O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 9551
To link to this article : doi:10.1016/j.indcrop.2011.11.034
URL : http://dx.doi.org/10.1016/j.indcrop.2011.11.034
To cite this version : Chabrat, Elodie and Abdillahi, Houssein and
Rouilly, Antoine and Rigal, Luc Influence of citric acid and water on
thermoplastic wheat flour/poly(lactic acid) blends. I: Thermal,
mechanical and morphological properties. (2012) Industrial Crops and
Products, vol. 37 (n° 1). pp. 238-246. ISSN 0926-6690
Any correspondance concerning this service should be sent to the repository
administrator: staff-oatao@listes-diff.inp-toulouse.fr
Influence
of
citric
acid
and
water
on
thermoplastic
wheat
flour/poly(lactic
acid)
blends.
I:
Thermal,
mechanical
and
morphological
properties
E.
Chabrat
a,b,∗,
H.
Abdillahi
a,b,
A.
Rouilly
a,b,
L.
Rigal
a,baUniversitédeToulouse,INPT,LCA(LaboratoiredeChimieAgro-industrielle),ENSIACET,4AlléeEmileMonso,BP44362,31030ToulouseCedex4,France bINRA,LCA(LaboratoiredeChimieAgro-industrielle),France
Keywords: Citricacid Starch Flour Poly(lacticacid) Extrusion
Wheatflourwasplasticizedwithglycerolandcompoundedwithpoly(lacticacid)inaone-step twin-screwextrusionprocessinthepresenceofcitricacidwithorwithoutextrawater.Theinfluenceof theseadditivesonprocessparametersandthermal,mechanicalandmorphologicalpropertiesofinjected samplesfromthepreparedblends,wasthenstudied.
CitricacidactedasacompatibilizerbypromotingdepolymerizationofbothstarchandPLA.Foran extrusionwithoutextrawater,theamountofcitricacid(2partsfor75partsofflour,25partsofPLA and15partsofglycerol)hastobelimitedtoavoidmechanicalpropertiesdegradation.Water,added duringtheextrusion,improvedthewholeprocess,minimizingPLAdepolymerization,favoringstarch plasticizationbycitricacidandthusimprovingphasesrepartition.
1. Introduction
Wheatflourismainlyconstitutedofstarchandproteins(Saiah, 2007). Starch, which is a natural renewable polysaccharide, is consideredtobeapromisingrawmaterialfortheproductionof bioplastics.However,itneedsintensetransformationtodisruptits nativestructureandtobecomethermoplastic.Itisalsopossibleto plasticizeflourtomakethermoplasticflour.Inlowmoisture con-ditionsusedforthermoplasticprocessing,theinfluenceofwheat proteinshasbeenshowntobeminor,theproteicphaseisdispersed inthestarchmatrix(Chanvrieretal.,2007)andnosignificant differ-enceswerenoticedintermsofmechanicalandthermalproperties betweenflourandstarch(Leblancetal.,2008).Thismaterialshows verysimilarpropertiesandthesamelimitsasthermoplasticstarch. Thermoplasticstarch(TPS)isindeedaveryinterestingproductfor
Abbreviations: DMTA,dynamicmechanicalthermalanalysis;DSC, differen-tialscanningcalorimetry;FTIR,Fourier Transform infraredspectroscopy;PLA, poly(lacticacid);SEM,ScanningElectronicMicroscopy;TPS,thermoplasticstarch; UTS,ultimatetensilestrength.
∗ Correspondingauthorat:Agromat–sitedel’ENIT,47Avenue d’Azereix– BP1629,65016TarbesCedex,France.Tel.:+330562446084;
fax:+330562446082.
E-mailaddresses:elodie.chabrat@ensiacet.fr(E.Chabrat),
houssein.abdillahi@ensiacet.fr(H.Abdillahi),antoine.rouilly@ensiacet.fr
(A.Rouilly),luc.rigal@ensiacet.fr(L.Rigal).
makingnon-durableobjects.However,its watersensitivity and limitedmechanicalpropertiesmakeitonlyusefulforspecific appli-cations(Forsselletal.,1999).Citricacidcouldbeagoodcandidateto increasetherangeofreachableproperties.Toourknowledge,there isnostudyconcerningtheadditionofcitricacidinthermoplastic wheatflour.Nevertheless,ithasbeenaddedtothermoplasticstarch indifferentstudies.
Severalauthors(Shietal.,2007;Wangetal.,2007a,2009)have reportedthatcitricacidcouldformester bondwithstarch.The esterificationcouldtakeplacebetweenthecarboxylgroupson cit-ricacidand thehydroxyl groups onstarch.Nevertheless when glycerolwaspresent,itreactedpreferentiallywiththehydroxyl groups of the glycerol (Wang et al., 2007a). The formation of glycerolcitrateestershasbeenstudiedbyDifferentialScanning Calorimetry(DSC) and Fourier Transform InfraredSpectroscopy (FTIR)(Holser,2008).Evenifnoesterbondwasformedbetween citricacidandstarch,ithasbeenreportedthatcitricacidcould formstronghydrogenbondinteractionswithstarch,strongerthan glycerol.Thethermalandwatersensitivityofthermoplasticstarch wasthenimprovedandretrogradationwasinhibited(Holser,2008; Shietal.,2007;Yuetal.,2005).Crosslinkingofstarchfilmshas beenperformedusingcitricacidandacatalyst(sodium hypophos-phite)(ReddyandYang,2010),citricacidbearingthreecarboxyl groups.Freecitricacid,whichwasnotinvolvedinany crosslink-inginteractioncouldactasaplasticizer(Shiet al.,2008).Citric acidhasalsobeen addedto thermoplasticstarch tomodifyits
physicalperformancebycontrolleddegradationofstarchthrough anacid-catalyzedhydrolysisoftheetherlinkagesinthe polysac-charidechains(Carvalhoetal.,2005;Hirashimaetal.,2005;Wu etal.,2010).Theviscosityofstarchwasthenreducedwithout rele-vantchangesinwateraffinityorindynamicmechanicalproperties (DaRozetal.,2011).
Citricacidhasbeenreportedtoincreasetheplasticizationand meltprocessingpropertiesofTPS(Ronasietal.,2010;Shietal., 2007).It mightaccelerate thefragmentationand dissolution of starch granules. It could help starch plasticization, even when additivessuchasmontmorilloniteareaddedwhichnormally hin-dertheplasticization(Wangetal.,2009).ThefluidityofTPSwas alsoimprovedusingcitric acid.Thisdecreasewasexplainedby someauthorsbyanacidhydrolysisofstarch(KeandSun,2003). So,co-plasticizingstarchwithamixtureofglycerol/citricacidis interestingbecauseitincreasesstarchplasticization,partial ester-ificationcanhappen,andchainswithlowermolecularweightare obtained.Inwheatflour,additionofcitricacidcanleadtoadecrease inthecross-linkingdegreeoftheproteinnetworkduring extru-sion.Ithasbeenstated,accordingtoastudyofthermo-mechanical behaviorofwheatglutenmaterials(Gomez-Martinezetal.,2009), thatanacidicenvironmentmightpreventaggregationofgluten protein.
Blendingpoly(lacticacid)(PLA)withthermoplasticstarchhas becomewidespreadinthebioplasticscommunityinthelast10 years,inordertoreachdifferentpropertiesanddecreasetheprice ofbioplastics.ButPLAandTPSareknowntobenon-miscible,and non-compatibilizedblendsexhibit weakproperties (Martin and Avérous,2001).
TheinfluenceofcitricacidonTPS/PLA blendshasthusbeen reportedindifferentstudies(KeandSun,2003;Wangetal.,2007b, 2010).Ithasbeenestablishedthatevenwithoutwater, plasticiza-tionofstarchwithglycerolwaspossiblewhencitricacid(0–4%) waspresent.Whenwaterwaspresent,theblendwasmore homo-geneousbutthermalstabilitywasdecreased.Inbothcases,abetter interactionbetweenPLA and TPS hasbeen found(Wanget al., 2010).
Citricacid hasalso been used as an additive tostarch-PVA (poly(vinylalcohol))films(Parketal.,2005).Itwasreportedthat addingcitricaciddecreasedthestrengthofthefilmsbutprovided betterstrengththanglycerol-addedfilms.Thiswasattributedto thebetterhydrogenbondingbetweencitricacidandstarch-PVA moleculesthanwithglycerol.Citricacidiscomposedofcarboxyl and hydroxyl groups which increase the different interactions betweenthecomponentsoftheblend(Yoonetal.,2006;Yunetal., 2006).Whenaddedtothermoplasticstarch/LDPEblends,citricacid improvedstarchplasticizationandincreasedthemechanical prop-ertiesoftheblend(Wangetal.,2007b).
CitrateestersareknowntoplasticizePLA(KeandSun,2003; Labrecqueetal.,1997).Moreover,citricacidisinexpensive, non-toxicandconsiderednutritionallyharmlessasapprovedbyFDA. Indeed,it isa metabolicproductofthebody(Krebs cycle). Cit-ricacid,whenconcentrationislessthan20%,showsnosignificant toxicityeffectonthecellproliferation(Shietal.,2008).
Inordertoimprovethermoplasticwheatflourproperties,and sotoreachnewapplicationfields,itwaschosentobeblendedwith asmallamountofpoly(lacticacid).Threeadditiveswereusedfor thecompoundinginanindustrial-sizetwin-screwextruder:water, glycerolandcitricacid.Wateristhemostknownstarchplasticizer. Glycerol is commonly used as a plasticizer to obtain thermo-plastic starch. Citricacid, which is a natural occurring organic acid,waschosentoimprovestarchplasticizationandthephases miscibility.Theobtainedcompoundshavethen beenmoldedin dumbbell-shapespecimenstoassesstheirmechanical,thermaland morphologicalproperties.Theinfluenceofwaterandcitricacid,in awiderange,onmaterialpropertieshasbeenstudied.
2. Experimental 2.1. Materials
WheatflourwassuppliedbyGersFarine(France).Itismainly madeupofstarch(65%).Secondarycomponentswerewater(13%), proteins(13%), fibers,essentiallyhemicelluloses (2%)and lipids (1%).Poly(lacticacid)isanextrusiongrade.Glycerol(purity99%) wasusedasstarchplasticizerandwassuppliedbyGachesChimie (France).CitricacidwasobtainedfromSigma–Aldrich(France).
2.2. Thermoplasticstarch/PLAblendsextrusion
Inthisstudy,tendifferentblendswereextrudedinan industrial-size twin-screw extruder (EvolumHT53, Clextral(France) with L/D=36)atatemperatureof60–140◦Candscrewrotationalspeed
of250rpm.Thescrewprofilewasdividedintotwomainzones, aplasticizationzonecomposedofkneadingelementsandreverse screwelementsinthefirsthalfofthebarrelandamixingzone composedofkneadingelementsinthesecondhalfofthebarrel. Poly(lacticacid)wasintroducedat thebeginning ofthesecond zone,i.e.afterstarchplasticization.Acylindricaldiewith6holes wasfixedattheendoftheextruder.Thecompositionsoftheblends canbeunderstoodfromtheirnames.First,wheatflour(extrudedat itsequilibriumhumidity(13%)),poly(lacticacid)andglycerolratio werekeptconstantat75parts,25partsand15partsrespectively. Theonlyvariableswerewaterandcitricacidratios.Thegeneric namefortheformulationsisCAx.WhenaWispresentbeforethis name,itmeansthat10partsof extrawater waspresentin the formulationduringtheextrusion. Thiswaterwasaddedtohelp processingandtostudythebehaviorbetweenwaterandcitricacid. xindicatestheconcentrationofcitricacid,withvaluesbetween0 and20parts.TheformulationWCA5containedthus10partsof waterand5partsofcitricacid.
2.3. Injection-molding
Afterstabilizationat60%RHand25◦C,theplasticspelletswere
injection-moldedintodumbbell-shapedspecimensusingaNegri BossiVE160-720injectionmoldingmachine(Italy).Molding tem-peratureprofilewasdefinedasfollowing:80/125/130/145◦Cand
backpressurewas10bar.Theholdingpressurewas300bar dur-ing 1s. For experimental reasons, WCA2 was not injected; no results from injection-molded specimens are available for this formulation.
2.4. Tensiletesting
ATiniusOlsenH5kT(UK)universaltestingmachinewas oper-atedatacrossheadspeedof5mm/minandusedfortensiletesting. Young’smodulus,ultimatetensilestrength,elongationatbreakand toughnesswererecordedforeachspecimen.Toughnessrepresents theenergybyvolumeunitnecessarytobreakthedumbbell speci-men.Itwascalculatedfromtheareaundertheforce–displacement curve.Injectiondumbbellspecimenswereconditionedat60%RH and25◦Cfor3weekspriortesting.Eachmechanicalparameterwas
averagedfrom5to10specimens.
2.5. Dynamicmechanicalandthermalanalysis
Dynamicmechanicalandthermalanalyses(DMTA)ofthe sam-pleswereperformedwithaTritec2000DMA(TritonTechnology, UK)inamulti-frequencymodeoverthetemperaturerange−80 to 180◦C witha scanning rate of 2◦C/min.The amplitude and
frequencieswerekeptconstantat25mmand1and10Hz, respec-tively.Thegeometryusedwasthesinglecantileverbendingmode with one extremity of the bar fixed,while theother one was
bendedwiththeamplitudeandfrequenciesindicatedabove.On glycerol-plasticized starch-based materials,two relaxations are usuallyobservedattributedtoplasticizerrich phase(T1around −50◦C)andtostarchrichphase(T2around40◦C)(Lourdinetal.,
1997).Inthiswork,thefirstrelaxationwasnotobservedinthe analyticalconditionsusedandthediscussionisthenfocusedon thesecondone,appearingjustbeforetheglasstransitionofPLA.
2.6. ScanningElectronMicroscopy(SEM)
Thesectionsofthedumbbellspecimenswereexaminedusinga JEOLJSM-700F(Tokyo,Japan)scanningelectronmicroscope,with a5kVacceleratingvoltage.Thedumbbell-shapespecimenswere cooledin liquid nitrogenand thenbroken. The PLA phase was etchedbystirringthesampleinchloroform(99.5%purity)atroom temperature.Thesampleswerethenvacuum-coatedwith palla-diumforobservationtoavoidchargingundertheelectronbeam. ThesizeoftheholeswasdirectlymeasuredwiththeJeolsoftware. Averagevaluewascalculatedfrom10measurements.
2.7. Molecularweightdistribution
ADionex(VoisinsleBretonneux,France)SizeExclusion Chro-matography (SEC) equipped with a Iota2 refractive index (RI) detectorwasusedtomeasurethemolecularweightdistribution ofthesamples.
Bothphases werestudiedseparatelyand theextractionwas processedinaSoxhletapparatus.PLAphasewasextractedwith chloroformduring8handthensolventwasremovedby evapora-tion.ExtractedmasswascomparedtotheoreticalPLAmassinthe sampletogiveanextractedpercentageofPLA.
Toanalyzethestarchyphase,twoPLgel5mmmixed-Dcolumns, andaPLgelprecolumnwerepurchasedfromPolymer Laborato-ries(ChurchStretton,UK).Thecolumnswereconnectedinseries alongwiththeprecolumn.Thetemperatureofthecolumnswas setto 80◦C. DMSO was used as thesolvent for dissolving the
starchysample,andastheeluent.SampleconcentrationinDMSO wasapproximately5mg/mL.Sampleswerepreparedatambient temperatureandstoredinanovenat60◦Cforafewdaysforthe
dis-solution.Theliquidpartwasthenremovedwithasyringe,leaving theinsolublepartinthevial.Nomolecularweightwascalculated fromthisexperimentbecauseofthenon-linearstructureof amy-lopectinandofthelackoftotaldissolution.Somemoleculeswhich werenotsolubleinDMSO,werenotanalyzedinthesamples. Elu-tiontimesweredirectlyanalyzed,correspondingtohydrodynamic volumes.
ToanalyzethePLAphase,threePLgelcolumnswereassociated inseriesof103,500and100 ˚Aalongwithaprecolumn.The
temper-atureofthecolumnswassetto30◦C.Chloroformwasusedbothas
thesolventfordissolvingthePLAsampleandastheeluent.Sample concentrationinchloroformwasapproximately5mg/mL.Samples
werepreparedfromwhathasbeenextractedwithchloroformin Soxhletapparatus.PSstandardswereusedforthecalibration. 2.8. FTIRspectroscopy
FTIRspectrawerecollectedfromthepartoftheblendwhich cannotbedissolvedinchloroform.KBrdiskswerepreparedby mix-ingapproximately300mgofsampleinKBr.64scanswerecollected withaSpectrum65apparatus(PerkinElmer,USA)foreachsample between4000and400cm−1.
3. Results
3.1. Processparameters
Theextrusionofthedifferentblendsisreallydependentonthe citricacidratioandonthepresenceofextrawater.Different pro-cessparameterswerefollowed(Fig.1)throughouttheextrusion tounderstandtheinfluenceofcitricacid.Theseparameterswere theSpecificMechanicalEnergy(SME)neededfortheextrusion,the mattertemperatureintheplasticizationzone(reversescrew ele-ments)(plasticizationtemperature)whichwasfollowedtodetect aneventualwarm-upofthematterandthepressureinthedie.This lastparametergivesinformationaboutmoltenblendviscosity.
Globally,theSMEdecreasedsignificantlywithincreasingcitric acidconcentration(Fig.1a).However,forformulationsextruded withextrawater,SMEwasmaximumfor2partsofcitricacidand thendecreasedforhighercitricacidratios.Fortheformulations withoutextrawater,alocalmaximumofSMEwasobservedfor for-mulationswith10partsofcitricacid.Aspecificchangeoccurredat theseconcentrations,alsovisibleonthediepressurecurve(Fig.1c) whichcouldbeattributedtoaviscositychangeoftheblend.
Diepressure(Fig.1c)washigherfortheformulationextruded withoutwater.Itdecreasedfrom36barto13barwhenadding10 partsofcitricacidintheblendwithoutwater.Itdecreasedfrom 18barto9barwhen10partsofcitricacidwereaddedintheblend withextrawater.
Thebarreltemperature oftheplasticizationzone wassetto 100◦C.WhenlookingatFig.1b,thematterwasself-warmed-upof
10◦Cfortheformulationwithwater,andofmorethan20◦Cforthe
formulationwithoutextrawater.Thisconfirmstheideathatwater helpsfortheprocess,promotingstarchgelatinization.Addingcitric acidlimitsthisself-warmingup.Forformulationswith10partsof citricacid,thetemperatureintheplasticizationzonewas105◦C.
So,globally,asthecitricacidconcentrationincreased,the extru-sionparameters (SME,plasticizationtemperature,diepressure) decreased.
3.2. Materialsproperties
MechanicalpropertiesarepresentedinTable1.Forcomparison, mechanicalpropertiesofPLAandF75G25(75%ofwheatflour(at
Table1
Mechanical,DMTAandextractabilitypropertiesofthedifferentblendscomparedtoPLAandF75G25.
Blends Young’smodulus(MPa) Ultimatetensilestrength(MPa) Elongationatbreak(%) Tandeltashoulder Tandeltapeak %ExtractedPLA
CA0 709(33) 9.6(0.3) 7(1) 45 65 111 CA2 189(26) 3.1(0.2) 34(6) 33 66 84 CA5 46(9) 1.0(0.1) 69(17) 32 65 88 CA10 29(5) 0.4(0.1) 109(20) 33 66 63 CA20 3(1) 0.2(0.1) 57(11) 32 67 53 WCA0 715(20) 10.0(0.2) 6(1) 43 65 101 WCA2 n.d. n.d. n.d. n.d. n.d. 112 WCA5 66(18) 1.4(0.1) 41(8) 22 66 60 WCA10 69(18) 1.6(0.2) 58(4) 25 67 61 WCA20 24(7) 1.3(0.1) 64(12) 20 67 46 PLA 1806(74) 52.4(10.3) 4(1) n.d. n.d. 95 F75G25 15(3) 1.8(0.1) 58(10) n.d. n.d. n.d.
Fig.1.Processparametersreadforthedifferentblendswithincreasingcitricacidconcentration,with( )orwithout(N)extrawater.
itsequilibriumhumidity,i.e.13%)and25%ofglycerol)arealso pre-sented.Plasticizedflourcompositionisnotthesameastheoneof theplasticizedflourofthestudy(75/25forF75G25and83.3/16.7 forthestudy).Becausemechanicalpropertiesaredirectly depen-dentonplasticizerratio,comparison cannotbedone.However, valuesofF75G25aregiventopresentanorderofmagnitudeofthe mechanicalpropertiesofaplasticizedflour.Plasticizedwheatflour (F75G25)isaductilematerialwithaYoung’sModulusof15MPa andanelongationatbreakof58%,whereasPLAisbrittlewitha Young’sModulusof1806MPaandanelongationatbreakof4%. Adding20partsofPLAtoplasticizedwheatflour(83.3%ofwheat flour(atitsequilibriumhumidity)and16.7%ofglycerol)increased itsYoung’sModulusupto709MPaanddecreaseditselongationat breakto7%.Thetwoblendswithoutcitricacid(withorwithout water)presentthesamemechanicalproperties:Young’sModulus reached700MPa,ultimatetensilestrengthwascloseto10MPabut elongationatbreakwas6–7%.Thislowvaluecanbeexplainedby thelowglycerolratio(15partsoftheblend).
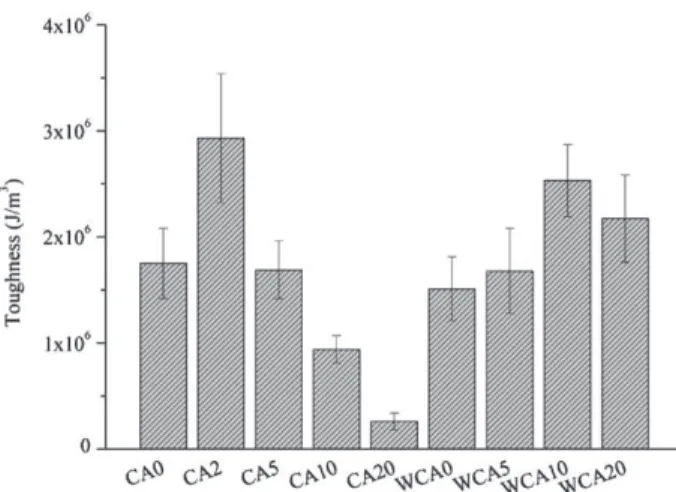
Assoonassomecitricacidwasadded(2parts),Young’s Modu-lusandultimatetensilestrength(UTS)decreaseddrastically.Their valueswereverylowassoonascitricacidratioreached5parts (Young’sModulusof46and66MPaandUTSof0.97and1.43MPa). However,thisdecreasewhenaddingcitricacidwasaccompanied byanimportantincreaseoftheelongationatbreak.With5parts ofcitricacid,theelongationatbreakwascloseto50%anditisthe samefor20partsofcitricacid.Elongationatbreakreachedeven valuescloseto100%when10partsofcitricacidwereaddedtothe blendextrudedwithoutextrawater.Therecouldbeacompetition betweenglycerolandcitricacidfortheplasticization.Toughness decreased a lotwhen adding citricacidin theblendsextruded
withoutwaterbutwasmorestablefortheblendswhichcontained extrawaterfortheextrusion(Fig.2).Thisisaninteresting obser-vation.Itisthenpossibletothinkthatthereisabetterdispersion intheblendswithwater.
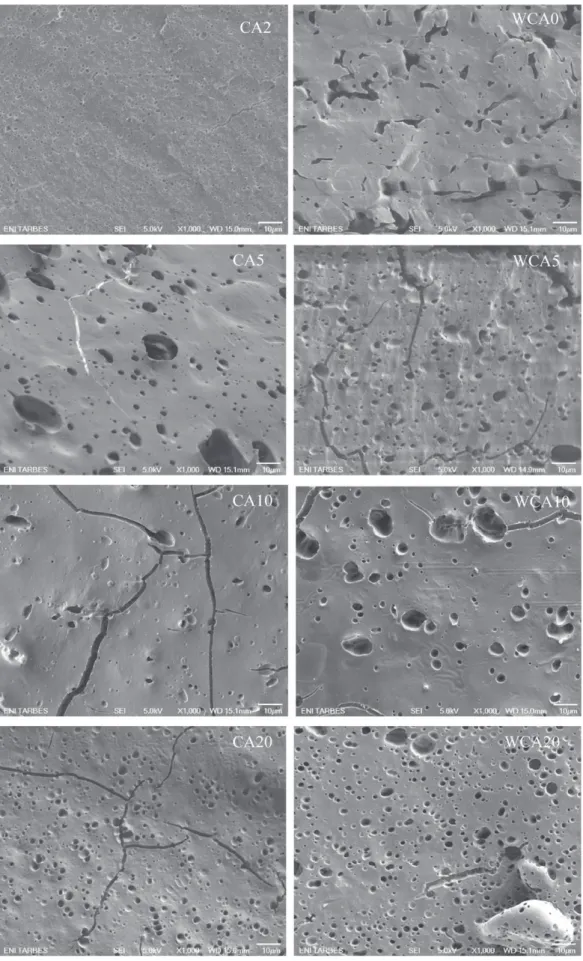
SEMimagesarepresentedinFig.3tobetterunderstandthe phases repartition (thermoplastic wheat flour and PLA) of the differentblends.PLAwhichwasetchedwithchloroformis repre-sentedbydarkholesontheimages.Theseobservationswillenable ustomakeacorrelationbetweenstructureandproperties.TheSEM imageofCA0(notshown)wassimilartothatofWCA0andwasnot assmoothastheonesoftheblendsinwhichcitricacidwasadded.
Fig.3.SEMimagesofthefracturedsectionafterPLAetchingforthedifferentblends(magnification1000×,left:blendswithoutwaterandright:blendsextrudedwithextra water).
PLAholeswerenotwell-definedholesbutmorelikecanals.When 2%ofcitricacidwasadded,thePLA repartitionwascompletely different.CA2presentsamultitude ofreallysmallholes witha diametercomprisedbetween1and5mm.Then,whenincreasing citricacidratioto5parts,therepartitionwasnotthesame.PLA holeswerebigger(6–30mm)andnotsowell-distributed.Thesize oftheholesdecreasedwhenthecitricacidratiowasincreasedto 10partsandto20parts.Alotofcracksbetweentheholeswere observablefortheblendwith20partsofcitricacid.
LookingattheSEMimages,therewasnotmuchstructural dif-ferencebetweentheblendsextrudedwithorwithoutextrawater. Fortheblendsextrudedwithwater(Fig.3),thestructurechange wasnearly thesameasthatpreviouslyobservedfor theblends withoutextrawater.Canalsweretransformedintoholeswith cit-ricacidandthesizeoftheholesdecreasedwhencitricacidamount wasincreased.Thisobservationisshiftedcomparedtotheblends extrudedwithoutextrawater.Fortheblendsextrudedwithextra water,PLAdomainswerestillsmallforWCA5andwerebiggerfor WCA10thensmallerforWCA20.Thedistributionoftheholesis finerfortheblendsextrudedwithextrawater(diameterofholesfor WCAx:2–6mm,whereasforCAx:3–22mm).SEMimageforWCA2 isnotavailable;duetoalackofpellets,WCA2wasnot injection-molded.
FTIR analyses of the non-soluble part in chloroform of the differentblends revealedthesame spectraas starch.The main absorptionbandswereobservedat3400–3450cm−1 (hydroxyls
groups),2880–2900cm−1 (C Hstretching)and1150–1085cm−1
(etherband)(DaRozetal.,2011).Oneabsorptionbandwas dif-ferentformstarch,theareaofwhichisincreasingwithcitricacid concentration:theabsorptionbandat1759cm−1.Itis
characteris-ticofthestretchingofthecarbonylbonds.Thepresenceofcarbonyl bondscanhave differentcauses:residualcitricacid, poly(lactic acid)presence,starchorglycerolesterification.
PLAwaseffectivelymoreandmorepresentinthepartofthe blendwhichhasnotbeendissolvedinchloroformwhencitricacid concentrationisincreased(Table1).Extractionratewascalculated fromtheoreticalPLAconcentrationresultingfromcompounding andisthennotveryaccurateresultinginvalueshigherthan100%. FortheformulationWCA10forexample,only61%ofthePLAwas extracted.The39%leftwerenotcarriedawaybychloroform. Inter-actionscouldthusformbetweenPLAand wheatflour.Therate decreasedwiththeincreaseofcitricacidratioandthisdecrease wasevenmoremarkedfortheblendsextrudedwithextrawater, fallingtoonly46%forWCA20.
Thereisapriorinofreeresidualcitricacidintheanalyzedpart becauseimportantvibrationbandsofcitricacidweremissing,for exampleat2635cm−1and2555cm−1.Citricacidstillintheblends
probablyparticipatesinlowenergyinteractionswiththe biopoly-mers.Fortheesterification,wedonothaveenoughcluetoargue aroundapossibleesterificationatthisstage,astheoneobserved byShietal.(2007)forexample.
4. Discussion
4.1. Citricacidinfluence
Citricacidcanbeseenasacompatibilizer(esterification, hydro-genbonding),asa starch plasticizerand asa depolymerization agentforstarchandPLAinthermoplasticwheatflour/PLAblends. Itcouldalsoplayaroleinglutendisaggregation.
Glutendisaggregationisassumed,becauseithasalreadybeen observedintheliterature(Gomez-Martinezetal.,2009)thatthe cross-linkingdegreeoftheproteinnetworkcanbedecreased dur-ingtheprocessing,byadding3%ofcitricacid.Thisphenomenon couldbeoneexplanationamongothersfortheviscositydecreasein
theextruder.However,sinceproteinsrepresentonly13%ofwheat flour,whichrepresentatmaximum65%oftheblenditself,proteins representatmaximum8%oftheblend.Asnospecificeffectofthese proteinscanbeseenoneitherthermalormechanicalanalyses,and asotherauthorshaveshowntheirminorparticipationinwheat flour-basedmaterialsproperties(Chanvrieretal.,2007;Leblanc etal.,2008),theirinfluencewillnotbespecificallydevelopedin thisstudy.Starchmolecularweightreduction,whichisprobably themainexplanationoftheviscositydecrease,isconsideredlater inthisdiscussion.
The compatibilization effect of citric acid is visible on the mechanicalproperties,especiallywhenlookingatthetoughness, whichrepresentstheenergyneededforthesampletobreak.When nowaterwasadded duringtheextrusion,citricacidseemedto beeffectiveonlyin smallamounts,whilewithwater,theeffect wasnoticedonthewholerangeofconcentration(Fig.2).Addinga smallamountofcitricacidchangedinaconsiderablewaythephase repartitionoftheblend,fromPLAcanalstoPLAholes(Fig.3).The sameeffectwasobservedbyWangetal.(2007a)onpureTPS/PLA blends.PLAwasalsolessextractablebychloroformforblends con-tainingcitricacid(Table1).Themorethecitricacid,thelessPLA wasextracted.Withhighratioofcitricacid,thenon-extractedPLA, whichissupposedtointeractmorewiththeothercomponentsof theblend,accountedfor40%andmore.Esterificationhasnotbeen provedbutalltheseobservationsatleastshowa betteraffinity betweenthephases.
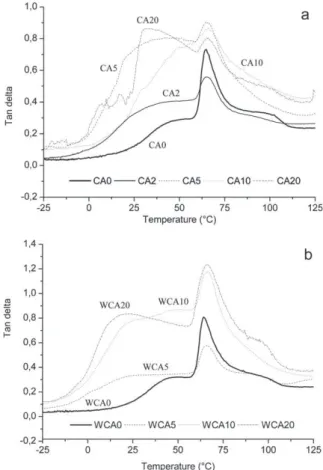
DMTAanalysesoninjectedsamplesclearlyshowedthe relax-ationpeakcorrespondingtoPLAglasstransitionaround65◦C.They
showedalsoanotherrelaxation shoulderbetween20 and45◦C
(Fig.4andTable1),whichhasbeenattributedtotheglass tran-sitionofthestarchbackboneorstarch-richphase(Lourdinetal., 1997).PLAglasstransitiontemperaturestayedconstantwhatever
Fig.4. Dampingfactortheblendsextrudedwithoutextrawater(a)andwithextra water(b).
theamountofcitricacidintheblendandthepresenceofwater duringtheextrusion. Citricaciddidnotplasticize PLAin these conditions.Moreover,citrateesters,theproposedproductofthe reactionbetweencitricacidandglycerol(Holser,2008),alsoknown asaPLAplasticizer(KeandSun,2003;Labrecqueetal.,1997),did notform.Also, thepossibleesterification ofglycerolwithcitric acidsasproposedbyShietal.(2007)toformcitrateestersdid notoccurinthisexperiment.However,dampingfactorintensity seemedtobeweaker forsmallamountsofcitricacid(CA2 and WCA5),meaningthatchainswouldbelessmobileandthat bet-terinteractionswerecreatedbetweenthechains.Onthecontrary, forhighercitricacidamount,mobility wouldbebetterbecause dampingfactorintensitywashigher.Theshouldercorresponding tostarch-backboneglasstransitiontemperature(T2)wasshiftedto lowervaluewhencitricacidwasaddedbutthevaluestayed con-stantwhateveritsconcentration(i.e.32–33◦CforCAxand20–25◦C
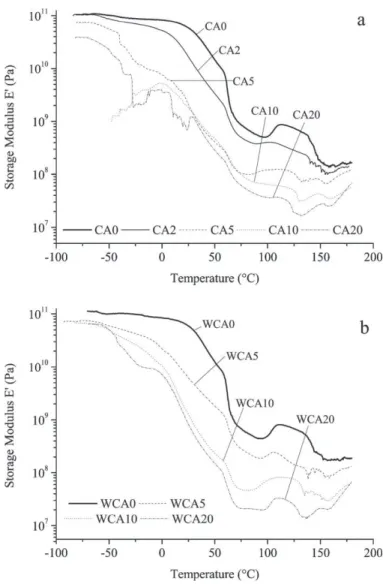
forWCAx).Thisshiftcouldbeduetotheplasticizingeffectofcitric acidoncarbohydrates,waterimprovingtheaccessinthepolymer networkthankstoaswellingeffectandloweringevenmorethe glasstransitiontemperature.Theexpectedbehaviorfor compat-ibilizedphases,involvingglasstransitionpeaksmovingtowards eachother,isnotobserved.Fig.5presentsthestoragemodulus oftheanalyzedblendsinalogarithmicscale.Blendswithout cit-ricacid(CA0andWCA0)showthehigherstoragemodulusforthe wholetemperaturerange.Storagemodulusiskeptatahighvalue
Fig.5. Storagemodulusfortheblendsextrudedwithoutextrawater(a)andwith extrawater(b).
Fig.6.Molecularmassdistributionofstarchforthedifferentblends.
forhighertemperaturecomparedtoblendswithcitricacid. How-ever,forsmallamountsofcitricacid(CA2andWCA5),theslope ofthedecreasingcurvebetween0and50◦Cissmallerthanthe
oneoftheblendswithoutcitricacid.Thisobservationconfirmsthe observationofthedampingfactorintensity;interactionsbetween chainsmaybemoreimportantwhenasmallamountofcitricacid isadded.Foralltheblends,storagemodulusincreasesalittlebit between100and110◦C.Thiscanbeduetowaterevaporationat
100◦Coracrystallizationevent.
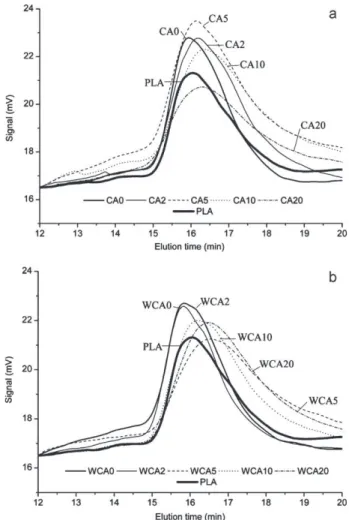
Anotherpossibleexplanationfortheshiftofthedamping shoul-der associated to the starch phase would be depolymerization (Wangetal.,2007a).Toconfirmthishypothesis,SECexperiments wereperformedseparatelyforthePLAphaseextractedby chloro-formandforthestarchyphasedissolvedinDMSO.BothPLAand starchshowedaloweringoftheirmolecularweightwhencitric acidwasaddedintheblends(Figs.6and7).
Fig.6 showsthemolecularmassdistributionofallthe com-ponentsoftheblendsolubleinDMSO.Amylose,amylopectinand proteins were the different polymers normally present in the analyzedsamples.Wheatflourwasanalyzedasareference.It pre-sented two mainpeaks that shouldcorrespondto amylopectin (11.28min)andamylose(14.20min)(Liuetal.,2010).Theintensity ofthepeaksdidnotcorrespondtotheamylopectin/amyloseratio of75/25forwheatstarch.Theamylopectinpeakwasverysmall.It iswell-knownthatthecharacterizationofamylopectinbySECis verydifficultbecauseofitslowsolubilityanditssize(Chenetal., 1997).Onlyasmallpartofamylopectinwasprobablydissolved, whichexplainsthesmallamylopectinpeak.Lookingatthetrace ofCA0(Fig.6a),extrudedformulationwithoutwaterandwithout citricacid,bothamylopectinandamylosepeakswerestillvisible.
Fig.7. MolecularmassdistributionofPLAforthedifferentblends.
Theywereneverthelessnotaswell-definedastheywerefornative wheatflour,andmorespreadovertime.Inspiteofthismolecular weightdispersion,apartoftheamylopectinwasstillpresent.As soonas2partsofcitricacidwereaddedtotheblend,molecular weightdecreased alot, andallthemoreas thecitricacid con-centrationwasincreased.Differentpeaksoflowersizethannative amylosewerevisible.
PLApeakwasshiftedtolongerelutiontime (smallerchains) (Fig.7)whencitricacidconcentrationincreasedforbothtypesof blends(withorwithoutwater).Thismolecularweightdecrease wasnotsufficienttoimpactthePLAglasstransitiontemperature (Table1).ItisinterestingtonotethatPLAmolecularweightwas keptwhen2partsofcitricacidareaddedtoablendextrudedwith extrawater(Table1).
Citricacidratioprobablyhadaneffectonthedepolymerization mechanism.Forlowcitricacidconcentration(i.e.<5parts),starch waspreferentiallyhydrolyzed,asalreadyseenonstarchglass tran-sitionshift(Fig.4),whereasforhigheramountsbothPLAandstarch molecularmassdistributionswerereduced.
4.2. Waterinfluence
Withoutcitricacid,bothblendsextrudedwithorwithoutextra watershowedthesameproperties(mechanical,thermaland mor-phological).Processingwasclearlyfacilitated(Fig.1);waterisa processaidwhichdidnotimpactonthematerialproperties.Starch depolymerizationmightbeweakerwhentheblendswereextruded withextrawater,asSECpeakcorrespondingtoamylosedidnot move(Fig.6).Unfortunately,weakamylopectinsolubilitydidnot allowacompleteinterpretationoftheSECtraces.
Withlowcitricacidamount,theuseofwaterdidnotinvolve specificPLAhydrolysis(Fig.7)assupposed.Onthecontrary,water mightbecompletelymobilizedwithinthestarchphase(swelling), facilitatingthecitricacidpenetrationandinducingaslightincrease of theSME during compounding. Citricacidpenetration in the starchyphasewasconfirmedbyalargerdecreaseofthedamping factorpeakforthestarchphase,whencomparedtoblendsextruded withoutextrawater(Table1).
With morecitric acid, water helpedto preservemechanical properties(Fig.2);materialelongationwashigherwithoutwater buttensilestrengthwaskeptatacceptablelevel.Thisshouldbe relatedtolesserextentofPLAdepolymerization(Fig.7).Moreover, thesizesofthePLAdomainsweresmallerforblendsextrudedwith water.
5. Conclusion
Wheat flour/PLAblendshave beensuccessfully produced on industrialequipments.Theuseofcitricacidpromotedthe com-patibility of the phases. Two different mechanisms have been enlightened dependingontheuseofextrawater ornotduring theextrusion.Withnoaddedwater,smallamountsofcitricacid tendedtoimprovematerialproperties,citricacidcausingstarch depolymerizationandimprovingthePLArepartitioninthematrix. Whenthecitricacidratiowasincreased,depolymerizationofPLA andstarchoccurredandelongationatbreakincreaseddrastically, buttensilestrengthdecreasedaswell,leadingtolesstough mate-rials. Adding smallquantity of water duringthe extrusionwas beneficialtothewholeprocess,facilitatingcompounding, improv-ingstarchplasticizationandloweringPLAdepolymerizationwhen smallamountsofcitricacidwereused.
Blendingthermoplasticwheat flour withPLAand citric acid led to highly deformable materials with an interesting tough-ness,thankstothePLA,makingthemaninterestingcompromise betweenbrittlePLAand very ductileglycerol-plasticized wheat flour.
Ourgroupiscurrentlyworkingonthedynamicvaporsorption andpermeabilitypropertiesoftheseblends.Theinfluenceofcitric acidonthesepropertieswillsurelybediscussedinanotherpaper.
Acknowledgements
WewouldliketothankNathalieAubezacandJoëlAlexis(LGP) fortheirhelpforSEMimagesandLaureCandyandJérôme Pey-decastaingfortheirhelpforSECanalyses.TheFrenchEnvironment andEnergyManagementAgency(ADEME)andthe“Conseilgeneral desHautes-Pyrénées”haveparticipatedtothisworkviafinancial support.
References
Carvalho,A.J.F.,Zambon,M.D.,Curvelo,A.A.S.,Gandini,A.,2005.Thermoplastic starchmodificationduringmeltprocessing:hydrolysiscatalyzedbycarboxylic acids.Carbohydr.Polym.62,387–390.
Chanvrier,H.,Uthayakumaran,S.,Lillford,P.,2007.Rheologicalpropertiesofwheat flourprocessedatlowlevelsofhydration:influenceofstarchandgluten.J.Cereal Sci.45,263–274.
Chen,Y.,Fringant,C.,Rinaudo,M.,1997.Molecularcharacterizationofstarchby SEC:dependenceoftheperformancesontheamylopectincontent.Carbohydr. Polym.33,73–78.
DaRoz,A.L.,Zambon,M.D.,Curvelo,A.A.S.,Carvalho,A.J.F.,2011.Thermoplastic starchmodifiedduringmeltprocessingwithorganicacids:theeffectofmolar massonthermalandmechanicalproperties.Ind.Crop.Prod.33,152–157. Forssell,P.M.,Hulleman,S.,Myllärinen,P.J.,Moates,G.K.,Parker,R.,1999.Ageingof
rubberythermoplasticbarleyandoatstarches.Carbohydr.Polym.39,43–51. Gomez-Martinez,D.,Partal,P.,Gallegos,C.,2009.Rheologicalbehaviourandphysical
propertiesofcontrolled-releasegluten-basedbioplastics.Bioresour.Technol. 100,1828–1832.
Hirashima,M.,Takahashi,R.,Nishinari,K.,2005.Effectsofaddingacidsbeforeand aftergelatinizationontheviscoelasticityofcornstarchpastes.Food Hydrocol-loids19,909–914.
Holser,R.A.,2008.Thermalanalysisofglycerolcitrate/starchblends.J.Appl.Polym. Sci.110,1498–1501.
Ke, T., Sun, S., 2003. Thermal and mechanical properties of poly(lactic acid)/starch/methylenediphenyldiisocyanateblendingwithtriethylcitrate.J. Appl.Polym.Sci.88,2947–2955.
Labrecque,L.V.,Kumar,R.A.,Davé,V.,Gross,R.A.,Mccarthy,S.P.,1997.Citrateesters asplasticizersforpoly(lacticacid).J.Appl.Polym.Sci.66,1507–1513. Leblanc,N.,Saiah,R.,Beucher,E.,Gattin,R.,Castandet,M.,Saiter,J.,2008.Structural
investigationandthermalstabilityofnewextrudedwheatflourbasedpolymeric materials.Carbohydr.Polym.73,548–557.
Liu,W.C.,Halley,P.J.,Gilbert,R.G.,2010.Mechanismofdegradationofstarch,ahighly branchedpolymer,duringextrusion.Macromolecules43,2855–2864. Lourdin,D.,Bizot,H.,Colonna,P.,1997.Antiplasticizationinstarch-glycerolfilms?
J.Appl.Polym.Sci.63,1047–1053.
Martin,O.,Avérous,L.,2001.Poly(lacticacid):plasticizationandpropertiesof biodegradablemultiphasesystems.Polymer42,6209–6219.
Park,H.R.,Chough,S.H.,Yun,Y.H.,Yoon,S.D.,2005.Propertiesofstarch/PVAblend filmscontainingcitricacidasadditive.J.Polym.Environ.13,375–382. Reddy,N.,Yang,Y.,2010.Citricacidcross-linkingofstarchfilms.FoodChem.118,
702–711.
Ronasi,S.,Hoppe,S.,Hu,G.H.,Six,J.L.,2010.Propertiesofpoly(lacticacid)and ther-moplasticstarchblends.In:ProceedingsofthePolymerProcessingSociety26th AnnualMeting.
Saiah,R.,2007.Propriétésphysiquesetcomportementdansletempsdes agro-matériauxvitreuxissusdelafarinedeblé.PhDThesis,RouenUniversity(France).
Shi,R.,Zhang,Z.,Liu,Q.,Han,Y.,Zhang,L.,Chen,D.,Tian,W.,2007. Characteriza-tionofcitricacid/glycerolco-plasticizedthermoplasticstarchpreparedbymelt blending.Carbohydr.Polym.69,748–755.
Shi,R.,Bi,J.,Zhang,Z.,Zhu,A.,Chen,D.,Zhou,X.,Zhang,L.,Tian,W.,2008.The effectofcitricacidonthestructuralpropertiesandcytotoxicityofthepolyvinyl alcohol/starchfilmswhenmoldingathightemperature.Carbohydr.Polym.74, 763–770.
Wang,N.,Yu,J.,Chang,P.R.,Ma,X.,2007a.Influenceofcitricacidonthe proper-tiesofglycerol-plasticizeddrystarch(DTPS)andDTPS/poly(lacticacid)blends. Starch/Stärke59,409–417.
Wang,N.,Yu,J.,Ma,X.,Wu,Y.,2007b.Theinfluenceofcitricacidonthe proper-tiesofthermoplasticstarch/linearlow-densitypolyethyleneblends.Carbohydr. Polym.67,446–453.
Wang,N.,Zhang,X.,Han,N.,Bai,S.,2009.Effectofcitricacidandprocessingonthe performanceofthermoplasticstarch/montmorillonitenanocomposites. Carbo-hydr.Polym.76,68–73.
Wang,N.,Zhang,X.,Han,N.,Fang,J.,2010.Effectsofwateronthepropertiesof ther-moplasticstarchpoly(lacticacid)blendcontainingcitricacid.J.Thermoplast. Compos.Mater.23,19–34.
Wu,W.S.,Tsai,Y.H.,Wei,C.I.,SunPan,B.,Huang,T.C.,2010.Effectsoforganicacids onthepastingpropertiesofriceflourfromwaxyandnonwaxyvarieties.J.Food Qual.33,137–154.
Yoon,S.D.,Chough,S.H.,Park,H.R.,2006.Propertiesofstarch-basedblendfilmsusing citricacidasadditive.II.J.Appl.Polym.Sci.100,2554–2560.
Yu,J.,Wang,N.,Ma,X.,2005.Theeffectsofcitricacidonthepropertiesof thermo-plasticstarchplasticizedbyglycerol.Starch/Stärke57,494–504.
Yun,Y.H.,Na,Y.H.,Yoon,S.D.,2006.Mechanicalpropertieswiththefunctionalgroup ofadditivesforstarch/PVAblendfilm.J.Polym.Environ.14,71–78.