To link to this article: DOI: 10.1016/j.msea.2011.06.044
URL :
ttp://dx.doi.org/10.1016/j.msea.2011.06.044
This is an author-deposited version published in:
http://oatao.univ-toulouse.fr/
Eprints ID: 5668
To cite this version:
Marder, Rachel and Chaim , Rachman and Chevallier, Geoffroy and
Estournès, Claude Densification and polymorphic transition of multiphase
Y2O3 nanoparticles during spark plasma sintering. (2011) Materials
Science and Engineering A, vol.528 (n° 24). pp. 7200-7206. ISSN
0921-5093
O
pen
A
rchive
T
oulouse
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers
and makes it freely available over the web where possible.
Any correspondence concerning this service should be sent to the repository
administrator:
staff-oatao@listes.diff.inp-toulouse.fr
Densification
and
polymorphic
transition
of
multiphase
Y
2
O
3
nanoparticles
during
spark
plasma
sintering
R.
Marder
a,
R.
Chaim
a,∗,
G.
Chevallier
b,
C.
Estournes
baDepartmentofMaterialsEngineering,Technion–IsraelInstituteofTechnology,Haifa32000Israel bCNRS,InstitutCarnotCirimat,F-31602ToulouseCedex9,France
Keywords:
Sparkplasmasintering Phasetransformation Densification Y2O3
a
b
s
t
r
a
c
t
Multiphase(MP)monoclinicandcubicY2O3nanoparticles,40nmindiameter,weredensifiedbyspark
plasmasinteringfor5–15minand100MPaat1000◦C,1100◦C,and1500◦C.Densificationstartedwith
pressureincreaseatroomtemperature.Densificationstagnatedduringheatingcomparedtothehigh shrinkagerateincubicsingle-phasereferencenanopowder.ThelimiteddensificationoftheMP nanopow-deroriginatedfromthevermicularstructure(skeleton)formedduringtheheating.Interfacecontrolled monoclinictocubicpolymorphictransformationabove980◦Cledtotheformationoflargespherical
cubicgrainswithinthevermicularmatrix.Thisresultedinthelossofthenanocrystallinecharacterand lowfinaldensity.
1. Introduction
Rapidsinteringanddensificationofceramicpowderstofull den-sityare nowadaysa routineprocedure, usingthespark plasma sintering (SPS) method.As wasnoted in many hot-press stud-iesincludingSPS,themaindensificationshrinkageofthepowder aggregatetakesplaceduringtheheatingbyparticlesliding,tothe close-packedarrangement[1–4].Furtherdensificationofthe pow-dercompactmaybeaccomplishedeitherbyplasticdeformation orbydiffusionalprocesses,attheparticlenecks.Nevertheless, dif-fusionalprocessesareinevitableduringthefinalstagesintering, whenisolatedporesformandcanbeeliminatedviabulkorgrain boundarydiffusion[5,6].Consequently,microstructureevolution duringtheSPSprocess,duetothechangeintheprocess param-eters,such astemperature,pressure,time,atmosphere, vacuum level, etc. mayaffectthe densificationmechanism. Many types ofphasetransformationsandtransitions associatedwithcrystal symmetrychangesinvolvechangesinthemicrostructureand mor-phology[7].Therefore,effectsofthephasetransformationsduring thedensificationbySPSmaybeofprimeimportancetothe densifi-cationprocess,aswellastothefinalphaseassemblageinthedense compact.Thephasecontentandassemblageoftenhavea consid-erableimpactonthefinalpropertiesofthesinteredceramic[8]. Inthisrespect,Takeuchietal.[9]investigatedthedensificationof
∗ Correspondingauthor.Tel.:+97248294589;fax:+97248295677. E-mailaddress:rchaim@technion.ac.il(R.Chaim).
submicrometersizetetragonalBaTiO3 powdersandfoundSPSto
beeffectiveforpreservationofthesubmicrometersizemetastable cubicBaTiO3atroomtemperature.Thepreservednanometricgrain
sizebySPSwasalsofoundtobeacauseforthecubicphase stabi-lizationatlowertemperatures[10,11].
Kumaretal.[12]tookadvantageofthehigheratomic mobil-ityneartheAnatasetoRutilephasetransformationtemperatureto enhancethedensificationoftheTiO2 nanoparticles.SPSof
mul-tiphaseTiO2 (70%Anatase and30% Rutile)with20-nmparticle
sizeat 62MPafor 5minand600◦C resultedincomplete phase
transformationtoRutile[13].Forcomparison,onlyannealingof the same precursor powder for 5min at 600◦C preserved the
multiphasecharacter ofthe powder.This exhibitstheeffect of theappliedpressureandpossiblytheelectricfieldonthephase transformationduringtheSPS.Fundamentalinvestigationofthe currenteffectonsolid-statereactivityduringSPSwasperformed onstackedMo–Si–Molayers[14].Nochangeinthereaction mecha-nismwasobserved,albeittheenhancedgrowthrateofthereaction productlayer(MoSi2),whichwasrelatedtotheenhancedmobility
orthechangeinthedefectconcentration.
The present paperfocuses on theeffect ofthe polymorphic phasetransitiononthemicrostructureanddensificationbehavior ofmultiphaseY2O3nanoparticlesduringtheSPS.
2. Experimental
Commercial pure (99%) multiphase (MP) Y2O3 nanopowder
(NeomatCo.,Riga,Latvia)withaverageparticlediameterof40nm
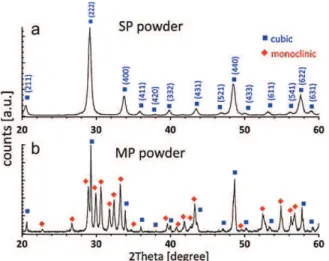
Fig.1.X-raydiffractionspectrafromtheY2O3nanoparticles.(a)Singlephase(SP)
cubic.(b)Multiphase(MP)cubic+monoclinic.
wasused.Asecondhighlypure(99.99%)nc-Y2O3powder(Cathay
AdvancedMaterials,China)with100%cubicphase,designatedas singlephase(SP),wasalsousedasareferencespecimen[15]. Con-stantamountofthepowdersamplewaspouredintothegraphite dieusinggraphitefoils(Grafoil)toseparatebetweenthepowder, thediewalls and theplungersurfaces.Thepowders were sin-tered(Dr.Sinter,SPS2080)atdifferentconditionsfor5–15minand 100MPaat1000◦C,1100◦C,and1500◦C.Thestartingtemperature
wasroomtemperatureforthe1000◦Ctreatment(designated‘cold
compaction’),but600◦Cforthe1100◦C and1500◦Ctreatments
(designated‘hotcompaction’).Theuniaxialpressurewasapplied eitherafewsecondaftertheprocessstartedorwhentheSPS tem-peraturewasreached.Inbothcases,thepressurewasincreased linearlywithtime,andheldconstantduringtheisothermal treat-mentattheSPStemperature.Theprocessdurationreferstothe isothermalSPStreatment.Aheatingrateof100◦C/minand
vac-uumlevelof3Pahasbeenused;thepulsedurationwas3.3ms.The SPSparameterswererecordedduringtheprocess.The tempera-turewascontrolledbyathermocouplefor‘coldcompaction’,while anopticalpyrometerwasusedabove600◦Cfor‘hotcompaction’
andathighertemperatures.Thefinalspecimendimensionswere 8mmindiameterand1.7–2.5mmthick.Theramdisplacements wereexpressedintermsofthelinearshrinkageandthetemporary relativedensity,followingthespecimenthicknessversustime, tak-ingintoaccountthethermalexpansion/shrinkagebehaviorofthe specimenandthegraphiteplungers[15].
Thephasecontentoftheas-receivednano-powdersandthe sin-teredspecimenswerecharacterizedbyX-raydiffractionusinga diffractometer(PhilipsPW3710)withmonochromaticCuKa
radi-ation (XRD),operatedat 40kVand 30mA.A scanningspeed of 0.5◦/minhasbeenused.Themicrostructureswerecharacterized
usingtransmission(TEM,FEITecnaiG2T20,operatedat200kV) andscanning(FEIE-SEMQuanta200,operatedat20kV)electron microscopes.Thespecimensfortheelectronmicroscopy observa-tionswereprepared bytheconventionalmethods.Thethermal stabilityofthenanopowderswascharacterizedusingdifferential scanningcalorimetry(Labsys1600,Setaram)upto1400◦CinArgon
atmosphere,ataheatingrateof5◦C/min.Thefinaldensityofthe
specimenswasdeterminedbytheArchimedesmethodfollowing ASTMstandardC20-92(±0.5%accuracy).
3. Results
X-raydiffractionspectrafromtheas-receivednanopowdersare showninFig.1.Thesinglephase(SP)Y2O3nanopowderwithcubic
symmetry(JCPDS41-1105)wascharacterizedindetailelsewhere [16](Fig.1a),whereasthemultiphase(MP)nanopowderrevealed polymorphswithcubicandmonoclinic(JCPDS39-1063) symme-tries(Fig.1b).Quantitative analysis ofthe spectrum inFig. 1b, assumingapowdermixture[17],resultedin30%cubicand70% monoclinicphaseinthenanopowder.Theseresultswerein agree-mentwiththe25:75cubictomonoclinicphaseratiodetermined byothers[18].
TEMobservationofthetwopowders(notshownhere)exhibited sphericalmorphologyforthemultiphasepowder,comparedtothe equiaxedpolyhedralshapeforthecubic,singlephasepowder[16]. Bothpowdersexhibitedlog-normalgrainsizedistributions,with averagegrainsizeof18±8nmand41±22nmforthesinglephase andthemultiphasepowders,respectively.
XRDspectrafromtheMPnanopowdercompacts,subjectedto SPSfor5minat100MPaandatdifferenttemperatures(Fig.2a), showedthe transformation of themetastable monoclinic poly-morphtothestable cubic phase tooccurat1100◦C. However,
further XRD characterization of the MP nanopowders sintered fordifferentdurationsat1000◦Cand100MPa(Fig.2b)revealed
the continuous nature of the phase transformation kinetics alreadyatthistemperature.Themonoclinictocubicpolymorphic phasetransformationwasnotcompleted,evenafter15minSPS duration.
Following the above phase transformation in the sintered specimens,thethermalstabilityofbothnanopowderswere char-acterizedbyDSC(Fig.3).Atthestagewherethefiner,cubicSP nanopowderwasrelativelystable,thecorrespondingcurvefrom
Fig.2.XRDspectraoftheas-receivedMPY2O3nanoparticles,aftersintering(a)for
Fig.3.DifferentialscanningcalorimetryfromnanocrystallineY2O3.(a)Singlephase
(SP)cubic.(b)Multiphase(MP)cubic+monoclinic.
theMPnanopowderexhibitedastrongexothermicpeakstarting at∼980◦C,withthemaximumaround1200◦C.Thisisin
agree-mentwiththeexpectedmonoclinictocubicphasetransformation reportedat∼950◦C[19],andinagreementwiththeXRDresults.
Therefore,specialattentionwaspaidtothisphasetransformation duringthedensificationbySPS.
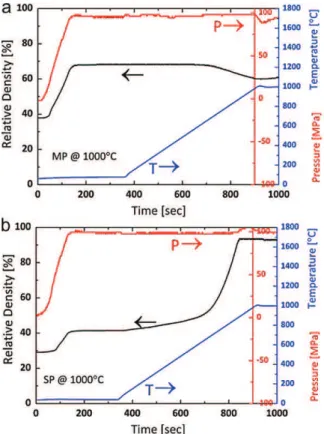
Followingthedensificationbehaviorofthetwonano-powders, severalimportantfeatureswereobserved.First,averyrapid com-pactionoftheMPpowderwasdirectlyassociatedwiththepressure whenappliedatroomtemperature(Fig.4a);thisroom tempera-turecompactionwiththepressureincreasepersistedforallSPS temperaturesinvestigated.However,furtherdensificationat ele-vatedtemperaturesdependedonthefinalSPStemperature.Inthe 1000◦C‘coldcompaction’treatment(Fig.4a),thedensityincreased
Fig.4.Relativedensity–time–temperature–pressuredependenciesduringSPSof (a)MPand(b)SPY2O3nanoparticlesat1000◦Cfor5minand100MPa.
simultaneouslywiththepressureincreasefromroomtemperature (i.e.SPSstartingtime),andleveledat67%within2min,whenthe pressurereacheditsmaximumvalue(100MPa).Sincethe temper-aturewasincreasedonlyafter5minfromtheSPSstart(Fig.4a), theobserveddensificationatroomtemperaturecanberelatedto particlesliding withnegligiblecontributionfromthediffusional processes.However,negativeramdisplacementwasrecordedat ∼600◦Cduringheatingataconstantpressure.Thisdisplacement
couldbeassociatedwithacertaindecreaseindensity,butasitis ceasedwhentheSPStemperatureof1000◦Cwasreached,itcan
berelatedtothethermalexpansionmismatchesbetweenthatof thegraphiteplungersandtheclosepackednano-particlenetwork. Apparently,theclosepackednano-particlesundergosurface dif-fusionduringtheheatingtoformarigidskeleton.Insuchacase, therigidskeletonmayopposefurther shrinkageunderthe con-stantpressure;ifthethermalexpansionofthisskeletonishigher thanthatofthegraphiteplunger,negativeramdisplacementmay occur. (Negativedisplacement due tothermal expansionofthe graphitemold,plungersandspacersisusuallyobservedwiththe increasingtemperatureusingblankspecimens.)Thisaspectwillbe discussedlaterindetail.Thedensitydidnotchangesignificantly duringtheSPSisotherm.Inaddition,increaseintheSPSduration to10 and15minresultedinhigherdensitiesof74.6and76.0%, respectively.
DensificationoftheSPreferenceY2O3nanopowderwasrecently
investigatedindetailundersimilarSPSconditions[20].Someof theresultswillbeusedhereforcomparison.ThedensityoftheSP referencespecimenat1000◦Ctreatmentincreasedwiththe
pres-sureincreaseatroomtemperature(Fig.4b),althoughitleveledoff atamuchlowerdensityof42%.However,incontrasttotheMP nano-powder,furtherincreaseinthedensitywasobservedduring theheatingprocess.Densificationwasacceleratedaround∼680◦C
andreacheditsmaximumvalueof93%around∼880◦C,beforethe
finalSPStemperatureof1000◦Cwasreached.
TheheatingratetotheSPStemperatureof1100◦Cwasof‘hot
compaction’anddifferedfromthatat1000◦C.Inthisrespect,a
heatingpulsewasusedtoreach600◦Cwithin3minunderthe
holdingpressureof 2MPa(Fig.5a).Thiswasfollowed by heat-ingto1100◦Cforadditional5min.Thenthepressurewaslinearly
increasedto100MPa,resultingin simultaneousincrease inthe density to its final value of 66%; nofurther densification was observedattheSPSisotherm.Similardensities(i.e.63.4and62.8%) were reached with further increase of the SPS duration to 10 and15min,respectively.Comparisonbetweenthedensities mea-suredattwoSPSregimes,i.e.at1000◦Cand1100◦C,revealedthat
theapplicationofpressure atthebeginning (‘coldcompaction’) resultedinhigherdensities.
ThecorrespondingSPspecimenexhibitedsignificantincrease in the density during theheating process between800◦C and
∼1050◦C, underthe2MPa holdingpressure only(Fig.5b)[20]. The maximum displacement/(shrinkage)rate was10−2mms−1.
Thisindicated thehigh capillaryforces in theSPto drive den-sification,incontrasttotheMPpowder,wherenodensification was recorded during the heating up. A higher densification rate(1.5×10−2mms−1)wasmeasuredwhen the pressure was
increasedtoitsmaximumvalue.Afinaldensityof93%,whichis higherthanthatoftheMPspecimen,wasreached.
Thedensificationbehavior intheMPspecimenat1500◦C is
shown in Fig.6, as in the other experiments,a very fast den-sification rate(1.5×10−2mms−1)wasrecordedsimultaneously
with the pressure increase. However, in this ‘hot compaction’ experiment(i.e.,aheatingpulseto600◦Cwasappliedbeforethe
pressurewasincreased),densificationceasedwhenthemaximum pressurewasreached.Densitywasstagnatedduringfurther heat-ing upto 1350◦C, where anadditional rapid densificationrate
Fig.5.Relativedensity–time–temperature–pressuredependenciesduringSPSof (a)MPand(b)SPY2O3nanoparticlesat1100◦Cfor5minand100MPa.
theperiodofdensity stagnation(i.e.,between200and650sin Fig.6), thepressureexperiencedtwodisturbancesexpressedby adecreaseof8–10MPaintherecordedpressure.Thesedecreases inpressureoccurredaround1030◦Cand1280◦C,andwere
recov-eredat 1130◦C and 1350◦C, respectively. The second pressure
recovery(increase)wasresponsiblefortherepeatedincreasein densificationat1350◦C,aswasmentionedabove.Thesepressure
disturbancesareassociatedwithincreaseintheresistanceofthe nano-particlestoundergosliding,whetherduetotheformation ofarigidskeletonorjammingoftheagglomeratednano-particles. Ineithercase,thermalexpansionsofboththegraphiteplungers andthespecimentogetherwiththelackofplasticityinthe speci-men,introduceinternalcompressivestresses.Sincetheexternal pressureappliedinthesystemisregulatedtoremainconstant, itsactualvalueshoulddecreaseinordertobalancetheinternal
Fig.6.Relativedensity–time–temperature–pressuredependenciesduringSPSof MPcubicY2O3nanoparticlesat1500◦Cfor5minand100MPa.
Fig.7.SEMimageshowingthehomogeneousdistributionofthesphericalgrains throughoutthespecimensinteredat1100◦Cfor5minand100MPausing
multi-phaseY2O3nanoparticles.
thermalpressuresformed.Thismayleadtothedisturbancesand decreasesobservedinthepresentSPSexperiments.
SEMimagesfromthespecimenssinteredat1100◦Cfor
differ-entdurations(i.e.5min,Fig.7)showedhomogeneousdistribution ofmanysphericalshapegrainsthroughoutthematrixoftheMP Y2O3 nanoparticles.Thelargestdiameterofthesphericalgrains
was∼15mmafter5min,and∼60mmafter10mindurations,with verywidegrainsizedistributions.Thesesphericalgrainsexhibited alowvolumefractionofthespecimensevenafter15minof sinter-ingat1100◦C.Thesesphericalgrainswereabsentaftersintering
at1500◦C;aregularpolyhedralshape grainmicrostructurewas
observed.
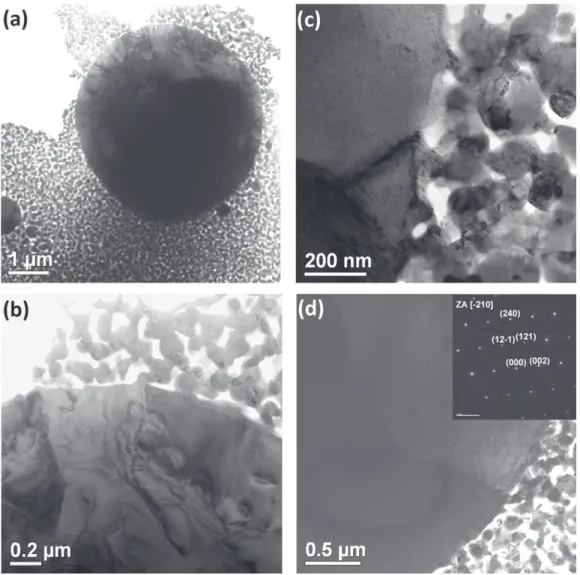
TEMimagesfromthespecimenssinteredatdifferent temper-aturesclearly revealedthe microstructureevolution duringthe SPS. First, at 1000◦C and 1100◦C, closeto the phase
transfor-mationtemperature,many polycrystallinesphericalparticles of submicrometerandmicrometer-sizeindiameterwereobserved withintheporousnanoparticlematrix(Fig.8a).Higher magnifica-tionprovedtheinternalstructureofthelargeparticles(Fig.8band c)tobecomposedofsub-grainsseparatedbydislocationnetworks; insomeoccasions,internalsubmicrometer-sizeporeswerealso observed.Thematrixwascomprisedofpartiallysintered nanopar-ticleswhichformedaporousnetworkresemblingthevermicular structure(theupperpartinFig.8b).Detailedexaminationofthe interfacebetweenthesphericalparticlesandthevermicular struc-turerevealedthattheformergrowontheaccountoftheporous nanoparticleskeleton(Fig.8c).Selectedareadiffractionpatterns confirmedthecubiccrystal symmetryof thesphericalparticles (Fig.8d).Therefore,itseemsthatthesphericalparticlespresent nucleationandinterface-controlledgrowthofthecubicgrainson theaccountofthemonoclinicnanoparticles.
Finally, TEM images from the 1500◦C treated specimen,
shown in Fig. 9, exhibit the micrometer size polyhedralshape grains with nanometric closed pores. Many grains were com-prisedofsub-grainsseparatedbydislocationnetworks(Fig.9b). A few nanometer size grains and closed pores were still visible along the sub-grain boundaries and at their corners (arrowed in Fig. 9b).This microstructure can beconsidered as analmostfullytransformedversionofthevermicular structure to thecubic grains. The microstructure evolution in the refer-ence single-phase cubic nanoparticle compactsduring the SPS were described in detail elsewhere [15,16,20]. At SPS temper-atures below 1100◦C, the nanometric character of the dense
Fig.8.TEMimagesshowingthe(a)nucleationandgrowthofthesphericalcubicY2O3grainsonaccountofthemultiphasenanocrystallinematrixat1000◦C.(b)Higher
magnificationof(a)showingthevermicularstructureofthematrixnanoparticles.(c)Theinterface-controlledgrowthofthesphericalcubicgrainsat1100◦C.(d)Selected
areadiffractionpatternconfirmedthecubicsymmetryofthesphericalY2O3grains.
compacts waspreserved. However, significant grain growthto micrometer-sizegrainswasobservedathighertemperatures[16]. The microstructural evolution associated withthe polymorphic phase transformationisthebasisfor theobserveddensification behavior ofthemultiphasenanoparticlesand willbediscussed below.
4. Discussion
Theobserveddensificationbehaviorofthetwonanopowders canbeexplainedbythemetastablenatureofthemonoclinic poly-morph.ThemonoclinicphaseisahighpressureversionofY2O3,
but can be retained in a metastable state at the atmospheric conditionswheninthenanoparticleform.Theseaspectsof poly-morphism,especiallyintheY2O3nano-particles,werediscussed
indetailelsewhere[19,21,22].Althoughbothpowdersexhibiteda closetonanocrystallineparticlesize(18-nmvs.41-nm),the sur-faceenthalpyofthemonoclinicpolymorphissignificantlyhigher (2.78Jm−2)thanthatofthecubicphase(1.66Jm−2)[19].
There-fore,thehighlyactivesurfacesofthemonoclinicnano-particles act as an efficient driving force for low-temperature sintering andneckformation.Thistypeofhighsinterabilityiswell char-acterized in transition alumina (g-alumina) and lead to rapid formationofa rigidporousskeletonbysurfacediffusionatlow
temperatures [23]; the resultant vermicular structure leads to low-densitysintered compacts.In this respect,consolidation of aluminananoparticlesbySPSshowedenhanceddensificationof the a-alumina compared to that of transition g-alumina [24]. Surprisingly, dense a-alumina was obtained at a considerably lowerSPStemperature,albeitoriginallywithalargerparticlesize. Thisbehaviorwasrelatedtog→aphasetransformationduring the SPS, which in turnled to the formation of the vermicular structure.
Thetheoreticaldensitiesofthecubic(c)andmonoclinic(m) Y2O3polymorphsare5.030gcm−3and5.468gcm−3,respectively [19]. Consequently, the polymorphic m→c phase transforma-tion is associated with∼8% volume increase. Nevertheless,the DSC results indicated the polymorphic transformation temper-ature to be around1000◦C, where a rigid skeleton of mY
2O3
hasbeendevelopedfairlywell.Ononehand,thekineticsofthis interface-controlled polymorphic phase transformation is more sluggish compared tothe SPSheating time scale, as evidenced byXRD analysis.Ontheotherhand, thepresenceof the nano-poreswithinthesphericalparticlesisanevidenceforaveryrapid interface-controlledprocess(i.e.nano-poreswerenotannealedby diffusion).Consequentlythechangeinthecompactvolume dur-ingtheheating,duetothephasetransformation,maybemarginal. SimilareffectswerereportedduringdensificationofSi3N4 with
Fig.9.TEMimagesshowingthe(a)micrometer-sizecubicgrainsformedat1500◦C.
Afewporesarevisiblealongthegrainboundaries.(b)Manysub-grainboundaries decoratedwithdislocationnetworksandnano-grainswerepresent(arrowed).
metallicsinteringadditivebySPS[25],wheresignificant shrink-ageoccurredbyparticlerearrangementandwhilealiquidphase wasformed.However,minorshrinkagewasobservedwhenphase transformationandgraingrowthviasolution-reprecipitationtook place.
Based on the microstructure developed in the multiphase nanoparticlecompacts,thefollowingprocessesmaybeconsidered. First, theapplication ofexternal pressure at roomtemperature enablesparticlesliding andrearrangement.Theearlyand rapid densificationstageceaseswhenreachingthemaximal pressure applied.Second,highlyreactivesurfacesof themnanoparticles enableenhancedneckformationandgrowthbysurfacediffusion duringtheheating.The partiallysinteredmnanoparticlesform a rigidand porous skeleton witha vermicular structure which opposesfurtherdensificationbyparticlesliding.Atandabovethe polymorphicm→cphasetransformationtemperature,nucleation ofthestablecphasetakesplace,homogeneouslythroughoutthe porousskeleton(thehomogeneous/heterogeneousnatureofthe nucleationeventwasnotinvestigatedhere).Furthergrowthofthe cubicnucleibythisinterfaced-controlledtransformationresults insphericalpolycrystallineparticles.Apparently,the8%volume
increaseaccompaniedtothetransformation,is notsufficientto overcomethevolumeconstraintsimposedonthegrowing spher-icalparticles withintherigid porousmatrix. Consequently,the elasticconstraintsmaybereducedbytheformationofdislocation networksandsub-grains,which,inturn,growontheaccountof them+cnanoparticles,attheirgrowingfront.Whentherapidly growingfrontofthesphericalparticlefacesalargecavityofthe vermicularstructure,itmaysurpassit,duetoinsufficienttimefor diffusion,resultinginoccludednano-pores.Athighertemperature, whenthephasetransformationisaccomplished,particlesliding, dislocationcreep,andgraingrowthmayberesponsibleforthelater stagedensificationclosetofulldensity.
Finally,theeffectofthevolumechangeduringthe polymor-phicphasetransformationtodensificationofthemultiphaseY2O3
nanoparticleswasnegligible.Nevertheless,themetastablenature andthehighsurface activityofthis polymorphwerethemajor causefortheformationofthevermicularstructure,whichinturn, inhibitedthedensificationduringtheheatingbySPS.The interface-controlledcharacterof thetransformation ledtotheformation ofverylargecubicgrains,responsibleforthelossofnanometric characterofthecompactsubjectedtodensification.
5. Summary
Sparkplasmasinteringofthemultiphasemonoclinicandcubic Y2O3 nanoparticles at 1000◦C exhibited limited densification
comparedtotherapiddensificationofthecubicsingle-phase coun-terpart.XRDofthemultiphasesinteredcompactsrevealedthatthe polymorphicmonoclinictocubicphasetransformationoccurred duringtheSPS around1000◦C, andwascompletedbyreaching
1100◦C.Themetastablemonoclinicphaseledtorapidneck
forma-tionduringtheheatingwhichresultedinavermicularnanometric matrixwithopenporositynetwork;thislimitedfurther densifi-cationofthemultiphasenanopowderandendedinverylowfinal densities.AtSPStemperaturesabovethepolymorphic transforma-tiontemperature,homogeneousnucleationofthesphericalcubic Y2O3grainswasobservedwithinthevermicularmatrix.This
inter-facecontrolledmonoclinictocubicphasetransformationresulted inthelossofthenanocrystallinecharacterofthecompact.SPSofthe multiphasenanoparticlesat1500◦Cshowedsimilardensification
behaviorasthepurecubicY2O3,resultinginadensemicrostructure
andcoarsemicrometer-sizepolyhedralshapedgrains. Acknowledgments
ThefinancialsupportoftheIsrael MinistryofScienceunder contract#3-3429isgratefullyacknowledged.WethankDr.Ori YeheskelfromNRC-NegevforsupplyingtheMPnanopowder. References
[1]S.-J.L.Kang,Sintering,Densification,GrainGrowth&Microstructure,Elsevier, Amsterdam,2005.
[2]J.Liu,D.P.DeLo,Metal.Mater.Trans.A32(2001)3117–3124.
[3]C.L.Martin,D.Bouvard,S.Shima,J.Mech.Phys.Solids51(2003)667–693. [4]R.Chaim,R.Reshef,G.Liu,Z.Shen,Mater.Sci.Eng.A528(2010)2936–2940. [5] E.Artz,M.F.Ashby,K.E.Easterling,Metall.Trans.A14A(1983)211–221. [6]R.Chaim,M.Margulis,Mater.Sci.Eng.A407(2005)180–187.
[7]D.A.Porter,K.E.Easterling,M.Y.Sherif,PhaseTransformationsinMetalsand Alloys,3rdedition,CRCPress,BocaRaton,2009.
[8]B.Li,X.Wang,L.Li,H.Zhou,X.Liu,X.Han,Y.Zhang,X.Qi,X.Deng,Mater.Chem. Phys.83(2004)23–28.
[9]T.Takeuchi,M.Tabuchi,H.Kageyama,Y.Suyama,J.Am.Ceram.Soc.82(1999) 939–943.
[10]X.Deng,X.Wang,H.Wen,A.Kang,Z.Gui,L.Li,J.Am.Ceram.Soc.89(2006) 1059–1064.
[11]J.Liu,Z.Shen,M.Nygren,B.Su,T.W.Button,J.Am.Ceram.Soc.89(2006) 2689–2694.
[12] K-N.P.Kumar,K.Keizer,A.J.Burggraaf,T.Okubo,H.Nagamoto,S.Morooka, Nature358(1992)48–51.
[13]Y.I.Lee,J.-H.Lee,S.-H.Hong,D.-Y.Kim,Mater.Res.Bull.38(2003)925– 930.
[14]U.Anselmi-Tamburini,J.E.Garay,Z.A.Munir,Mater.Sci.Eng.A407(2005) 24–30.
[15]R.Marder,R.Chaim,C.Estournes,Mater.Sci.Eng.A527(2010)1577–1585. [16]R.Chaim,A.Shlayer,C.Estournes,J.Eur.Ceram.Soc.29(2009)91–98. [17]C.Suryanarayana,M.GrantNorton,X-rayDiffraction,APracticalApproach,
PlenumPress,NewYork,1998,pp.223–236.
[18]I.Halevy,R.Carmon,M.L.Winterrose,O.Yeheskel,E.Tiferet,S.Ghose,J.Phys. Conf.Ser.215(2010)012003.
[19]P.Zhang,A.Navrotsky,B.Guo,I.Kennedy,A.N.Clark,C.Lesher,Q.Liu,J.Phys. Chem.C112(2008)932–938.
[20]R.Marder,R.Chaim,G.Chevallier,C.Estournes,J.Eur.Ceram.Soc.31(2011) 1057–1066.
[21]A.Camenzind,R.Strobel,S.E.Pratsinis,Chem.Phys.Lett.415(2005)193–197. [22]B.Guo,A.Harvey,S.H.Risbud,I.M.Kennedy,Phil.Mag.Lett.86(2006)457–467. [23]F.W.Dynys,J.W.Halloran,J.Am.Ceram.Soc.65(1982)442–448.
[24] R.S.Mishra,S.H.Risbud,A.K.Mukherjee,J.Mater.Res.13(1998)86–89. [25]G.H.Peng,X.G.Li,M.Liang,Z.H.Liang,Q.Liu,W.L.Li,ScriptaMater.61(2009)