razil L ombard et al. ( 2010b ) Ca . piauiensis CBS134849 KM395972 KM395885 KM396059 KM396142 Soil (tr opical rainforest) Serra d as Co n fusões, Piauí A lfenas et al. ( 2015 ) Ca . piauiensis CBS134850 KM395973 KM395886 KM396060 KM396143 Soil (Eucalyptus plantation) T eres ina, P iauí, B razil A lfenas et al. ( 2015 ) Ca . piauiensis CBS134851 KM395974 KM395887 KM396061 KM396144 Soil (t ropical rainforest) T eres ina , P iauí, B razil A lfenas et al. ( 2015 )
Ta b le 1 (continued) S p eci es 1 Isolates nb Gen B ank acces sion nr . 2 Subst rat e L oca lity Ref er enc e β -tubulin Elo ngation fa ctor Calmodulin His tone (TU B2) (TE F1 α )( CAL )( HI S3 ) Ca . pollizii CBS 125270 FJ972417 FJ972486 GQ26 7461 FJ972436 Call iste mon ci trinus Italy L ombard et al. ( 2010c ) Ca . pollizii CBS 125271 FJ972418 FJ972487 GQ26 7462 FJ972437 Call iste mon ci trinus Italy L ombard et al. ( 2010c ) Ca .pseudometr o si deri CBS 134843 KM395907 KM395819 KM395993 KM396081 Metr osider os polymorpha V içosa , M in as Ge ra is, B raz il A lf ena s et al . ( 2015 ) Ca .pseudometr o si deri CBS 134844 KM395908 KM395820 KM395994 KM396082 Eucalyptus sp. (leaf) A çailândia, Maranhã o, Brazil Alfenas et al. ( 2015 ) Ca .pseudometr o si deri CBS 134845 KM395909 KM395821 KM395995 KM396083 Soil (Eucalyptus plantation) Maceió, A lagoa s, Braz il Alfena s et al . ( 2015 ) Ca . pseudoscopa ria CBS 125255 GQ26 7227 GQ26 7347 GQ26 7439 GQ2 67276 Eucalyptus grandis Ecuador Lombard et al. ( 2010c ) Ca . pseudoscopa ria CBS 125257 GQ26 7229 GQ26 7349 GQ26 7441 GQ2 67278 Eucalyptus grandis Ecuador Lombard et al. ( 2010c ) Ca . pseudospathulata CBS 134840 KM395982 KM395895 KM396069 KM396152 Soil (t ropical rainforest) Minas G er ais, Brazil Alfenas et al. ( 2015 ) Ca . pseudospathulata CBS 134841 KM395983 KM395896 KM396070 KM396153 Soil (t ropical rainforest) Minas G er ais, Brazil Alfenas et al. ( 2015 ) Ca . pseudospathulata CBS 134842 KM395984 KM395897 KM396071 KM396154 Soil (t ropical rainforest) Minas G er ais, Brazil Alfenas et al. ( 2015 ) Ca . seminar ia CBS 136630 KJ46299 6 K J46288 3 K J4631 13 KJ46322 9 E. ur ophylla × E. grandis clone seedling leaf CERC Nursery , Guangdong , C hina Lombard et al. ( 2015 ) Ca . seminar ia CBS 136631 KJ46299 7 K J46288 4 K J4631 14 KJ46323 0 E. ur ophylla × E. grandis clone seedling leaf CERC Nursery , Guangdong , C hina Lombard et al. ( 2015 ) Ca . seminar ia CBS 136632 KJ46299 8 K J46288 5 K J4631 15 KJ46323 1 E. ur ophylla × E. grandis clone seedling leaf CERC Nursery , Guangdong , C hina Lombard et al. ( 2015 ) Ca . sil vic ola CPC 18766 KM395977 KM395890 KM396064 KM396147 Soil (tropical rainforest) Mucuri , B ahia, B razil A lfenas et al. ( 2015 ) Ca . sil vic ola CBS 134836 KM395975 KM395888 KM396062 KM396145 Soil (tr opical rainforest) Arap onga, M inas Gerais, B razil A lfenas et al. ( 2015 ) Ca . sil vic ola CPC 18741 KM395976 KM395889 KM396063 KM396146 Soil (t ropical rainforest) Arap onga, M in as Gerais, B razil A lfenas et al. ( 2015 ) Ca . spathulata CBS 1 12689 AF308 463 FJ918554 GQ26 7426 FJ918524 Eucalyptus viminalis Br az il Lombar d et al. ( 2010b ) Ca . spathulata CBS 555.92 GQ26 7215 GQ26 7331 GQ26 7427 GQ2 67261 Arauc araia angus tifo lia Br az il Lombar d et al. ( 2010c ) Ca . tetraramosa CBS 136637 KJ46301 2 K J46289 9 K J463129 KJ46324 5 E. ur ophylla × E. grandis clone seedling leaf CERC Nursery , Guangdong , C hina Lombard et al. ( 2015 ) Ca . tetraramosa CBS 136635 KJ46301 1 K J46289 8 K J463128 KJ46324 4 E. ur ophylla × E. grandis clone seedling leaf CERC Nursery , Guangdong , C hina Lombard et al. ( 2015 ) Ca . zul u ensi s CBS 125268 FJ972414 FJ972483 GQ26 7459 FJ972433 Eucalyptus grandis So uth A frica L ombard et al. ( 2010b ) Ca . zul u ensi s CMW 9896 FJ972415 FJ972484 GQ26 7460 FJ972434 Eucalyptus grandis So uth A frica L ombard et al. ( 2010b )
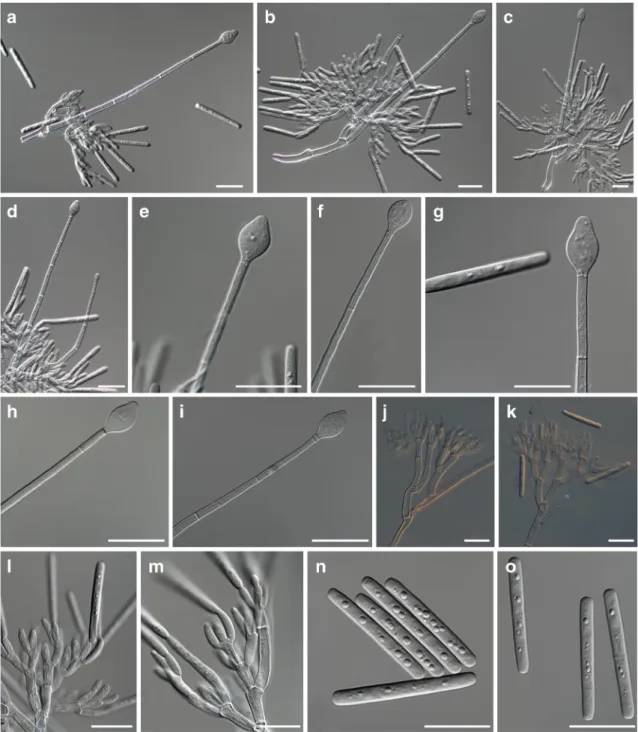
Calonectria fragariae R.F. Alfenas, O.L. Pereira & Crous, sp. nov.– MycoBank MB817706; (Fig.2).
& Etymology: In reference to the genus Fragaria, from which the fungus was isolated.
& Host: Fragaria x ananassa (Strawberry fruit). & Distribution: Brazil.
& Material examined: Brazil, Espírito Santo State, Santa Maria do Jetibá, from strawberry fruit collected from cv.
‘Oso Grande’ plantation, 2010, U.P. Lopes (holotype VIC 42833, culture ex-type CBS 133607 = LPP040).
Conidiophores penicillate consisting of stipe, and seta-like stipe extension terminated by a vesicle. Stipe was septate, hyaline, smooth, 48–65 × 5–7 μm. Stipe extensions septate, straight to flexuous, 114–159 μm long and 2–4 μm wide at apical septum; vesicle obpyriform to ellipsoidal 8–10 μm
Fig. 2 Morphological features of Calonectria fragariae. a-d: Conidiophores with seta-like stipe extensions terminated by vesicles,
e-i: Obpyriform and ellipsoidal vesicles, j-m: Conidiophores with three series of branches, n-o: septate macroconidia. Scale bars = 20μm
w i d e , la t e r a l s t i p e e x t e n s i o n s w a s n o t o b s e r v e d . Conidiogenous apparatus 43–75 μm high 64–111 μm wide; primary branches, 14–21 × 4–5 μm, secondary branches, 13– 18 × 3–4 μm, tertiary branches, 8–15 × 3–4 μm. Phialides in groups of 2–6, which range from doliiform to reniform, 7– 11 × 2–4 μm, periclinal thickening and inconspicuous collarette visible. Conidia cylindrical, rounded at both ends, straight, (35–) 38–41 (−46) × 3–5 μm (av. = 39 × 4 μm), 1-septate, without abscission scar, held in parallel cylindrical clusters by a colourless slime. Sexual morph unknown. Mega and microconidia were not seen.
Culture characteristics: Colonies have rapid growth rate (50–55 mm diameter after 10 days at 25 °C on MEA medi-um). Aerial mycelia and sporulation were sparse.
Notes: Calonectria colombiana and Calonectria pseudospathulata are phylogenetically (Fig.3) and morpho-logically close to Ca. fragariae. Calonectria colombiana
[ m a c r o c o n i d i a s i z e ( 3 3–)35–39(−40) × 3–4 μm; conidiogenous apparatus with four branches], Ca. fragariae [macroconidia size (35–) 38–41 (−46) × 3–5 μm; conidiogenous apparatus with three branches], and Ca. pseudospathulata [(macroconidia size (35–)41–44(−50) × 3– 5 μm; conidiogenous apparatus with three branches] were morphologically similar, but they can be distinguished by ves-icle shape, numbers of branches, macroconidia size and phy-logenetic analyses (Table2).
DNA sequence comparisons and phylogenetic
analysis
Amplicons of approximately 450 base pairs (bp) for HIS3 and 500 bp for TUB2, TEF1α and CAL were generated. The con-gruency analyses revealed no conflicts in tree topologies, so the sequences of TEF1α, TUB2, CAL and HIS3 were
Fig. 3 Consensus tree obtained by Bayesian inference using the combined sequences ofβ-tubulin, histone H3, translation elongation factor 1α and calmodulin of Calonectria species. The support values (BS and PP) are indicated at the nodes. The tree was rooted to Ca. chinensis (CBS 112744) and Ca. colombiensis (CBS 112220), and ex-type strains are indicated in bold
combined for analyses. The combined dataset had 370 parsi-mony informative characters. Analysis of the 370 parsiparsi-mony informative characters yielded 32 equally parsimonious trees (TL = 832, CI = 0.790, RI = 0.893, RC = 0.706).
The BI analysis stopped with 2045.000 generations (anal-ysis stopped because convergence diagnostic hit stop value) and the consensus tree (Fig.3) and posterior probabilities (PP) were calculated from 3069 trees sampled. In the consensus tree, a small clade was formed with Ca. fragariae and Ca. pseudospathulata with a BS = 75 and PP = 0.92 support (Fig.3). Alfenas et al. (2015) does not suggest monophyly for these species, however, after include a new taxon (Ca. fragariae) the phylogenetic relationships changed. This spe-c i e s f o r m e d a spe-c l a d e w i t h C a . s p a t h u l a t a , C a . pseudospathulata, Ca. colombiana and Ca. fragariae (Fig.
3). In addition, the single nucleotide polymorphisms (SNP’S) were determined for the small clade formed by Ca. spathulata, Ca. pseudospathulata, Ca. colombiana and Ca. fragariae. Ten unique fixed nucleotide were only present on Ca. fragariae isolates (2 on TEF1α, 4 on HIS3 and 4 on CAL). This supports that Ca. fragariae is a new phylogenetic line-age. Nevertheless, the evolution history of this clade remains uncertain, thus it is necessary to collect more isolates and generate further analyses with additional gene regions.
Pathogenicity test
Strawberry fruits dipped into a conidial suspension showed a slight leakage of liquid on the first day after inoculation, and had a darker red colour. On the second day, mycelium was observed on the fruit surface. On the third day, the natural colour of the fruit was altered and the fruit covered by mycelium (Fig.4).
The single eucalyptus plant inoculated with Ca. fragariae developed clear symptoms of disease at three days after inoc-ulation (Fig. 5 a), with severe twig dieback and leaf spots, typical of Calonectria (Fig.5b and c, respectively). In straw-berry plants, the symptoms (leaf spots) were observed one week after inoculation (Fig.5d) in both cultivars. Spots were first small and necrotic, but became prominent and defined after fifteen days. Spots showed greyish brown centres with a dark margin (Fig.5f), very similar to leaf spots caused by Mycosphaerella in strawberry plants. No differences were ob-served among the symptoms in the three cultivars. The fungus was reisolated from fruit rot and leaf spots of strawberry and from leaf spots and twig die back of eucalyptus tissues. The strawberry and eucalyptus plants and strawberry fruits used as controls remained healthy.
Discussion
An unknown fungus causing strawberry fruit rot is described in this study as Calonectria fragariae. This new species
Table 2 Morphological characteristics of Calonectria spp. cl osel y rel ate d to C a. fr agariae Species C onidiogenous apparatus S tipe exte ntio n V es icle Macroconidia R eference Siz e (μ m) Br anc h es Siz e (μ m) D iam (μ m) Sh ape S ize (μ m) Septation L ength/Diam ra tio Calonectria colombian a 38 –11 5 × 3 5– 91 4 143 –173 × 5– 78 –12 obpyriform to ellipsoid al (33 –)35 –39( − 40) × 3– 41 1 0– 12 Lombard et al. ( 2010b ) Ca . pseudos pathulata 60 –100 × 30 –70 3 145 –190 × 2– 47 –10 obpyriform (35 –)41 –44( − 50) × 3– 51 9– 11 A lf en as et al . ( 2015 ) Ca . spathulata 60 –100 × 30 –70 3 150 –300 × 3– 46 –10 ellip soid to obpyriform to cl av at e (48 –)75 –90( − 100) × (4 –)5 –6( 1– )3( − 6) 12 –18 C rous ( 2002 ) Ca . fragariae 64 –11 1 × 4 3– 75 3 1 14 –159 × 2– 48 –10 obpyriform to ellipsoid al (35 –)3 8– 41 (− 46) × 3– 51 7– 10 This study
belongs to Ca. candelabra species complex, which consists of 27 species where 18 of them occur in South America (Alfenas et al.2013b,2015; Crous2002; Lombard et al.2010b,2015; Schoch et al.1999). Species of Ca. candelabra complex are characterized by having obpyriform to ellipsoidal vesicles and uniseptate conidia (Schoch et al.1999). However, species representing this complex are not easily distinguished based on their morphology alone, and a comparison of their DNA sequence data is required to facilitate accurate identification (Alfenas et al.2015; Lombard et al.2010c).
Calonectria are grouped in 10 well defined species, known as Ca. brassicae complex, Ca. candelabra complex, Ca. colhounii complex, Ca. cylindrospora complex, Ca. kyotensis complex, Ca. mexicana complex, Ca. naviculata complex, Ca. pteridis complex, Ca. reteaudii complex and Ca. spathiphylli. Identification of species complexes can be easily distinguished by morphological features. However, for spe-cies identification inside the complex, multigene analysis is necessary (Alfenas et al.2013b; Lombard et al. 2010c). Based on phylogenetic comparisons, Ca. fragariae ap-pears to be most closely related to Ca. colombiana, Ca. pseudospathulata and Ca. spathulata. Morphologically, it is more similar to Ca. colombiana, Ca. polizzii, Ca. pseudospathulata, and Ca. zuluensis (Alfenas et al.
2015; Lombard et al. 2010b). However, Ca. fragariae can be distinguished from Ca. polizzii and Ca. zuluensis based on its vesicle shape: Ca. fragariae has obpyriform to ellipsoidal vesicles (8–10 μm diam) and Ca. polizzii and Ca. zuluensis have broadly clavate to obpyriform
vesicles. Although Ca. fragariae is morphologically very similar to Ca. colombiana and Ca. pseudospathulata, it can be distinguished based on the width of the apical septum in the stipe extension (2–4 μm diam), the absence of a sexual morph, and by phylogenetic inference.
Hirooka et al. (2009) reported Calonectria canadiana (as Cy. canadense) causing damping-off and leaf blight in straw-berry, however this species is morphologically and phyloge-netically distant to Ca. fragariae. While Ca. canadiana is characterized by its pyriform to sphaeropedunculate vesicle shape and macroconidia size of (38–)48–55(−65) × 4(−5) μm (Kang et al.2001), Ca. fragarie has obpyriform to ellip-soidal vesicles and macroconidia size of (35–) 38–41 (−46) × 3–5 μm. Although several rots on strawberry fruits are known, the Calonectria rot can be differentiated from the other due to the characteristic symptoms. Fruits affected by Calonectria show a slight leakage of liquid and the presence of mycelium with clearly defined hyphae containing a mass of conidio-phores and conidia within colourless mucilaginous matrix. The rots caused by G. candidum, Mucor spp., and R. stolonifer leads to the fruit tissue disintegration. Fruits af-fected by B. cinerea present a grey mass of aerial conidia, while fruits affected by Phytophthora show mycelium sparse-ly. In rots caused by Pestalotiopsis spp. acervuli producing conidia held in dark conidial cirrhi is verified (Lopes2011). On the other hand, in rots caused by Neofusicoccum species, the sporulation is less common, and an extensive mycelial growth on the fruit surface leads to the mummification (Lopes et al.2014).
Fig. 4 Strawberry fruits inoculated by dipping into the conidial suspension of Calonectria fragariae, showing the development of disease symptoms at one (a), two (b), three (c) and four days (d) after inoculation
The lesions on leaves of strawberry plants inoculated with Ca. fragariae are indistinguishable to those observed in Mycosphaerella leaf spot, an important strawberry dis-ease in Brazil (Costa et al. 2003; Tanaka et al. 2005; Ruaro et al.2014).
Calonectria fragariae infected and caused symptoms on an eucalyptus plant. In the farm where the isolates were obtained, strawberry and eucalypt plantations are close. This finding is important, since eucalyptus plantations are common in Brazil, and often in close proximity to commercial strawberry plan-tations, mainly in the Espírito Santo and Minas Gerais states. These results suggest that nearby eucalyptus should be sur-veyed for which species of Calonectria are causing leaf blight. Calonectria leaf blight is currently one of the main impedi-ments to eucalyptus cultivation in Brazil, and various species of Calonectria have been associated with this disease (Alfenas et al.2015). However, Ca. fragariae has not yet been detected. Based on morphological and phylogenetic analysis, the fungus was distinct, and is described here as Ca. fragariae sp. nov. This finding provides additional information for the better understanding of strawberry diseases in Brazil, enabling
the development of more effective strategies of management of these diseases. Further studies will be required to quantify the damage caused by this pathogen and to evaluate its impli-cations in strawberry crops, thereby increasing our under-standing of this pathosystem.
Acknowledgements The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico– CNPq, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior– CAPES and Fundação de Amparo a Pesquisa do Estado de Minas Gerais– FAPEMIG for finan-cial support.
References
Alfenas RF, Pereira OL, Ferreira MA, Jorge VL, Crous PW, Alfenas AC (2013a) Calonectria metrosideri, a highly aggressive pathogen causing leaf blight, root rot, and wilt of Metrosideros spp. in Brazil. Forest Pathol 43(4):257–265.https://doi.org/10.1111/efp. 12035
Alfenas RF, Pereira OL, Jorge VL, Crous PW, Alfenas AC (2013b) A new species of Calonectria causing leaf blight and cutting rot of Fig. 5 Symptoms of the leaf spots
in eucalyptus (clone 9882) (a-c) and strawberry plants (cv.‘Oso grande’) (d-f) inoculated with Calonectria fragariae. (a-c) three days after inoculation; (d-f) fifteen days after inoculation
three forest tree species in Brazil. Trop Plant Pathol 38(6):513–521.
https://doi.org/10.1590/S1982-56762013000600007
Alfenas RF, Lombard L, Pereira OL, Alfenas AC, Crous PW (2015) Diversity and potential impact of Calonectria species in eucalyptus plantations in Brazil. Stud Mycol 80:89–130.https://doi.org/10. 1016/j.simyco.2014.11.002
Antunes LEC, Peres NA (2013) Strawberry production in Brazil and South America. Inter J Fruit Sci 13(1-2):156–161.https://doi.org/ 10.1080/15538362.2012.698147
Capobiango NP, Pinho DB, Zambolim L, Pereira OL, Lopes UP (2016) Anthracnose on strawberry fruits caused by Colletotrichum siamense in Brazil. Plant Dis 100(4):859–859.https://doi.org/10. 1094/PDIS-10-15-1121-PDN
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3): 553–556.https://doi.org/10.2307/3761358
Castellani A (1939) Viability of some pathogenic fungi in distilled water. J Trop Med Hyg 42:225–226
Costa H, Zambolim L, Ventura JA (2003) Manejo integrado das doenças do morangueiro. In: Zambolim L (ed) Manejo integrado de pragas e doenças: fruteiras tropicais. Editora UFV, Viçosa pp 131–164 Crous PW (2002) Taxonomy and pathology of Cylindrocladium
(Calonectria) and allied genera. APS Press, St. Paul
Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hywel-Jones NL (2004) Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Stud Mycol 50:415–430
Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD (2006) Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Stud Mycol 55:213–226.https://doi.org/10. 3114/sim.55.1.213
Henz GP, Reis A, Silva KCC, Pereira SF (2008) Incidência de doenças de pós-colheita em frutos de morango produzidos no Distrito Federal (Boletim Técnico13). Embrapa Hortaliças, Brasília
Hirooka Y, Ishikawa S, Takeuchi J, Horie H, Nakayama K, Koitabashi M, Okuda S, Natsuaki KT (2009) New cylindrocladium diseases of strawberry and coral bells caused by Cylindrocladium canadense. J Gen Plant Pathol 75(1):83–86. https://doi.org/10.1007/s10327-008-0136-y
Kang JC, Crous PW, Schoch CL (2001) Species concepts in the Cylindrocladium floridanum and Cy. spathiphylli complexes (Hypocreaceae) based on multiallelic sequence data, sexual compat-ibility and morphology. Syst Appl Microbiol 24(2):206–217.https:// doi.org/10.1078/0723-2020-00026
Katoh K, Toh H (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol and Evol 30(4):772–780.https://doi.org/10.1093/molbev/mst010
Lombard L, Rodas CA, Crous PW, Wingfeld BD, Wingfeld MJ (2009) Cylindrocladium species associated with dying Pinus cuttings. Persoonia 23(1):41–47. https://doi.org/10.3767/ 003158509X471052
Lombard L, Crous PW, Wingfield BD, Wingfield MJ (2010a) Species concepts in Calonectria (Cylindrocladium). Stud Mycol 66:1–13.
https://doi.org/10.3114/sim.2010.66.01
Lombard L, Crous PW, Wingfield BD, Wingfield MJ (2010b) Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Stud Mycol 66:15–30.https://doi.org/10. 3114/sim.2010.66.02
Lombard L, Crous PW, Wingfield BD, Wingfield MJ (2010c) Phylogeny and systematics of the genus Calonectria. Stud Mycol 66:31–69.
https://doi.org/10.3114/sim.2010.66.03
Lombard L, Chen SF, Mou X, Zhou XD, Crous PW, Wingfield MJ (2015) New species, hyper-diversity and potential importance of Calonectria spp. from eucalyptus in South China. Stud Mycol 80: 151–188.https://doi.org/10.1016/j.simyco.2014.11.003
Lopes UP (2011) Podridões em pós-colheita de morango: Etiologia e efeito de produtos alternativos. Universidade Federal de Viçosa, Viçosa
Lopes UP, Zambolim L, Lopes UN, Pereira OL, Costa H (2010) First report of Pilidium concavum causing tan-brown rot in strawberry fruits in Brazil. Plant Pathol 59(6):1171–1172.https://doi.org/10. 1111/j.1365-3059.2010.02331.x
Lopes UP, Zambolim L, Pinho DB, Barros AV, Costa H, Pereira OL (2014) Postharvest rot and mummification of strawberry fruits caused by Neofusicoccum parvum and N. kwambonambiense in Brazil. Trop Plant Pathol 39(2):178–183.https://doi.org/10.1590/ S1982-56762014000200009
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol 7(1):103–116.https://doi. org/10.1006/mpev.1996.0376
O’Donnell K, Kistler HC, Cigelnik E, Ploetz RC (1998) Multiple evolu-tionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealo-gies. P Natl Acad Sci USA 95(5):2044–2049.https://doi.org/10. 1073/pnas.95.5.2044
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14(9):817–818. https://doi.org/10. 1093/bioinformatics/14.9.817
Ronquist F, Heulsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574.
https://doi.org/10.1093/bioinformatics/btg180
Ruaro L, Mio LLM, Costa H (2014) Doenças do morangueiro. In: Zawadneak MAC, Schuber JM, Mógor ÁF. (Ed) Como produzir morangos. UFRP, Curitiba, pp 159–194
Schoch CL, Crous PW, Wingfield BD, Wingfield MJ (1999) The Cylindrocladium candelabrum species complex includes four dis-tinct mating populations. Mycologia 91(2):286–298.https://doi.org/ 10.2307/3761374
Serrato-Diaz LM, Latoni-Brailowsky EI, Rivera-Vargas LI, Goenaga R, Crous PW, French-Monar RD (2013) First report of Calonectria hongkongensis causing fruit rot of rambutan (Nephelium lappaceum). Plant Dis 97(8):1117–1117.https://doi.org/10.1094/ PDIS-01-13-0008-PDN
Silva GS, Cutrim FA, Ferreira FA (2001) Mancha foliar e podridão de frutos da acerola causadas por Calonectria ilicicola. Fitopatol Bras 26(1):101.https://doi.org/10.1590/S0100-41582001000100021
Sivapalan A, Metussin R, Hamdan F, Zain RM (1998) Fungi associated with postharvest fruit rots of Durio graveolens and D. kutejensis in Brunei Darussalam. Australas Plant Pathol 27(4):274–277.https:// doi.org/10.1071/AP98033
Swofford DL (2002) PAUP* 4.0: phylogenetic analysis using parsimony (*and other methods). Sinauer associates, Sunderland
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol and Evol 30(12):2725–2729.https://doi.org/10.1093/molbev/mst197
Tanaka MAS, Betti JA, Kimati H (2005) Doenças do morangueiro. In: Kimati H, Amorim L, Rezende JAM, Bergamin Filho A, Camargo LEA (eds) Manual de fitopatologia: doenças das plantas cultivadas. Agronômica Ceres, São Paulo, pp 489–499
View publication stats View publication stats