A remarkable change of the spectrum of the magnetic Of?p star HD 148937 reveals evidence of an eccentric, high-mass binary
Texte intégral
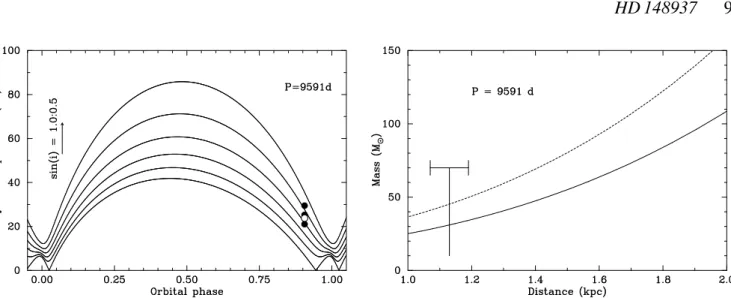
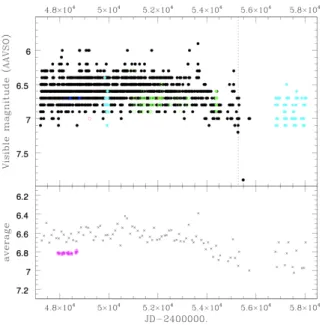
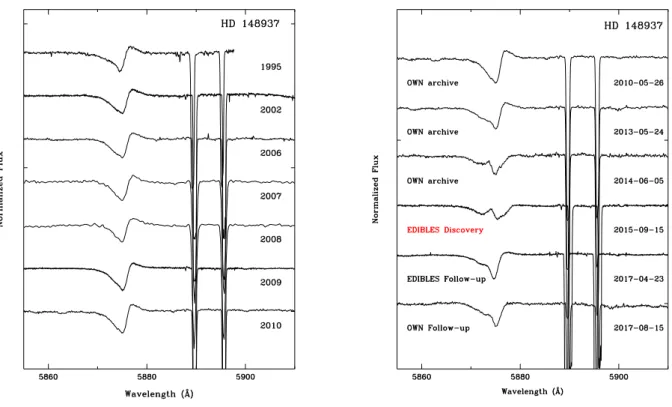
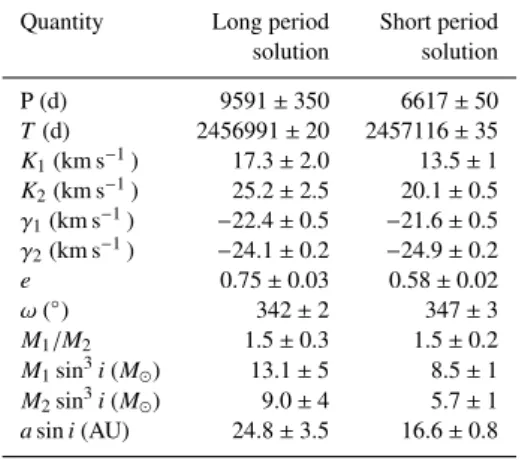
Figure


Documents relatifs
Testing commenced with the base case design and continued in pursuit of an optimized design. Throughout the testing program, the effects of several key variables
The goal of this course is to present a coherent variety of acces- sible examples of modern mathematics where intelligent com- puting plays a significant role and in doing so
In its turn, sample clean-up involves two steps: fat digestion (by acid silica reaction or by size exclusion) and fractionation between Dioxins and PCBs (using Alumina or
The ratio (percentage) of cells expressing the O4 oligodendroglial marker to the total cell number evaluated by Hoechst-stained nuclei (blue) showed a significant deficiency ( *** , P
ةحارب ذيملات ا رعشت ةيعامج ا باع لأا نأ ىلع نوقفتم ةيبلغلأا نأ دج بس ا ذه للاخ نمو ةيدرف ا باع لأاب ةراقم رب أ... هارملا
The algorithm produces an interannual variability that is similar to the variability observed in burned area statistics for some regions (e.g.. Schultz Title Page Abstract
Dans le domaine des biomarqueurs, les puces à protéines ne sont pas uniquement utilisées pour la découverte et la validation de biomarqueurs, elles peuvent
If the active substance contained in the medicinal product is not listed on the EURD list, the MAH should continue to submit PSUR according to the condition in the MA if any,