READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Pore structure formation during hydration of fly-ash and slag cement
blends
Feldman, R. F.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=c8c9e6df-44d5-4ce3-9949-3f83338fdfb2 https://publications-cnrc.canada.ca/fra/voir/objet/?id=c8c9e6df-44d5-4ce3-9949-3f83338fdfb2
- 2
National Research
Conseil national
I
I*
Council Cagada
de recherches Canada
PORE STRUCTURE FORMATION DURING HYDRATION OF FLY-ASH AND SLAG CEMENT BLENDS
by R.F. Feldman
Appeared in A N A L Y Z E D
Effects of Flyash Incorporation in Cement and Concrete
Proceedings, Symposium N Materials Research Society
Annual Meeting, Boston, Massachusetts November 16
-
18,1981p. 124
-
133Reprinted with the permission of Materials Research Society
DBR Paper No. 1071
Division of Building Research
T r o i s melanges, un ciment de r s f e r e n c e , un d l a n g e contenant 35% de cendres v o l a n t e s e t un d l a n g e contenant 70% de l a i t i e r , o n t 6 t b d u r c i s d a n s l ' e a u B 21, 35 e t 55°C. avec un r a p p o r t eaulciment a 0.45. Apr'es 2, 7, 14, 28, 90, 180, 365 e t 550 j o u r s , on a dbtermin'e l a teneur e n Ca(OH)2, l a porosit'e, l a t a i l l e d e s pores e t l e u r d i s t r i b u t i o n , l a r e s i s t a n c e a l a compression, l e module de Young e t l a microduret'e d e s produits. Les logarithmes d e c e s p a r a d t r e s mecaniques o n t BtS repr'esent'es graphiqwment e n f o n c t i o n de l a porosit'e. Une tempstature p l u s Blev'ee augmente l a v i t e s s e d e r h c t i o n du ciment de r'eflrence pendant l e s deux premiers j o u r s , p u i s l a r e t a r d e . C e t t e pgriode d9acc&16ration e s t
consid'erablement p l u s longue pour l e s d l a n g e s . Les
propri'etes mecaniques correspondant B une porosit'e n u l l e s o n t g'en'eralement m e i l l e u r e s l o r s q u e l ' h y d r a t a t i o n d e s melanges s e f a i t B l a tempgrature de l a p i k e ; e t t o u s l e s melanges o n t d e s pores p l u s f i n s e t une t e n e u r e n p l u s f a i b l e que l e ciment de reference.
PORE STRUCTURE FORMATION DURING HYDRATION OF FLY-ASH AND SLAG CEMENT BLENDS
R.F. Feldman
D i v i s i o n o f Building Research, N a t i o n a l Research Council o f Canada,
Ottawa, Canada K1A OR6
ABSTRACT
Three mixes, a r e f e r e n c e cement, one f l y - a s h hlend- c o n t a i n i n g 35% f l y - a s h , and one w i t h 70% s l a g , were c u r e d i n w a t e r a t 21, 35 and 5 5 * ~
a t a w/c = 0.45. A f t e r 2, 7, 14, 28, 90, 180, 365 and 550 days
Ca(OH)2 c o n t e n t , p o r o s i t y , p o r e s i z e d i s t r i b u t i o n , compressive s t r e n g t h , Young's modulus and microhardness o f t h e p r o d u c t s were determined. The l o g a r i t h m s of t h e s e mechpnical p r o p e r t i e s were p l o t t e d a g a i n s t p o r o s i t y . Higher t e m p e r a t u r e i l l c r e a s e d t h e r a t e o f r e a c t i o n o f t h e r e f e r e n c e cement f q r up t o two d a y s , t h e n r e t a r d e d i t . T h i s a c c e l e r a t i o n p e r i o d was c o n s i d e r a b l y extended f o r t h e cement b l e n d s . Mechanical p r o p e r t i e s a t z e r o p o r o s i t y were g e n e r a l l y
improved i n b l e n d s hydrated a t room t e m p e r a t u r e ; a n d a l l b l e n d s had a
f i n e r p o r e and lower Ca(OM)2 c o n t e n t t h a n t h e cement specimen.
I
1
I n t r o d u c t i o nI
F u l l y h y d r a t e d p o r t l a n d cement c o n t a i n s about 20% c a l c i u m hydroxide, b o t h a s f a i r l y l a r g e c r y s t a l s and a s a small q u a n t i t y i n a n amorphous s t a t e . The c o n t r i b u t i o n o f t h i s m a t e r i a l t o t h e s t r e n g t h of 1:he p a s t e body and t o t h e d u r a b i l i t y o f c o n c r e t e h a s been t h e s u b j e c t of much s t u d y (1.-3). It i s known t h a t t h e a d d i t i o n o f s l a g s o r pozzolans ( i n c l u d j n g f l y - a s h ) improves
t h e performance of cements exposed t o a g g r e s s i v e s o l u t i o n s ( 4 - 7 ) . T h e i r
g r e a t e r r e s i s t a n c e h a s been a t t r i b u t e d t o lower Ca(Oll)2 c o n t e n t i n t h e blended cements.
I t h a s been shown (8) t h a t nlthough f l y - a s h and slag-cement b l e n d s may have h i g h e r t o t a l p o r o s i t i e s , t h e y have f i n e r p o r e d i s t r i b u t i o n t h a n t h e r e f e r e n c e
cement p a s t e a f t e r 180 days of h y d r a t i o n . I t h a s a l s o been shown t h a t t-he
Ca(OH)2 c o n t e n t of hydrated blended cement p a s t e i s much lower t h a n t h a t o f
hydrated p o r t l a n d cement p a s t e . These a r e major f a c t . o r s i n r e s i s t a n c e t o a t t a c k by s o l u t i o n s o f compounds such a s MgC12.
F u r t h e r a n a l y s i s h a s noLC been c a r r i e d o u t u s i n g r e s u l t s o b t a i n e d f o r extended p e r i o d s o f h y d r a t i o n . Mechanical p r o p e r t i e s a r e a l s o r e l a t e d t o p o r o s i t y a t v a r i o u s t e m 2 e r a t u r e s o f h y d r a t i o n .
Experimental Eilaterials
Type I p o r t l a n d cement was mixed w i t h a f l y - a s h (85% o f t h e p a r t i c l e s between 45 and 12 ym ( 9 ) ) t o g i v e a blend m i x t u r e c o n t a i n i n g 35% o f t h e a d d i t i o n . A second m i x t u r e was made w i t h Type I cement and 70% s l a g . A r e f e r e n c e mix c o n t a i n i n g no a d d i t i o n was a l s o made. Composition o f t h e m a t e r i a l s i s p r e s e n t e d i n T a b l e I , shovjng t h a t t h e f l y - a s h and s l a g c o n t a i n much h i g h e r q u a n t i t i e s o f s i l i c a but lower q u a n t i t i e s of CaO t h a n t h e
p o r t l a n d cement.
Hydration: Cubes 50 x 50 mm were made from t h e t h r e e mixes u s i n g a water/ s o l i d r a t i o o f 0.45. The cubes were cored a f t e r c u r i n g f o r 24 h and s l i c e d i n t o d i s c s 32 mm i n d i a m e t e r and 1,.3 mm t h i c k . Measurements were t a k e n a f t e r h y d r a t i o n p e r i o d s o f approximately 2 , 7 , 1.4, 28, 90, 180, 365, and 550 days; h y d r a t i o n was c a r r i e d o u t a t 21, 35 and 5 5 " ~ .
Met hod I
P o r o s i t y and p o r e s i z e d i s t r i b u t i o n : Pore s i z e d i s t r i b u t i o n was o b t a i n e d by mercury porosimetry, u t i l i z i n g a p r e s s u r e of up t o 408 MPa. American Instrument Company equipment was used. The volume of mercury i n t r u d e d a t t h e
maximum p r e s s u r e was c o n s i d e r e d t o be t h e t o t a l p o r o s i t y (10, 1 1 ) . I I I A p r e v i o u s paper (8) showed t h a t measured by mercury p o r o s i m e t r y
( u s i n g t h e volume o f mercury i n t r u d e d a t t h e maximum p r e s s u r e ) gave t h e same o r a g r e a t e r p o r o s i t y than- methods i n v o l v i n g s a t u r a t j - o n w i t 1 1 CH30M o r He comparison pycnonletry. The r e s u l t s r e p o r t e d i n t h i s work a r e based on mercury porosimetry.
Compressive s t r e n g t h , Young's modulus and microhardness: S t r e n g t h was measured i n compression on cubes, two cubes f o r each p r e p a r a t i o n . Young's modulus was measured on d i s c s , a t l e a s t f o u r d i s c s f o r each v a l u e . The
procedure involved measuring t h e d e f l e c t i o n o f a s a t u r a t e d specimen loaded a t i t s c e n t r e and supported a t t h r e e p o i n t s on t h e c i r c u m f e r e n c e o f a c i r c l e 25 mm i n d i a m e t e r . A I . c i t z microhardness t e s t i n g machine w i t h a V i c k e r ' s i n d e n t e r was used f o r t h e microhardness measurements o f d i s c s used f o r modulus o f e l a s t i c i t y . Measurements were a l s o c a r r i c d o u t a t s a t u r a t e d c o n d i t i o n s ; each v a l u e was t h e average of r e a d i n g s t a k e n on f o u r d i s c s (9).
T A B L E I C h e m i c a l c o m p o s i t i o n o f c e m e n t s a n d a d d i t i o n s u s e d C h e m i c a l F l y - a s h S l a g T y p e I B C e m e n t S i O g 5 5 . 5 8 3 1 . 5 0 2 0 . 6 6 A 1 2 0 3 2 2 . 6 6 1 5 . 9 8 6 . 2 2 F e 2 0 3 4 . 3 2 < 1 2. 16 C a O 1 3 . 3 2 3 6 . 0 6 4 . 4 5 MgO 2 . 3 5 1 2 . 9 8 1. 28 I g n l o s s 0 . 6 4 - - 0 . 8 1 O3 0 . 16 2 . 8 3 . 0 2
Degree o f hydrati.on and calcium hydroxide c o n t c n t : TGA and. 1)SC thernlograms were o b t a i.ned f o r each specimen between room' t e m p e r a t u r e and 1000 and 600GC, respectively. Non- evaporable water was e s t i m a t e d a t v a r i o u s t i m e s by t a k i n g t h e weight l o s s from t h e TGA between 100 and 1000°C. A d i f f e r e n t i a l s c a n n i n g c a l o r i m e t e r (DSC) c e l l , s u p p l i e d a s a
module t o t h e Du Pont 990 thermal. a n a l y s i s system, was used t o
determine t h e r e l a t j . v e a r e a s o f t h e thermal peaks r e s u l t i n g from Ca (OH) 2 decomposition. I n each experiment
20 mg o f sample \(as h e a t e d i n a i r a t a r a t e of 2 0 " ~ / m j n . For c a l i b r a t i o n
purposes Ca(OH)2 was mixed with a-A1203 i n d i f f e r e n t p r o p o r t i o n s and t h e endothermal peak a r e a s were p l o t t e d a g a i n Ca(OH)2 c o n c e n t r a t i o n (12).
R e s u l t s and Discussion
The i n f l u e n c e of time and temperature of hydration on Young's modulus, compressive s t r e n g t h , microhardness and p o r o s i t y of t h e cements i s presented i n Figs. 1 t o 3.
Hardened Cement P a s t e
Changes i n mechanical p r o p e r t i e s and p o r o s i t y a r e p r e s e n t e d a s a f u n c t i o n of t h e square r o o t of time i n hours i n Fig. 1. A t about two days t h e
Young's modulus a t t a i n e d by t h e specimen was g r e a t e s t a t 35°C and t h e lowest a t 21°C. Beyond two days, t h e Young's modulus increased a t a g r e a t e r r a t e a t 2 1 " ~ : a t a r a t e of i n c r e a s e i n t h e o r d e r of 21° > 3S0 > 55°C. The v a l u e a t 21°C exceeds t h a t a t 55°C a f t e r two t o t h r e e days and t h a t a t 3 5 " ~ a f t e r
10.7 days.
The r e s u l t s f o r micro- T I M E , d a y s
hardness and compressive
2
2 6 7 14 28s t r e n g t h (Fig. 1, B and
C) a r e s i m i l a r t o t h o s e o A
f o r Young's modulus. V)
-
a
Below about f o u r days t h e
2
o 2 1 ° cr a t e o f development of 0 35OC 5 5 ° C both microhardness and
s t r e n g t h i n c r e a s e s with "l temperature, but a f t e r "l w, 30 Z I eleven days t h e v a l u e s
:
2 a r e g r e a t e s t a t 21°c, i n zo t h e o r d e r of 0 L 21" > 35" > 55°C.5
- 5 10 I The r a t e of decrease o i n p o r o s i t y (Fig. lD) rn i n c r e a s e s with i n c r e a s e 1 s w "l i n temperature up t o~f
ao about seven days. A f t e r-
a w z t h i r t e e n days t h e poro- s u E E s i t y f o r t h e specimen tA made a t 2 1 " ~ i s lowest o and t h e r a t e s of decrease a r e i n t h e o r d e r of 21" > 35" > 5S°C. A s such, p o r o s i t y r e s u l t s a r e c o n s i s t e n t with t h o s ez
f o r development of o a mechanical p r o p e r t i e s . 10 10)Fly-ash B - Cement Blend o I I I I I I I I I 1
I
o 20 40 60 -
m
Ur]In Fig. 2A t h e r a t e of
i n c r e a s e of Young's OF H Y D R A T I O N . ' h 1 I 2
modulus f o r t h e f l y - a s h
B
-
cement blend F I G U R E 1specimen i s shown t o EFFECT OF T E M P E R A T U R E A N D H Y D R A T I O N TIME O H
i n c r e a s e with temperature M E C H A N I C A L P R O P E R T I E S OF C E M E N T P A S T E
which p o i n t Young's modulus a t 3S°C exceeds t h a t measured a t 5S°C. The
Young's modulus measured a t 3 5 " ~ exceeds t h a t measured a t 21°C a t a l l p e r i o d s of h y d r a t i o n . I n t h i s r e s p e c t t h e s e r e s u l t s d i f f e r from t h o s e f o r cement p a s t e i n which i n c r e a s i n g t e m p e r a t u r e s of h y d r a t i o n c a u s e r e t a r d a t i o n , a l t h o u g h i n c r e a s i n g t h e r a t e o f mechanical p r o p e r t y development a t e a r l i e r t i m e s .
P r e v i o u s work (13) h a s suggcstcd t h a t r e t a r d a t i o n i s due t o an accumula- t i o n o f dense h y d r a t i o n p r o d u c t ( c o n s i s t i n g o f C-S-H and Ca(OH)2) around t h e cement g r a i n a", h i g h e r t e m p e r a t u r e s . Perhaps t h e i n t e r a c t i o n o f Ca(OH)2 with f l y - a s h p a r t i a l l y b r e a k s t h i s i m p e r m e a b i l i t y around t h e unhydrated cement g r a i n , a l l o w i n g more r a p i d r e a c t i o n . R e t a r d a t i o n t a k e s p l a c e a t l a t e r t i m e s f o r i n c r e a s e d t e m p e r a t u r e s due t o e v e n t u a l b u i l d - u p o f C-S-1-1 around g r a i n s . T h i s phenomenon o c c u r s t o a g r e a t e r e x t e n t when i n i t i a l r e a c t i o n i s r a p i d .
The r e s u l t s f o r microhardness and compressive s t r e n g x h a r e a g a i n similar t o each o t h e r , b u t d e v i a t e somewhat from t h e Young's modulus r e s u l t s i n t h a t t h e microhardness developed a t 21°C exceeds t h a t developed a t 3S°C a f t e r about 180 days, and exceeds t h a t a t 5S0c a f t e r about 28 d a y s . The compressive
T I M E , d a y s 2 4 7 14 28 90 180 365 560 I I I -
--:-
- a 0 21°C - a 3 5 ° C 0 5 5 ° C ( A ) m Y a 2 60 - V)&====-
6 s- & w c 4 0 --
E:
I w/:"d
"
"-6
-
I C I 0 -*
"
-"-.,
- 0_g
- > +z
30.---:--:
0- - 0 E2
20.- - FIG. 2 10 - ( D ) - Effect cf temperature I I I I I I and h y d r a t i o n t i n e on 0 I t 1 1 a 20 40 60 80 IOC mechanical p r o p e r t i e s o f f l y - a s h B + cementf l z
O F H Y D R A T I O N , hl" b l e n ds t r e n g t h developed a t 2 1 ' ~ exceeds t h a t developed a t 35 and a t 55°C a f t e r 90
and 28 days, r e s p e c t i v e l y , while t h e p o r o s i t y decrease observed a t 21°C exceeds t h a t a t 35 and a t 55°C only a f t e r 51 and 37 days, r e s p e c t i v e l y ; t h e r a t e o f d e c r e a s e of p o r o s i t y i s g r e a t e s t a t 55°C i n t h e f i r s t two days. A s was observed f o r cement, p o r o s i t y r e s u l t s a r e c o n s i s t e n t w i t h r e s u l t s f o r development of mechanical p r o p e r t i e s .
B l a s t Furnace S l a g - Cement Blend
Measurements on a s l a g
-
cement blend a t 21 and 3 5 " ~ a r e p r e s e n t e d i n Fig. 3. I n t h i s c a s e t h e i n c r e a s e i n r a t e of development of s t r e n g t h and Young's modulus with temperature i s s i g n i f i c a n t up t o 28 days and i n p o r o s i t y f o r about 90 days; a t g r e a t e r ages t h e d i f f e r e n c e f o r any of t h c p r o p e r t i e s a t t h e two temperatures i s n o t s i g n i f i c a n t . The reason f o r t h e discrepancybetween microhardness and s t r e n g t h i s u n c l e a r . P o r o s i t y
-
Mechanical Property R e l a t i o nThe dependence o-f mechanical p r o p e r t y on
p o r o s i t y is presented i n T I M E . days
Fig.
4 f o r ~ i u n ~ ~ s2 4 7 14 28 90 1W 365 5M I
modulus and i n Fig. 5 w 3 I I I I I I I i
3 f o r compressive s t r e n g t h .
2~
The logarithm of 0 3 2 - z r a mechanical p r o p e r t y was 0 21'C - p l o t t e d a g a i n s t p o r o s i t y v 3s0c ( A ) according t o eq. (1) where A r e p r e s e n t s mechanical p r o p e r t y , t h e v a l u e a t zero A. p o r o s i t y , b i s a c o n s t a n t and P i s p o r o s i t y . A l l curves i n t h e s e p l o t s d i s p l a y an a b r u p t change i n s l o p e between 35 t o 40% p o r o s i t y , where degree of h y d r a t i o n i s about 50%; using v a l u e s a t lower p o r o s i t i e s t h e curves have beene x t r a p o l a t e d l i n e a r l y t o zero p o r o s i t y . The v a l u e of t h e i n t e r c e p t a t t h i s p o i n t i s A,.
The change i n s l o p e has been observed p r e v i o u s l y
(14j and has been
explained a s a change i n composition from predominantly high d e n s i t y , unhydrated m a t e r i a l t o lower d e n s i t y , hydrated m a t e r i a l . I I I
I
D 1 I I I L I I 0 M 40 60 80 100 O F H Y D R A T I O N , h l f 2 F I G U R E 3 E F F E C T O F T E M P E R A T U R E A N D H Y D R A T I O N T I M E O N M E C H A N I C A L P R O P E R T I E S O F S L A G + C E M E N T B L E N D100 l I I E I ] I I I I I Feldman and Beaudoin (15)
- - p l o t t e d d a t a from a v a r i e t y of
- -
sources and found Eo t o be
- -
0 C E M E N T
- - 3.2 x
lo4
MPa f o r cement p a s t e . Io F L Y - A S H B B L E N D Dyczek and P e t r i (16) found Eo's
- -
I
v S L A G B L E N D of tobermorite, x o n o t l i t e and I I - - C-S-13 ( I ) t o b e 50.1, 0.97 and I 7 . 6 xlo4
MPa, r e s p e c t i v e l y . - m 0 The p o r o s i t y v a l u e s i n t h i s workA were, however, measured by water
X
-
ca a d s o r p t i o n and t h u s a r e exces-
a
a s i v e l y high. Beaudoin and
- W
-
Feldman (9) found t h e s e v a l u e s f o r Eo f o r an autoclaved cement- v, 10 - - _1 s i l i c a with 30% mixture t o be 3 - - n - 3.8 x 104 MPa; t h i s v a l u e 0 5 - - i n c r e a s e d f o r o t h e r specimens v, - - with lower s i l i c a c o n t e n t s i n 0 Z - - 3 t h e mixture b e f o r e autoclaving. 0 > - - In t h e p r e s e n t work, f o r h y d r a t i o n a t 21°c E, v a r i e d from - - 2.5 t o 3 . 2 xl o 4
MPa f o r a l l p r e p a r a t i o n s w i t h cement p a s t e , t h e v a l u e being a t t h e lower-
-end, and t h o s e f o r cement blends f a l l i n g
a t
t h e h i g h e r end of the spread. A t 3 5 O ~ t h e v a l u e s ofEo were about 1 . 9 x
l o 4
MI1a.1 1 1 1 1 1 1 1 1 1 1 1
o 10 20 30 40 50 6 0 Fagerlund (17) c o r r e l a t e d P O R O S I T Y , % compressive s t r e n g t h d a t a f o r
p a s t e using a d i f f e r e n t equation
F I G I J R E 4 and found So t o be about 500hIPa. Y O U N G ' S M O D U L U S V S P O R O S I T Y F O R H Y D R A T E D Danyushevsky and D j abarov (18), C E M E N T P A S T E A N D C E M E N T B L E N D S AT 2 1 ° C howcvcr, uscd t h e same equation
with a v a r i c t y of p r c p a r a t j o n s and ;'ound t h a t So v a r i e d between
150 and 300 MPa. Again, Feldman
and Beaudoin (15) p l o t t e d d a t a f o r p a s t e s froln t h c l i t e r a t u r e according t o eq. ( I ) , f i n d i n g So t o be around 200 MPa. They found t h a t So f o r an auto- claved 30% silica-cement mixture was 290 bIPa ( 9 ) . Jn t h e p r e s e n t work So v a r i e d from 140 t o 260 MPa f o r h y d r a t i o n products a t 21°C, w i t h cement p a s t e a t t h e lower end and cement blends a t t h e higher end of t h e spread. A t 3S°C So f o r a l l t h e m a t e r i a l s was i n t h e range of 120 t o 150 MPa.
These r e s u l t s suggest t h a t cement blends produce a s t r o n g e r m a t r i x than does t h e r e f e r e n c e cement p a s t e a t normal temperatures. T h i s may be due t o t h e lower C a ( 0 H ) 2 and higher C-S-H c o n t e n t i n t h e blends.
P o r o s i t y and Pore D i s t r i b u t i o n
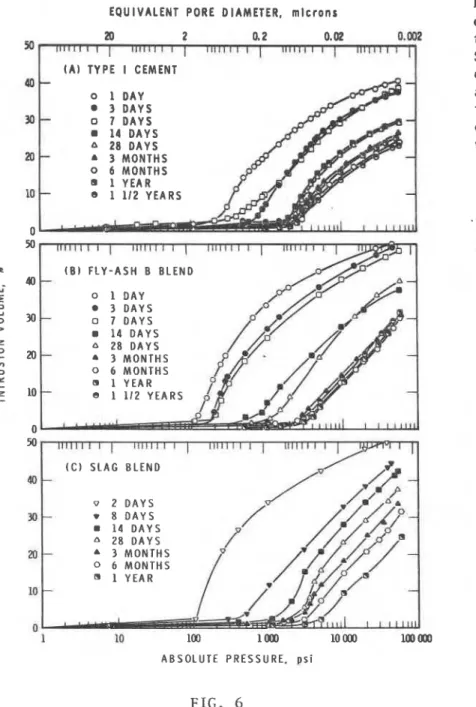
I t was shorin i n e a r l i e r work (8) t h a t t o o b t a i n a t r u e measure of t h e t o t a l p o r o s i t y of cement blends a high p r e s s u r e mercury i n t r u s i o n method (408 MPa, 60,000 p s i ) should be used. Fig. 6 shows t h e v a r i a t i o n of pore d i s t r i b u t i o n with hydration time a t 21°C. The d i f f e r e n c e i n t h e shape o f t h e d i s t r i b u t i o n curve f o r cement p a s t e (Fig. 6A) and cement b l e n d s (Fig-G, B and C) i s c l c a r . Methanol cannot e n t e r a l a r g e p o r t i o n of t h e pores intruded by mercury a t 408 IvlPa, i n d i c a t i n g t h a t some d i s r u p t i o n of t h e pore s t r u c t u r e
occurs owing t o d i f f e r e n t i a l 1000
-
I ~ I ~ I ~ I ~ I ~ I p r e s s u r e c r e a t e d during mercury - - i n t r u s i o n . T h i s i n d i c a t e s t h a t - 0 C E M E N T mercury e n t e r s l a r g e r pores by - 0 F L Y - A S H B B L E N D c r e a t i n g o r opening f u r t h e r - 9 S L A G B L E N D very narrow e n t r a n c e s t h a t - cannot b e p e n e t r a t e d even bymethanol. Thus, t h e mercury i n t r u s i o n curves may n o t
r e p r e s e n t a t r u e pore s i z e l o o -
:\
%**
L,d i s t r i b u t i o n a t t h e high 5 + -
**.
\,p r e s s u r e r e g i o n s (low p o r e s i z e I -
'*
I- -
range). Some of t h e pores a Z -
e n t e r e d h e r e may be q u i t e l a r g e , Y nz - + but o r i g i n a l l y c l o s e d o r V) A Y p a r t i a l l y c l o s e d . The volume . > - o f mercury should be c r e d i t e d V) VI - W t o a l a r g e r pore s i z e . oz n Nevertheless, t h e p o r e s , I 0 although c l o s e d , s t i l l a 1 0 c o n t r i b u t e t o mechanical - - p r o p e r t i e s . - + Although t o t a l p o r o s i t y and c l o s e d p o r e s a r e important i n c o r r e l a t i n g mechanical p r o p e r t y r e s u l t s , t h i s i s n o t n e c e s s a r i l y t h e c a s e with some o t h e r p r o p e r t i e s a s s o c i a t e d 1 with d u r a b i l i t y , f o r example, 0
m
1 0 2 0 3 0 4 0 5 0 6 0 p e r m e a b i l i t y (19). I t has been P O R O S I T Y . % suggested (19) t h a t only r e l a t i v e l y l a r g e p o r e s F I G U R E 5 c o n t r i b u t e t o poor d u r a b i l i t y . C O M P R E S S I V E S T R E N G T H V S P O R O S I T Y F O R Consequently, p o r o s i t y H Y D R A T E D C E M E N T A N 0 C E M E N T B L E N D S A T 2 1 ° Ccomprising pores g r e a t e r than 880
A
(13.6 MPa i n t r u s i o n p r e s s u r e ) and excluding closed pores were considered a sp o r o s i t y , and t h e s e v a l u e s a r e
t a b u l a t e d i n Table 11. Also included i s t h e t o t a l uncombined Ca(Ol-1)2 c o n t e n t f o r t h e sample a t each p o r o s i t y value.
D u r a b i l i t y Aspects
D u r a b i 1 j . t ~ of blended cements exposed t o a concentrated c h l o r i d e s o l u t i o n c o n t a i n i n g MgC12 was r e p o r t e d e a r l i e r ( 8 ) . P e r t i n e n t f i n d i n g s o f t h i s work can be explained by t h e r e s u l t s t a b u l a t e d i n Table 11. In a d d i t i o n , some p r e d i c t i o n s a r e p o s s i b l e .
1. Fly-ash B - cement blend cured a t room temperature f o r 14 days contained 13.8% l a r g e pores and 14.1% Ca(OH)2. T h i s m a t e r i a l showed poor d u r a b i l i t y . On t h e o t h e r hand, curing f o r s i x months a t 2 1 " ~ produced a product containing 1.4% l a r g e p o r e s and 6.5% Ca(011)2 t h a t proved t o be more d u r a b l e . The b e n e f i - c i a l e f f e c t of c u r i n g t h i s blend a t s l i g h t l y e l e v a t e d temperatures i s demon- s t r a t e d by i t s hydration a t 3 5 " ~ f o r 14 days; both p o r o s i t y (2.0%) and Ca(OlQ2 c o n t e n t (7.9%) a r e g r e a t l y reduced i n r e l a t i o n t o v a l u e s following hydration a t 2 1 " ~ . A mzjor i n c r e a s e i n d u r a b i l i t y would be p r c d i c t c d . Curing a t higher temperatures ( 5 5 " ~ ) may not be so b e n e f i c i a l , s i n c e p o r o s i t y (9.2%) i s much
E Q U I V A L E N T P O R E D I A M E T E R , mlcrons 20 2 0.2 0.02 O.m l l l l , l 1 1
,
1 1 1 1 , 1 1 1 1 1 1 1 1 1 1 I 1 1 1 1 1 1 1 r I ( A 1 T Y P E I C E M E N T 0 1 D A Y 3 D A Y S 0 7 D A Y S 1 4 D A Y S 4 2 8 D A Y S 3 M O N T H S 0 6 M O N T H S 0 1 Y E A R 1 112 Y E A R S 0 1 D A Y 3 D A Y S 0 7 D A Y S 1 4 D A Y S 4 2 8 D A Y S 0 1 Y E A R - 1 112 Y E A R S ( 8 ) F L Y - A S H B B L E N D L 0 1 D A Y 3 D A Y S 0 7 D A Y S 14 D A Y S I a 2 8 D A Y S 3 M O N T H S 0 6 M O N T H S 0 1 Y E A R1,
,,ez
l f
)#
* h i g h e r and Ca(OH)2 c o n t e n t (7.8%) i s about t h e same a s a t 3S0c. S i m i l a r e f f e c t s o f c u r i n g m o r t a r s of f l y - a s h b l c n d s a t e l e v a t e d t e m p e r a t u r e s have been observed by o t h e r workers (20).
2. Type I cement p a s t e specimens c u r e d f o r 14 d a y s and 6 months 'have l a r g e p o r e - - p o r o s i t i e s o f 5.4 and 1.8%, r e s p e c t i v e l y , and c o n t a i n l a r g e amountso f Ca(OH)2. They were
n o t d u r a b l e , althougll t h e r e was some improve- ment w i t h t h e lower p o r o s i t y specimen. 3. The s l a g - cement blend i s d u r a b l e a f t e r 1 4 d a y s and more s o 50 afte;
G
months. T h i s ( C ) S L A G B L E N D i s p r o b a b l y due mainly da t o t h e v e r y low Ca(Of1) v 2 D A Y S c o n t e n t . R e s u l t s f o r M v 8 D A Y S samples c u r e d a t 3 S ° C 14 D A Y S"
2 8 D A Y S f o r 7 d a y s (Table 1 1 ) 20 A 3 MONTHS i n d i c a t e t h a t t h e y 0 6 M O N T H S Q 1 Y E A R should a l s o produce a 10 d u r a b l e p r o d u c t .The low Ca (Oil) 2
0
1 10 100 lrn 10 OOO moo0 c o n t e n t o f t h e hydrated
A B S O L U T E P R E S S U R E . p s i s l a g
-
cement blend(Table 11) i s a r e s u l t of t h e low p r o p o r t i o n
FIG. 6 of r o r t l a n d cement i n
Change i n p o r e s i z e ' d i s t l i b u t i o n o f cement p a s t e t h i s blend and low CaO and cement b l e n d s w i t h h y d r a t i o n time a t 21cC c o n t e n t i n t h e s l a g
(compared w i t h t h a t i n c e ~ n e n t )
.
I t a l s o i n d i c a t e s t h a t some Ca(OH)2 formed from p o r t l a n d cement r e a c t s d u r i n g h y d r a t i o n o f t.lle s l a g .Conclusions
1 . When cured a t h i g h e r t e n ~ p e r a t u r e s , cement p a s t e shows a n i n c r e a s e d r a t e of
s t r u c t u r a l change and mechanical p r o p e r t y development a f t e r t h e f i r s t tr<o d a y s
,
of h y d r a t i o n o n l y . These r a t e s reduce w i t h i n c r e a s e d t e m p e r a t u r e a f t e r two days o f h y d r a t i o n .2. The p e r i o d d u r i n g which t h e r a t e o f s t r u c t u r a l and mechanical p r o p e r t y developinent i s i n c r e a s e d by ten1:peratui.e i s extended f o r cement - f l y - a s h and s l a g b l e n d s ( r e l a t i v e t o t h e p e r i o d f o r cement a l o n e ) .
Effect o f t e m p e r a t u r e a n d t i m e o n p o r o s i t y a a n d Ca(OHI2 c o n t e n t
Cment Fly-ash B Slag
lime 21°C 35°C 55°C 21°C 5 ° C 55°C 21°C 35°C 1 2 1 2 1 2 1 2 1 2 1 2 1 2 1 2 Zdays 20.0 16.8 11.5 10.8 10.9 12.9 35.4 10.9 25.5 6.36 16.1 10.7 38.3 3.29 2 8 8 2 8 7 days 7.6 18.6 7.7 13.3 8.0 14.9 30.0 12.3 7.3 7.20 1 2 7 8.6 17.5 2 8 2 5.3 2 3 14days 5.4 19.7 4 5 13.2 4.4 15.3 13.8 14.1 2.0 7.90 9.2 7.8 7.2 3.13 5.4 2.3 28days 3.4 19.6 3.6 12.5 3.3 17.6 8.8 9.6 2.5 7.37 9.6 8.0 3.5 2.60 2.1 2.3 3months 1.5 15.0 1.7 12.8 0.7 17.2 1.4 7.9 2 3 6.60 2.3 2.80 1.5 2.3 6 months 1.8 16.0 1.8 15.5 1.4 6.5 3.5 7.37 2.0 2.95 1.2 2.6 1 year 1.1 16.1 1.1 15.6 1.3 4.7 2 0 5.78 0.9 2.97 0.9 1.5 year 17.9 1.3 6.5
* 1 - Porosity above 880 diam (51 2 - Ca(OHI2 content. (51
3 . Zero p o r o s i t y v a l u e s f o r mechanical p r o p e r t i e s a r e somewhat improved by t h e a d d i t i o n o f f l y - a s h o r s l a g , b u t reduced by i n c r e a s e i n t h e t e m p e r a t u r e o f h y d r a t i o n .
4 . D u r a b i l i t y c a n be p r e d i c t e d by t a k i n g i n t o c o n s i d e r a t i o n t h e p o r o s i t y v a l u e o f p o r e s g r e a t e r t h a n 880
fi
and Ca(Otl)2 c o n t e n t s .Acknowledgement
The a u t h o r would l i k e t o ackrlowledge t h e f i n e experj.menta1 work of
A. J
.
Charron.
T h i s p a p e r i s a c o n t r i b u t i o n from t h e D i v i s i o n of Building Research, N a t i o n a l Research Council of Canada, and i s p u b l i s h e d w i t h t h e approval of t h e D i r e c t o r o f t h e D i v i s i o n .
Referenccs
1. R.L. Berger and J . D . McGregor. The i n f l u e n c e o f admixtures on t h e morphology o f calcium hydroxide formed d u r i n g t r i c a l c i u m s i l i c a t e h y d r a t i o n . Cem. Conc. Res. - 2, 43-55 (1972).
2. A. Grudemo. Microcracks, f r a c t u r e mechanics and s t r e n g t h o f t h e cement p a s t e m a t r i x . Cem. Conc. Res. 9, 19-34 (1979). -
3 .
F.V.
Lawrence, J . F . Young and R.L. Berger. Hydration and propert.j.es o fcalcium s i l i c a t e p a s t e s . Cem. Conc. Res.
-
7, 369-377 (1977).4. H. M i g a i r l , R. Faukawa and K. S a i t e e . The i n f l u e n c e o f chemical
coniposition o f g r a n u l a t e d b l a s t - f u r n a c e s l a g and p o r t l a n d cement c l i n k e r of v a r i o u s p o r t l a n d - s l a g cements on r e s i s t a n c e t o s e a w a t e r . Review o f 2 6 t h General Meeting of Cement Assoc. o f J a p a n , 73-75 (1975).
5. G.L. Kalousek, L.C. P o r t e r and E . J . Benton. Concrete f o r long-time s e r v i c e i n s u l p h a t e environment. Cem. Conc. Res. 2, 78-89 (1972). -
6.
J.T.
Dikeou. Fly ash increases resistance of concrete to sulfate attack.U.S. Bureau of Reclamation, Res. Rept. No. 23, p. 17 (1970).
7.
W.
Riedel. Corrosion resistance of cement mortars in solutions ofmagnesium salts. Zement-Kalk-Gips - 6, 286-296 (1973). I
I
8. R.F. Feldman. Durability of blended cements to high concentration chloride
solutions. 5th Int. Symp. Concrete Tcclmol., Monterrey, Mexico, -262-288
(1981).
I
9. J.J. Beaudoin and R.F. Feldman. A study of mechanical properties of
1
autoclaved calcium silicate systems. Cem. Conc. Res. 5, 103-118 (1975).
-
10. R.F. Feldman. Density and porosity studies of hydrated portland cement.
Cement Technol. 3, 5-14 (1972). -
11. J.J. Beaudoin. Porosity measurements of some hydrated cementitious
systems by high pressure mercury-intrusion - microstructural limitations.
Cem. Conc. Res. 9, 771-781 (1979).
+
12. V.S. Kamachandran and R.F. Feldman. Time-dependent and intrinsic .
characteristics of portland cement hydrated in the presence of calcium
chloride. I1 Cemento 75, 311-322 (1978). -
13.
D.L.
Kantro,S.
Brunauer and C.H. Weise. Development of surface in thehydration of calcium silicates: solid surfaces and the solid-gas interface. Advances in Chemistry Series, No. 33, Amer. Chem. Soc., 199-219 (1961).
I
14. I. Soroka and P.J. Sereda. The structure of cement stone and the use of compacts as structural models. Proc. 5th Int. Symp. Chem. Cement, Tokyo, Vol. 111, 67-73 (1968).
15. R.F. Feldman and J.J. Beaudoin. Micro-structure and strength of hydrated
cement. Cem. Conc. Res. 6, 389-400 (1976). -
16. J. Dyczek and M. Petri. The mechanical properties of calcium silicate
hydrates existing in autoclaved cement-quartz materials. Proc. 6th Int. Congr. Chem. Cement, Moscow, Vol. 11, Book 2, 159-161 (1974).
17. G. Fagerlund. Strength and porosity df concrete. Proc. Conf. "Pore Structure and Properties of Materials," Prague, Vol. 11, D-53-D-73 (1973). 18. V.S. Danyushevsky and K.A. Djabarov; Interrelation between pore structure
and properties of hydrated cement pastes. Proc. Conf. "Pore Structure
and Properties of Materials," Prague, Vol. 11, D-97-D-114 (1973).
19. P.K. Mehta. Durability of concrete in marine environment - a review.
ACI SP-65, 1-20 (1980).
20. J.A. Dalziel. The effect of curing temperature on the development of strength of mortar containing fly ash. 7th Int, Congr. Chem. Cement, Paris, Vol. 14, 93-97 (1980).
This publication is being distributed by the Division of
Building R e s e a r c h of the National R e s e a r c h Couelcil of Canada. I t shouldnot b e r e p r o d u c e d i n whole o r i n p a r t without p e r m i s s i o n of the original publisher. The Di- vision would be glad to b e of a s s i s t a n c e i n obtaining s u c h permiseion.
Publications of the Division m a y b e obtained by m a i l - ing the a p p r o p r i a t e r e m i t t a n c e (a Bank, E x p r e s e , o r P o s t Office Money O r d e r , o r a cheque, m a d e payable t o the R e c e i v e r G e n e r a l of Canada, c r e d i t NRC) t o t h e National R e s e a r c h Council of Canada, Ottawa. K1A OR6. Stamps a r e not acceptable.
A l i s t of a l l publications of the Division i s available and m a y b e obtained f r o m the Publications Section, Division of Building R e s e a r c h , National R e s e a r c h Council of Canada, Ottawa. KIA OR6.