T
T
H
H
È
È
S
S
E
E
En vue de l'obtention du
D
D
O
O
C
C
T
T
O
O
R
R
A
A
T
T
D
D
E
E
L
L
’
’
U
U
N
N
I
I
V
V
E
E
R
R
S
S
I
I
T
T
É
É
D
D
E
E
T
T
O
O
U
U
L
L
O
O
U
U
S
S
E
E
Délivré par l'Université Toulouse III - Paul Sabatier
Discipline ou spécialité : Biologie moléculaire, cellulaire et du développement
JURY
Jean Antoine Lepesant (DR) Elzbieta Pyza (Prof.) Catherine Soula (Prof.)
Alain Vincent (DR) Zbigniew Dabrowski (Prof.) Maria Slomczynska (dr hab.)
Ecole doctorale : Biologie, Santé, Développement Unité de recherche : Centre de Biologie du Développement Directeur(s) de Thèse : Alain Vincent et Zbigniew Dabrowski
Rapporteurs : dans le Jury
Présentée et soutenue par Joanna Krzemien Le 27 octobre 2008
Titre : "Control of larval hematopoiesis in Drosophila; microenvironment, precursors and
“Control of larval hematopoiesis in Drosophila;
microenvironment, precursors and cell lineage".
Joanna Krzemień
PhD thesis made in “co-tutelle”
at Jagiellonian University in Cracow
and Université Paul Sabatier in Toulouse
Acknowledgements
Taking adventage from the opportunity that gives me this manuscript, I would like to thank to everyone who has helped me to live the experience of PhD studies. It is a very difficult task, which I will probably not fulfil perfectly. Sometimes though, even a smile from a person that You’ve just met, can give You plenty of joy and force to go further.
These four years of my PhD were rich in experience and interesting rencontres. I’ve met many people who more or less had impact on my life. I’ve learnt plenty of things about the surrounding world. I had the chance to have excellent guides at the scientific tracks and wonderful company to enjoy my time. There were a lot of people, who, even if not directly involved in the scientific part of my PhD, made it possible for me to study in Toulouse and in the same time, stay attached to my Jagiellonian University in Krakow.
Without too much introduction, I would like simply and very sincerely to thank:
to Tomek Skalski, who was actually a key person at the beginning of my PhD. He contacted me with Alain Vincent and (see below ;) and encouraged me a lot in my choices.
to My Dear Profesor Zbigniew Dąbrowski, who let me free, who helped me at each step of my international experience, who was always there when I needed a piece of advice, a joke or just to talk; who was my Friend and Teacher.
to Alain Vincent for plenty of things; first of all for adopting me into CBD family, then for discussions, ideas, jokes, corrections, all the time that he spent for his students, among them lucky Joanna; for patience and formation; for support and enthusiasm. Thank You!
to Michele Crozatier a master of flies, for guiding me in the world of Drosophila and fly hematopoiesis, for teaching me so many things! For being always there, when I need her, for tiramisu, honey when I had a throat ache and for taking care of me ☺
to Rami, a “brother in PhD” for all the patience that he had for me ☺, for all knowledge that he shared with me, for discussions not always very scientific ;) for giving me the confidence.
to Delphine, for staying with us, for making me laughing, for together dissecting, for nice time that we have had with Maeva and Gaylord who were funny, who were laughing and who were always there for me when I needed a good company.
to Nico for the joy that he spreads all around, for always good mood, for big heart and all parties that we enjoyed together
to Hadi for making me smile, for supporting polish canoeing for having fun in the lab and for being a good man
to Monika, Bruno, Carmen, Virginie, Jonathan, Justine people who accompanied me in the laboratory life. Each of them completely different made me learn a lot about respect for others and about the joy of life.
to Brice, Julian, Phiphi, Serge, Christian, Eric, Yannick, Daniel, Mohamad, Dani, Thomas, Alex, my boys, for smiles, nice words, bisous, parties, ambiance and actually, just for being there ☺
to Brice and Aurelie for showing me world through the microscope, for patience, help and experience that I got thanks to them and to Bruno, for coping with complicated informatic problems and curing my computer
to Murielle, Elea, Aisha, Gaelle, Gwenaelle, Helene, Cathy, and Aurelie for them, simply
to Mylene, Elizabeth and Philippe for taking care of me ☺
to all my Friends and colleagues from CBD for four years, which I spent in Toulouse and even if sometimes I had difficult moments, I could always, find someone to smile to me ☺
to prof. Marian Lewandowski and doc. Maria Slomczynska, who made it possible for me to get through the meanders of administration and exams; always friendly and helpful
to Monika, my polish Friend, discovered in Toulouse, who was my Small Poland in France, who made me feel like at home
to my Polish Friends Sławek, Kama, Kasia, Kamila, Monika, Paweł, Mariusz and Kuba (Your names and all which I haven’t written here, are written in my heart) for being my joy!; for all hello and goodbye parties that I could always count on when I was coming back home. for Your support and warmth that helped me live far away from home and for making out me a smiley person.
to my Parents, my Brother and the whole Family who were always with me even being far away
Abbreviation list:
A1 - A8 - abdominal segments ael - after egg laying
AGM – aorta - gonad – mesonephros AMPs - antimicrobial proteins Antp - Antennapedia
Bam - Bag of marbles Bc - Black cells
BMP – Bone Morphogenetic Protein BrdU - bromodeoxyuridine
cc – crystal cells Cg25C - Collagen25C CNS - central nervous system
COE - Collier/Olfactory-1/Early B cell Factor Col - Collier
CRQ - crocquemort CXC - C-X-C chemokine Cyc - Cyclin
Cyo - Curly of Oster CZ - cortical zone
DBD - DNA binding domain Dome - Domeless
DoxA3 - Diphenol oxidase A3 Dpp - Decapentaplegic
Dscam - Down syndrome cell adhesion molecule dSR – CI – Drosophila scavanger receptor EBF – Early B-cell factor
ECM - extracellular matrix EH - embryonic hemocytes EL - egg length
ESC - escorting stem cell Esg - Escargot
fbn - facial branchiomotor neurons Flp - Flipase
FOG - Friend of GATA
FRT - Flipase Recombination Targets
GAT - gastrointestinal associated lymphoid tissue Gbb - Glass-bottomed boat
Gcm - Glial cells missing GFP – green fluorescent protein GMC – ganglion mother cell GSC - germline stem cell H3P - phosphorylated Histone 3 Hh - Hedgehog
HLH - helix-loop-helix Ho - Hoechst
Hop - Hopscotch
Hop Tum-l – Hopscotch Tumorous-lethal Hs - heat shock
HSC - hematopoietic stem cell Ig - immunoglobulin
Imd - immune deficiency IP - intermediate progenitors
IPT - Ig-like domain shared by Plexin and Transcription factor ISC - intestine stem cells
JAK/STAT - Janus Kinase/Signal Transducer and Activators of Transcription L1, L2, L3 – firt, second, third instar larvae
LG - lymph gland
Lin- - lack of lineage differentiation markers Lz - Lozange
MHC - Major Histocompatibility Complex MZ - medullary zone
N - Notch NB - neuroblast Neur - Neuralised NimC1 - Nimrod NK - natural killer cell Odd – Odd skipped
PDGF - Platelet-derived growth factor PGRP - Peptidoglycan recognition protein PNS - peripheral nervous system
PO - Phenoloxidase Pon - Partner of Numb proPO - Prophenoloxidase
PRRs - pattern recognition receptors Prx – Peroxidasin
PSC – posterior signalling center Pvf - PDGF- and VEGF-related factor
Pvr - Drosophila homolog of receptor for cytokines of platelet-derived growth factor (PDGF) family and vascular endothelial growth factor (VEGF) receptor
RGC - radial gliall cells RNAi – RNA interference Rpr –Reaper
S1 – signal 1 S2 – signal 2
Sca - stem cell antigen SCZ - subcortical zone Ser - Serrate
Shh – Sonic hedgehog
SNSD - self non-self discrimination SOP - sensory organ precursor cells Sp - Sternopleural
Srp - Serpent
SSC - somatic stem cells SVZ - subventrical zone T1-T3 – thoracic segments
Tep - Thioester-containing protein UAS - Upstream Activating Sequence Upd - Unpaired
Ush - U-shaped
VEGF - Vascular Endothelial Growth Factor Vkg - Viking
VLP - virus like particles W - White
Wg - Wingless Wnt – Wint wt – wild type Y-Yellow
Contents
Introduction………....13
The Immune system in Metazoa ... 15
Hematopoiesis in Drosophila melanogaster... 19
The Drosophila immune response... 19
The Drosophila hemocytes... 23
Plasmatocytes ... 23
Crystal cells ... 27
Lamellocytes ... 29
Embryonic hematopoiesis ... 31
Larval hematopoiesis ... 33
Control of larval hematopoiesis... 43
Collier, a transcription factor with multiple developmental functions ... 45
Osteoblasts, hematopoietic stem cells and their niche... 49
The hematopoietic stem cells ... 53
Drosophila melanogaster stem cell niches as a model for vertebrate systems. ... 57
Hematopoietic stem cells in Drosophila? ... 63
Materials, methods and experimental design………65
Antibodies and reagents... 67
Fly strains ... 67
BrdU pulse-chase experiments... 69
Mitotic index measurements... 69
Notch ts experiment... 71
Clonal analysis... 71
Results……….75
Introduction... 77
Ser expression in PSC cells is involved in maintenance of col expression. ... 79
The JAK/STAT pathway is activated in the MZ ... 81
Wasp infestation switches off JAK/STAT signalling in the MZ and induces massive differentiation of prohemocytes. ... 83
PSC cells extend numerous filopodia... 85
Looking for stem cells. ... 87
Waves of mitosis during development and after wasp infestation ... 95
Cell lineage analyses ... 99
Lamellocytes and crystal cells share a common progenitor ... 103
Notch signalling and cell fate restriction... 107
The extracellular matrix of the lymph gland... 109
Discussion……….111
The PSC as a model of hematopoietic niche... 113
Collier expression in the lymph gland, a matter of golden mean. ... 119
Role of the PSC in immune response against the parasitoid wasps... 119
Are there hematopoietic stem cells in the lymph gland?... 123
Proliferation versus differentiation... 127
The origin of larval hemocytes... 131
What more could we learn with clonal analysis? ... 135
Concluding remarks... 137
References……….141
Appendix………...155
The immune system in Metazoa
All Metazoans, the multicellular animals, are capable to keep self-integrity by recognising “non-self’ tissue thanks to their immune system. The immune system must make specific distinctions so that it can eliminate the pathogen without destroying the body’s own tissues. In the “self non-self discrimination” (SNSD) theory the recognition receptors are central to immunity, however it seems not to be sufficient. Actually, it is very important to recognise what poses harm to the body among self and non-self tissue, in order to avoid the detrimental reaction against foreign but non-dangerous agent, like for example food, commensal bacteria or just naturally changing self-tissue. The “theory of Danger” proposed by Matzinger suggests that damage rather then “foreignness” is what initiates an immune response. In this model the alarm signal is released from the body’s own cells and triggers the immune reaction (Janeway 1992; Matzinger 1994; Kvell, Cooper et al. 2007).
Two branches of the immune response have evolved in the Metazoans: innate and adaptive immunity. Innate immunity is germline encoded and considered as non-specific, nonanticipatory, nonclonal, whereas the adaptive response is specific, anticipatory, clonal and somatic (rewiewed in (Kvell, Cooper et al. 2007). Innate immunity is shared by all metazoans and can be grouped into cellular (that uses specific cells as, e.g., phagocytes) and humoral (mediated by secreted factors, like antimicrobial peptides) compartments. A fundamental experiment, showing the cellular aspects of innate immunity, was made in 1882 by Metchnikov. Upon inserting a horn into the body of a starfish larva he could observe that many mononuclear cells were “attacking” and engulfing the horn in a process that he termed phagocytosis. This finding brought him a Nobel price in 1908 (Petranyi 2002).
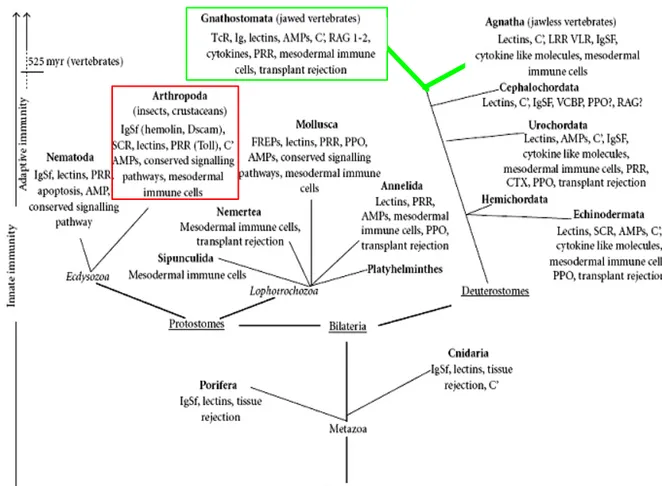
Immune-related innate functions like phagocytosis or cytokine secretion were already developed in pre-bilaterian times, 700 million years ago, in sponges and higher aquatic invertebrates. The innate immune response, although very efficient lacks, the memory function that appeared only with vertebrates. Around 500 milion years ago, primitive fishes developed jaws and thanks to this invention got access to a true predatory life, enjoying great advantage over agnathas. However, they also began to be subjected to a higher rate of infections by microbes and parasites in their gastrointestinal tract. The infections were the result of increased physical injuries caused by swallowed animals containing scales and skeletal elements. Additionally new prey–predator relationships created new food chains, through which microbes, e.g. viruses, could spread crossing the species barriers.
Fig. 1. Phylogenetic tree of the animal kingdom highlighting the evolution of key immunological elements.
Innate immunity operates in the entire animal kingdom. Adaptive immunity appeared with jawed vertebrates about 500 million years ago (green frame) (after Kvell et al.. 2007). The position of Drosophila in the animal kingdom is marked by a red frame.
The development of jaws was followed by establishment of a new immune system, called gastrointestinal associated lymphoid tissue (GAT), containing T-lymphocyte-like cells. Generation of the complex repertoire of immune receptors in lymphocytes through somatic gene rearrangement emerged 350-400 million years ago and the system antigen presenting proteins called MHC (major histocompatibility complex) appeared around 300 milion years ago. This resulted in development of adaptive immune response characterised by a remaining memory that permits a shorter and stronger secondary response thanks to the clonal expension of activated lymphocytes (Matsunaga and Rahman 1998; Petranyi 2002; Kvell, Cooper et al. 2007).
Figure 1 shows the phylogenetic tree of animal kingdom underlining the evolution of key immunological elements.
Millions of invertebrate species rely uniquely on the innate immunity and only around 45 000 today vertebrate species use also an adaptive response. It is postulated that the generally small size of invertebrates does not allow them to come up with the large number of cells required for operation of the adaptive immune system. Not only the number of cells but also the number of cell types (complexity), raised during the evolution of Metazoa are highest in vertebrates. Only vertebrates could afford to run such a costly system without the risk that the costs would outweigh benefits (Klein 1997; Kvell, Cooper et al. 2007). Needless to say, invertebrates cope very well with immune challenge and innate immunity is efficient for their vital needs. Since innate immune responses are both relatively simple and many mechanisms are highly conserved during evolution, invertebrates have become the subject of very extensive studies.
One of the best described model organisms is Drosophila melanogaster, called a fruit fly. Drosophila is a holometabola insect whose life cycle is composed of the embryonic stage, three larval stages and a pupal stage from which the imago finally emerges. Drosophila spends most of its life cycle in decaying organic matter such as rotting fruit, an environment enriched in microorganisms, that constantly challenges the immune system (Tzou, De Gregorio et al. 2002).
In Drosophila melanogaster (as in all other invertebrates), specific cells called hemocytes, the analogues of vertebrate blood cells, are key players in the response to immune challenges. These cells are formed during fly development in a process called hematopoiesis. During my PhD work, I studied the control of this complex process at cellular and molecular levels.
A B C
Fig. 2. The three types of Drosophila hemocytes
(A) Crystal cell containing crystals of proPhenylOxidase; (B) Plasmatocyte, the macrophage-like phagocyte; (C) Lamellocyte, the encapsulating hemocyte.
Hematopoiesis in Drosophila melanogaster
Hematopoiesis of Drosophila melanogaster, thanks to the great interest that this model organism has received for many years, starts to be very well described. Currently, we know that it occurs in two spatially and temporally distinct phases (Evans, Hartenstein et al. 2003; Holz, Bossinger et al. 2003; Meister and Lagueux 2003). The first phase, which is often compared to the vertebrate primitive hematopoiesis, takes place during embryogenesis in the head mesoderm. The second phase takes place during the larval stages, in a specific mesodermal organ, the lymph gland. It has been proposed to represent a model for the vertebrate wave of definitive blood formation. As a result of hematopoiesis, three hemocyte types form in Drosophila: plasmatocytes and crystal cells that are produced both in embryo and larvae and lamellocytes which are specific of the larval stages (Fig.2 A-C) (Meister and Lagueux 2003). In contrast, to vertebrate blood cells, Drosophila hemocytes participate in neither the transport of oxygen, since there exists a tracheal system which supplies the gazes to each body area, nor the nutrition or hormone transport, because the hemolymph circulation system is open. The main hemocyte tasks are to perform efficient immune responses and assure the proper development of the organism.
The Drosophila immune response
As for probably all metazoans, including vertebrates, the immune system of Drosophila is based on two main branches: the humoral response and the cellular response. The humoral response is the secretion, mainly by fat body and epithelia, of antimicrobial proteins (AMPs) that degrade the invader and other immune effectors (ex. reactive oxygen species, opsonins, clotting factors). Epithelia are the first barrier against the invading microorganisms and successfully protect the animal from the infection. The fat body is the major immune-responsive tissue. Thanks to its large size and broad localization, it is an efficient organ for the secretion of peptides into the hemolymph where they reach easily the proper concentration. The fat body is believed to be somewhat analogous to the mammalian liver (Lemaitre and Hoffmann 2007). The humoral response allows protection against a broad range of bacteria, fungi and viruses. Infection by Gram positive or Gram negative bacteria and
++
Antimicr obial peptides
opsonins (Teps), clotting factors stress factors
Bacteria Gram+ Fu ngi
Bac teria Gram
-A B To ll pathway T oll PGRP-LC Imd pathway J AK/STAT pathwa y D om eless Fat b od y
Fig. 3. Schematic overview of Drosophila host defenses.
Detection of pathogens induces specific immune responses in specialised tissues depending on the type of pathogen, via activation of various signalling pathways (after Lemaitre and Hoffmann 2007, modified).
fungi induces different patterns of AMP expression in the fat body, suggesting that the microbial recognition mechanism can discriminate between these various classes of invaders (Lemaitre, Reichhart et al. 1997). Recognition of microorganisms is a crucial step for the efficient immune response and it involves the series of proteins known as pattern recognition receptors (PRRs) (that can be soluble or membrane bound) and recognise microbial molecules (Wang and Ligoxygakis 2006). The recognition of pathogen can lead to activation of specific signalling pathways: Fungi and Gram+ bacteria trigger the Toll signalling pathway, Gram negative bacteria lead to Imd (immune deficiency) pathway activation and some viruses engage the JAK/STAT signalling pathway (Fig. 3A) (Lemaitre and Hoffmann 2007).
The massive production of AMPs that occurs following infection is primarly regulated at the transcriptional level. Characterisation of promoters of inducible immune genes revealed that ĸB-like recognition motifs were involved in the activation of AMPs. The three Drosophila members of transcription factor ĸB (NF- ĸB/Rel) family: Dorsal , Dif and Relish were shown to contribute to the response to infection and signal through Toll and Imd pathway by translocating to the nucleus and act as the switch of AMPs expression upon infection (Engstrom, Kadalayil et al. 1993; Han and Ip 1999; Stoven, Ando et al. 2000; Uvell and Engstrom 2007). Some AMPs are very stable and are still detected in hemolymph several weeks after immune challenge (Uttenweiler-Joseph, Moniatte et al. 1998).
The JAK/STAT signalling pathway has been also proposed to be involved in the fat body humoral response, where it triggers the expression of the complement-like protein tep1 and a stress gene turandotA of unknown function. JAK/STAT deficient flies are however resistant to bacterial and fungal infection and they show normal AMP profile (Lagueux, Perrodou et al. 2000; Ekengren and Hultmark 2001; Ekengren, Tryselius et al. 2001; Ekengren, Tryselius et al. 2001; Agaisse and Perrimon 2004; Lemaitre and Hoffmann 2007).
The humoral response has been extensively studied and is a subject of many excellent recent reviews (Ferrandon, Imler et al. 2007; Lemaitre and Hoffmann 2007; Uvell and Engstrom 2007). Although it was believed that insects were devoid of the immune specificity and memory, last few years have brought evidence that they could exist in Drosophila (Watson, Puttmann-Holgado et al. 2005; Pham 2007). Possibly the future will bring us more surprises in this area.
The second aspect of the immune response, that is the cellular aspect, is less well known. Cellular immune responses rely upon three types of hemocytes that participate in three main immune processes: plasmatocytes are engaged in phagocytosis, crystal cells are
responsible for melanisation and lamellocytes are required for encapsulation of foreign bodies (Rizki and Rizki 1984) (Fig. 3B).
The Drosophila hemocytes
Plasmatocytes
Plasmatocytes are the “professional phagocytes”, usually compared to vertebrate macrophages. With the exception of some glial cells that can function as macrophages in the developing central nervous system (CNS) (Sonnenfeld and Jacobs 1995), they are the only cells responsible for the removal of apoptotic cells and microorganisms in embryo, larvae and adult flies (Wood and Jacinto 2007). One other critical role of plasmatocytes is to produce and secrete extracellular matrix (ECM) molecules. These are the main source of the ECM covering all cell surfaces being in contact with the hemolymph. Plasmocytes are highly motile cells: from stage 10 of embryogenesis, they migrate from their place of origin (head mesoderm, see below) and disperse into the embryo, following specific migratory routes (Tepass, Fessler et al. 1994). This migration is possible thanks to very well developed actin rich filopodia and lamellopodia, that allow not only the moving of plasmatocytes but also their exploring of the environment (Wood, Faria et al. 2006). One of the main migratory routes leads along the ventral midline to the CNS, where the plasmatocytes via secretion of the ECM play a critical role in the proper condensation of the developing nervous system (Oloffson 2005). This developmentally programmed migration of hemocytes depends on the expression of the Pvr (receptor) in the plasmatocytes and the Pvf2 and Pvf3 (ligands) by other embryonic tissues (Cho, Keyes et al. 2002; Wood, Faria et al. 2006). The Pvr is the only Drosophila homolog of a vertebrate receptor for cytokines of platelet-derived growth factor (PDGF) family and vascular endothelial growth factor (VEGF) receptor. The fruit fly genome contains three genes coding for putative Pvr ligands: pvf, pvf1 and pvf3. The Pvr signalling pathway is also involved in plasmatocyte survival in embryos and plasmatocyte specification and proliferation in larvae (Munier, Doucet et al. 2002; Bruckner, Kockel et al. 2004; Jung, Evans et al. 2005).
The plasmatocytes are capable of chemotactic movements toward a tissue wound where they participate in damage healing. The chemotaxis is however independent of the Pvr
signalling (Stramer, Wood et al. 2005). The main function of plasmatocytes is phagocytosis. During development, removal of apoptotic corpses and cellular debris is essential for the correct placement and shape of newly forming organs. By the end of embryogenesis up to 90% of all plasmatocytes may contain at least one apoptotic body (Tepass, Fessler et al. 1994). The recognition of apoptotic cell requires the function of specific receptors present at the embryonic phagocyte membrane : Croquemort (CRQ) and Draper (Franc, Dimarcq et al. 1996; Manaka, Kuraishi et al. 2004). The engulfing of invading microorganisms is another phagocytic function of plasmatocytes that engages several types of receptor proteins, such as Eater, a EGF-domain phagocytosis receptor expressed exclusively on plasmatocytes. It binds to and leads to internalisation of a broad range of bacteria (Kocks, Cho et al. 2005). Recently, Nimrod (NimC1) was described as a receptor present only on larval phagocytes which increases remarkably the efficiency of bacteria engulfing (Kurucz, Markus et al. 2007). The scavenger receptor, dSR-CI, is a receptor capable of recognizing both gram-negative and gram-positive bacteria (Ramet, Pearson et al. 2001). Extremely interesting is the case of the Dscam proteins (Down syndrome cell adhesion molecule), that belong to the immunoglobulin (Ig) superfamily. Alternative splicing offers the theoretical possibility of up to 19 000 Dscam different isoforms. Dscam are expressed inter alia at the hemocyte membrane and take part in the phagocytosis of bacteria (Watson, Puttmann-Holgado et al. 2005).
Lastly, it was reported that the plasmatocyte mediated phagocytosis could participate in a kind of “immune memory”, a process so far specific only for vertebrates. Pham and colleagues have shown that flies primed with a sublethal dose of bacteria (S. pneumoniae) are protected against a lethal dose of the same bacteria when challenged one week later and this memory depends on plasmatocytic phagocytosis (Pham 2007).
The phagocytic capacities of plasmatocytes are reinforced by the presence of opsonins belonging to the family of Thioester-containing proteins (Teps). In the Drosophila genome there are six different genes coding for Teps. Teps contain several domains shared with the α2-macroglobulin, and complement (C3) family of proteins, which in vertebrates act as protease inhibitors and opsonin respectively (Dodds and Law 1998; Lagueux, Perrodou et al. 2000; Blandin and Levashina 2004). It was also suggested that Dscam molecules could bind directly to bacteria and potentially opsonise them (Watson, Puttmann-Holgado et al. 2005).
Finally, there is another function of plasmatocytes worth to be mentioned as it is linked to the humoral response. Plasmatocytes have the capacity to secrete the cytokine upd3 that activates JAK/STAT pathway in the fat body, leading to secretion of immune effectors (Agaisse and Perrimon 2004).
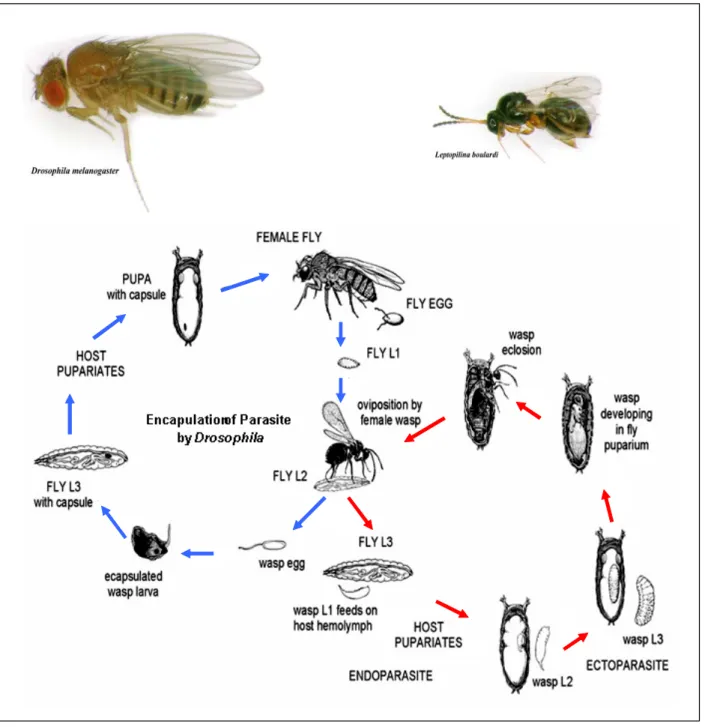
Fig. 4. The life cycles of Drosophila melanogaster and Leptopilina boulardi.
Females of L. boulardii lay eggs in Drosophila larvae. Larvae mount a cellular immune response against parasitoid wasp egg, resulting in encapsulation and melanisation of the egg. The succesful neutralisation of the parasite, permits the development of fly (blue arrows). In case where the Drosophila immune response fails, wasp larvae develop (red arrows) (Photos M.Crozatier).
Crystal cells
Crystal cells are generated both during embryonic and larval hematopoiesis. Their role in the embryo remains unknown (Wood and Jacinto 2007), while adult flies are devoid of this cell type (Lanot, Zachary et al. 2001). In the larval hemolymph, crystal cells make about 5% of circulating hemocytes They owe their name to the big crystals of zymogen proPhenylOxydase (proPO) present in their cytoplasm and necessary for the process of melanisation. Melanisation leads to the deposition of a black pigment that contributes to the clot at the site of the wound or around foreign bodies in the hemocel (Ashida M. 1997). Crystal cells function as a storage for large amount of proPO. Once disrupted they release their content into the hemolymph where the enzymes can function (Meister 2004). ProPO is cleaved and turned to its active form - phenoloxidase (PO) by the action of a cascade of serine proteases. PO carries the monophenol mono-oxygenase activity that converts tyrosine to melanin. Many of intermediate compounds formed during the melanin synthesis are cytotoxic and could participate into the killing of pathogens (Meister and Lagueux 2003; Meister 2004). The Drosophila genome contains three genes coding for proPO: doxA1, doxA3 and cg8193, that are expressed in crystal cells with doxA3 being expressed also in lamellocytes (Irving, Ubeda et al. 2005). Melanisation at the site of injury in larvae is mediated exclusively by crystal cells. It is impaired in mutants affecting crystal cell development such as lozenge, or affecting the release of proPO such as Black cells (Bc) (Rizki T. M. 1980). The source of PO in the adult where the crystal cell are no longer present is unknown (Lanot, Zachary et al. 2001; Lemaitre and Hoffmann 2007).
Crystal cells do also participate in clot formation. Clotting is critical in limiting hemolymph loss and creating an immune barrier during wound healing in insects. Clot is a quickly forming barrier against infection that immobilises bacteria, prevents their spreading and promotes their killing. The first step of clot formation– preliminary soft clot - is independent of the crystal cell action, as it can occur even in Bc, or lz mutant larvae. However, the PO originating from crystal cells acts during clot maturation to produce the hard clot by cross-linking the preliminary soft clot that tightens the grip of bacteria and thereby reducing the risk of post-injury infection (Bidla, Lindgren et al. 2005; Lemaitre and Hoffmann 2007).
Finally, melanisation mediated by crystal cells is engaged in the encapsulation process that critically requires the third hemocyte type, the lamellocytes.
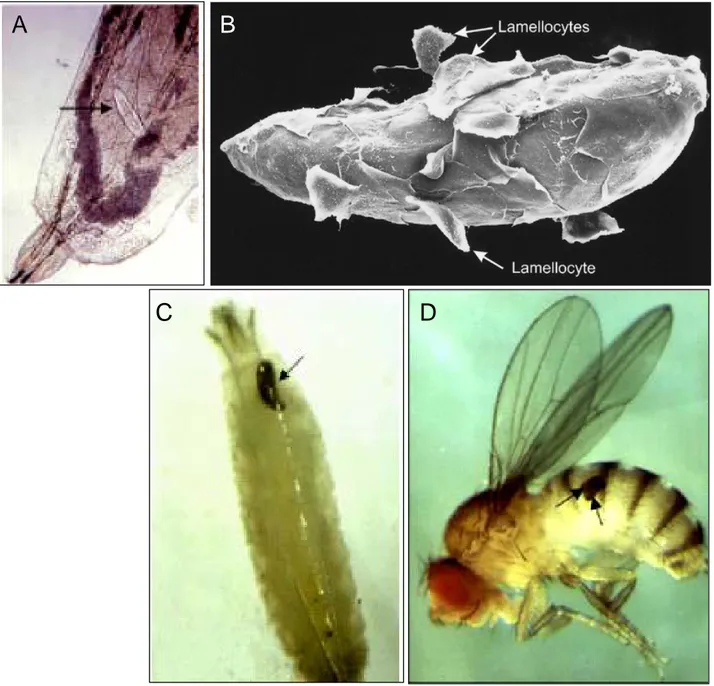
Fig. 5. Encapsulation and melanisation of the parasitoid wasp Leptopilina boulardi in Drosophila larvae
(A) Wasp egg (arrow) in the body cavity of Drosophila melanogaster, soon after deposition; (B) Encapsulated egg. Lamellocytes are clearly observed on the egg surface (arrows); (C) Melanised capsule (arrow) surrounding a wasp egg in a Drosophila larva; (D) Adult fly with two encapsulated wasp eggs (arrows) in its abdomen (after Vass and Nappi 2000 and Carton et al. 2005).
A
B
Lamellocytes
The lamellocytes of Drosophila are flat, large, adhesive cells only produced in the larval hematopoietic organ – the lymph gland. They are rarely observed in healthy larvae but differentiate in very large number upon specific challenge like parasitoid wasp egg laying into the larva. In the nature at least around 50 hymenopterae species are the parasites of Drosophila (Carton 1986). One species widely used in laboratories to study the Drosophila immune response is Leptopilina boulardii. Wasp females lay eggs into the hemocel of young Drosophila larvae and use the host body as an environment for the developing offspring (Fig.4). The presence of the parasitoid egg triggers a cellular immune response. It is proposed that the wasp egg is recognised in the larval body by circulating plasmatocytes (Russo, Dupas et al. 1996). Then the signal of danger is sent to the lymph gland and massive differentiation of lamellocytes occurs (Crozatier, Ubeda et al. 2004). The lamellocytes are released into the hemolymph where they stick to the egg and form a multilayered capsule around the invader in a process called encapsulation (Fig.5). Encapsulation engages all three hemocyte types as the crystal cells are involved in the melanisation of the capsule. The egg is finally killed, probably by the effect of cytotoxic free radicals or quinons, although the real reason of its death is not entirely clear (Vass and Nappi 2000; Meister 2004). Once the egg is encapsulated and melanised it stays as a scar in the larva and adult (Fig. 5 C,D) but the fly can develop and hatch normally.If the fly immune response fails, a wasp hatches from the pupa at the expense of the fly (Fig. 4). Parasitoid wasps use different strategies that can “cheat’ the host response. In some wasp species like Asobara tabida, the eggs attach easily to the host tissue thanks to a sticky chorion and finally get embedded in a host site inaccessible to hemocytes (Prevost, Eslin et al. 2005). Another way to escape is an active suppression based on specific substances introduced into host by the female wasp at the time of oviposition. For example, L. heterotoma and L. boulardi inject virus like particles (VLP) produced by the so-called long glands. The proteins present in the VLP inhibit the encapsulation by changing the morphology of the lamellocytes, resulting in their diminished adhesive ability (Rizki and Rizki 1984; Rizki and Rizki 1990; Rizki and Rizki 1990; Rizki, Rizki et al. 1990; Lemaitre and Hoffmann 2007; Schlenke, Morales et al. 2007). Species devoid of VLP, such as Asobara citri could possibly directly affect the hematopoietic organ, the lymph gland and induce its degradation (Moreau, Eslin et al. 2003).
The relationship between Drosophila and parasitoid wasps is an example of the co-evolution that pushes the species to reciprocal improvement of the physiological
prohemocytes
Stage 11
Stage 17
plasmatocytes crystal cells
Stage 5 crystal cells plasmatocytes
A
srp gcm, prx prx doxA3 doxA3B
prohemocytes Stage 11 Stage 17plasmatocytes crystal cells
Stage 5 crystal cells plasmatocytes
A
srp gcm, prx prx doxA3 doxA3B
prohemocytes Stage 11 Stage 17plasmatocytes crystal cells
Stage 5 crystal cells plasmatocytes
A
srp gcm, prx prx doxA3 doxA3B
Fig. 6. Embryonic hematopoiesis
(A) Blastoderm fate map of the embryonic hemocytes (EH). The EH anlage is restricted to 70-80% EL (egg length) (red); (mesoderm is in yellow) (after Holz et al. 2003, modified)
(B) overlapping of gcm and srp expression in stage 5 embryos, showing the domain of origin of the prohemocytes. (C, E) Plasmatocytes stained with peroxidasin (prx) located in the anterior part of the embryo at stage 11 (C) and dispersed within the whole embryo at stage 17 (E). (D, F) Crystal cells, stained with doxA3 do not migrate but stay localised in the anterior segments in stage 11 (D) and 17 (F). (after Bataille 2006, PhD thesis)
capacities. However initiating and maintaining the immune system can be energetically expensive and have an impact on the fitness of an animal. Parasites and pathogens can influence the life history of host (Sandland and Minchella 2003). It was reported that Drosophila melanogaster that successfully defended itself against Asobara tabida exhibited reduced feeding and thinner puparian walls, but also decreased survival as adults under conditions of starvation and desiccation (Fellowes A. 1999; Hoang 2001; Tien 2001). The immunological costs can be masked or revealed depending on environmental conditions. Additionally, although immunological response might be initiated at a particular developmental stage, the results can be exhibited at later point in ontogeny (Sandland and Minchella 2003). Similarly to parasitoidal, bacterial infection can affect the fitness of the fly in sub-optimal conditions and it was even suggested that the cost of immunity might be important factor limiting the evolution of resistance in food-limited environments (McKean, Yourth et al. 2008).
Embryonic hematopoiesis
Embryonic hematopoiesis takes place in the head mesoderm and leads to formation of an almost invariant number of plasmatocytes (~700) and crystal cells (~30), 95% and 5% of all hemocytes respectively (Bataille, Auge et al. 2005). The anlage of the embryonic hemocytes is defined by the expression of the transcription factor Serpent (Srp), a member of the GATA-binding transcription factors family. Srp is expressed in all hemocyte precursors and is required for the development of plasmatocytes and crystal cells. Loss of srp leads to the complete depletion of embryonic hemocytes (EH) (Rehorn, Thelen et al. 1996).
Elegant, homotypic single–cell transplantation experiments done by Holz et al. restricted the anlage of the EH to a precise region located between 70-80% EL (the posision on the egg in the anterioposterior axis is given as per cent of Egg Length, where 100% EL is the anterior tip and 0% the posterior), within the mesoderm corresponding to srp cephalic expression (Fig. 6A,B). They were also able to show that EH are already specified at the blastoderm stage (Holz, Bossinger et al. 2003).
During embryogenesis, a fraction of hemocytes migrates from its place of birth by specific paths to seed the whole embryo. These cells are the plasmatocytes, the dedicated phagocytes. A much smaller fraction of prohemocytes do not migrate and remains localised
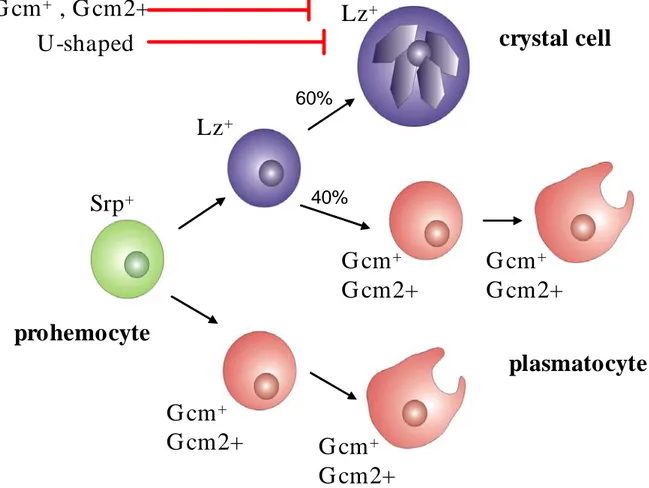
Fig. 7. The model for the generation of the hematopoietic lineages in the Drosophila embryo
Srp is the earliest expressed gene in the embryonic hemocyte pecursor pool. Most of Srp+ cells begin to express Gcm and differentiate as plasmatocytes. A smaller fraction of Srp+ cells begin to express Lz. ~60% of Lz+ cells maintain the expression of Lz and become crystal cells and the rest will become plasmatocytes. Gcm/Gcm2 and U-shape antagonise the crystal cell fate (after Lebestky et al.. 2000, Bataille et al. 2005, modified).
Srp
+Lz
+Lz
+G cm
+G cm2+
G cm
+G cm2+
G cm
+G cm2+
G cm
+G cm2+
U-shaped
crystal cell
plasmatocyte
prohemocyte
G cm
+, G cm2+
60%
around the proventriculus. These hemocytes are the crystal cells (Fig. 6 C-F) (Tepass, Fessler et al. 1994; Lebestky, Chang et al. 2000).
The molecular control of embryonic hematopoesis has now been well described. Several transcription factors and signalling pathways have been reported to be involved in this process. Two zinc-finger containing transactivators: Gcm ( glial cells missing) and Gcm2 are required for plasmatocyte development. The crystal cell fate needs the expression of the transcription factor Lozange ( Lz) (Lebestky, Chang et al. 2000; Waltzer, Ferjoux et al. 2003). In a detailed work, Bataille et al. showed that Gcm is coexpressed with Srp in all prohemocytes in early embryos (stage 5) (Fig.6B). Only later (stage 6), the anterior-most cells of the prohemocyte cluster downregulate gcm thereby allowing the expression of Lz. Only 60% of Lz+ cells will be able to maintain Lz through an autoregulatory loop and acquire a crystal cell fate, the rest becoming plasmatocytes. In these 40%, it is residual Gcm that interferes with lz expression and promotes plasmatocyte differentation (Fig.7) (Bataille, Auge et al. 2005). The fact that gcm, when ectopically expressed, can induce differentiation of all the prohemocytes into plasmatocytes, (Lebestky, Chang et al. 2000) and that, in absence of gcm/gcm2, lz can transform all of the hemocytes into crystal cells suggests that embryonic prohemocytes are bipotent progenitors (Bataille, Auge et al. 2005). Additionally it was shown that another Drosophila transcription factor, U-shaped (Ush), the homolg of vertebrate Friend of Gata (FOG), is expressed from stage 8 in plasmatocytes and antagonises crystal cell development. Figure 7 summarises the molecular control of the embryonic hematopoiesis.
Larval hematopoiesis
Unlike embryonic hematopoiesis, larval hematopoiesis occurs in a specific organ, called the lymph gland (LG). Although this nomenclature from the literature will be used here, we have to keep in mind that the insects have no lymph but rather hemolymph, therefore we should call the hematopoietic fly organ the “hemolymph gland”. Larval hematopoiesis is more flexible than embryonic hematopoiesis in terms of the total number of cells released and proportion between plasmatocyte and crystal cell fates. It can also give rise, in specific conditions, to a third cell type – the lamellocyte.
The lymph gland forms in the embryo, independently of the embryonic prohemocytes. The LG primordium originates from the cardiogenic mesoderm (as the other Drosophila
Fig. 8. Ontogeny of the lymph gland
The earliest known marker of the developing anlage of the larval LG is the gene collier.(A) Expression of col is detected in two discrete clusters of cells in the dorsal mesoderm of the thoracic segments T2 and T3 starting at embryonic stage 11 (germ band extension stage) (black arrows). (B, C) The Col+ clusters migrate toward each other between stage 12 and early 13 and coalesce to form the paired lobes of the lymph gland. (D-F) Expression of col becomes restricted at stage 14 and at stage 16 it marks only a specific posterior region of the LG, the PSC (black arrowheads) (Crozatier et al.. 2004).
(G,H) Expression of the transcription factor Odd indicates that the primordium of the embryonic LG likely comes from three paired clusters of cells located in the thoracic segments T1, T2 and T3. (G-J) Expression of the homeotic gene antp is restricted at 11-12 stage to T3 in the LG (Mandal et al.. 2007).
E
F
G
J
I
H
Stage 11 Stage 12/13circulatory system cells: cardioblasts and pericardial cells) that is specified within the mesoderm by signalling pathways, Decapentaplegic (Dpp), Wingless (Wg) and FGF (Cripps and Olson 2002; Han, Yi et al. 2006). On the blastoderm fate map, it was initially mapped between 50 and 55% EL in the thoracic lateral mesoderm ((Holz, Bossinger et al. 2003); however the cells of the LG primordium are not determined to a specific fate before the second post-blastoderm mitosis. The earliest marker of developing anlagen of the LG is the gene collier, the expression of which is detected in two discrete clusters of cells in the dorsal mesoderm of the thoracic segments T2 and T3 starting in embryonic stage 11 (germ band extension stage). The Col+ clusters migrate toward each other and coalesce to form the paired lobes of the lymph gland. Afterwards, Collier expression becomes restricted and, at stage 16, it marks only a specific posterior region of the hematopoietic organ (see below) (Fig. 8 A-F). Srp is not detected in the LG precursor prior to stage 12. In srp mutant embryo col specific expression in LG progenitors can be observed, indicating that these cells are specified independently of srp (Crozatier, Ubeda et al. 2004). Although expression of collier suggests that the LG origins from two cell clusters, expression of another transcription factor Odd (Odd-skipped) indicates that the primordium of the embryonic LG more likely comes from three paired clusters of cells located in the thoracic segments T1, T2 and T3 at stage 15 (Fig.8 G-J) (Mandal, Banerjee et al. 2004). Odd is a zinc-finger protein expressed in segmental clusters in the dorsal mesoderm of segments T1-A6 (Ward and Skeath 2000). Expression of the homeotic gene Antennapedia is already restricted at stage 11-12 to the T3 and A1 segment in the cardiac tissue (Fig. 8 G-J) (Mandal, Martinez-Agosto et al. 2007). Antp colocalizes with Collier in the posterior region of the LG at stage 14.
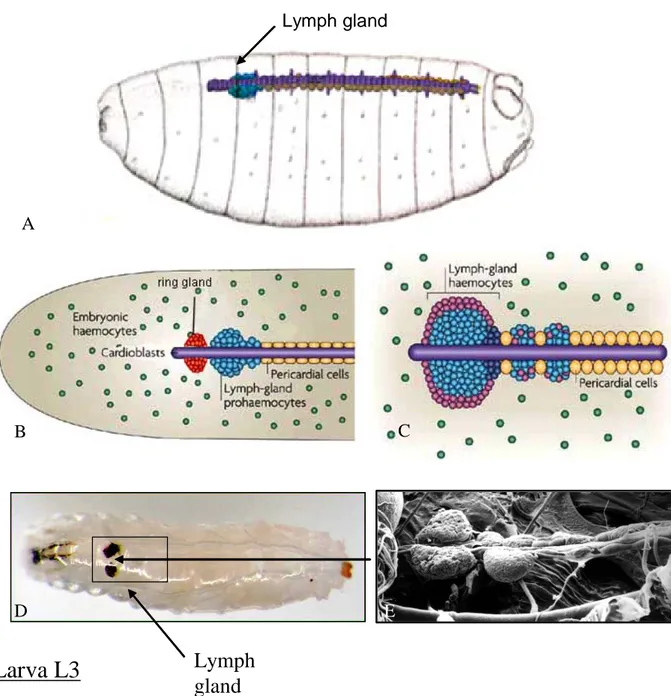
The developing LG is localised in the dorsal part of the embryo along the cardiac tube (Drosophila heart), just posterior to the hormone-secreting organ, the ring gland (analogue of vertebrate pituitary)(Fig 9A, B). During larval development, the LG grows in size and when fully developed in third instar larvae, it consist of about 5000 cells per lobe (compared to ~30 at the end of the embryogenesis). In addition to the anterior lobes, which starts to form in the embryo and become the main Drosophila hematopoietic site, variable numbers of posterior lobes form during larval development (Fig. 9 C). Although their function is not well characterised it has been proposed that they constitute a reservoir of prohemocytes. At the onset of metamorphosis, in response to the ecdysone secretion peak, the lymph gland disperses and hemocytes are released into circulation (Sorrentino, Carton et al. 2002). It is the first developmental time when larval hemocytes can be used by Drosophila. Before that, all circulating hemocytes originate from the embryonic wave of hematopoiesis. The embryonic
Fig. 9. Development of the lymph gland
(A,B) The lymph gland (LG) is localised in the dorsal part of the embryo in direct contact with the anterior part of the cardiac tube, posterior to the ring gland. (C) During larval stages, the LG grows in size and in the late L3 larva is composed of a pair of anterior lobes, the main hematopoietic site, and several posterior lobes. Circulating embryonic hemocytes (in green) persist throughout embryogenesis and larval development until the adult stage (after Wood and Jacinto 2007). (D) Localisation of the LG in larvae (E) image of the LG in scanning electron microscopy (from Meister and Lagueux 2003).
Larva L3
Lymph
gland
A B C D E Lymph glandhemocytes in larval circulation divide actively growing in number from ~200 cells in L1 up to ~2500 in L3 larvae and survive larvae and up to the adult stages (Lanot, Zachary et al. 2001). A majority of larval hemocytes circulates but a fraction, called sessile hemocytes (containing both plasmatocytes and crystal cells), stays attached to the integument. Therefore, the hemolymph of the imago contains a mixture of embryonic hemocytes and larval hemocytes released from the lymph gland. Until now, no haematopoietic site has been described in the adult fruit fly (Holz, Bossinger et al. 2003).
The detailed work of Jung et al. revealed a very interesting organisation of the anterior-most lobes of the fully developed L3 LG. Already in the light microscopy one can observe two different regions: a more rough zone at the periphery of the lobe and a smooth area inside. The outside zone is called the cortical zone (CZ) and is occupied by differentiated plasmatocytes and crystal cells. The inner part is called a medullary zone (MZ) and contains non-differentiated hemocytes (Fig. 10A) Additionally, the Posterior Signalling Center (PSC) resides in the posterior part of the lobe (Fig.10C) (Lebestky, Jung et al. 2003; Jung, Evans et al. 2005).
The medullary zone can be visualised by the expression of tep4 and a domeless-gal4 transgene that was isolated from a P-insertion screen for identifying X-linked essential genes (Fig10 B,C) (Bourbon, Gonzy-Treboul et al. 2002; Irving, Ubeda et al. 2005; Jung, Evans et al. 2005). Domeless (Dome) is the Drosophila receptor of the JAK/STAT (Janus Kinase(JAK)/Signal Transducer and Activators of Transcription (STAT) pathway (Fig. 10D). The fact that Dome is expressed in the MZ is very interesting, knowing that the JAK/STAT pathway was previously described to be involved in larval hemocyte formation. The JAK/STAT pathway was first described in vertebrates as the signalling pathway responding to interferon (Schindler, Fu et al. 1992; Darnell, Kerr et al. 1994). Then in mammals, large families of cytokines and single-pass transmembrane receptors, named type I cytokine receptors have been identified (for review (Taga and Kishimoto 1997; O'Shea, Gadina et al. 2002; Heinrich, Behrmann et al. 2003; Kristensen, Kalisz et al. 2005). The JAK/STAT pathway mediates intra-cellular signalling. JAK kinases are anchored to the intracellular part of signalling receptors. Binding of the cytokine induces conformational changes in the latter that bring two JAKs in close proximity. This allows JAK trans-phosphorylation and phosphorylation of the receptor, thereby creating a docking site for STAT transcription factors. STATs become in turn phosphorylated, leading to their dimerisation and translocation into the nucleus where they function as transcriptional regulators. The JAK/STAT signalling pathway regulates numerous aspects of development and tissue differentiation. Altered
Fig. 10. Morphology of the lymph gland.
(A) Nomarski view of the LG in third instar larvae, showing two distinct regions within the primary lobes: a rough cortical zone (CZ) and a smooth medullary zone (MZ) (red arrows). Black arrows indicate the boundary between the CZ and MZ. 1° primary, 2° secondary lobe (from Jung et al.. 2005) (B) The MZ can be visualised by the expression of tep4 (purple). (C) Confocal microscopy view of the LG anterior lobes: the MZ containing prohemocytes is marked with GFP (dome-gal4/UASmCD8GFP) (green), the CZ containing differentiated cells is stained for the proPO (red) and the PSC is stained with anti-Col (blue). (D) Schematic representation of the components of the JAK/STAT signalling pathway in Drosophila (from Arbuzova and Zeidler 2006).
1°lobe 2°lobe CZ (Cortical zone) differentiatied cells MZ (Medullary zone) prohemocytes
Col,dome,ProPO
1º
2º
tep4
A
D
C
PSC(Posterior Signaling Center)
B
1º
2º
JAK/STAT activity has been associated with several human diseases including leukaemia, myocardial hypertrophy and asthma while knock-out studies in mice point to a central role of JAK/STAT signalling in hematopoeisis and regulation of immune functions (Shuai and Liu 2003; O'Shea and Murray 2008). In contrast to mammals, only one receptor, Domeless (Dome) (Brown, Hu et al. 2001; Chen, Chen et al. 2002) one JAK (Hopscotch, Hop) (Binari and Perrimon 1994) and one STAT (Stat 92E or Marelle (Hou, Melnick et al. 1996; Yan, Small et al. 1996) have been characterised in Drosophila, while three cytokines, Unpaired (Upd), Upd2 and Upd3 have been shown to act upstream of JAK-STAT signalling (Harrison, McCoon et al. 1998; Brown, Hu et al. 2001; Chen, Chen et al. 2002; Agaisse, Petersen et al. 2003; Gilbert, Weaver et al. 2005; Hombria, Brown et al. 2005).
Thanks to the conservation during evolution on the one hand and relative simplicity on the other, Drosophila has become a model of choice for investigating this signalling pathway. One of the first evidences that JAK/STAT signalling could be involved in Drosophila hematopoiesis came from analysis of dominant-gain of function mutations of hop. Two thermosensitive alleles Tum- and T42 were isolated, where the kinase is constitutively active. hop Tum-l larvae display numerous circulating lamellocytes and melanotic pseudotumors in absence of wasp infection when kept at restrictive temperature. The effect seems to be LG autonomous since hop Tum-l LG when transplanted in a wild type host, provoke an overproliferation of hemocytes and a melanisation phenotype (Hanratty and Dearolf 1993; Harrison, Binari et al. 1995; Luo, Hanratty et al. 1995; Agaisse and Perrimon 2004). Conversely, loss of function of hop leads to a complete absence of lamellocyte differentiation upon wasp infestation. Again, the phenotype seems to be related to hop function in the LG since no effect on the number on circulating cells was observed (Sorrentino, Melk et al. 2004). Aside, while one of the JAK/STAT ligands, upd3, was previously reported to be secreted by circulating plasmatocytes upon septic injury, Jung et al. have shown that an upd3gal4 construct drives the expression of the reporter gene in the MZ and in the PSC. Together these data suggested that the JAK/STAT pathway could be active in the LG in controlling hematopoiesis (Jung, Evans et al. 2005). Function of tep4, another gene specifically expressed in the MZ, remains unknown (Irving, Ubeda et al. 2005).
The cortical zone (CZ) is occupied by differentiated hemocytes and is defined mainly by
expression of markers of mature hemocytes and lack of expression of MZ markers (Fig. 10C). By elegant cell tracing experiments, Jung et al have shown that the cortical zone cells originate from the medullary zone. In second instar larvae, all hemocytes are in a precursor
Fig. 11a. col is required for the formation of the PSC
(A,C,E) wild type, (B,D,F) col1 LG. The col1 mutant phenotype is the loss of the PSC. Expression of PSC markers, Ser-lacZ and Ser is lost (arrowhead in C,E) while the expression of Ser in scattered cells (arrows E,F) remains unchanged. Bar:50μm (from Crozatier et al. 2004).
Fig. 11b. PSC plays an instructive role in lamellocyte production
(A,C,E) wild type, (B, D, F) col1 LG. (A,B) col1 lymph glands show increased numbers of crystal cells (stained with doxA3) in comparison to wt. (E,F) When challenged by parasitoid wasps, col1 larvae are unable to produce lamellocytes (stained with specific antibody against an integrin αPS4), while wt larvae lymph glands are full of newly differentatied lamellocytes Bar: 50μm (from Crozatier et al. 2004).
state and reside in the MZ. CZ forms with the progressive downregulation of domeless and differentiation of hemocytes in third instar larvae (Jung, Evans et al. 2005). The exact mode of the transition from the medullary zone toward the cortical zone remains a challenging question. The cell lineage of larval hemocytes has not been documented up to now and still needs to be resolved.
The Posterior Signalling Centre (PSC) was described for the first time by Lebetzky and
colleagues in 2003, when they observed that Serrate (Ser), a Notch (N) ligand was expressed in a small population of lymph gland cells, localised in the posterior part of the anterior lobe. This is where the name “PSC” comes from. Ser expression could be seen with the use of an antibody or expression of Ser-lacZ reporter gene. In addition to the PSC, the antibody revealed the expression of Ser in scattered cells throughout the gland (Fig. 11a, C-F), (Lebestky, Jung et al. 2003). Notch signalling is an evolutionary conserved signalling pathway that regulates cell fate decisions in numerous developmental systems and involves directcell-cell interaction(for review see (Bolos, Grego-Bessa et al. 2007; Carlson, O'Connor et al. 2007; Fiuza and Arias 2007; Nichols, Miyamoto et al. 2007). Several reports have documented the involvement of N signalling in crystal cell formation. In Nts larvae (Nts is a termosensitive allele of Notch receptor), raised at restrictive temperature (29ºC) no crystal cell forms (Duvic, Hoffmann et al. 2002). Based on this mutant phenotype and Ser expression in the late LG, Lebetzky et al. proposed that Ser+ cells migrate from the PSC and seed the lobe where they instruct the neighbouring cells to become crystal cells. The proposition that the PSC controls proper larval hematopoiesis was also based on the observation that Ser and not Delta (the other Drosophila Notch ligand), is necessary for crystal cell formation. Unfortunately, not all of the PSC hypothesis turned to be right. In 2004, Crozatier and colleagues showed that collier which codes for a transcription factor, orthologous to vertebrate Early B-cell Factor (EBF) is expressed specifically in the PSC of the lymph gland. One of the phenotypes displayed by col1 mutant larvae (col1 is a null allele of collier), is the loss of the PSC. While, as a consequence, Ser expression is lost from the PSC, it remains unchanged in scattered cells in the anterior lobes of col1 mutant LG (Fig. 11a). Likewise, crystal cells form correctly (Fig. 11b) (Crozatier, Ubeda et al. 2004). The differentiation of crystal cells in col1 therefore showed that it is Ser activity in the scattered cells and not in the PSC that is necessary for crystal cell formation. However Lebetzky’s idea of a signalling center was correct, as it turned out that the PSC controls other aspects of larval hematopoiesis. The loss of the PSC in col1 has indeed dramatic consequences in terms of hemocyte formation
S1 PSC Col lamellocytes L S2 pro-hemocyte wasp egg circulating plasmatocytes L
Fig. 12. A model for the induction of lamellocyte differentiation in response to wasp parasitation.
Col enables the PSC cells to respond to a primary signal (S1), that is likely emitted by plasmatocytes after their contact with the wasp egg.
As a result, the PSC sends a secondary signal (S2) to prohemocytes, inducing their massive differentation into lamellocytes (after Crozatier et al. 2004, modified).
(see below). The first observation was that PSC-depleted larvae are unable to mount a cellular immune response, i.e. there is no differentiation of lamellocytes in response to egg-laying by the parasitoid wasp Leptopilina boulardii (Fig.11b). In wild type larvae, col-expressing cells remain restricted to the PSC upon wasp infestation, while lamellocytes differentiate from the MZ, showing that PSC cells are not the precursors of lamellocytes but rather that they play an instructive role in orienting prohemocytes to differentiate into lamellocytes. The following model of cellular response upon infestation was proposed: Col enables PSC cells to respond to a primary signal (S1) likely emitted by plasmatocytes upon their encounter of a parasitoid egg. Upon receiving S1, PSC cells send a secondary signal (S2) that orients prohemocytes to develop into lamellocytes (Fig. 12) (Crozatier, Ubeda et al. 2004).
Later work showed that the Posterior Signalling Center can first be identified in the embryo by the expression of Antennapedia in stage 11-12 when the primordium of the lymph gland starts to form. The homeodomain cofactor Homothorax (Hth) is initially expressed ubiquitously in the embryonic LG but is soon downregulated in the Antp+ cells. The mutually exclusive expression pattern of Antp and Hth specifices two LG areas: Antp specifies the PSC cells and Hth, the rest of the gland (Mandal, Martinez-Agosto et al. 2007). In parallel, collier expression starts to be restricted to the PSC around stage 14 where it persists up to the end of L3 (see above) (Crozatier, Ubeda et al. 2004). Then Serrate starts to be expressed in the PSC in second instar larvae (Lebetzky) as does hedgehog (Fig. 13), while downstream components of the Hh signalling pathway, the receptor Patched (Ptc) and transcription factor Cubitus interruptus (Ci) are expressed in the medullary zone. Together, these led to the proposal that Hh produced in the PSC diffuses and signals through activated Ci in the medullary zone, keeping the MZ cells in a precursor state (Mandal, Martinez-Agosto et al. 2007).
Control of larval hematopoiesis
Contrary to embryonic hematopoiesis, the control of Drosophila larval hematopoiesis has not been characterised in details. In 2004, when I started my PhD work, the information in the literature was still very fragmentary and there was not a clear image of overall regulation of hemocyte formation in the lymph gland. Only few links were made between the embryonic and larval waves of hematopoiesis. One of them was the transcription factor Lozenge (Lz)
Fig.13. Expression of PSC markers during LG development
(A) The Posterior Signalling Center can first be identified in the embryo by the expression of Antennapedia (Antp) in stage 11-12 when the primordium of the lymph gland starts to form. The homeodomain co-factor Homothorax (Hth) initially expressed ubiquitously in the embryonic LG is downregulated in the Antp+ cells. (B) Collier expression starts to be restricted to the PSC at around stage 14 where it persists up to the end of L3. (C,D) Serrate (Ser) and Hedgehog (Hh) start to be expressed in second instar larvae.
that is required for crystal cell specification and seems to be common to both embryonic and larval phases of hematopoiesis, since lz mutant embryos and larvae lack this cell type (Lebestky, Chang et al. 2000). Otherwise, for example Notch signalling that has been shown to be necessary for crystal cell formation in larvae, is apparently not required during the first hematopoietic wave (Duvic, Hoffmann et al. 2002; Bataille, Auge et al. 2005). It should be noted, however, that even though we know that N controls the larval crystal cell fate, the temporal window of this requirement has not been defined. The embryonic plasmatocyte fate specific factors Gcm and Gcm2 are not expressed in the lymph gland (Bataille, Auge et al. 2005). The only information about requirement for larval plasmatocyte specification comes from the studies of Jung and colleagues showing that Pvr signalling specifically controls the differentiation of phagocytes (Jung, Evans et al. 2005). Support for the involvement of JAK/STAT signalling in larval hematopoiesis has already been presented above. Interestingly a phenotype similar to gain-of-function phenotype of hop (hop Tum-l), that is a massive differentiation of lamellocytes in absence of immune challenge, was described for hyperactivation of the Toll pathway. This cellular phenotype appears to be independent from Toll function in the humoral immune response in the fat body (Crozatier M. personal communication, (Qiu, Pan et al. 1998). A more precise view of control of larval hematopoiesis has started to emerge with the characterisation of the PSC.
Collier, a transcription factor with multiple developmental functions
Crozatier et al. in 2004 showed that the transcription factor Collier (Col) is crucial for the proper function of the PSC. The gene coding for this protein was first characterised by the group of A. Vincent in 1996 and since then, it has been at the center of interest of the team (Crozatier, Valle et al. 1996). Col is a founding member of the family of COE transcription factors, COE for Collier/Olfactory-1/Early B cell Factor isolated from Drosophila, rat and mouse respectively (Hagman, Belanger et al. 1993; Wang and Reed 1993; Crozatier, Valle et al. 1996). While there is no evidence for coe genes either in protozoa, fungi or plants, identification of a coe gene in cnidarians and sponges suggests that the coe genes appeared with Metazoa (Pang, Matus et al. 2004). While up to four ebf paralogs have been identified in vertebrates, a single coe member has been identified in all other animals for which genome sequence is available (NCBI, mars 2008), indicating that expansion of the coe gene family
Fig. 14. Schematic representation and alignment of mouse EBF/COE1 and Drosophila Col.
The regions corresponding to the DNA Binding Domain (DBD) and the IPT domain are shown in red and yellow, respectively. The ancestral Helix-Loop-Helix (HLH) motif is represented by two separate black boxes (helices H1 and H2a) and the H1-H2 linker in green. The duplicated helix (H2d) specific to the vertebrate proteins is indicated by a blue box and the C-terminal transactivation domain is in grey. EBF and Col show very significant sequence identity; 86% in the DBD domain and 89% in the IPT domain. (from Daburon et al. 2008).
occurred at the origin of vertebrates (Garel, Marin et al. 1997; Wang, Tsai et al. 1997; Wang, Betz et al. 2002; Daburon, Mella et al. 2008). Biochemical dissection of mouse EBF identified three functional domains: i) an amino-terminal DNA binding domain (DBD), that became the signature of the COE family of transcription factors, ii) a Helix-Loop-Helix (HLH) dimerisation motif showing limited sequence similarity with HLH motifs described in basic helix-loop-helix proteins (b-HLH), iii) a transcription activating domain. An Ig-like/Plexin/Transcription factor (IPT) domain with unknown function is also present between the DBD and HLH domains (Bork, Doerks et al. 1999). Comparison between Col and EBF showed that the DBD, IPT and HLH domains have been particularly well conserved during evolution with one noticeable exception that in vertebrates one of the α-helix of the HLH, has been duplicated (Fig 14) (Dubois and Vincent 2001; Daburon, Mella et al. 2008). EBF and Col bind to DNA as homodimers and homodimer formation is mediated by the HLH domain (Daburon, Mella et al. 2008).
Collier was first described as a regulator of head patterning in Drosophila (Crozatier, Valle et al. 1996); ebf(1) was first characterised as a crucial factor for B-lymphocyte specification and differentiation (Travis, Hagman et al. 1993; Rothenberg 2000) and olf-1 was isolated as a gene coding for an olfactory neuron specific transcription factor (Kudrycki, Stein-Izsak et al. 1993). Further studies of the function of col and ebfs showed that these genes are involved in the development of several different tissues. In Drosophila in addition to the head, Col is expressed and required in a specific somatic muscle (muscle DA3), the wing, and subsets of neurons in the central and peripheral nervous system. (Crozatier, Valle et al. 1996; Crozatier, Valle et al. 1999; Crozatier and Vincent 1999; Vervoort, Crozatier et al. 1999; Dubois, Enriquez et al. 2007; Crozatier and Vincent 2008). Finally it is also required to specify the PSC (see above) in the lymph gland (Crozatier, Ubeda et al. 2004). During mouse embryogenesis, ebf genes are expressed in the limb buds, immature olfactory neuronal precursors, mature neurons of adult olfactory epithelium, developing nervous system (ebf1-3) (Garel, Marin et al. 1997; Wang, Tsai et al. 1997; Mella, Soula et al. 2004), progenitors of facial branchiomotor neurons (fbn) (ebf2,3), mature fbn (ebf1), the genital bulbs and the harderian gland (ebf4); ebf3 and 4 were detected in human placenta (Garel, Garcia-Dominguez et al. 2000; Pattyn, Hirsch et al. 2000; Mella, Soula et al. 2004; Asai, Yamaki et al. 2008). Of particular interest in the context of my studies, is the recent finding that mouse ebf2 is expressed in immature osteoblasts that constitute a major component of the HSC niche (Kieslinger, Folberth et al. 2005; Wilson and Trumpp 2006). This expression of ebf2 in cells controlling the hematopoiesis in bone marrow in the vertebrates raised the fascinating
Endosteal
niche
vascular
niche
A
B
C
D
sinusoidsEndosteal
niche
vascular
niche
A
B
C
D
sinusoidsFig. 15. HSC niches in vertebrates
(A-C) Schematic organisation of a long bone and (C-D) localisation of stem cell niches. The endosteal niche, of which the main components are the osteoblasts is located in the inner surface of the bone at the interface between the bone and bone marrow. The vascular niche is made by endothelial cells of blood vessels – sinusoids. (D) Both niches express regulatory components that influence stem cell functions (after Shiozawa et al..2008, Kiel and Morrison 2008).