Publisher’s version / Version de l'éditeur:
Polymer Engineering and Science, 49, 4, pp. 666-672, 2009-02-19
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1002/pen.21359
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Preparation and properties of epoxy nanocomposites. Part 1. The effect of pre-mixing on dispersion of organoclay
Ngo, T.-D.; Ton-That, M.-T.; Hoa, S. V.; Cole, K. C.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=2d1e5d4c-43d9-41ad-ab4f-5e29ad1417a3 https://publications-cnrc.canada.ca/fra/voir/objet/?id=2d1e5d4c-43d9-41ad-ab4f-5e29ad1417a3
Preparation and Properties of Epoxy Nanocomposites Part 1: The Effect of Pre-Mixing on Dispersion of Organoclay
T.-D. Ngo1, M.-T. Ton-That2, S. V. Hoa1,K. C. Cole2
1
Department of Mechanical & Industrial Engineering, Concordia University 1455 De Maisonneuve Blvd., Montreal, Quebec, Canada H3G 1M8
2
National Research Council Canada, Industrial Materials Institute 75 De Mortagne Blvd., Boucherville, Quebec, Canada J4B 6Y4
ABSTRACT
The effect of pre-mixing clay with the epoxy on the dispersion of organoclay in epoxy nanocomposites was studied using different mixing approaches: hand stirring, conventional mechanical stirring, a homogenizer, and a microfluidizer. The epoxy resin and the hardener were EPON™ 828 from Resolution Performance Products and Hunstman Jeffamine® D-230 (an amine-terminated polyoxypropylene diol). The organo-clay Cloisite 30B (montmorillonite treated with a quaternary ammonium intercalant) was used. Epoxy and its nanocomposites were cured either at room temperature for 2 days or at 120°C for 2 hours, with subsequent post-cure at 140°C for 2 hours in both cases. The quality of dispersion and intercalation/exfoliation of organoclay in epoxy after pre-mixing (before adding hardener) was analyzed by means of X-ray diffraction (XRD) and rheological measurement. The dispersion and intercalation/exfoliation of organoclay in the epoxy nanocomposites (ENCs) after curing were characterized by XRD, field emission gun scanning electron microscopy (FEGSEM) and transmission electron microscopy (TEM). To quantify the dispersion, image analysis was also performed. The results indicate that both the pre-mixing and curing steps play a determining role in the dispersion and intercalation/exfoliation of organoclay. During curing, the organoclay can be further intercalated by the epoxy resin quite easily and the intercalation and exfoliation process can continue further or not depending on the curing rate. Although full exfolia-tion of clay cannot be achieved at the pre-mixing stage, this step appears to be very important in controlling the micro-dispersion and thus affecting the further intercalation and exfoliation that take place during the curing step. A well dispersed and well intercalated/exfoliated state of organoclay in ENC can be achieved with a non-solvent-assistance method by using a homogenizer and controlling the curing process.
Key words: mixing, dispersion, nanoclay, epoxy, nanocomposites.
INTRODUCTION
Dispersion of organoclays in epoxy is a complex process, which takes place during the pre-mixing step and the curing step. Both pristine and commercially treated clays tend to form stacks rather than individual platelets due to their layer structure and the strong van der Waals force between the layers. In most cases, the stacks combine and form large aggregates due to such strong secondary interaction. As a result, it is very difficult to break down such interaction in order to disperse the clay layers individually in the epoxy
matrix, especially when the two phases are incompatible. During the fabrication of an epoxy nanocomposite, normally the clay is first pre-mixed with the liquid epoxy resin. In the absence of the curing agent, the system can have an extended shelf life at certain elevated temperatures. Therefore, different means such as mechanical shear and thermo-dynamic force, etc., can be used to facilitate the dispersion. At the end, the system is cured by the use of hardener. At this curing stage, sufficient external shear stress may not be applied, but further dispersion, more specifically intercalation and exfoliation, can continue to take place at the beginning of the curing as the hardener and/or intermediate molecules are still mobile enough to diffuse into the clay galleries; the motion of these molecules is governed by thermodynamic forces. In general, it is easier to achieve good clay dispersion in the pre-mixing step than in the curing step. Dispersion process parameters mainly include pre-mixing temperature, speed and time, power of ultrasonic tooling, shearing forces etc. Direct mixing of organoclay and epoxy with mechanical mixing and sonication is widely used to disperse nanoclay in epoxy 1-5. However it is not enough to produce a high degree of dispersion of clay in epoxy. Yasmin et al. 6 used a three-roll mill to disperse the clay nanoparticles in an epoxy matrix and improved the distribution of the particles. Chen and Tolle 7 achieved fully exfoliated layer silicate epoxies by shear mixing in the presence of acetone. Liu et al. 8-10 used a high-pressure mixing method with assistance of acetone solvent to improve the dispersion of clay in epoxy and observed significant improvement in fracture toughness of epoxy nanocomposites. However, the large amount of solvent required meant that considerable time was required for removing it. There is still work to be done to develop nanocomposites with fine dispersions and well-exfoliated morphologies. Achieving such morphologies with epoxy-based nanocomposites is a challenge, since high shear is always required but epoxy resin typically has rather low viscosity. In this paper, the exploration of a different way to generate the shear in order to improve the quality of dispersion and intercalation/exfoliation of clay in epoxy is discussed.
EXPERIMENTAL
Epoxy and clay were pre-mixed together by means of different methods and devices. The first one was a room temperature process performed without mechanical shear (Rm), in which the clay and epoxy were stirred at room temperature by hand at 100 rpm for a few minutes then kept at room temperature for 1 hour. The second one was a “high temper-ature without shear” process (Tm), in which the clay and epoxy were stirred at 120°C by hand at 100 rpm for a few minutes then kept in an oven at 120°C for 1 hour. The third method was a “high temperature with low-speed mixing” process (TM), in which the clay and epoxy were stirred at 120°C for 1 hour by a mechanical stirrer at 1000 rpm. The fourth one was a “room temperature and high-speed mixing” process (RS), in which the clay and epoxy were stirred at room temperature by means of a homogenizer at high speed (24000 rpm) for 1 h. The fifth method was a “high temperature and high-speed mixing” process (TS), in which the clay and epoxy were stirred at 120°C by means of a homogenizer at high speed (24000 rpm) for 1 h. The sixth one was a high-pressure process (HP), in which organoclay was first dispersed in acetone (at a level of about 8%) with a microfluidizer ( 15000 psi or 103 MPa) to form a suspension, and then the suspension was added into the epoxy resins according to the approach used by Liu et al.
8-10. The desired amount of paste of organoclay and acetone was added to epoxy resin and then the mixture was stirred by hand at room temperature. When the epoxy was visibly dispersed, the mixture was mechanically stirred at 1000 rpm in a fume hood at room temperature for 30 minutes, followed by slow heating to 80°C for 1 hour. Finally, the mixture was degassed under vacuum at 95°C for 30 minutes. For curing, the required amount of hardener was mixed with neat epoxy resin or epoxy-clay mixtures at room temperature for 5 min and the mixture was then subjected to vacuum. Samples were cured either at room temperature for 2 days or at 120°C for 2 hours, with subsequent post cure at 140°C for 2 hours in both cases.
To evaluate the intercalation/exfoliation of the nanoclay in the polymer matrix, X-ray diffraction (XRD) patterns were obtained from the surface of the samples with a Bruker Discover 8 powder X-ray diffractometer with CuK radiation. A Hitachi-S4700 FEGSEM was used to observe the dispersion of clay in the epoxy matrix at the micro-level. For clay dispersion at the nano-level, ultra-thin (50 to 80 nm) sections of nanocom-posite samples were prepared with a cryoultramicrotome and supported on a copper 200 mesh grid for observation with a Hitachi H9000 TEM.
RESULTS
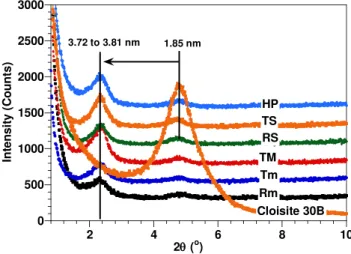
The X-ray diffraction curves of EPON828-C30B mixtures after pre-mixing by means of different methods are shown in Figure 1. A summary of the first peak‟s position (d001) in
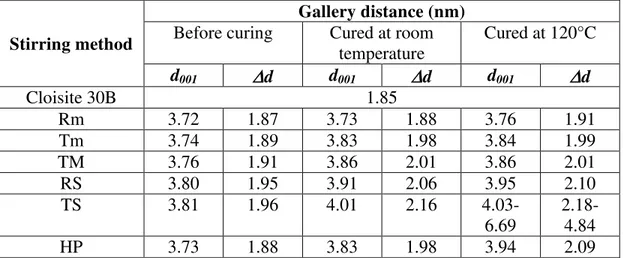
the XRD curves of the epoxy-clay mixtures, as well as the difference d between the positions of these peaks and that of the peak of C30B, is given in Table 1.
0 500 1000 1500 2000 2500 3000 2 4 6 8 10 In ten si ty ( C o u n ts) 2(o) Cloisite 30B HP TS RS TM Tm Rm 3.72 to 3.81 nm 1.85 nm
Figure 1. X-ray diffraction curves of EPON828-C30B mixtures prepared by means of different methods.
Generally speaking, although higher temperature and shear rate tend to give slightly higher d-spacings, the differences among the various mixtures are very small. All the mixtures give a value in the range of 3.72 to 3.81 nm, which is considerably larger than the initial clay spacing of 1.85 nm. It is surprising that the basal spacing d of organoclay
in epoxy did not obviously increase even with high temperature and high-speed aids. Moreover, the presence of the identical peaks in the XRD curves for the epoxy-clay mixtures clearly indicates that the clay was not fully exfoliated at the pre-mixing step. It would appear that the main driving force is thermodynamic and that a stable state is reached well short of full exfoliation, regardless of the mixing technique applied.
Table 1. Summary of XRD results for EPON828-C30B mixtures and their ENCs prepared by means of different methods
Stirring method
Gallery distance (nm)
Before curing Cured at room
temperature Cured at 120°C d001 d d001 d d001 d Cloisite 30B 1.85 Rm 3.72 1.87 3.73 1.88 3.76 1.91 Tm 3.74 1.89 3.83 1.98 3.84 1.99 TM 3.76 1.91 3.86 2.01 3.86 2.01 RS 3.80 1.95 3.91 2.06 3.95 2.10 TS 3.81 1.96 4.01 2.16 4.03-6.69 2.18-4.84 HP 3.73 1.88 3.83 1.98 3.94 2.09
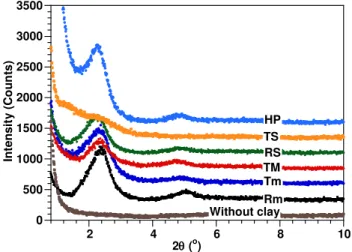
X-ray diffraction curves of the cured epoxy and its nanocomposites based on C30B made by different pre-mixing methods are illustrated in Figures 2 and 3. A summary of the first peak‟s position (d001) in the XRD curves of the ENCs, as well as the difference d
between the positions of these peaks and that of the peak of C30B, is also given in Table 1. In all the nanocomposite samples, the clay layer separation (degree of intercalation) is considerably higher than in the original C30B. Clays have been further intercalated somewhat by the epoxy matrix for curing at both room and high temperatures.
0 500 1000 1500 2000 2500 3000 3500 2 4 6 8 10 In ten si ty ( C o u n ts) 2(o) Without clay HP TS RS TM Tm Rm Cloisite 30B 1.85 nm 3.73 to 4.01 nm
Figure 2. X-ray diffraction curves of neat EPON828-D230, C30B clay, and their nanocomposites at 2 wt% C30B made by different methods and cured at RT.
When samples were cured at room temperature (RT), there was no significant difference between the positions of the peak in the XRD curves. However, the intensity (area under the peak) changed depending on the pre-mixing method. At the same loading level of 2 wt% C30B, the ENC made by the high temperature method (Tm) shows better intercalation than the ENC made by the room temperature method (Rm). The intensity of the XRD peak for pre-mixing with Tm is lower than for pre-mixing with Rm. This means that pre-mixing at high temperature leads to better delamination of clay than pre-mixing at room temperature. On comparing “high speed at high temperature” (TS) with “high speed at room temperature” (RS), the same phenomenon is observed. “High speed at high temperature” pre-mixing (TS) also shows better intercalation/exfoliation than mechanical pre-mixing (1000 rpm) at high temperature (TM) and just high temperature (Tm). This indicates the effect of shear on the level of intercalation/exfoliation of clay in epoxy nanocomposites. 0 500 1000 1500 2000 2500 3000 3500 2 4 6 8 10 In te n s it y ( Co u n ts ) 2(o) Tm Rm TM RS TS HP Without clay
Figure 3. X-ray diffraction curves of neat EPON828-D230, C30B clay, and their nanocomposites at 2 wt% C30B made by different methods and cured at 120°C for 2 h. Curing at high temperature (120°C) leads to better intercalation/exfoliation than curing at room temperature. With the same pre-mixing method, the intensity of the XRD peak is lower for curing at 120°C than for curing at room temperature. This can be explained by the fact when the temperature increases, the mobility of epoxy and hardener molecules increase and because of this, they can diffuse more easily into the clay galleries and further intercalate or exfoliate the clay [4, 11]. A similar effect can be seen here on the intercalation/exfoliation of clay in the epoxy matrix when high temperature and high speed are used in the pre-mixing step. The intensity of the peak for the ENCs decreased when high temperature and speed were used in the pre-mixing step. The orders of intercalation/exfoliation are now TS RS, TM Tm Rm, TS TM Tm and RS Rm. It should be noted that there is little difference in peak intensity between Tm and TM. It can also be seen that there are two peaks in all the XRD curves for ENCs prepared by the Rm, Tm, TM, RS, HP and TS methods. For ENCs prepared by Rm, Tm, TM and RS, the two peaks are at around 2.3° and 4.8°. However, the peaks shifted to lower angles for the TS method. They are located at 1.3° and 2.2°, corresponding to d-spacings of 6.69 nm and 4.03 nm, respectively. The TS method shows better intercalation/exfoliation than
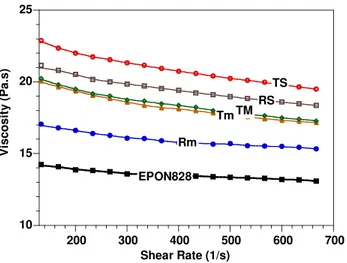
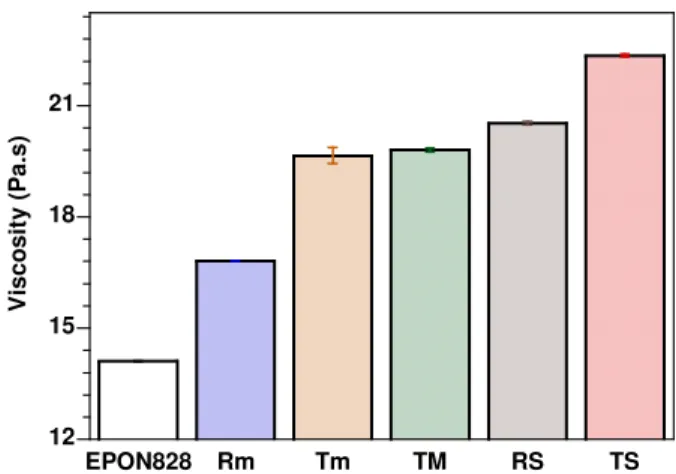
all the other methods. The intensity of XRD peaks of ENC made by the HP method is lower than for the RS method. In addition the XRD peak is more asymmetric for the ENC made by the HP method than for the one made by the RS method. It is believed that the HP method gives better intercalation/exfoliation than RS, although the d-spacing is a little lower for HP than for RS. In general, it can be noted here that the level of intercalation and exfoliation of clay in epoxy nancomposites is in the following order: TS HP RS TM Tm Rm. The explanation for the better intercalation/exfoliation at this step for the ENC prepared with the “high speed and high temperature” pre-mixing method is that the high speed and temperature helped to break down the clay aggregates to smaller sizes and thus improve the dispersion of the clay in the epoxy resin (more homogenous dispersion) during the pre-mixing step. This has an indirect subsequent effect on the intercalation/exfoliation of clay in epoxy matrix at the curing step. With smaller aggregates and a more uniform distribution, the epoxy resin and hardener have more chance to diffuse into the clay galleries and further expand the distance between clay platelets than for the case of large aggregates and non-uniform distribution, especially with high temperature curing where the mobility of the molecules increases. Figure 4 shows curves of viscosity at 25°C versus shear rate for the epoxy EPON 828 and its mixtures with organoclay C30B made by different pre-mixing methods. The effect of pre-mixing methods on the viscosity at a fixed shear rate of 167 s-1 is shown in Figure 5. It can be seen here that the viscosity of the suspensions changes according to the pre-mixing condition and it corresponds to the following order: TS RS TM Tm Rm
(Figures 4 and 5). High temperature and high shear both show a positive effect on the viscosity of the suspension. For example: Tm Rm, TS RS (temperature effect) and
RS Rm, TS Tm (speed effect). Again the result here confirms that the clay has a
better dispersion in epoxy resin when increased temperature and high speed are used in the mixing process.
10 15 20 25 200 300 400 500 600 700 V isc o si ty (P a. s) Shear Rate (1/s) EPON828 Rm Tm TM RS TS
Figure 4. Viscosity vs. shear rate curves of EPON828 and its mixtures with C30B prepared by means of different methods.
12 15 18 21 EPON828 Rm Tm TM RS TS V is c o s it y ( P a .s )
Figure 5. Viscosity of EPON828 and its mixtures with C30B after being pre-mixed using different methods for 60 minutes, measured at a shear rate of 167 s-1.
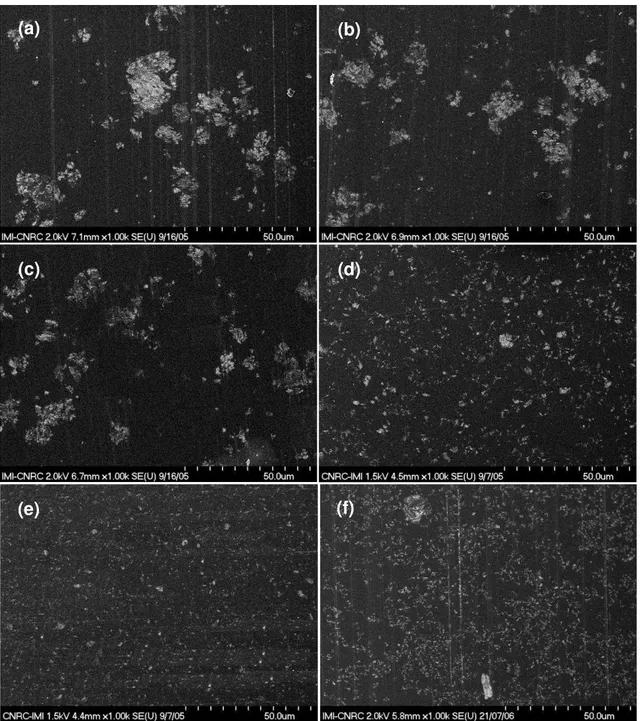
The microstructures of ENCs based on C30B and made by different pre-mixing methods were examined by SEM and are illustrated in Figure 6. The bright spots on the back-scattered images correspond to clay aggregates. Apparently, a portion of the clay remains at the micro-scale level with different size populations depending on the pre-mixing conditions. However, given the resolution limitations of the SEM, one should not rule out the possibility that some exfoliation does take place. The size of aggregates is reduced significantly with high speed (Figure 6d) as compared to mixing by hand at room temperature (Figure 6a) or even mechanical mixing at high temperature (Figure 6c). The size of aggregates became much smaller when both high speed and temperature were introduced at the pre-mixing step (Figure 6e). The result also confirms that the micro dispersion of ENC made by different pre-mixing methods follows the order TS HP RS TM Tm Rm.
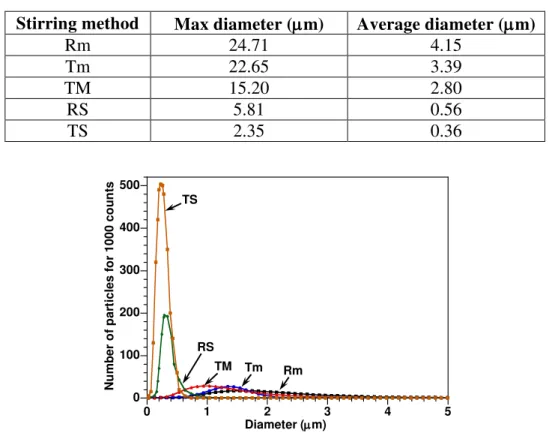
From the SEM images, the distribution of agglomerates of clay in the epoxy as shown in Figures 6a, 6b, 6c, 6d, and 6e was analyzed by means of Image Pro Analysis Software. A summary of the average diameter, the maximum size and the distribution of clay particles in epoxy nanocomposites made by different methods is given in Table 2 and Figure 7. The TS method leads to much smaller aggregates (average size < 1 µm) and a narrow size distribution. The result is much better than for the direct mixing method with mechanical stirrer (average size around 3 µm and many large aggregates). During pre-mixing, shear forces broke down the clay aggregates to smaller sizes. This has an indirect beneficial effect on intercalation/exfoliation of clay in epoxy at the curing step, especially at high temperature.
(c) (d)
(e)
(a) (b)
(f)
Figure 6. SEM micrographs of ENCs based on EPON828 and D230 with 2 wt% C30B made by different pre-mixing methods: (a) Rm, (b) Tm, (c) TM, (d) RS, (e) TS and (f)
Table 2. The maximum size and average diameter of clay particles in epoxy.
Stirring method Max diameter (m) Average diameter (m)
Rm 24.71 4.15 Tm 22.65 3.39 TM 15.20 2.80 RS 5.81 0.56 TS 2.35 0.36 0 100 200 300 400 500 0 1 2 3 4 5 Nu mb e r o f p a rt ic le s f o r 1 0 0 0 c o u n ts Diameter(m) RS TS TM Tm Rm
Figure 7. Clay size distribution for different pre-mixing methods
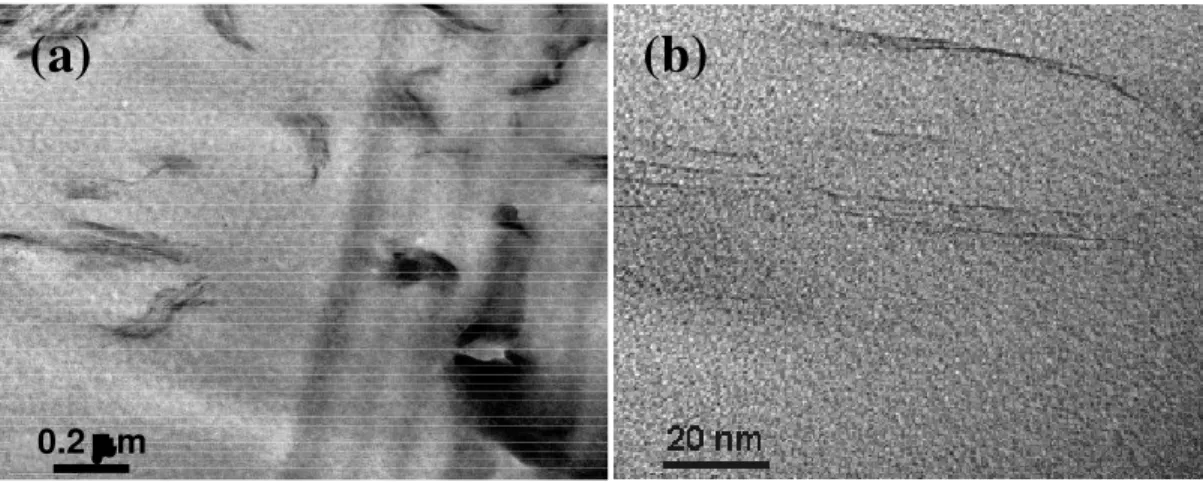
Figure 8 shows TEM photos of nanocomposite prepared by the TS technique (120°C and 24000 rpm for 1 hour) at different magnifications. It can be seen in Figure 8a that the clay has been well dispersed in the epoxy matrix. The size of the small aggregates or clay stacks is less than 0.5 µm. At higher magnification (Figure 8b), the dark lines indicate the individual silicate nanolayers. Although the clay particles were not completely exfoliated into individual platelets, there are many regions showing single, double and triple clay silicate nanolayers. So far, the results from XRD, SEM and TEM combine to indicate that with high speed and temperature assistance, one can obtain a fine dispersion at the micro-scale, good distribution of clay, and good intercalation/exfoliation of clay in the epoxy system.
0.2 m
(a)
(b)
Figure 8. TEM photo of 2 wt% Cloisite 30B prepared by TS method CONCLUSIONS
Well dispersed and well intercalated/exfoliated epoxy nanocomposites have been produced with a non-solvent-assistance method. With this method, high shear force can be generated and applied to the clay particles during the pre-mixing step. This leads to small well-dispersed tactoids with high surface area. The advantages of this method are that it is simple, fast and easy to apply. This method also avoids the use of solvent for dispersing the clay in epoxy. Consequently it is economical with respect to time and cost in addition to being environmentally friendly.
ACKNOWLEDGEMENTS
We acknowledge the Vietnamese Government for a scholarship to T.-D. Ngo. We also would like to thank the Natural Sciences and Engineering Research Council of Canada for funding for this project (grant N00784 – Development of Polymer Nanocomposites, and grant N00004 – Analysis and Vibration of Elastic and Viscoelastic systems).
REFERENCES
[1] Wang M. S. and Pinnavaia T. J. „„Clay-Polymer Nanocomposites Formed from
Acidic Derivatives of Montmorillonite and an Epoxy Resin‟‟. Chem. Mater., Vol. 6, No. 4, pp 468 - 474, 1994.
[2] Lan T. and Pinnavaia T. J. “Clay-Reinforced Epoxy Nanocomposites”. Chem. Mater.,
Vol. 6, No. 12, pp 2216 - 2219, 1994.
[3] Kornmann X., Lindberg H. and Berglund L. A. “Synthesis of epoxy–clay
nanocomposites: influence of the nature of the clay on structure”. Polymer, Vol. l. 42, No. 4, pp 1303-1310, 2001.
[4] Kornmann X., Lindberg H. and Berglund L. A. “Synthesis of epoxy–clay
nanocomposites. Influence of the nature of the curing agent on structure”. Polymer, Vol. 42, No. 10, pp 4493-4499, 2001.
[5] Massam J. and Pinnavaia T. J. “Clay nanolayer reinforcement of glassy epoxy
polymer”. Mater. Res. Soc. Symp. Proc. Spring Meeting, San Francisco, CA (US), Vol. 520, pp 223-232, 1998.
[6] Yasmin A., Abot J. L. and Daniel I. M. “Processing of clay/epoxy nanocomposites by shear mixing”. Scripta Materialia, Vol. 49, No. 1, pp 81-86, 2003.
[7] Chen C. and Tolle T. B. “Fully exfoliated layered silicate epoxy nanocomposites” J. Polym Sci Part B: Polym. Phys., Vol. 42, No. 21, pp 3981-3986, 2004.
[8] Liu W. P., Hoa S. V. and Pugh M. “Morphology and performance of epoxy
nanocomposites modified with organoclay and rubber”. Polym. Eng. Sci., Vol. 44, No. 6, pp 1178-1186, 2004.
[9] Liu W. P., Hoa S. V. and Pugh M. “Organoclay-modified high performance epoxy nanocomposites”. Compos. Sci. Technol., Vol. 65, pp 307-316 (2004).
[10] Liu W. P., Hoa S. V. and Pugh M. “Fracture toughness and water uptake of high- performance epoxy/nanoclay nanocomposites”. Compos. Sci. Technol., Vol. 65, 2364-2373 (2005).
[11] Ngo T.-D., Ton-That M.-T., Hoa S. V. and Cole K. C. “Curing Kinetics and Mechanical Properties of Epoxy Nanocomposites Based on Different Organoclays”,