Publisher’s version / Version de l'éditeur:
Macromolecules, 25, 10, pp. 2651-2656, 1992-05-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1021/ma00036a015
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Viscoelastic properties of immiscible telechelic polymer blends : effect
of acid-base interactions
Charlier, Pascal; Jerome, Robert; Teyssie, Philippe; Utracki, L. A.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=b193d94f-839e-4bf6-8e07-c9c3ebeddb71 https://publications-cnrc.canada.ca/fra/voir/objet/?id=b193d94f-839e-4bf6-8e07-c9c3ebeddb71
1992, 25,
Viscoelastic
Properties of Immiscible Telechelic Polymer
Blends:
Effect of
Acid-Base Interactions
Pascal
Charlier,f
Robert Jéróme,*andPhilippe
TeyssiéLaboratoryof Macromolecular Chemistryand Organic Catalysis, University ofLiége,
Sart-Tilman B6,B-4000 Liége,Belgium L. A.
Utracki
Industrial MaterialsResearchInstitute, NationalResearch Council ofCanada, Boucherville,
Québec, CanadaJ4B6Y4
ReceivedOctober7, 1991;RevisedManuscriptReceivedJanuary27, 1992
ABSTRACT: Whenatelechelic polyisoprene(PIP)end-cappedwithtertiaryamine groupsisblendedwith
an immiscible telechelic polystyrene (PS) terminated with either sulfonicor carboxylicacid functions, a
proton transferoccurs fromthe acid to the amine end groups. Viscoelastic propertiesoftheseblendssupport theformation of eitherammonium sulfonateor ammonium carboxylate linkages betweenPSandPIP ina manner analogous to block copolymerization. Due to the lowstability ofammonium carboxylates, the related
polyblendshave beeninvestigated up to100°CandtheyshowTeofeachpolymeric partner. WhenPSand
PIPchainendsare associatedthroughmore thermallystableammonium sulfonates, thethermal
depend-ences ofstorageandloss shearmodulishowtwoadditional relaxationprocessesat leastinsome rangeof
PSandPIPmolecular weights. Byanalogywith SAXSanalysisof similarpolyblends, these relaxationshave been assignedtoaclassicorder-disordertransition similartothatseen in.traditional blockcopolymers and totheirreversible dissociationofthesulfonate salts at higher temperatures, respectively. Theseadditional transitionswhich involveachangeinphasemorphologyhavebeenconfirmedby the“Cole-Cole”logG"vs
logG' plots.
Introduction
Nowadays,thefinetailoring ofmultiphase polymersis
one ofthe most efficient ways to generate new organic materials ofhighperformance.1"9 That opportunityhas been highlighted for thefirst time by the
poly(styrene-b-diene-b-styrene) triblock copolymers which behave as thermoreversibly cross-linked rubbersand are commer-cializedasthermoplasticelastomers.4 Comparedto block copolymerization, melt blending of available polymers
usingtraditionalprocessingequipmentis a more conve-nientway to producemultiphasepolymericmaterials.1"3,5^
Although inthese systemseachofthepartners retains its own properties, the overall physicomechanical behavior
is only satisfactory when two demanding structural
requirements are met: (i) a controlled microphase sep-arationinrelation to aproper interfacialtension and/or appropriate processing conditions; (ii) an interphase
adhesionstrongenough toassimilatestressesandstrains
withoutdisruption oftheestablished morphology. Several strategies have been proposedwiththe viewofreaching
these targets.5 Creating a practically irreversible mor-phology either in the polymerizationprocess itself(e.g.,
HIPS andABS resins)10 or in the blending processis a
first possible approach.5·7·8 A more general strategy
consistsofusingdiblockcopolymersoftailored structure andmolecular weightasinterfacialagents.1·5·11"24 These
polymeric additivescan lead toexcellent propertiesofthe finalmaterialseven when usedinratherlowamounts(ca. 1-2 wt %). Mutual interaction of functional groups
randomly attached,one on eachimmiscible polymerofa binary blend, isanothermeans of controlling interfacial tensionandinterphaseadhesion. Severaltypes of inter-action can be promoted, e.g., acid-base, dipole-dipole,
ion-ion,andion-dipoleinteractions, hydrogen bonding,
* Towhom correspondence shouldbeaddressed.
1Present address: Machelen Chemical Technology Center,Exxon ChemicalInternationalInc.,2,NieuweNijverheidslaan,B-1831
Ma-chelen,Belgium.
charge-transfercomplexes, andmetal-ligand coordination
complexes.25
The beneficial effectofmutual acid-base interactions on themaincharacteristicsofpolyblendshas beenreported
in the scientificliterature.25"30 Forinstance,ionic cross-interactionsbetweenoriginallyimmiscible polymers are
generatedbyaproton transfer fromacid groupsattached
to one polymer to amino groups present on the second
polymer. For that transfer to be quantitative,pKa’sof
theacid andaminogroups havetobedifferentby atleast
3units.25 This isthe reason why sulfonic acid/aromatic amine(ApKa 4) isacommonlyusedacid/basepair. The effect promoted by the mutually interacting groups dependson theirconcentration. Belowacriticalvalue(5 mol%withrespect to the monomericunitsofthemodified
polymer),polystyrene/poly(ethylacrylate) and polystyrene/
polyisoprene blends are opaque and show two Tg’s, although theirmechanicalpropertiesare improved. Above the critical ionic content, blends are transparent and exhibitauniqueTgwhichis,however, observedasabroad relaxationpeakby mechanicalmeasurements, suggesting that miscibilitydoesnot existat ascale smallerthan 10
nm.25 Spectroscopicandmechanicalstudieson blendsof sulfonicacidcontaining polystyreneandpolyurethanealso showthataproton transfertakes placefrom the sulfonic
acid groups to the urethanemoieties.31"33 Interestingly enough, when used at a rate ofatleast 13 mol %, car-boxylic acid and pyridineunitsare ableto promote the
miscibility of poly(methyl methacrylate) (PMMA) and poly(butyl acrylate).34 A positive effect of hydrogen
bonding has also been reported forblends of
poly(2-vi-nylpyridine) and poly(ethylene-co-methacrylic acid).35 Finally,carboxylic acid-urethane interactions are likely
to occur sincemechanical propertiesof polystyrene/poly-urethane blendsare improvedwhenpolystyreneis mod-ified by carboxylicacidgroups.36·37
Proton transfer fromacid end groupsofone telechelic polymerto dimethylamine end groupsofan immiscible
0024-9297/92/2225-2651$03.00/0 © 1992American Chemical Society
2652 Charlieret al. Macromolecules, Vol. 25, No. 10, 1992 telechelic partnerhasbeeninvestigatedas amodel system
for the more complex situation in which intermolecular ionic bonding or hydrogen bonding is promoted by interactive moieties distributed at random along the polymericbackbones.38-42 Since the spontaneoustrend
oftheblendedpolymers to demixiscounterbalancednow
by cross-interactions between the chain ends of each
polymeric component, these mixtures are expected to mimicclosely thephasemorphologyand general properties
ofthecorrespondingmultiblockcopolymers. Thatanalogy hasbeen substantiated byseveraltechniques. IR
spec-troscopy38·41 supports the occurrence ofproton transfer from carboxylicacid to dimethylamine end groupswith
theformationof “interchain”ammonium carboxylate ion pairs (eq 1). Optical microscopy shows that the phase
-PA—COOK + R2N~^PB«
-PA—COO R2NH*~PB- (1)
separationofPA and PB is controlled by the ion pairs
bridging the chain extremities toalevelthatdepends(i) on the nature ofPA and PB and their intrinsic
immis-cibility,(ii)on the ion pair content, in relation to
molec-ular weightofPAandPB,and(iii)on the strengthofthe
ionpairs, ammonium sulfonatesbeing more effectivein
fightingtheimmiscibility ofPAandPB than ammonium
carboxylates.41 Tgmeasurementsare in qualitative agree-ment with the general pattern reported for multiphase blockpolymers.38·41 Thesolution viscosityofPA andPB telechelic polymerblendsinacommon, apolar solventis
significantly modified by the ionic bondingbetweenthe polymers and the possible dipolar interactions of the bridging ion pairs.41 Finally a temperature-dependent
small-angle X-ray scattering study has shown that the morphology ofthepolyblendsclosely resemblesthatseen in covalentlybonded blockcopolymers.39 When ammo-nium sulfonate ion pairsare concernedanddependingon themolecular weightofPAand PB,aclassicorder-disorder
transitionisobserved andeven possibly followed by the effectofacriticalsolution temperature. Thispaper deals
withthe viscoelastic properties ofblends of a,w-bis(di-methylamino)polyisoprene [PIPfNRzk] and
,
-dicar-boxy-or -disulfopolystyrene [PS(COOH)2or PS(S03H)2]. The effectofthebridging ion pairsandtheirstrengthand
content willbeconsidered, andthe dynamic mechanical behaviorwillberelatedasmuchaspossibletothephase
morphologypreviously investigated by SAXS.
Experimental
SectionSamplePreparation. Telechelic polymers were prepared
by anionicpolymerization. Pure andcarefully driedmonomers
and solventswere used. Isoprene and styrenewere driedover
CaH2atroom temperature anddistilledunder reduced pressure justbefore use. Isoprene andstyrene were mixed with rc-bu-tyllithiumandlithium fluorenyl, respectively, and againdistilled beforepolymerization.
Polymerization was performed in previously flamed and
nitrogen-purged flasks equippedwith rubberseptums. Hypo-dermicsyringesandstainless-steel capillarieswere usedtohandle
liquidproducts underanitrogenatmosphere. a,o)-Bis(dimeth-ylamino)polyisoprenewas anionicallypreparedinTHF,at-78 °C, using sodium naphthaleneas adifunctional initiator, ,
-Disulfopolystyrenewas prepared under thesame experimental
conditions exceptforthe counterionoftheactivespecieswhich
was Li insteadofNa. Theinitiatorsresultedfromthereaction ofnaphthalenewitha slightexcess oftheappropriate alkaline metalinTHF at room temperature.
The living macrodianions were deactivated by anhydrous
carbondioxide,43 1,3-propane sultone,44and l-chloro-3-(dime-thylamino)propane45withtheformation of ,
-dicarboxypoly-TableI
Molecular Characteristics of Telechelic Polymers
sample M„,X10"3 Mw/Mn Mt,°X10"3 Z6 PIP(NMe2)218K 18.0 1.2 19.5 1.85 PIP(NMe2)238K 38.4 1.3 41.5 1.86 PS(COOH)213K 13.2 1.4 13.5 1.96 PS(S03H)29K 9.1 1.3 10.0 1.82 PS(S03H)215K 14.6 1.2 15.8 1.85
0Mt= molecular weight determined bytitrationoftheend groups.37
bf = functionality= 2MJMt.
TableII
Compositions andCodeNamesof Blends of Telechelic Polymers
blends0 codename
PIP(NMe2)238K-PS(COOH)213K M38-13C PIP(NMe2)238K-PS(S03H)2 9K M38-9S PIP(NMe2)238K-PS(S03H)215K M38-15S PIP(NMe2)218K-PS(COOH)213K M18-13C PIP(NMe2)218K-PS(S03H)2 9K M18-9S PIP(NMe2)218K-PS(S03H)215K M18-15S “PIP/PS= 1/1 (mol/mol).
styrene [PS(COOH)2], , -disulfopolystyrene [PS(S030H)2], and a,ü>-bis(dimethylamino)polyisoprene [PIP(NMe2)2],
respec-tively.
The microstructure ofthe polyisoprene was found to be a
mixture of1,2/3,4inaratio of35/65. Molecularweightsofthe telechelicswere controlledbyadjustingthemonomer toinitiator molar ratio. The molecular weight and polydispersity ofthe telechelic polymerswere measured by size-exclusion
chroma-tography(SEC),asreportedelsewhere.45 Thefunctionalitywas
calculatedfrom theM„value (SEC) and from thepotentiometric
titration ofthe end groups. Dimethylamino groups and acid groups (COOH and S03H)were titratedwith perchloricacid and tetramethylammoniumhydroxide, respectively,ina9/1toluene/ methanolmixture. Polyisoprenewas carefullystabilized byan
antioxidant(1wt % ofIrganox1010).
The main molecular characteristicsoftelechelic polymersare
reported in Table I.
Polymer blendswere prepared bymeans ofsolvent-casting
techniques. Separate solutionsofeachpolymerina9/1toluene/ methanolmixturewere prepared. Afterhaving mixed the proper volumesofeachsolution(sothatthe acid/amine molarratiowas
1), the solutions were allowed to stir for24 h before casting. Filmswere preparedbyaslowevaporation ofthesolvent followed byvacuum dryingto constant weight at 70 °C (ca. 1 month).
Samplesfordynamic mechanical measurementswere prepared
by compressionmoldingat2MPaand140°Cfor20min. Table II summarizesthe compositionsofthe blends investigated in thisstudy togetherwith theircodenames.
Differential Scanning Calorimetry(DSC). DSC thermo-gramswere recorded, from-40 up to +200 °C. Sampleswere
previously annealed at200°Cfor15min. APerkin-Elmer
DSC-4 was usedataheating rateof20°C/minunderaconstantflow ofdrynitrogen.
Dynamic MechanicalMeasurements. A Rheometrics
me-chanical spectrometer (RMS 605S) was used with a
parallel-plate geometry. The 25-mm-diameter specimenin a 1.4-mm-wide gap was tested under a constant flow of dry nitrogen. Measurementwere carried out ataconstant frequency(t-= 1Hz)
and a constant heating/cooling rate of2 °C/min. Isothermal frequencyscans atfrequenciesfrom0.016to16Hzwere conducted forM38-13C and M18-15S blends.
Results and Discussion
Effect of
Strength
of the Ion Pairs and of Their Mutual Interactions. Figures 1-3illustratethethermaldependenceofthestorage(GOandloss(G")shearmoduli,
measured atafrequency of1rads-1forblendscontaining PIP(NR2)2 38K (where 38K refers to molecular weight)
associated to PS (SOsH^ 15K, PS(S03H)2 9K, and PS (COOH)2 13K, respectively.
Macromolecules, Vol.25, 10,
Figure 1. Storage(GOandloss(G") shearmodulivs
temper-ature (1 Hz) for theM38-15S blend (TableII).
Figure2. Storage(GOandloss (G'O shearmodulivs
temper-ature (1 Hz)for theM38-9Sblend (TableII).
Figure3. Storage (GOandloss(G") shearmodulivs
temper-ature (1 Hz)for theM38-13Cblend (TableII).
All
theG"curves showamaximumin the temperaturerangeof 70-80 °C. Such arelaxationhas been assigned totheglasstransitionof polystyrene(orpolystyrene-rich)
phases. Abovethat transitiontemperature, the material clearly starts to flow. That interpretationassumes that the investigated blends are phase separated. This has beenconfirmed elsewhere41and shown in this study by
differentialscanningcalorimetry(DSC). Indeed,twoTg’s
are reported (Table
III)
whichcomparedtoTgoftheparent PIP and PS are characteristicofphasesof that nature.The difference in the Tg’sforeach type ofphase isalso
mentioned inTableIII. ATgissmaller byca. 10°Cwhen
Viscoelastic Properties Telechelic Polymer Blends TableIII
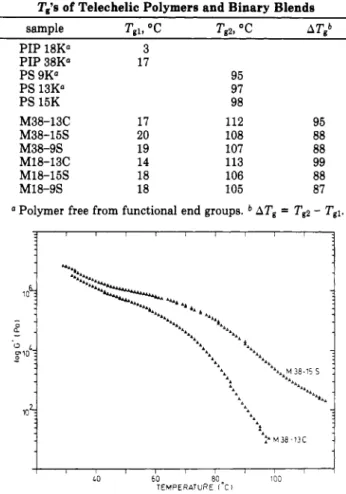
Tg’sof Telechelic PolymersandBinaryBlends
sample Tgi, °C Tg2,°C ATtb
PIP 18K“ 3 PIP38K° 17 PS9K“ 95 PS13K“ 97 PS15K 98 M38-13C 17 112 95 M38-15S 20 108 88 M38-9S 19 107 88 M18-13C 14 113 99 M18-15S 18 106 88 M18-9S 18 105 87 a
Polymerfreefromfunctionalend groups.6
ATf= “
T$l.
Figure4. Comparisonofthe log G'vstemperaturecurve (1Hz) forM38-15S and M38-13C blends (TableII).
thechainendsare held together through ammonium
sul-fonate ion pairs rather than carboxylate ones. This indicatesthatan increaseinthe strengthofthe bridging ion pairs is in favor of an improved miscibility, all the other conditionsbeing unchanged.
Figure4 comparesthe G' curves fortwo blends M38-15SandM38-13C which essentiallydifferon thenature
oftheacid end groups.
Theintermediate plateau below70-80°Cextendsover alargertemperaturerange, and G'of thatplateauishigher
when PSis end-cappedbyasulfonicacid. These
obser-vations mean that ammonium sulfonates are stronger dipoles than theircarboxylatecounterpartsandthattheir
mutual interactions stabilize a stronger chain network. Indeedthe telechelic polymerblendsunder investigation can be depicted not only as an ionically bonded block copolymerbutalsoas atwo-component(PS andPIP) ion-omer ofthesulfonic type. In thisregard,itiswell-known
thatthemutualassociationofsulfonateion pairs improves
thehigh-temperature properties oftheionomer muchmore effectively than carboxylate dipoles do.46
At
70°C, theM38-13C blend starts to flow rapidly which reflectsthe fast disruption ofthemutual association oftheammonium carboxylate ion pairsand mostlikelythe thermalrupture oftheion pairsthemselveswith release ofthe carboxylicacid and dimethylamineend groups.It isworth pointingoutthataPS(COOH)213Ksamplehas beenpreviously neutralizedwith triethylamineandthen
heatedat130°Cundervacuum. Thebackproton transfer
has been observed as supported by the quantitative elimination oftriethylamine47,48 (eq 2). Incontrastto
M38-130°C
PS(COO~ +HNR3)2 —
PS(COOH)2+ 2NR3 (2)
2654 Charlieret al. Macromolecules, Vol.25, No. 10, 1992
TableIV
MolarandWeightCompositionsofBinaryBlendsof Telechelic Polymers
pip_
ps so3-n+:codename mol%< wt % mol %° wt % mol%°
M38-15S 79.3 71.7 20.4 28.3 0.28
M38-9S 86.3 80.9 13.4 19.1 0.31
M18-15S 64.4 54.5 35.1 45.5 0.49
M18-9S 74.9 66.7 24.5 33.3 0.57
0Onthebasisofthemonomer content.
Figure5. Storage (G') andloss (G")shearmodulivs
temper-ature(1 Hz) for theM18-15S blend (TableII).
13C, the occurrence ofthe flowregime isdelayed in the M38-15SblendandthedecreaseinG'atincreasing tem-peratureismuchslower. Forinstance,thecomplex shear modulusofM38-15Siseasily measured at 150°C (Figure
1)whereasitisalready too small tobemeasured accurately at100°Cfor theM38-13Cmixture(Figure3). Thisisthe
consequence of the highest thermal stability of the ammonium sulfonate ion pairscomparedto the
carbox-ylate ones.47’48
Effect
oftheIonPair
Content. Inthe previous blends, the molecular weight of PStSOsHh associated toPIP-(NH2)2 38K was changed from 15K to 9K and no substantialmodificationintheviscoelasticbehaviorwas noted(seeFigures1and2). Itmust,however,bestressed
thattheion pair content remained essentially unaffected
ascalculatedinTableIV. Thisisthereason whyasecond
seriesofblendswaspreparedinwhich the molecularweight
of PIP(NH2)2 was made to decrease by a factor of 2. Accordingly, the ion pair content was increased by ca.
80% withrespecttosamplesofthefirstseries(TableIV). Figures5and6showtheG' andG"curves forthe
M18-15Sand M18-9S mixtures whichare basically different from those reported for the related blends containing PIP(NR2)2ofa twice as high molecular weight (Figures
1and2). Actually, two additional transitionsare observed on the high-temperature side. In order to assign the relaxationprocessesobservedin the investigated temper-ature range, it is worthreferringto TgdataofTable II. Inspiteofasubstantialincreaseintheionpaircontent, the telechelic polymer blends are still phase separated
and the Tg of each phase as measured by DSC is not significantly modified. According to the G" curves of Figures 5 and 6,the first maximumon the low-temper-ature sideisobservedat 65°C andthenext one at
120-130°C. Oneofthesemaxima shouldbeattributedtothe Tgofpolystyrene-rich phases. The decrease in the PIP molecular weight together withthe increasein thecontent oftheionpairs whichhaveto accumulateintheinterfacial
regionisexpectedtoenhancethe polymermiscibilityand
TEMPERATURE ( C!
Figure6. Storage (G') andloss(G") shearmodulivs
temper-ature (1 Hz) forthe M18-9S blend (TableII).
Figure7. SchematicphasediagramofblendsofPIP(NR2)2 and PS(COOH)2or PS(S03H)2.35
move Tg’s closer together. On that basis, the glass
transitionofthePS-richphasesshouldbeatthe originof
therelaxation at65°C. If thisisso,dynamic mechanical
measurements conclude to a decrease in Tg ofthe rigid
phaseswhenthePIP molecular weightisdecreasedfrom 38K to18K. The apparent contradictionwithDSCdata (Table
III)
will be discussed later.Theadditionalrelaxations observed at 120-130 and 160-180°C, respectively, havesomethingto dowiththe
sul-fonateion pairsandtheir association, sincepolystyrene
ionomersof the sulfonic type exhibitan ionicrelaxation inthat range oftemperature.49 It is,however, ofgreat
significancetorefernow toapreviousstudyon thethermal
dependence ofthe SAXS profile of blends comprising PIP(NR2)218Kanda,w-disulfopoly(a-methylstyrene)of
various molecularweights.39 Fromthatstudy, the general phasediagramschematizedin Figure7hasbeen proposed whereUCSTistheuppercriticalsolution temperature of
the constituent telechelic polymers, MST is the
mi-crophase-separationtemperatureoftheioniccopolymers, Tgistheglasstransitionofthehardphases,andT¡ isthe
25, Viscoelastic Properties Telechelic Polymer Blends
block copolymers are disrupted. T¡ depends on the strengthoftheionpairs, theintrinsic immiscibility ofthe
polymerpair,andthe molecular weightofeachofthem.
When Tiisaboveboth TgandtheMSTcurve (represen-tativesystemA),anorder-disordertransitionoccurs when
the microphase-separated system goes across the MST curve and a uniform melt is observed. At higher tem-peratures, i.e.,greater thanT¡,the effectivehomogeneous
copolymer melt is broken up into the constituent
ho-mopolymersand phaseseparationofthe homopolymers occurs. The two additional relaxation mechanisms of Figures5and6couldnow beaccounted for. The maximum
in G" at 120-130 °C should correspond to the classical
order-disordertransitionseen incovalentlybondedblock
copolymers. In the investigated blends (M18-15S and
M18-9S), that transition is actually triggered by the
relaxationofthe ionpairassociation.50"53 Inotherwords,
themutual interactionsoftheammonium sulfonate ion pairs are unable to stabilize a microphase-separated morphology at temperatureshigher than120-130 °C. As
longasthe ion pairsare stable, chainsretaina “blocky” structure and the system remains homogeneous at the microscopiclevel. Athigher temperatures(160-180 °C)
thethermalbreakupoftheammonium sulfonate ion pairs occurs togetherwithareleaseofsulfonic acid andtertiary amine end groups,resulting ina macrophase separation.
Thisissupported by the elsewhere-reported observation that ammonium sulfonates attached at the end of a polystyrene chainare unstable above 180 °C.47,48 Since
it
iswell-knownthataliphatic sulfonicacidsare unstable at such high temperatures, the ionic block copolymerstructureisirreversiblylost.
It
isnow worthmentioningthatthe samples analyzedbyDSCinthis studyhave been
previouslyannealedat200°Cin order tomakethemfree
from any residual solvent. That pretreatment might
explain the discrepancy inTgvaluesdetermined byDSC anddynamic mechanicalmeasurements. Whenthe high-temperaturepretreatmentisnot applied, data provided bythe two methodsare consistent.41 TheG' andG"curves reported inFigures1-3can alsoberationalizedbyreferring to thephase diagram ofFigure 7 where B is the repre-sentativesystem. Since T¡ is below Tg, as soon as Tg is reached, the components ofthe ionic block copolymer behaveas individualhomopolymer moleculesbelow the UCSTandcoarse phaseseparationensues. Thus,above Tg of the rigid PS phases, no additional relaxation is expectedtooccur,asisactuallyobserved. Figure8 sche-matizes the three main phase situations which can be observed when ion pairs associate the chain ends of immiscible telechelic polymers.
Transitions observed at 120-130 and 160-180 °C in
Figures5and6havebeenassigned torelaxationprocesses thatinvolveachangein thephasemorphology: an order-disorder transition and a macrophase separation of a
homogeneous system. Very interestingly, Han etal.54-56
have proposedamethodwhichhasprovento be
fruitful
in giving prominence to the order-disordertransitionin poly(styrene- -isoprene-b-styrene) copolymers.57 Themethodconsistsof firstrecording the isothermal
depend-ence ofG'andG"on frequencyandthenplottinglogG" versus logG'. These graphsare actually Cole-Cole plots on a log-log scale. As long as the morphology remains
unchanged in the investigated temperature range,
iso-thermallogG"vslogG'curves superimposewithformation
ofasingle“mastercurve”.54 Incontrast,whenthephase
morphology is modified, the superposition failsat least until it becomes againtemperature independent. Onthe
basisof that criterion, theCole-ColeplotofFigure9shows thatthemorphology oftheM38-13Cmixtureispreserved
from40up toca. 100°C,i.e.,up to theoriginoftheviscous
'·...I-Figure8. Representationofthemainphasesituationsobserved in blends of immiscible telechelic polymers, where
sche-matizesanammonium carboxylate (~COO"R2+NH-)or sulfonate
(-SC>3"R2+NH-) ion pair. 7 6 4 3 2 3 4 5 6 7 logG' (Pa)
Figure9. logG'vslogG"curves fortheM38-13Cblend (Table
II). 6 , 4 3 2 2 3 4 5 6 logG' (Pa)
Figure 10. Cole-Coleplot forthe M18-15S blend (Table II). flowregime. Thus,as longas TgofthePSphases isnot
exceeded, the two-phase structure is stabilized by that rigid PS componentinagreement with Figure7 (repre-sentative system B). When the M18-15S blend is
con-cerned,thesituationradicallychanges, as illustratedby Figure10. The superpositionofthe logG"vslogG'curves
does not fit in two temperature ranges. The first one
extendsapproximately from 115to 155 °C,which
corre-sponds satisfactorily to the second relaxation seen in Figure5. The Cole-Coleplotgivesaccordingly credit to
2656 Charlier etal.
the assignment ofan order-disorder transition to that relaxation. The same conclusion can be drawn for the
secondtemperaturerangein which superpositionofthe
Cole-Coleplotdoesnotwork(i.e.,aboveca. 165°C),since it fits the third relaxation observed in the viscoelastic
curves ofFigure5 andsubstantiates the attribution ofa
macroscopicphase demixing.
Inconclusion, thereisaverygood agreement between
the viscoelastic properties of blendsof
a,o>-bis(dimeth-ylamino)polyisopreneand , -dicarboxy-or
-disulfopoly-styrene and the phase morphology which has been
determined by SAXSfor analogousblends.39 The relative positions of Tg of the PS phases, the order-disorder transitiontemperatureofthebridging ion pairs(T¡), and the MST curve play a key role. That situation can interestinglybecontrolled by (i) theintrinsicstrengthof theion pairs(ammoniumcarboxylates against sulfonates)
whichaffectsthepartial
miscibilitity
ofPSandPIP (thus Tgof thePS phase),the strength of themutual ion pairassociation (thus theMSTvalue), andT¡and by (ii) the molecular weight of the telechelic polymers, i.e., the
thermodynamic repulsion of PS and PIP which acts adversely onto the electrostatic interactions of the am-monium cation andsulfonate (or carboxylate) anion, as wellas on theirmutualassociation. Asaresult,itisquite possible togothroughthe whole phasediagramshownon Figure7andtochangedeeply theviscoelasticbehaviorof the polyblends.
Acknowledgment. P.C., R.J., andPh.T.are very much indebted to the “ServicedelaProgranimationdela Poli-tique Scientifique” and the “Fonds National de la Re-chercheScientifique” (FNRS) for financial support. P.C. is very much indebted to IMRI for the opportunity of
spendinga 3-monthstayintheir facilities. Theskillful technical assistanceofP. Sammut (IMRI) for dynamic mechanicalmeasurementsisverymuchappreciated.P.C. also thanks the
“Instituí
pour l’Encouragement de la RechercheScientifiquedansl’lndustrieet Agriculture” (IRSIA)forafellowshipand the FNRS for having awarded him a grant to visit the Industrial Materials ResearchInstitute (IMRI) ofMontreal. Referencesand Notes
(1) Teyssié, Ph.;Fayt,R.;Jéróme, R.Makromol. Chem.,
Macro-mol. Symp. 1988, 16, 41.
(2) Utracki, L.A.Int. Polym.Process. 1987, 2, 3.
(3) Schreiber, P.;Latham,J.Polym.News 1987, 13,19.
(4) Legge,N.R.,Holden,G., Schroeder, . E., Eds.Thermoplastic Elastomers;Hanser: Munich,1987.
(5) Paul, D. R., Newman, S., Eds. Polymer Blends; Academic Press: NewYork,1978;Vols.1 and2.
(6) Olabisi,0.; Robeson, L. M.; Shaw, . T. Polymer-Polymer
Miscibility;Academic Press: NewYork, 1979.
(7) Walsh, D. J.; Higgins,J.S.PolymerBlendsand Mixtures;
Ma-connachie,A., Ed.; NATOAdvancedStudyInstituteSeries;
MartinusNijhoff: Dordrecht, The Netherlands,1985. (8) Utracki, L.A.Polymer Alloys andBlends,Thermodynamics
andRheology; Hanser: Munich, 1989.
(9) Utracki, L.A., Weiss,R.A., Eds.MultiphasePolymers: Blends
andlonomers; ACS Symposium Series395;AmericanChemical Society: Washington,DC,1989.
(10) Bucknall,C.B. Toughened Plastics;AppliedScience: London,
1977.
(11) Fayt,R.;Jéróme, R.; Teyssié, Ph.J. Polym.Sci.,Polym. Lett.
1981, 19,79; 1986, 24, 25.
(12) Fayt,R.; Jéróme, R.; Teyssié, Ph.J. Polym.Sci., Polym. Phys. Ed.1981, 19, 1269; 1982,20, 2209; 1989, 27, 775.
(13) Fayt,R.;Jéróme, R.; Teyssié, Ph.Makromol.Chem. 1986,187, 837.
Macromolecules, Vol. 25,No. 10, 1992 (14) Fayt, R.; Jéróme,R.;Teyssié, Ph. Polym.Eng. Sci.1987,27,
328.
(15) Fayt, R.; Jéróme, R.; Teyssié, Ph. J.Appl.Polym. Sci. 1986,32, 5647.
(16) Fayt,R.; Teyssié, Ph. Macromolecules 1986, 19, 2077. (17) Ouhadi,T.; Fayt,R.;Jéróme, R.; Teyssié, Ph.J. Polym.Sci.,
Polym.Phys. Ed. 1986,24, 973.
(18) Ouhadi,T.; Fayt,R.;Jéróme, R.; Teyssié, Ph. Polym. Commun. 1986, 27, 212.
(19) Ouhadi,T.; Fayt,R.;Jéróme,R.;Teyssié, Ph. J.Appl. Polym.
Sci. 1986,32, 5647.
(20) Heuschen, J.; Vion,J. M.;Jéróme, R.; Teyssié, Ph. Polymer
1990,31, 1473.
(21) Barlow,J. W.; Paul, D. R.Polym.Eng. Sci.1984,24, 525. (22) Anastasiadis,S.H.; Gancarz,I.; Koberstein,J.T.
Macromol-ecules 1989, 22, 1449.
(23) Shull, K. R.; Kramer, E. J.; Hadziioannou, G.; Tang, W. Macromolecules 1990,23, 4780.
(24) Cho, K.; Brown, H. R.;Miller, D. C. J. Polym.Sci.,PartB;
Polym.Phys. 1990,28, 1699.
(25) Smith, P.; Kara, M.;Eisenberg, A. A Review ofMiscibility Enhancement via Ion-Ion and Ion-Dipole Interactions. CurrentTopicsin PolymerScience; Hanser: NewYork,1987; Vol.2,Part6.
(26) Eisenberg, A.;Smith,P.;Zhou,Z.L.Polym.Eng. Sci. 1982,22, 1117.
(27) Smith,P.;Eisenberg, A.J. Polym.Sci., Polym.Lett.Ed.1983, 21, 223.
(28) Zhou, Z. L.; Eisenberg, A. J. Polym. Sci.,Polym. Phys. Ed. 1983,21, 595.
(29) Bazuin,C.G.;Eisenberg, A.J. Polym.Sci.,Polym.Phys. Ed. 1986,24, 1021.
(30) Murali,R.; Eisenberg, A. J.Polym. Sci.,Polym.Phys. Ed.1988, 26, 1385.
(31) Rutkowska,M.;Eisenberg, A.Macromolecules 1984,17,821. (32) Tannenbaum,R.;Rutkowska,M.;Eisenberg, A. J.Polym. Sci.,
Polym.Phys. Ed. 1987,25, 663.
(33) Natansohn, A.; Murali,R.; Eisenberg, A. Makromol. Chem.,
Makromol. Symp. 1988, 16, 175.
(34) Jo, W.H.;Lee,S. C.;Lee,M.S.Polym.Bull. 1989, 21, 183. (35) Lee,J.Y.;Painter,P. C.;Coleman, . M. Macromolecules1988,
21, 954.
(36) Hsieh,K. H.; Wu,M. L. J.Appl.Polym.Sci. 1989, 37, 347. (37) Hsieh,K. H.;Chou,L. M.;Chiang, Y.C.Polym. J. 1989,21,1. (38) Horrion,J.;Jéróme, R.;Teyssié, Ph.J. Polym. Sci., Polym.
Lett.Ed. 1986,24, 69.
(39) Russel, T. T.;Jéróme, R.; Charlier, P.; Foucart, M.
Macro-molecules 1988,21, 1709.
(40) Horrion, J.; Jéróme, R.; Teyssié, Ph.; Vanderschueren, J.; Corapci,M. Polym.Bull. 1989,21, 627.
(41) Horrion,J.;Jéróme, R.; Teyssié, Ph. J. Polym. Sci., Polym.
Chem.Ed.1990,28, 153.
(42) Charlier, P.;Jéróme, R.; Teyssié, Ph.;Utracki, L. A.Polym.
Networks Blends 1991,1,27.
(43) Broze, G.;Jéróme,R.;Teyssié, Ph. Macromolecules1982,15, 920.
(44) Foucart, M.Ph.D. Thesis,University ofLiége,Belgium,1988. (45) Charlier,P.;Jéróme, R.; Teyssié, Ph. Macromolecules 1990,23,
1831.
(46) Lundberg,R.D.;Makowski, H.S.Adv.Chem.Ser. 1980,187, 21.
(47) Charlier,P.Ph.D. Thesis,University ofLiége,Belgium,1990. (48) Charlier,P.;Jéróme, R.;Teyssié, Ph.;Prud’homme,R. E., to
bepublished.
(49) Bazuin,C. G.;Eisenberg, A.Ind.Eng. Chem. Prod.Res.Dev. 1981 20 271
(50) Chung,C.I.;Lin, . I.J. Polym.Sci.,Polym.Phys. Ed.1978, 16, 545.
(51) Widmaier,J.M.; Meyer,G. C.J. Polym.Sci.,Polym. Phys. Ed. 1980, 18, 2217.
(52) Roe,R.J.;Fishkis,M.;Chang,J. C.Macromolecules 1981,14, 1091.
(53) Kraus,G.;Hashimoto,T.J.Appl.Polym.Sci.1982,27, 1745. (54) Han,C.D.; Kim,J.J.Phys. Sci.,Polym.Phys. Ed. 1987,25,
1741.
(55) Kim,J.;Han,C.D.J. Polym.Sci.,Polym.Phys. Ed.1988, 26, 677.
(56) Han,C.D.;Kim,J.;Kim,J.K.Macromolecules1989,22, 383. (57) Han,C.D.; Back, D.M.;Kim,J.K. Macromolecules1990,23,