OATAO is an open access repository that collects the work of Toulouse
researchers and makes it freely available over the web where possible
Any correspondence concerning this service should be sent
to the repository administrator:
tech-oatao@listes-diff.inp-toulouse.fr
This is an author’s version published in: http://oatao.univ-toulouse.fr/20891
To cite this version:
Murillo, Carlos
and Berdouzi, Fatine
and Olivier, Nelly
and Gabas,
Nadine
Deviation propagation analysis along a cumene process by using
dynamic simulations. (2018) Computers & Chemical Engineering, 117.
331-350. ISSN 0098-1354
Official URL:
https://doi.org/10.1016/j.compchemeng.2018.06.010
Open Archive Toulouse Archive Ouverte
Deviation
propagation
analysis
along
a
cumene
process
by
using
dynamic
simulations
Murillo
Carlos,
Berdouzi
Fatine,
Olivier-Maget
Nelly,
Gabas
Nadine
∗Laboratoire de Génie Chimique, Université de Toulouse, CNRS, Toulouse 31030, France
a
b
s
t
r
a
c
t
ThedynamicresponseofbenzenealkylationprocesstoasetofdeviationsisanalyzedwithAspenPlus Dynamics.Aquantitativerisk assessmentisdeveloped throughsimulationsofdeviation scenarios.The processcomprisesareactorandthreedistillationcolumnswitharecyclestream.Thesimulation scenar-iosaredeterminedaccordingtolessonslearntfromaccidents.Thisstudyunderlinestheconditionsthat induceanoverpressureorafloodinginadistillationcolumn.Threescenariosareproposed:feedflowrate variations,coolantflowratereductionandcoolingofthereboilersteam.Thereafter,theresultsallow cal-culatingasetofriskindexesrelatedtofloodingandoverpressurephenomena.Thisstudyunderlinesthe deviationpropagationeffectsthatcanbeexpectedinalltheprocessequipment.Moreover,itrepresentsa significantcontributiontothe definitionoftheprocesscontrolstrategyand thenecessarysafety barriers.
1. Introduction
The analysisofconsequences that canbe causedby a process deviationis usually performedwitha risk assessment methodol-ogy. Recently, quantitative or semi-quantitative approaches have emerged due to increasing requirementsof process and technol-ogydevelopments(Tianetal.,2015).Nowadays,itisadmittedthat riskassessmentshouldbeenvisagedasacomplementaryapproach inwhich thetechnical expertiseissupported by simulationtools (Berdouzietal.,2016).Inthisorder,dynamicsimulationsimprove theknowledgeandunderstandingofthedynamiccharacteristicsof a chemicalprocess byrelatingits dynamicoutput responseto an input disturbance(Inghametal., 2007). Inthis manner,it allows estimatingvariouscontrolstrategiesaswellastheoptimizationof thecontrolsettingsoftheprocess.
The objective of dynamic simulations is to predict how fast variableschangeintheeventofanoperatingemergencyor equip-ment failure (Luyben, 2012a). This analysis determines the time period to reach critical limits (safety response time) and per-mitstheengineer toquantitativelydesigneffectivesafetysystems (Luyben, 2012b). Therefore, dynamic simulations can contribute to the characterization of process deviations in future risk anal-yses (Tian et al., 2015). In the literature, numerous papers deal
with dynamic simulation-based HAZOPanalyses (Isimite and Ru-bini, 2016; Tian et al., 2015). In this manner, risks are quanti-fied thanks to the knowledge of the process abnormal behavior (Gabbar et al., 2003). This description of the dynamic evolution of the operating parameters allows recommendingthe appropri-ate safetybarriers (Luyben, 2012a) andevaluating newsafeguard methods(Bodizsetal.,2015).
Our study envisages the dynamic simulation of an industrial plant with ASPEN PLUS DYNAMICS 9.0 in order to analyze the propagationofasetofspecificdeviations.Apreliminarystudywas carried out to show thefeasibility of the method(Murillo etal., 2017). Firstly, a casestudy is modeled under steady-state condi-tions and a list of possible disturbances is defined according to lessons learntfromprevious accidentsoccurredinthedistillation columns (ARIA,2016;Kister, 1997, 2003).Next,the dynamic sim-ulation underlines how each deviation might lead to hazardous eventsandtheir associatedrisks.Moreover, themethodallowsto knowthebehavioroftheprocessduringthedegradedmodesand toquantifytheseverityofaccidentalscenarios.Thus,thefinalgoal is to recommend the required safetybarriers (nature, sizing and responsetime).
2. Casestudy
Thechemicalprocess thatisconsideredforthiscasestudy en-visages the industrial alkylation of benzene by propene to iso-propylbenzene,whichisalsodenominatedascumene(C9H12).This
chemical process can be developed continuously with a vapor-phase orliquid-phasereaction. Before1990,the former
manufac-Fig. 1. Process flowsheet of the industrial alkylation of benzene with propene to cumene.
turingrouteprevailedovertheliquid-phasealternative. Neverthe-less, the development of the alkylation reaction withzeolites as catalysts has allowed considering the latter as a more appropri-ate technique (Bellussi et al., 1995; Corma et al., 2000). Besides the productionofcumene,thealkylationprocess alsoimpliesthe production ofpolyalkylates andpolyisopropylbenzenesas well as propyleneoligomersduetoasetofconsecutivereactions.Forthis reason,thealkylationreactionsarecarriedoutwithlargepore ze-olites(e.g.MCM-22)andamolarfeedratioofbenzene/propylene over4:1.Theseoperatingconditionsprovideamajorsignificanceof theproductionofthecumeneandthediisopropylbenzene(C12H15)
with regard to other by-products. Further information about the physicochemical properties ofthe zeolites andtheir influence on theselectivity ofthereactioncanbe consultedinthe experimen-talstudydevelopedby(Cormaetal.,1995).
2.1. Generaldescriptionoftheprocess
The workpresentedinthispaperconsiders themanufacturing process ofcumenefrombenzeneandpropeneintheliquidphase withzeolites ascatalysts.Previous studieshave characterizedthe cumeneproductioninthevaporphaseaswellasitscontrol struc-ture (Geraet al., 2013;Luyben, 2010). In thisstudy, the produc-tionisintheliquidphaseandtheprocessshowninFig.1,which iscomposedofaPackedBedReactor(PBR)andatrain of distilla-tioncolumns(C-1toC-3).Theseparationequipmentrecovers ben-zene, which is the excess reagent, to be recycled to the reactor. Additionally, thecolumnsrecoverandpurifycumenealongwitha by-productoftheprocess(diisopropylbenzene).These characteris-ticsconstitutean importantsimulationcomplexitysincethe recy-clepromotesthepropagationofoperatingparameterdeviations.
The casestudyis an industrialplantthat produces 87.6 ktons per yearwithaglobalmanufactureefficiencyof95%(Fig.1).The dynamic simulation isdeveloped accordingto theoperating con-ditionsspecifiedbyDimianandBildea(2008)forthePBRandthe distillation columns.Initially,a makeup stream of91.3 kmol·hr−1
Table 1
Global apparent kinetic parameters of the benzene alkylation to cumene and diisopropylbenzene ( Dimian and Bildea, 2008 ).
Chemical reaction Chemical reaction rates Benzene alkylation C6 H 6 + C 3 H 6 → C 9 H 12 r1 = 6510 exp( −52564
RT ) C C3H6
Cumene alkylation C9 H 12 + C 3 H 6 → C 12 H 15 r2 = 450 exp( −50000
RT ) C C3H6
ofbenzeneisfedalongwithamakeupstreamof100.9kmolhr−1
of aliphatic hydrocarbons: propane and propene. This stream is mainlycomposed ofthealkene whosemolarfractionis99%. Ad-ditionally,bothstreams aremixedwiththerecycleofthesecond distillation columninorder tohavea benzene/propylene ratioof 7:1inthereactorfeed(RF).Thisratioensuresthedesired selectiv-itytocumeneatthereactoroutlet(RO).
The process consistsof a reaction unit and a separation unit thatrecoverstheunreactedcompounds andpurifiesthealkylated hydrocarbons.Thefirstunitcarriesout thealkylationreactions in thePBR.Thisisatubularadiabaticequipmentwhosediameterand lengthare1.3and10mrespectively.Thisreactorisfilledwiththe bedofthesolidcatalyst(dp equalto2.4mm)toconstituteavoid
fractionequal to 0.4. This reactorconfiguration defined the sim-ulationschemeproposed by DimianandBildea(2008)aswell as thekineticparametersofthecumeneanddiisopropylbenzene pro-ductionaccordingtotheoperatingconditionsoftheprocess equip-ment.Thisschemeposesapseudo-first-orderreactionadjustedto thetemperaturerange oftheindustrial reactionunit (150-230°C) anda highbenzene/propylenefeedratio(5-8:1).Thus,the Arrhe-nius expressionsthat are listed inTable1 areobtained by calcu-latingtwo apparent kinetic constants that take into account not onlythechemicalreactionbutalsothemasstransferphenomena occurringintheliquidphase.Theactivationenergiesofthe Arrhe-niusequationsareexpressedinkJ·kmol-1andthereactionratesin kmol·m−3·s−1.
Table 2
Design specifications of the distillation columns of the separation unit.
Design specification C-1 C-2 C-3
Separation specification Aliphatics in distillate: Benzene in distillate: Cumene in distillate:
Recovery: 99.0% Recovery: 99.9% Recovery: 99.5%
Purity: 99.9% Purity: 99.0% Purity: 99.0%
Condenser Total (Logarithmic mean temperature
difference) Total (Logarithmic mean temperaturedifference) Total (Logarithmic mean temperaturedifference)
Condenser pressure (bar) 12.0 3.0 1.0
Reboiler Kettle
Constant steam temperature
Kettle
Constant steam temperature
Kettle
Constant steam temperature
Height (m) 3.9 6.0 5.0
Number of stages 13 19 18
Feed stage 6 10 9
Reflux ratio 44.52 0.157 0.325
Theperformanceofthereactionunitisestablishedbythe con-versionofpropene,whichis93.1%atsteady-stateconditions. Sim-ilarly, the selectivity of the alkylation reactions is established as the ratio of the moles of the mono and disubstituted hydrocar-bons that areproduced inthepacked bedreactor.The high ben-zene/propene ratio determines a steady-state operation with the productionof 25.8moles ofcumeneper moleof diisopropylben-zene.Subsequently,theproductsofthereactorareseparatedinthe secondunit,whichconsistsofaseriesofthreedistillationcolumns packed with pall rings. First, they are fed to a distillation tower (C-1) thatseparates thealiphaticcompounds (propane and unre-actedpropeneinD1) fromthearomatichydrocarbons,which are concentratedatthebottomofthecolumn(B1).Thisseparation de-mandsahighrefluxratio(Table2)becausethefractionofaliphatic compoundsisnegligiblewithregardtothatofaromatic hydrocar-bons.Then,thearomaticcompoundsarefedtoasecondtower (C-2) that separatesthe unreacted benzene (D2) from the alkylated hydrocarbons(B2).Thereafter,therecoveredbenzeneisrecycledto thereactor.Finally,thedistillateofthethirdcolumn(C-3)is com-posedof highpuritycumene(D3) whereas the bottoms are con-stituted bythe remaining diisopropylbenzene (B3).Moreover, the industrialprocesscanaddanothercolumntopurifythis hydrocar-bonfromotherhighmolecularweightby-products.Theseparation sequenceofthedistillationtowers isdefinedtoensureagood re-covery of thelight compound fed to each tower. The designand separationspecificationsofthecolumnsareshowninTable2.
Additionally, Fig. 1 alsopresents the heatexchangers that are included in the flow diagram of the chemical process (E-1 to E-4). The exchangers E-1andE-2 are positioned ina network that preheatsthereactive mixturewiththe outletstream ofthe reac-tor and medium pressure steam (MPS) respectively. On the con-trary,theothertwoexchangersreducethetemperatureofthefeed streams of the columns C-2 and C-3 with a coolant fluid as a utility. Finally,two pumps (P-1andP-2) are implementedin the chemical processtopressurizethemakeup streamsandthe recy-cleofthecolumnC-2until 36bar. Thispressurizationlevelisset inordertoavoidaphasechangeofthereactivemixturewithinthe reactor.Similarly,thevalves(V-1toV-3)areincludedtoadjustthe feedpressure ofthedistillation columns.Theseelementsallowed equalizingthe pressureofeach feedstream tothe corresponding pressureofthefeedstage.
The conditionsare determinedby a steady-statesimulationof the chemical process that is developed withthe softwareASPEN PLUS9.0TMunderasequentialmodularapproach.Thisscheme
im-plies the definition of unit block operations that are solved one at a time in sequence by the simulator by considering the inlet stream variables anda set ofspecified parameters (Smith, 2016). For thisstudy, the thermodynamic modelis based on thePeng– Robinsonequationsincethesystemisonlycomposedof hydrocar-bonswhosecriticalpressures rangebetween24.5bar
(diisopropy-lbenzene) and 48.9bar (benzene). These values are considerably higher than the operating conditions of the process equipment withvapor-liquidequilibria.
2.2. Controlstructureoftheprocess
Theplantwidecontrolstructureofthechemical processisalso presentedinFig.2:
It is divided into three nodes: reactor, intermediate heat ex-changers,andcolumns.Inthefirstgroup,thetotalfeedflowrateof the reactorisregulated by manipulatingtheflowrateofthe ben-zene makeup stream(FC),which isthe chemicalcompound with thehighestflowrateintherecyclestream.Inthesamemanner,the temperature of the feedstream of the reactor iscontrolled with theutilitytemperatureoftheheatexchangerE-2(TC).Thesecond group consists of the temperature controllers of the other inter-mediate heat exchangers(E-3 andE4).The objectiveisto reduce thefeedstreamtemperatureofthedistillationcolumnsC-2and C-3. This regulationisachievedbymanipulatingthecoolantflow of each heat exchanger (TC). The control parameters ofthese nodes arelistedinTable3.
Additionally,thethirdgroupofcontrolloopsregulatesthe per-formanceofthedistillationcolumns.Fivecontrolloopsareusually considered forthistypeofequipment(Luyben,2006;Wangetal., 2016).Firstly,thecondenserpressureisregulatedbymanipulating thecoolantflowrate(PC).Theliquidlevelsoftherefluxdrumsare controlled by adjusting the distillate flowrates (LC). In the same manner,theliquidlevelsofthesumpsarecontrolledwiththe ma-nipulation ofthe bottoms flowrates(LC). Besides,there isa tem-peraturecontrol inaspecificstage ofeachcolumn(TC).Itadjusts thetemperatureoftheheatingsteamintheboilerinorderto sta-bilizethestagewiththehighestvariationinthetemperature pro-file of thevessel. Thisstrategy isproposed by Luyben (2006) for the temperaturecontrol of a distillation column. The last control loop correspondsto aratiocontrol(X)betweenthefeedflowrate and the reflux rate for each column of the separation unit. The controlparametersofthedistillationcolumnsarelistedinTable4. The dynamicsimulation isdevelopedbyexporting the steady-state simulationtoASPEN PLUSDYNAMICS9.0TM asaflow-driven
mode. This definitionis determined by considering that the out-let temperatures ofthe process streams inthe intermediate heat exchangers are below the corresponding boiling points. To make a dynamic simulation, it is necessary to define the models that representtheprocessequipment.Forinstance,alogarithmicmean temperature difference is used forthe condensers and the inter-mediate heat exchangers (E-1 to E-5in Fig.1). The modelling of the reboilers ofthe columns iscommonlybased onthe constant temperature model. Then, the control loops described above are implemented in the dynamic simulation in order to analyze the response of the control structure after the occurrenceof a given
Fig. 2. Aspen plus dynamics flowsheet of the cumene process.
Table 3
Control parameters of the packed bed reactor and the intermediate heat exchangers.
Position (control loop) Action Type Controlled variable Manipulated variable Reactor Feed Flow (BENZENE_FC) Reverse PI Feed flowrate Benzene flowrate
Heat exchanger E-2 (E-2_TC) Reverse PID with dead time Feed temperature Temperature of heating steam Heat exchanger E-3 (E-3_TC) Direct PI with dead time Outlet temperature Cooling fluid flowrate Heat exchanger E-4 (E-4_TC) Direct PID with dead time Outlet temperature Cooling fluid flowrate
Table 4
Control parameters of the distillation columns.
Position CONTROL LOOP ACTION TYPE Controlled variable Manipulated variable REFLUX DRUM C-1_CondPC Direct PI Pressure Cooling fluid flowrate
C-2_CondPC C-3_CondPC
REFLUX DRUM C-1_DrumLC Direct P Level Distillate rate
C-2_DrumLC C-3_DrumLC
SUMP C-1_SumpLC Direct P Level Bottoms rate
C-2_SumpLC C-3_SumpLC
REFLUX FtoR_C-1 Direct (Multiplier) X Reflux to feed ratio Reflux rate FtoR_C-2
FtoR_C-3
COLUMN Reverse PID with dead time Temperature Temperature of heating steam C-1 - Stage 03 C-1_TC
C-2 - Stage 15 C-2_TC C-3 - Stage 16 C-3_TC
disturbanceordeviation.All theparametersofthecontrollersare tunedaccordingtoaconventionalmethodsuchasZiegler-Nichols. Some details about thesimulation settings have been added but for more information, the reference of our works in this field (Berdouzi,2017)iscitedinthetext.
2.3. Processsafetyvalves
Figs. 1and2showtheprocess safetyvalvesPSV-1,PSV-2, and PSV-3, whicharepositioned atthetopofeach distillationcolumn asasafetybarrier,upstream tothecondenser.AsPSVdevicesare essentialfortheprocesssafety.Inordertosimulatethereal func-tioningoftheprocesssafetyvalves,itispossibletorepresenttheir opening and closingby a schematic lift-pressure diagram that is commonlycalledasPSVhysteresis.ThecharacteristicPSV
parame-Table 5
Hysteresis and design parameters of the process safety valves. Design specifications PSV-1 PSV-2 PSV-3 Set pressure – SP (bar) 14.40 3.60 2.00 Primary lift valve position - PLVP (%) 10 10 10 Full lift pressure, opening -FLO (bar) 15.12 3.78 2.10 Full lift pressure, closing -FLC (bar) 14.40 3.60 1.30 Reseating pressure – RP (bar) 13.68 3.42 1.10 Reseat valve position - RVP (%) 50 50 50
tersarementionedinFig.3andTable5.ThesesettingsallowaPSV response that constitutesan immediateresponse to an overpres-surewithoutgeneratingoscillationsduetotheaction ofpressure controllers.
Fig. 3. Hysteresis diagram of a process safety valve.
Fig. 3illustrates thefunctioning ofa PSV. At thepressure set SP,theprocess safetyvalve graduallyopensuntil thePrimaryLift Valve Position PLVP (10%). At the Full Lift OpeningPressure FLO, the process safety valve totally opens. It remains open until the FullLiftClosing PressureFLC.Then,theprocess safetyvalve grad-ually closesuntil the ReseatValve Position RVP (50%).When the pressure isequalto theReseating PressureRP,the processsafety valves are totally closed. For the cumene process, the different thresholdsarechoseninaccordancewiththerecommendationsof the DIERSmethod(DesignInstitute forEmergency ReliefSystem) constitutedbytheAIChEengineers(AmericanInstituteofChemical Engineers).Thesizingofthepressuresafetyvalvesisperformedby estimatingtheoverheadvaporrateofaventrelease.Thedifferent diametersoftheprocesssafetyvalvesaredeterminedaccordingto theDIERSmethod(EtchellsandWilday,1998;Gustin,2006,2009). Thesizingcriterionofthismethodcanbeassociatedwithfailures of thecontrol valves,reflux, power orcoolantsupply oreven an exteriorfire.Thescenarioconsideredforthispurposecorresponds toatotallossofheatremovalinthecondenserssinceitrepresents themajorincreasesinthetoppressure.Itisinaccordancewiththe ventsizingstrategyrecommendedbyKister(1990).Thebore diam-eterisdeterminedaccordingtoastandardcode(API520-I,2008). Table5givesthespecificationsofthePSV.
3. Simulationscenarios
The simulationscenarios ofthisstudyare definedinthis sec-tionaccordingtoareviewofaccidentalreports.Thisanalysis eval-uates the factors that can generate an abnormal operation in a distillationcolumn. Then,thesimulationofthesescenarios allows evaluatingtheirpotentialeffectsonthechemicalprocess.
3.1. Literaturereview
AccordingtoKister(2003),thenumberofmalfunctionsin dis-tillationcolumnsisincreasinginspiteoftheadvancesinthe distil-lationtechnology.Thelessonslearntfromaccidentsarenotalways effectivelycommunicated andthe undesiredmalfunctions are in-cessantlyrepeated. Kister (1997) has listed themain failuresand deviationsthat causeabnormaloperationsinthe distillation tow-ers. The failure concerns the malfunction of a device whereas a deviationconsiders anychangefromthenormaloperating condi-tions.
The obtained data associate the operating problems mainly with troublesome column internals and operational difficulties. Thesecausesarefollowedbyinstrumentationandcontrolfailures
and facility mishaps.These four factors represent60% ofthe re-ported cases andare the mostcommon malfunctions considered bythetroubleshooters.Mostoftheremainingcasescorrespondto designmistakes,startup/shutdowndifficulties,andheatexchangers failures.Theknowledge ofthesefactorsis determiningsincethey can represent 27% of the reported malfunctions. Finally, the re-mainingcasesarelinkedtothetrayanddowncomerlayout, foam-ingandreliefproblems.
Thedynamicresponseoftheplant-widestructurecanbe char-acterized by a setofscenarios that describesthebehavior ofthe manufacturing process.Thisanalysisconstitutesagreatadvantage inthedevelopmentofariskassessmentsinceitallowsevaluating thebehaviorofdifferentprocessvariablesatthesametime. How-ever, itisnecessary todefine aclearscope intheanalysisofthe simulation results since manyelements can be omitted dueto a generalstudyofthechemicalprocess.Forthisreason,this discus-sionenvisagesthedynamicresponseofthecumeneprocess equip-mentbutfocusesonthenegativeeffectsoccurredintheseparation unit. This case study mainly considers scenarios associated with columnfloodingandoverpressure.Thesephenomenaareanalyzed because they mayresult in a hydrocarbons leak in the columns. The abnormal operation of a distillation columnunderthese cir-cumstancesisdescribedasfollows:
• Flooding: This inoperability condition is caused by an
exces-siveretentionofliquidinsidethecolumn.Thisissueisduetoa highvapor/liquidloading(GorakandSchoenmakers,2014).This abnormal operation is mainly associated with changes in the tanks levels, increases in the pressure dropand deterioration ofthemasstransferefficiency(GorakandSchoenmakers,2014; Treybal,1980).
• Overpressure: The deviationsandfailures that canlead to an
overpressure of a distillation column depend strictly on its design and operating conditions.However, the mostcommon sources andcauses can be grouped in the checklist that was proposedbyKister(1990).ThesereasonsarelistedinTable6. 3.2. Lessonslearntfrompreviousaccidents
Theaccidentsoccurredindistillationcolumnarerelatedto dif-ferentcausesaccordingtotheaccidentsreports.Thelessonslearnt from accidentsstudy is a commonpractice andplays a key role intheimprovementofsafetyofindustrialprocesses(Kletz,2009). Thereareseveralaccidentdatabasesavailableforthispurpose.For example, we can quote the European MARS (Major Accident Re-porting System), the American CSB (Chemical Safety Board) and the FrenchARIA(Analysis,ResearchandInformationonthe Acci-dents).Thelatterdatabaseischosenforthisstudysinceitis well-documentedontheaccidentcausesandalsotakesintoaccountthe foreignaccidents.
A review of the lessons learnt in the ARIA database is per-formed inthisstudy.Thisanalysis onlyconsidersthe eventsthat led tomajor lossesbetween1990andApril 2017(explosion, fire, leaks, and vent to reliefs or flares). The causes and the conse-quences ofthe81 accidentsfoundinthe ARIAdatabaseare ana-lyzed inFig.4.Thehumanerrors(22.9%)andthematerial(20.8%) represent the main causes. Similarly, the survey established that fire (58.3%)andventrelease(20.8%)arethemostfrequent conse-quences whereasa minorfractionresults inavapor cloud explo-sion(5.2%).
Thesurveysandlessonslearnthaveshownthatsometimesthe column flooding oroverpressure lead to an accident dueto vent releasesorleaks.Anexampleofthissituationisillustrated inthe investigationreportoftheBPTexasrefineryaccidentthatoccurred in 2005 (US ChemicalSafetyBoard, 2007).The bottoms of a raf-finate splitter tower heated up its feed stream in an exchanger
Table 6
Factors responsible for the distillation column overpressure ( Kister, 1990 ).
Failures and deviations Causes
Utility failure • Loss of coolant (heat removal) • Loss of electric power • Loss of steam • Loss of instrument air
Controller failure or Human error under manual operation • Failure of the steam controller • Failure of the pressure controller • Failure of the feed controller
• Failure of the reflux (or pumparound) controller (or
pump)
Extraneous sources • Valve opening to an external pressure source • Loss of heating in an upstream column • Failure of exchanger
• Exterior fire
Internal sources • Accumulation of noncondensables • Chemical reaction
• Closes column outlets
Transient sources • Pockets of water in a hot tower • Steam hammer
• Internal explosions
Fig. 4. Causes and consequences of malfunctions in distillation columns from ARIA database (1990–2017).
network. The failure of one of the level controls of this column caused its excessive level increase. This abnormal operation also augmented the temperature of the liquid stored in the column. Then, the interaction ofthe energy network caused that the hot bottoms also heated up the feed flow to hazardous levels. Both failures caused the flooding of the column and the subsequent overflowing(Kalantarniaetal.,2010;MancaandBrambilla,2012). This deviation initiated a sequence of events that led to the re-leaseofan explosive vaporcloud, whichlater ignitedandcaused 15deathsand170injuries.Certainly,thiscaseillustratesthe influ-enceofthevariationsofthefeedconditions(operationaldifficulty) andthefailuresofthe controlsystems.Thiscasealsoshowsthat thedisturbancesinadistillationcolumnmightbecomemore rele-vantwhenthechemicalprocessisinteractiveduetothepresence of a recycle stream (Smith and Corripio, 1985). The interactions amongtheprocessunitsenhancethepropagationofadisturbance; hence,theymustbetakenintoaccountfortheevaluationof con-sequencesduringariskassessment.
Likewise, other computational characterizations of the con-trol structures of distillation columns (Bezzo et al., 2004; EbrahimzadehandBaxter,2016;Luyben,2012a)havealsoanalyzed thedrasticcoolantflowratereductionasthemainhazardousevent duetoahighpressurizationofthesystemsinwhichthisdeviation hasoccurred.Anexampleofthissituationisregisteredonthe ac-cident reportsof thedatabase ofthe Bureauof Analysis ofRisks andIndustrial Pollution (BARPIARIA, abbreviated inFrench). The reportN° 45,345 ofthisdatabaseregisterstheleakage of55tons ofmethanolonMarch16,2013,inabiodieselproductioncenterin Limay,France.Themaincauseisattributedtothefailureofa mo-toroftherefrigerationsystemthatincreasedthetemperatureand the pressure of a distillation column. This fact led to the subse-quentreleasethrougharupturedisk.Thisexampledescribeshow therefrigerationlossemerged froman electricfailurethat affects thecoolantsupply.
Previousdynamic analyseshavesimulatedcoolingsystem fail-ures by setting the pressure control to a manual mode and
re-ducing theflowrateofthecorresponding coolant.Inthismanner, all thecoolingfactorsthat maycausean overpressureofthe sys-temcanberepresented.Thissituationhasalreadybeenassociated with accidents occurredin the industry. For instance, the report N° 22,626oftheBARPIARIA(2016)reportsalossofcontainment in a gasoline stabilization columnof a refinerylocated in Feyzin (France)in 2002.The releaseofa pressurizedhydrocarbons mix-turewascausedbytheopeningofaprocesssafetyvalve.Thefinal report attributed the accident to an overheating occurredduring thestartupofthecolumnwhenthepressurewas manually regu-latedbytheoperator.
3.3. Definitionofthesimulationscenarios
The literature and lessons learnt from accidents established how a change in the feed conditions and the heat removal fail-ure might lead to flooding or overpressure in a distillation col-umn. These deviations are simulated in the benzene alkylation process in order to estimate their propagation effects. Addition-ally, Kister(1990) listedthe failuresanddeviations leading to an overpressureinadistillationcolumnandtheirpotentialassociated causes(Table6).Oneofthesedeviationsisalsoevaluatedinorder toestimate its potentialimpacts onthechemicalprocess aswell. Forthiscase,anextraneoussourcerelatedtotheheatingefficiency is studied.In accordancewiththisdefinition,this studyanalyzes three differenttypes ofscenarios according toa deviation ofthe followingprocessvariables:
• Flowrateofthemakeupstreams(feedconditions). • Coolantflowrateofeachcondenser(heatremoval).
• Steamtemperatureofthereboilers(heatinginanupstream
col-umn).
The direct influence of thesevariables on the performance of thetrainofdistillationcolumnsisestablishedwiththedescription of thedeviation effects on thechemical process units aswell as the potentially hazardous consequences. Subsequently, the flood-ingandoverpressurerisksassociatedwiththesedeviationsare es-timatedaccordingtothesimulationresults.Forthispurpose,each deviationisimplementedinasimulationscenarioasastepchange inoneoftheprocess variableslistedabove.Thedeviationofeach scenario occursaftera steady-state periodof6 h.Thereafter, the dynamic responseofthesystemisanalyzedforan additional pe-riod of24h.Inthismanner,itis possibletoestablishtheeffects ofthedeviationpropagationwithadescriptionofthetransient be-haviorofsomekeyprocessvariables.Thedynamicsimulationsare developedwithoutconsidering somecomplementary safety barri-ers such asalarms, operator’s response or integrated safety sys-tems.Thus,theprotectionlayersanalyzedinthisstudyonly corre-spondtobasicprocesscontrolsystemsandpressurereliefdevices.
4. Variationoftheflowrateofthemakeupstreams(scenarios 1to4)
The first type ofsimulation scenarios deals withthe flowrate variationofthemakeupstreamcomposedofpropeneandpropane. Thesteady-statevalueofthisvariableis100.9kmolhr−1.The
de-viationsaresimulatedbysettingastepvariationoftheflowrateof thestream“ALIPHATI” (Fig.2).Forthisanalysis,fourscenariosare simulatedwithdifferentvaluesofthismakeupstreamflowrate:
• Scenario1:Aliphaticflowratereducedby15% • Scenario2:Aliphaticflowratereducedby5%
• Scenario3:Aliphaticflowrateincreasedby5% • Scenario4:Aliphaticflowrateincreasedby15%
4.1. Effectsofthedeviationontheperformanceofthepackedbed reactor
The actionoftheflowcontrollerduetotheflowratedeviation ofaliphatichydrocarbonsmodifiesthepropene/benzeneratio.This fact causesan importantchangeoftheconversionandselectivity ofthealkylationreactions.Fig.5showsthatasurgeofthepropene flowrateincreasesits conversiondueto ahigherconcentrationof thishydrocarboninthereactivemixture.However,italsoenhances theproductionofdiisopropylbenzene,whichrepresentsadecrease of the chemical reactions selectivity. This fact allows concluding that thedeviationsoftheflowrates haveoppositeeffectson con-versionandselectivity(Fig.5).Itcanalsobenotedthattheprocess reachesanewsteadystateaftereachdisturbance.
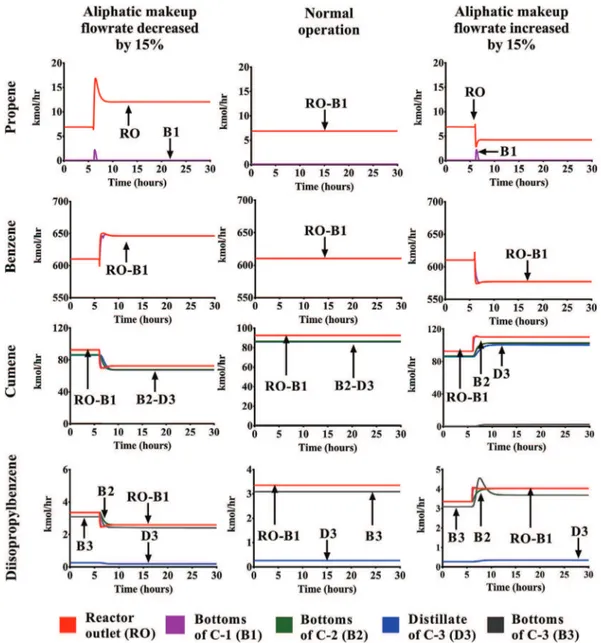
Furthermore, the variations ofthe benzene alkylation ratesin the packed bed reactor induce the propagation of the deviation throughtheseparationunit.Thisfactcanbeexplainedbythe con-version resultsinthereactor.Thecumeneanddiisopropylbenzene flowrates change accordingto the benzene variation. As a result, thefeedflowratesofthethreedistillationcolumnschangeaswell. These effects can be clearly observed by analyzing the flowrates ofthe followingstreams:Reactor outlet(RO), bottomsofC-1,C-2 andC-3andthedistillateofC3.Forthispurpose,theresponsesof thesevariablestothegreatestpropeneflowratevariations (±15%) arecomparedwiththenormaloperationvaluesinFig.6.
Fig. 6 showsthat the makeup streams variations have an ef-fect onthechemical processbehavior.Theeffectsobservedinthe reaction conversion and selectivity represents a significant varia-tion in theflowrates of each hydrocarbonalong the process. De-spitethe factthat thedeviationonlymodifiesthepropenemolar fractioninthereactorfeedbetween0.10(−15%)and0.14(+15%), thereisanimportantvariationinthecumeneand diisopropylben-zene flowrates: adecrease of22% (−15%) andan increase of18% (+15%).Thesedeviationsarenottemporarybutpermanent;hence, they induce anothersteady-statecondition fortheindustrial pro-cess.Theseresultsshowthatthedeviationpropagationcaninduce magnified effects along the process units. Thus, it is also neces-sarytoanalyzetheperformanceoftheseparationequipment.This analysisallowsidentifyingthehazardouseventsthatmightemerge duetoasignificantvariationofthecompositionofthereactorfeed stream.
4.2. Effectsofthedeviationontheperformanceofthedistillation columns
The only scenario that generates an overpressure of one of the distillation columnscorresponds to an increase of15%ofthe aliphaticmakeup stream.ThecolumnC-3is submittedtoa pres-sure increase of0.34bar whenthis deviationoccurs(Fig.7). The pressurecontrolloopofthiscolumndeterminesanewsteady-state pressure atthetopofthecolumn. Thisoverpressureisbelowthe setpressure(SP)andtheprimarylift(PL)pressurespecifiedforthe valve PSV–3inTable 5. Therefore,thesafetyvalve doesnot open andthetopvaporsarenotventedforthisdeviation.Thisresult es-tablishesthat anincrease of15%inthepropenemakeup flowrate doesnot constitutean accident byitself butitcontributes signif-icantly to a pressure increase atthe top ofthe columnC-3. This abnormal operation mightresult inan accident if theeffects are enhanced byanotherhazardousevent(e.g.failure inadistillation temperaturecontrol).
Furthermore, the flooding of a distillation column represents an uncontrollable accumulation of liquid in the packed bed that makes a continuous operation of the column more difficult
Fig. 5. Evolution of the propene conversion and the cumene selectivity during the deviation.
Fig. 7. Pressure increase of the distillation column C-3 for the scenario 4.
(Kister,1990).Thisabnormaloperationdemandsnotonlythefeed flowrate regulation but also the action of the temperature and pressure controllers inorder to regulate the heat transfer in the reboilers and thecondensers andadjust the liquid/vapor ratioin the column. Theflowrate variationsalso affectthe hydraulic per-formance ofthe distillation columns.Indeed,flooding or entrain-mentcanemergeinthecolumniftheliquidorgasratesare exces-sive. Forthisreason,columnsthat operatewithnon-foaming liq-uidsare usuallydesignedforgasvelocitieslower than80%ofthe critical flooding velocity (Treybal, 1980). The columnstage flood-ing factorisdefinedastheratiobetweenthegasvelocityandits criticalvalueinthistheoreticalstage.
Let us consider the case where the flowrate represented by ‘propeneandpropanemakeup´inFig.1changes±15%(scenarios1 &4).Thesedeviationspropagateinseveralstages:
• The packedbedreactor isthe firstequipment affectedby the
deviation.The conversionandselectivity ofthechemical reac-tionschangeinaccordancewiththereagentratio.The compo-sitionofthereactoroutlet(ROinFig.1)changes.
• Then,columnC-1isfedbytheoutletofthereactor.In
conse-quence,theseparationinthiscolumnismodifieddueto vari-ationsinthevapor-liquidequilibria.Theflowratesand compo-sitions ofthedistillateandbottoms ofthiscolumn(D1 &B1) varywithregardtothenormaloperatingconditions.
• AsimilarpropagationeffectisobservedincolumnsC-2and
C-3.
• Moreover, the propagation is enhanced by the presence of a
recyclestream,which corresponds tothe distillateflowrateof columnC-2(D2).Theloopgeneratesasecondaryeffectonthe reactor feed (RF). This fact increases the propagation effects listedinthepreviousbullets.
In orderto illustrate these propagations,Fig. 8represents the flooding factors inthe distillation columns duringthe deviations (scenarios1&4).Theirdynamicresponsesareclassifiedintothree differentbehaviorsaccordingto theinfluence ofthehydrocarbon withthehighestflowrateinC-1andC-2(benzene):
Firstly,thefloodingfactorsofthecolumnC-1evidenceadrastic changeduringaperiodof1.5happroximately.Thisobservationis dueto thesurgeofbenzene whenpropeneflowrate isdecreased (Fig.8A)orduetothe surgeofthealkylated hydrocarbonswhen itisincreased(Fig.8D).Thisabnormaloperationrepresentsan im-portantdeviationthatiscausedbythehighrefluxratio(44.5)that is requiredtoseparate the smallamountsof thealiphatic hydro-carbons. However, the flooding variations are temporary due to the regulationofthe flowcontroller that isinstalled on thefeed streamofthepackedbedreactor.
Secondly,the changesofthe flooding factorsofC-2 issmaller but sustained. Fig. 8B and E show that the second column is moderately affected. Indeed, it normally separates the unreacted
benzene (600 kmol·hr-1 approximately). Therefore, the distillate flowrate only varies between-4.4% for thescenario 4 and+4.7% forthe scenario1.Asa result,no significanteffectisobserved in theliquidholdup.
Finally, Fig.8C andF show a greater variationin thelast col-umn duetothechangesofthefeedflowratesofthealkylated hy-drocarbons(Fig.6).Thisresultunderlineshowthedeviation prop-agates through all theprocess equipment andinduces a flooding phenomenon.
Finally, Fig.9 showsthe influenceof thedeviation magnitude on the numberof floodedstagesof the distillation columns.The extent ofa deviationhasa radicalinfluenceonitscorrection and the systembehavior (Labovský et al., 2007). Forthis reason, the initial and final numbers of flooded stages in the most affected columns (C-1 and C-3) are compared forthe±5% and±15% sce-narios. The simulation allows establishing the critical values for the process deviationby analyzingthedynamic response inboth columns.Fig.9Ashowsthatthetemporaryabnormaloperation as-sociated withthe partialflooding ofthe columnC-1is enhanced by theincrease ofthebenzene flowrate.The reductionof−5%to −15%impliesthechangefromonetosixfloodedstages.Therefore, the equipment is submitted to a longer period of instability due totheflooding.Similarly,Fig.9Bshowsthattheaffectationofthe increase ofthealiphaticcompoundsfrom+5%to+15%represents thefloodingofsevenadditionalstages.
This section of the paper only discusses the deviation effects ontheflooding levelofthedistillation columns.Nonetheless, this analysis canbe completed bythe studyoftheevolution ofother processvariables.Forexample,considerthesteamconsumptionin thedistillationcolumnC-1whenthealiphaticmakeupflowrateis decreasedby15%.Forthisdeviation,thepropeneflowrate diminu-tion implies the increase of the benzene flowrate at the reactor outlet. Thisfactleadstoagreaterconcentrationofthiscompound at the feed of the column. The greater presence of heavy com-pounds in this column altersthe vapor-liquid equilibria. Thus, it isnecessarytohaveahighersteamconsumption(+20%)inorder toregulatethetemperatureofstage3ofcolumnC-1.
5. Coolantflowratereductioninoneofthedistillation columns(scenarios5to7)
The second type ofsimulationscenarios envisagesthecoolant flowrate reduction in the condenser of one of the distillation columns in accordance with the lessons learnt discussed in Section 3.2.For this purpose,three additional scenarios are pro-posedfortheanalysisofthedynamicsimulations.Thesimulation resultsdescribetheresponseofthechemicalprocesstothe reduc-tionofthecoolantflowrateinoneofthedistillationcolumns.The followingdeviationsaresimulatedbysettingapressurecontroller tomanualmodeandadjustingthevalueofthecoolantflowrateto 20%ofthesteady-statevalue.
• Scenario5:Coolantflowratereducedby80%inC-1 • Scenario6:Coolantflowratereducedby80%inC-2 • Scenario7:Coolantflowratereducedby80%inC-3
5.1. Effectsofthedeviationontheperformanceofthedistillation columns
The heatremovalreduction representsavariation ofthe ther-malloadsinallthedownstreamheattransferequipment.Initially, the failure of the cooling unit increases the outlet temperature of the remaining coolantbut the action isinsufficient. In conse-quence, the column modifies its vapor and liquid flowrate pro-files anddeteriorates its separation efficiency. This fact enhances the propagationofthedeviationsincethe feedcompositionsand
Fig. 8. Variations of the flooding factors of the distillation columns caused by the changes of the makeup flowrates.
Fig. 9. Influence of the makeup flowrates variation on the column flooding (C-1 and C-3).
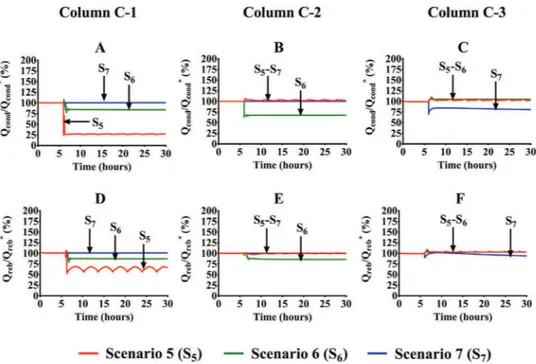
flowrates of the downstream equipment are determined by the vapor-liquid equilibriainside thecolumnwiththedeviation. This resultcanbeobservedinFig.10,whichshowsthevariationsofthe thermal loadsthat arepredictedby thedynamic simulations. For this purpose,Fig. 10A–C show theratio betweenthe heat trans-ferred ineach condenser (Qcond) andits initial steady-state value
(Q∗
cond). Likewise, Fig. 10D to F show the ratio betweenthe heat
transferred ineachreboiler(Qreb) anditsinitialsteady-statevalue (Q∗
reb).
Inaccordancewiththeheattransfervariations,thedynamic re-sponseobservedineachscenarioisdescribedasfollows:
• Scenario5:ThecoolantflowrateisreducedinC-1from29,132
to 5826kg hr−1 (−80% inFig. 10A).The effects are not
com-pletely corrected by the action of thetemperature controllers ofthedistillation columns.Thecontrol ofthecolumnC-1 im-mediately reducesthesteam temperatureinorder toregulate thetemperatureprofileofthecolumn. However,the deviation
remains duringtherestofthesimulationtime,whichalso im-plies a continuous overheating in the column. Both contrary effects induce the periodic oscillations that are observed in Fig.10D.Thisphenomenonaffectsthetemperatureand compo-sitionoftheproductsofthecolumn,whichcausesthe propaga-tionoftheperiodicbehaviortotheothertwocolumns(Fig.10B andCandEandF).However,theamplitudeisinferiorinthese columns dueto the actionof morecontrol loops through the mainstreamsoftheprocess.
• Scenario 6: The coolant flowrate is reduced in C-2 from
195,664 to39,133kghr−1 (−80% inFig.10B). Inconsequence,
the upstream anddownstreamcolumns areaffected since the heat transfer is notably reduced inC-1 andslightly increased in C-3.Thisfactcorroborates againthehighinteractivity level ofthe systemthatiscaused bytherecyclestreamofthe sys-tem.Thereductionoftheseparationlevelofthecolumnaffects the makeup flowrates ofbenzene andaliphatic hydrocarbons. In fact, the decrease ofthe heat transfer in C-2 is associated
Fig. 10. Heat transfer variations occurred after the coolant flowrate reduction in one of the distillation columns.
withareductionofthebenzenefedtothecolumn,whichalso reducesthepropenedilutioninthereactingmixture.This ab-normalconditionenhancesthereactionrate(pseudofirst-order kinetics)butdiminishesthecumeneselectivity.Thisincreaseof thediisopropylbenzeneproductionexplainstheincreaseofthe thermalloadsinC-3.
• Scenario7:ThecoolantflowrateisreducedinC-3from42,165
to8433kghr−1(−80%inFig.10C).Theeffectsofthisdeviation
onthethermalloads oftheseparationequipmentare negligi-ble inthe upstream columns in spite ofthis reduction. Thus, thesystemonly manipulatesthe steamtemperatureinC-3 to regulatethetemperatureprofileofthiscolumn.
Thesimulationresultsdiscussedaboveshowthatthedeviations ofthecondensersofC-1andC-2involvegreaterrisksthanthe C-3 condenser deviation due to their highpropagation levels. This resultcanbeexplainedbyconsideringtheflowrateprofilesshown inFig.11.
The main issues emerged from the failuresof the condensers ofC-1andC-2are associatedwiththeundesiredfeedofpropene to the column C-2 (bottoms ofC-1) andbenzene to thecolumn C-3 (bottomsof C-2).These abnormalconditions implythe pres-ence of light hydrocarbons in the low-pressure equipment. This fact deterioratestheperformance andseparationefficiencyofthe distillation columns in both scenarios. However, the controllabil-ityof thethermalloads afterthe deviationofthe C-1condenser indicates a different dynamic response for the benzene flowrate at the bottoms of C-2. The C-1 condenser has a periodic behav-ior whereas the deviation inC-2 defines a sustainedincrease. In consequence, both scenarios differ notably regardingthe severity magnitude. The difference of the severity levels of both scenar-ioscanbe illustratedinFig.11withthevariationsofthereagents flowrates.Themiddlecolumnofthischartshowsthatthebenzene andpropeneflowrateshaveadrasticdecreaseaftertheoccurrence ofthedeviationinC-2.Obviously,thedistillateofC-2hasan im-portant reduction in its flowrate that cannot be compensated by theflowcontrolofthereactorfeed.Thisresultexplainsthe reduc-tionofthethermalloadsthatisdiscussedabove(Fig.10).
Moreover, the flowrate variations through the process can re-sultincertainhazardouseventsassociatedwiththeliquid
contain-mentinothervesselsoftheseparationunit.Forinstance,Fig.12A– C show thatthereduction ofthecoolantflowrateofacondenser causes an immediate decrease of the liquid level in the corre-spondingrefluxdrum.Thisfactresultsinthedamageofthereflux pump dueto aninsufficientliquidfeedrate. Inthesamemanner, thesumpsofC-1andC-2haveaslightlevelincrease(Fig.12Dand E) whereasthesumpofC-3iscompletelyfilled(Fig.12F).Thisfact inducesanoverfillingatthebottomofthecolumnthatreachesthe inlet nozzleofvapor comingfromthereboiler. Theseissues con-firm thenecessityofsettinguphigh-levelandlow-levelalarmsin therefluxdrumsandthebottomsofthedistillationcolumns.
This result illustrates the necessity of implementing a set of protection layers in the distillation columns. The departure from normal conditions of the liquid levels, temperature or pressure activates the corresponding alarm in order to alert the operator. However, greater deviation levels require the action of override systemstomaintaintheprocessoperation.Inthiscase,safety bar-rierssuch astheactivationofredundantequipmentorutilitiesto increase reliability canbe added.Additionally, the interlock shut-down systems can be included in order to avoid events that af-fecttheintegrityofthesystem.Theseprotectionlayersarenotthe subjectofthispaper.However,their sizingwillbepartofa com-plementarystudy.
5.2. Ventreleasesinthedistillationcolumns
The severityof thethree simulatedscenarios can alsobe dis-criminated by the overpressure levels reached in the distillation columns.Thedynamicresponsesoftheseprocessvariablescanbe describedasfollows:
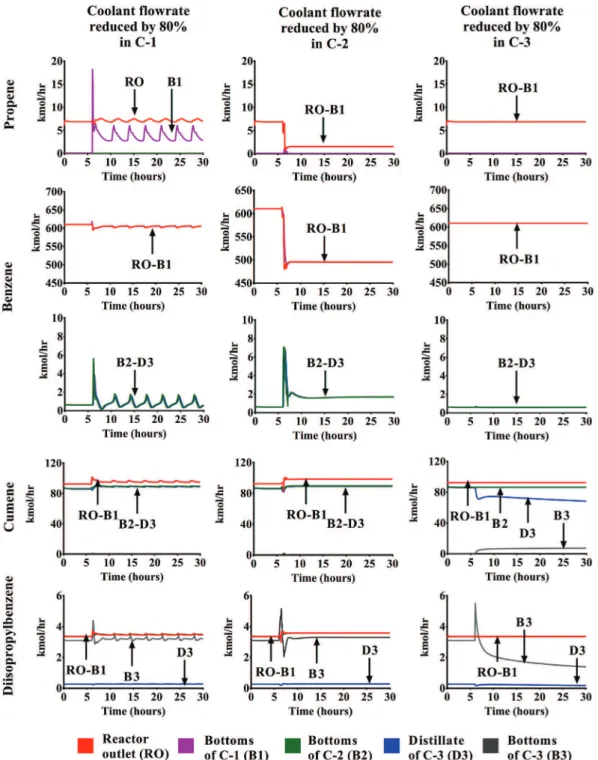
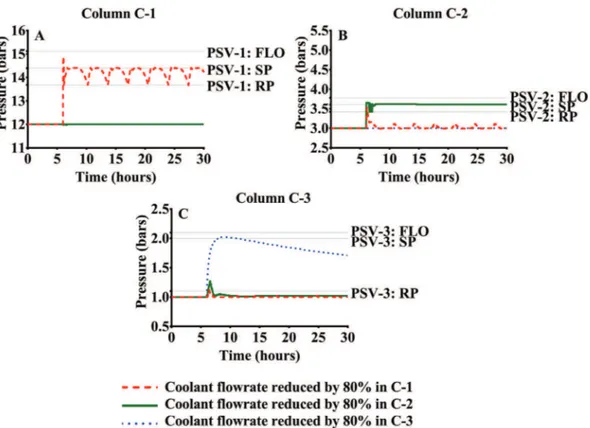
• Scenario5:Thedeviationimmediatelycauses anoverpressure
inthethreedistillationcolumns.Theoverpressuresofthe sim-ulatedscenarioarecharacterizedbyaperiodicbehaviorineach one oftheseparationequipment.Fig.13Ashowsthatthe ma-jor increase (2.5bar) is established in the column with the deviation whose top stage overcomes the set pressure of the process safetyvalve (14.4bar). In accordance withthis result, Fig.14Aindicatesahighventreleaseinthistowerafterthe oc-currence ofthe deviationthat is followed by periodicsmaller
Fig. 11. Influence of the coolant flowrate reductions on the molar flowrates.
vent releases. On the contrary, the other columns have pres-sureincreasesbelow0.5bar. Duringthesimulation,the maxi-mumoverpressuresofC-1andC-2arebelowthesetpressures oftheir relief devices.Therefore,the simulationdoesnot pre-dictanyventreleaseforthesecolumnsinthisscenario.
• Scenario6: Fig.13B showsthat the reduction ofthe heat
re-movalinthecondenserofC-2hasasimilareffectasthe devia-tioninC-1duringthefirst2.3hofabnormaloperation. There-after,theventflowratebecomessteadyaswellasthe overpres-surelevelatthetopofthecolumn(Fig.14B).Thisresultis ob-servedbecausethecolumnisdesignedtoremovetheunreacted benzene.Theanalysisoftheventflowratedefinesthisdeviation asthemostseverescenarioduetothecontinuoushydrocarbon leakage atthe top of C-2. This fact underlines that a process
safetyvalveisnotenoughtopreventahazardousoverpressure causedby afailure inthe condenser.Therefore,itisnecessary toimplementacomplementarysafetybarriertoavoidthe sus-tained feedtothecolumnC-2.Generally,an emergency inter-lockshutdownisaddedtocutthefeedtothechemicalreactor (Kister,1990).
• Scenario 7:Fig.13Cshowsthat the deviationoccurredin the
columnC-3causesanoverpressurewithalowerseveritywith regard to the two previous scenarios. In fact, the maximum pressureatthetopofC-3justreachesthesetpressure(2.0bar). Thus,thevalvepositionoftheprocesssafetyvalveonlyshows a low opening during a short time lapse. Moreover, this sce-nario only predicts an overpressure in C-3. Since the column
Fig. 12. Influence of the coolant flowrates reductions on the liquid levels in the reflux drums and sumps.
Fig. 13. Pressure increase in the distillation columns after the coolant flowrate reduction in one of the condensers FLO: full-lift opening pressure SP: set pressure RP: reseating pressure.
C-3isoutoftherecycleloop,thedeviationeffectsdonothave interactiveconsequencesontheothercolumns.
6. Lossofheatinginanupstreamcolumn(scenarios8&9)
Inthispart,thelossofheatinginanupstreamcolumnis stud-ied. Thisdeviation hasbeen previously discussedin the research work of Kister (1990). This author shows that the reduction of the heating efficiency ina distillation tower can inducean over-pressureindownstreamequipment.Thisfactisduetothe signifi-cantdecreaseofthevaporflowrateintheupstreamcolumn,which
reduces its liquid holdupand generatesits weeping or dumping. Thereafter, the pressure drop of this column is considerably af-fected along with the separation efficiency of the equipment. In consequence, thefeed rateofdownstream equipment,as well as the fractionoflight compoundsentering toit,aresubmitted toa drastic increase.Theoccurrenceofthiseventmayincrease exces-sivelythepressureofthedownstreamdistillationcolumnsbecause ofthepresence oflight hydrocarbonsinthelow-pressure separa-tionequipment.Thepropagationeffectsassociatedwiththis devi-ationareconsideredinthissectionthroughtwoscenarios:
Fig. 14. Vent flowrates generated by the coolant flowrate reduction in one of the condensers A. Column C-1 (Scenario 5) B. Column C-2 (Scenario 6).
• Scenario8:Steamtemperaturedecreasedby15°CinC-1 • Scenario9:Steamtemperaturedecreasedby15°CinC-2
6.1. Effectsontheperformanceofthedistillationcolumns
The analysis of the propagation effects of the simulated sce-nariosisinitiallybasedonthecomparisonoftheflowrateprofiles withthoseofthesteady-stateoperation.Forthispurpose,the sim-ulationresultsshowninFig.15areconsideredtodescribethe dif-ferentdynamicresponsesofthechemicalprocess:
• Scenario8:Thevariationofthesteamtemperatureconstitutes
a permanent reduction of 12% in the heat transfer ofthe re-boilerofC-1.Theeffectsofthisdeviationareshownintheleft columnofFig.15.Thisdeviationinducesatemperature diminu-tion inthecolumn. As a result,the aliphaticcompounds con-centrate atthebottom ofthe columninsteadofthe distillate. Forthisreason,an importantincrease ofthepropeneflowrate inthebottomsstream(0to 12kmolhr−1)canbe observedin
Fig.15A.Inaddition,theresultsunderlinethatthepropagation of this deviationalso increases the flowrate of this hydrocar-bon from 6to 13 kmol hr−1 at the outletof the packed bed
reactor. Therefore, it ispossible to identify a magnifiedeffect in the propagationthrough C-1 since a poor propene separa-tionisaccompaniedbyahigherpropenefeedrate.This abnor-maloperationcanbeconsideredasasevereconditionsincethe bottoms propene flowrate should be approximately zero. The variationsofthepropeneflowrateshaveadirectimpactonthe benzeneflowrates(Fig.15BandG).Evidently,thealiphatic hy-drocarbonsfed toC-2are recoveredatthetop ofthecolumn. This factimpliesa lower benzene recovery inthe distillate of thistower along withthe increase of its concentration inthe bottoms stream.Inconsequence,a highamount ofbenzeneis temporarily fed to the column C-3 and the benzene makeup flowratediminishes permanently. Finally,Fig.15JandM show that theflowratesvariationsofthealkylated hydrocarbonsare not significant. The rise of the benzene feed to C-3 increases temporarilytheamountofcumeneinthebottomsofC-3. How-ever,thispropagationeffectisnotpermanentduetotheaction ofthecontrollers.Therefore,theproductionofcumeneand di-isopropylbenzenedoesnotpresentsignificantchanges.
• Scenario9:Thevariationofthesteamtemperatureconstitutes
a temporary reduction of 5.5% in the heat transfer ofthe re-boiler of C-2. The effects of this deviation are shown in the rightcolumnofFig. 15.Thisscenario differs notablyfromthe scenario 8becausethe deviationpropagationonly hasan im-portant effect on one of the columns. This result can be ob-servedbycomparingFig.15GwithI.Thereductionofthesteam temperatureinC-1causesatemporaryincreaseofthebenzene flowrateatthe bottoms ofC-2.However, thereduction ofthe steaminC-2columnmakesitpermanent.Thesesimulation
re-sults allow establishingthat the systemiscapable toregulate the benzeneflowrateinB2 whenthefailure isinC-1 butnot when it isin C-2.Additionally, scenario 9doesnot constitute a significant variation ofthe propeneflowrates. Thisresult il-lustratesthat thelocationoftheprocessdeviationinduces dif-ferent issues inthe chemicalprocess operation. Evidently,the presenceofarecyclestreamconstitutesamitigationfactorfor the benzene flowrate regulationand a propagation factor for thepropeneflowrate.
Furthermore, the response of the column flooding factors in scenarios 8 and 9 is similar to that observed in the makeup flowratevariationsscenarios.TheseeffectsareshowninFig.16.
Thesimulationresultsshowthatthedynamicresponsesofthe floodingfactorsofthedistillationcolumnsaredeterminedbytheir positionwithregardtotheoriginoftheprocessdeviation.Forthis reason, theanalysisofthesekey processvariables isdivided into thefollowingcategories:
• Columnswiththedeviation:Fig.16AandEshowthattheloss
ofheatinginareboilerreducethefloodingfactorsofthestages of a distillation columnthat are located below the feed inlet in adistillation columnwhereas theupper stageshavean in-crease.Theseeffectscorresponddirectlytotheaccumulationof thelight hydrocarbonsatthebottomofthecolumncausedby thelowerboilingratesinthesecolumns.
• Downstream columns:Fig.16B andFshow thatthebehavior
ofthefloodingvariablesinthisequipmentismainlyassociated with the benzene feed rates. The temporary variation that is observed with thedeviation inC-1 andthe permanent varia-tionthatisobservedinthedeviationoccurredinC-2decrease thefloodingfactorsofthedownstreamcolumns.Thesechanges correspond to the reduction of the gas velocity through each distillationcolumn.Apriori,anincreaseofthesefactorswould be expecteddueto the inlet ofthe light hydrocarbons. How-ever, this is not the case because the densities of the rising vapors decreasebecause of thechemical compositions. In ad-dition, the hydrocarbons that should be recovered in the dis-tillates(benzeneforC-2andcumeneforC-3)arenow concen-trated at lower stages.This fact represents an increase of the down-coming liquid flow aswell. The characteristics that are discussedabovearealsoobservedinthelastcolumn(Fig.16C). Therefore, it is possible to establish that the decrease of the flooding factorsisobserved witha lowerintensityforthelast equipment.
• Upstreamcolumn:Inspiteofthepresenceofarecyclestream
inthechemicalprocess,thefloodingfactorsofthecolumnC-1 are not significantly affected whenthe heating ofthe column C-2isdecreased(Fig.16D).
The simulation results show that the column flooding should not be considered as a severe negative consequence of the two
Fig. 15. Influence of the loss of heating in a distillation column on the molar flowrates.
consideredscenarios.Forthisreason,thistypeofeffectsismainly associatedwiththeventreleasesthatarecausedbythe overpres-suresatthetopoftheseparationequipment.
6.2. Ventreleasesinthedistillationcolumns
The deviation propagations on the reagent flowrates induce different overpressure levels in the distillation columns. Initially, Fig.17showsthat thedeviationoccurringinC-1resultsina per-manent overpressure at the top of C-2 and a transient increase atthetopofC-3.Theoverpressureemerged fromthe heating re-ductioninC-1isassociatedwiththepermanentpropeneincrease at the bottoms of C-1and the transientbenzene increase atthe
bottoms of C-2. Similarly, the heating reduction in C-2 induces an overpressure in C-3 by the presence of benzene in its feed stream.However, bothscenariosdifferfromeach othersinceonly the deviation in C-1 causesan overpressure that reachesthe set pressure of a process safetyvalve (Fig. 17A).In consequence,the only hazardous eventistriggered by the decrease ofthe heating steam temperatureinC-1(Fig.18). Theprocess safetyvalve PSV-2 is opened, which induces a vent release of hydrocarbons.This flowrateiscontinuouslyincreasing duetotheinfluenceofthe re-cyclestream.Indeed,thepropenelossthroughtheventgenerates adecreaseofthebenzenemakeupstreambytheactionofthefeed control ofthechemical reactor(BENZENE_FC).Inconsequence,an
Fig. 16. Variations of the flooding factors of the distillation columns caused by the loss of heating in an upstream column.
Fig. 17. Pressure increase of the distillation columns after the loss of heating in a distillation column FLO: full lift opening pressure SP: set pressure RP: reseating pressure.
Fig. 18. Vent flowrate established in the column C-2 for the scenario 8.
additionalamountofnon-reactedpropenewillbe ventedthrough PSV-2aswell.Thisphenomenonwillinduceasnowballeffect dur-ingthedeviationpropagation.Thispropeneincreaseislowduring the initial stage of the deviation(0.69 h after the occurrence of thedeviation).Then,thisescalatingabnormaloperationleadstoa surge of propenefromC-1 to C-2 that isobserved through a 6% reduction of thedistillate flowratein C-1.Moreover, the increase
oftheventflowrateisalsoduetothehysteresischaracteristicsof PSV-2(Fig.3), whichcontributestothediscontinuityobserved in Fig.18.
This result establishes that this scenario must be prioritized overthedeviationinC-2duetoitshigherpotentialnegative con-sequences. However, the loss of heating in C-2 also describes a pressureincreaseinthecolumnC-3(Fig.17B).Thus,itisalso nec-essaryto considerthisscenario inthe risk assessmentbecausea greater reduction ofthe steam temperaturein C-2 mayresult in anotherventreleaseinthecolumnC-3.
6.3.Effectsofthedeviationontheperformanceofthepackedbed reactor
Fig.19showsthatscenario8developsanewsteady-state oper-ationthatisdefinedbyareductionof5%intheconversionrateof propeneandadecreaseinthecumene/by-productratio.Previously, itisdiscussedhowthisdisturbanceresultsinahigherfeedrateof propenetotheseparationunit.Inaccordancewiththisstatement, itispossibletoanalyzethepropagationprocessbyconsideringthe factorsthataffectnegatively theconversioninthepackedbed
re-Fig. 19. Influence of the loss of heating in a distillation column on the conversion of propene and the selectivity of the reaction.
actor. The simulation results show that the presence ofaliphatic hydrocarbons inthedistillateofC-2 reducesthetoptemperature of thiscolumn from120.8°C to 106.3°C.This fact diminishes the feedtemperatureofthe reactorfrom170°Cto 161.3°Cand there-forethekineticsofthechemical reactionisnegativelyaffected.In thismanner,itispossibletoexplainthesynergiceffectthatis dis-cussedabovebyconsideringtheeffectsontheyieldofthe alkyla-tionreactions.
Upstream the reactor the permanenttemperature decrease of the streams indicates that the temperature andflow controls do nothaveasufficientresponsetothistypeofdeviations.The reac-tor feedremains ata low temperatureevenifthe heating steam oftheexchangerE-2reachesthemaximummanipulatedpressure. This resultis due to the propeneflowrate increase and thenew vapor-liquidequilibriaofthecolumnC-2.Thisabnormaloperation canonlybestabilizedbyacontinuousventandanexcessive recy-cleoflighthydrocarbons(Fig.18).
7. Discussionoftheprocessdeviationresults
The threetypesofsimulation scenariosthat areconsidered in thisstudydiffernotablyinthe severityandlocation ofthe nega-tive effectsassociated withthepropagationofthe process devia-tions. Forthisreason, ariskassessmentiscarried outinorderto ranktheabnormaloperationscenarios.Riskanalysisisa decision-making tool that allows performing a tolerability judgment. This can be achievedby analyzing theevolution of an operating vari-able. Forthis study, the risks associatedwith flooding and over-pressurephenomenaarediscussed.Scenarios2and3donot gen-eratetheserisks.Thus,theyarenotconsideredinthissection. 7.1. Pressuredropratio
The pressuredroppermeterineach distillation column(
1
Pr)is determinedasthe ratiobetweenthepressure dropin the col-umn(
1
P)anditsheight(H).Thisoperatingparameterdetermines acomparativebasisofthefloodingorweepinglevels.Forthis rea-son,it istakenintoaccount fortherisk assessmentofthisstudy. The dynamic analysis considers the scheme shown in Fig. 20 to establish themaximum variation ofthe pressuredrop ratioafter the occurrenceofthe deviation(1
Pr). Likewise, the analysisalsodetermines the time thatis requiredby the columntoreach the maximumvariation(
1
t).Theresponsesofthethreedistillationcolumnstoeachprocess deviationareshowninTable 7andFig.21.Forinstance,the ma-jor variations of the pressure drop ratio in the column C-1 are observedfortheincrease ofthe aliphaticflowrateandthe reduc-tionofthesteamtemperatureinitsreboiler.Thesebehaviorsagree with the surge of the flooding approaches in Fig. 8A as well as
Fig. 20. Determination of the maximum deviation and its corresponding time lapse ( 1t).
their rapiddecreaseinFig.16A.Similarly,thisanalysiscan be ex-tended forthe analysis ofconsequences in the columns C-2 and C-3.
The risk assessment presented in this section evaluates the floodingorweepingrisksemergedfromallthedeviations.Forthis purpose,aseverityindexisproposedwiththefollowinglevels:
1. Lowseverityindex(valueequalto1):Maximumvariationofthe pressuredropratiolesserthan10%ofthesteady-statevalue. 2. Mediumseverityindex(valueequalto2):Maximumvariationof
the pressure drop ratio between 10% and 20% of the steady-statevalue.
3. Highseverityindex(valueequalto3):Maximumvariationofthe pressuredropratiogreaterthan20%ofthesteady-statevalue. Likewise,thedynamicresponse(Fig.21)indicatesthetime pe-riod duringwhich thepropagations reach its maximumor mini-mum values.Italsoestablishes thetime periodduringwhichthe operators can react effectivelyto theoccurrenceof aspecific de-viation(Berdouzi,2017).Forexample,theincreaseofthealiphatic makeupflowratereachesapressuredropratioof5.3mbarm−1 in
the columnC-1 after39min(0.65h)ofabnormaloperation.The flooding phenomenon dynamicsis takenintoaccount witha sec-ondindexwhoselevelsarebasedonthetimeelapsedtoreachthe maximumvariation:
1. Slowdynamicsindex(valueequalto1):Pressuredroppeakvalue reachedinmorethan30min(0.5h)aftertheoccurrenceofthe deviation.
2. Moderatedynamicsindex(valueequalto2):Pressuredroppeak valuereachedbetween10min(0.17h)and30min(0.5h)after theoccurrenceofthedeviation.
Table 7
Pressure drop ratio in each distillation column.
Deviation C-1 C-2 C-3
1Pr (mbar ·m −1 ) 1t (hours) 1Pr (mbar ·m −1 ) 1t (hours) 1Pr (mbar ·m −1 ) 1t (hours)
Scenario 1 5.32 0.65 3.85 1.54 2.11 15.65 Scenario 4 3.76 0.34 3.37 1.38 4.35 5.62 Scenario 5 2.97 0.43 3.10 0.08 3.80 1.12 Scenario 6 3.27 0.78 1.94 0.72 3.70 6.09 Scenario 7 4.30 – 3.59 – 2.17 0.14 Scenario 8 3.27 0.40 3.29 1.32 3.79 2.68 Scenario 9 4.37 0.98 3.22 0.38 3.61 0.35
Fig. 21. Variations of the pressure drop per meter generated by the process deviations.
Table 8
Flooding risk assessment risk levels: low or slow (normal) – medium or moderate (italic) – high or quick (bold).
Deviation C-1 C-2 C-3
Severity index Dynamics index Risk index Severity index Dynamics index Risk index Severity index Dynamics index Risk index
Scenario 1 3 1 3 1 1 1 3 1 3 Scenario 4 2 2 4 1 1 1 3 1 3 Scenario 5 3 2 6 2 3 6 2 1 2 Scenario 6 3 1 3 3 1 3 1 1 1 Scenario 7 1 1 1 1 1 1 3 3 9 Scenario 8 3 2 6 1 1 1 2 2 2 Scenario 9 1 1 1 2 2 4 1 2 2
3. Quick dynamics index (value equal to 3): Pressure drop peak valuereachedinlessthan10min(0.17h)aftertheoccurrence ofthedeviation.
Table 8liststhe risk indexesestimatedforthiscasestudy ac-cording to the pressure dropratios andthe response times. The risk levels prioritize the simulation scenarios that have drastic changesinthemechanicalperformanceofthedistillationcolumns. The scenarioranking shown in Table8 is based on a risk index, which corresponds to the multiplication of the severity and
dy-namicsindexes.Thisindexallows classifyingtheriskaccordingto thefollowingcriteria:
1. Low-levelrisk:Riskindexbetween1and3. 2. Medium-levelrisk:Riskindexbetween4and6. 3. High-levelrisk:Riskindexbetween7and9.
Theclassificationbasedonthemaximumandminimumvalues includes a majority of the scenarios into the medium and high-levelrisks.Forexample,theanalysisofthecolumnC-1alsoallows consideringthereductionsofthecoolantflowratesinthecolumn C-1andC-2ashigh-levelrisksituationsinspiteoftheirshort
du-Table 9
Comparison of the vent releases generated by the most severe scenario of each process deviation risk levels: low (normal) – medium (italic) – high (bold).
Deviation C-1 C-2 C-3
Overpressure (bar) Max. vent flowrate Risk index Overpressure (bar) Max. vent flowrate Risk index Overpressure (bar) Max. vent flowrate Risk index
Scenario 1 – – 1 – – 1 – – 1 Scenario 4 – – 1 – – 1 0.34 – 2 Scenario 5 2.89 204.8 3 0.53 – 2 0.12 – 2 Scenario 6 – – 1 0.65 211.3 3 0.27 – 2 Scenario 7 – – 1 – – 1 1.02 1.0 3 Scenario 8 – – 1 0.60 42.0 3 0.57 – 2 Scenario 9 – – 1 – – 1 0.48 – 2
ration orminoreffectson theoperation ofthecolumn. In accor-dancewith thetotal risk indexes, the major risk associatedwith theoperatingstabilityofthedistillationequipmentisobservedfor the coolant flowrate reduction in C-3 (scenario 7). This criterion provides a better scenario description since ittakes into account thechemicalprocessdynamics.
7.2. Columnoverpressureandventrelease
Theprevioussectionshowsthattheconsequencesofthe prop-agationofaprocessdeviationaredeterminedbyitscapacityto af-fect theinternalvaporflowrates ofthedistillation columns.Thus, it is also possible to prioritize the scenarios according to a risk analysis basedon the columnoverpressure. Inthis study,a clas-sificationisproposedaccordingtothedatalistedinTable9,which compares the scenarios that develop the highest overpressure in thethreetypesofdeviations.Theriskindexisdefinedinthiscase accordingtothefollowingcriteria:
1. Low-leveloverpressurerisk(valueequalto1):Nooverpressure inthedistillationcolumn.
2. Medium-leveloverpressure risk (valueequalto2):Insufficient overpressuretoopentheprocesssafetyvalve.
3. High-level risk overpressure (value equal to 3): Overpressure thatactivatesthePSVopening.
Theresultsindicatethatthehighestoverpressurelevelsaredue tothecooling systemmalfunctions whereastheminoreffectsare mainly associated with the changes in the compositions of the reactor feed. This result establishes a greater risk level for the coolant flowrate reduction since it constitutes the major instan-taneous vent release. Therefore, it represents a greater potential to forman explosive atmospheredueto hydrocarbons accidental leakage.
This comparative analysis can be extended to any simulated scenarioinordertoprovideadetailedcomparisonofthe propaga-tionfactorsandthenegativeeffectsofeachprocessdeviation.Asa result,thesubjectivityinthedeterminationofthehazardousevent severityandlikelihoodduringaHAZOPanalysiscanbediminished (IsimiteandRubini, 2016). In thismanner, the simulationresults canprovidethenecessarypredictiveinformationthatallows eval-uating theresponse ofacontrol structure andthepressure relief devices.
8. Conclusions
Thisworkshowsthatthedynamicsimulationisan interesting tool tostudythepropagationofprocessdeviation, usefuland es-sentialforprocessriskassessment.Thegoalofthisresearchwork isto assessthemagnitudeanddynamicsofthedeviationeffects. The feasibility of this methodology is demonstrated through the simulationofacomplexcasestudy.Thesimulationresultsare di-rectly linked to the sizing and control strategies. Therefore, the dynamic response to each deviation is obviouslyassociated with
thesesimulation settings.Thatiswhyitisimportantto establish anappropriaterepresentationofthesystem.Thus,anexperimental studyinnormalanddegraded modesisalsonecessarytovalidate thesystemmodel.
Wehaveimplementedthedynamicsimulationmethodologyon benzene alkylation process, which is composed of a reactor and a setofthree distillationcolumnsconnected bya recyclestream. Thereafter, westudythedeviationpropagationalong thisprocess. Based onlessonslearntfrompreviousaccidents, we focuson de-viations that can result in a pressure increase of the distillation columns.
The simulation results put in evidence the deviation propa-gation effects through the alkylation reactor and the distillation columns. The simulation scenarios identify the abnormal condi-tions inwhich an overpressureisfeasible aswellasthe flowrate profilesoftheeventualventreleases.Thedeviationscanresultina periodic orpermanentoverpressure ifthe mitigationsystemdoes notincludeacomplementarysafetysystem.Thisadditionalcontrol must respond directly to the disturbance or stop completely the operation of thechemical process. Thus, itis compulsoryto take intoaccount themaincharacteristicsofthefeasible process devi-ationsaswell astheoperatingconditionsofanindustrialprocess duringthedeterminationoftherequiredsafetybarriers.
Moreover,therecyclestreamcanbeapropagationfactorin ac-cordancewiththecharacteristicsofthemanufacturingprocess.For instance, the reduction ofthe steamtemperature on thereboiler ofthecolumnC-2doesnothaveanysignificant effectonthe up-stream equipment.Onthecontrary,thereduction ofthisvariable in C-1 constitutes not only the overpressure of the downstream distillationcolumnsbutalsoadecreaseof5%onthepropene con-versiontocumene.
Finally, thecomparisonofthe simulationresults isconsidered to propose a scenario classificationbased on themostsevere ef-fects that are observed inthe dynamic simulations ofthe chem-ical process. Inaccordancewiththiscriterion, thecasestudy de-fines thedrastic decrease of the coolantflowrate ina condenser as the most critical event whereas the changes of the makeup streamsrepresenttheminornegativeeffects.Nonetheless,the lat-terscenariomustalsobeconsideredbecauseitdescribesan exces-sive flooding inthecolumns.Forthisreason, thenegative effects shouldalwaysbedeterminedforeachprocessunitinthedynamic analyses inordertoestablishproperlyall theriskscausedbythe deviationpropagation.
Ofcourse,thismethodologyshould beapplied toother devia-tions, inordertobeabletoprioritize scenariosthat canresultin an industrial accident. This work contributes to the definitionof theworst-casescenariosandtherequiredsafetybarriers.The sim-ulation toolcan determinegloballythepotential effectsofa pro-cess variation andprovide predictive informationto validate the natureandthesizingofsafetybarriers.Thevalidationstepconsists in checkingthat theresidualrisk that remainsaftertheaction of safetybarriersisacceptable.