D. Q u i n t a n a r - G u e r r e r o E. All6mann
E. Doelker H. Fessi
A mechanistic study of the formation
of polymer nanoparUcles
by the emulsification-diffusion
technique
Received: 30 October 1996 Accepted: 27 March 1997 D. Quintanar-Guerrero PharmapeptidesInteruniversity Centre Geneva-Lyon 74166 Archamps, France E. All,mann (1~)" E. Doelker School of Pharmacy University of Geneva Quai Ernest-Anserrnet 30 1211 Geneva 4, Switzerland H. Fessi School of Pharmacy
University Claude Bernard, Lyon I Avenue Rockefeller 8
69373 Lyon, France
A b s t r a c t The mechanism of formation of polymer nanoparticles prepared by the emulsification- diffusion method was evaluated under different preparation conditions and by turbidimetry measurements. Biodegradable poly (D,L-lactic acid) was used as the polymer model. The results show that each emulsion droplet will form several nano- particles and that the interfacial phenomena during solvent diffusion determine the size properties of the resulting colloid particles. These phenomena cannot be entirely explained by the convection effects caused by interfacial turbulence. We suggest that nanoparticle formation is due to diffusion alone, and we
propose a mechanism based on the "diffusion-stranding" mechanism for spontaneous emulsification. In this mechanism, the diffusion of solvent causes local supersaturation near the interface, and nanoparticles are formed, due to the phase trans- formation and polymer aggregation that occur in these regions. This interpretation is supported by the turbidity measurements made at different polymer concentrations and stirring rates.
K e y w o r d s P o l y m e r - n a n o p a r t i c l e s - e m u l s i f i c a t i o n - d i f f u s i o n method - spontaneous emulsification - interfacial turbulence - diffusion stranding
Introduction
During the last two decades, polymeric biodegradable colloidal carriers have shown promise as controlled drug delivery dosage forms for specific sites in the body. In general, biodegradable nanoparticles (nanospheres or nanocapsules) can be prepared by two methods: either by a polymerization of dispersed monomers or from a disper- sion of preformed polymers. Extensive investigations have been made on polyalkylcyanoacrylates nanoparticles and their close derivatives, prepared by emulsion-polymeriz- ation processes [1, 2]. Due to the potentially toxic effects of residual substances present after a polymerization reaction, in addition to probable cross-reactions with the
drug, nanoencapsulation techniques using preformed, well-defined polymers are preferable [3, 4].
Recently, a new method for manufacturing nano- spheres from preformed polymers, namely, emulsifica- tion-diffusion, has been described [-5, 6]. This technique presents clear advantages over other existing methods, such as (a) the use of pharmaceutically acceptable organic solvents, (b) no need of high-pressure homogenizers or ultrasonication, (c) high yields, (d) high reproducibility; (e) easy to scale up and (f) rapid purification by cross-flow filtration. The process involves the formation of an o/w emulsion with a partially water-soluble solvent, which contains the biodegradable polymer, and with an aqueous phase containing a stabilizer such as poly(vinyl alcohol). The subsequent addition of water to the system causes the
D, Quintanar-Guerrero et al. 641 Mechanistic study of formation of polymer nanoparticles
diffusion of the solvent into the external phase, with the subsequent aggregation of the polymer held in the globules to form nanospheres, as a product of the newly generated nonsolvent phase. The mechanism by which the diffusion of solvent from the globules induces polymer aggregation and hence nanosphere formation, has been attributed to the formation of new nanometer-sized globules, during diffusion [6], but the exact mechanism remains unclear. Thus, a better understanding of this phenomenon is im- portant to control and optimize the variables involved. The aim of this work was to study the effect of varying conditions of the emulsification diffusion process such as polymer/stabilizer ratio, internal phase/dispersion me- dium ratio, viscosity of the dispersion medium, addition rate of the dilution medium, presence of electrolyte during diffusion and stirring rate, in order to understand better the mechanism of formation of polymeric nanoparticles. Poly(D,L-lactic acid) (PLA) was used as a polymer model. Turbidimetric evaluations during the diffusion stage were carried out, to determine the globule transitions from PLA solution to solid particles.
Materials and methods Materials
PLA (Medisorb| 100
D,L)
with an average molecular mass of 130550 and an inherent viscosity of 0.72dlg -1 in chloroform was supplied by Medisorb Technologies L.P. (Cincinnati, OH, USA). Propylene carbonate (PC) of analytical grade was chosen as a partially water-soluble solvent (Fluka, Buchs, Switzerland). Poly(vinyl alcohol) (PVAL) with an average molecular mass of 26000 was used as supplied (Mowiol| 4-88, Hoechst, Frankfurt-am- Main, Germany). Dichloromethane and acetone of H P L C grade were obtained from Fluka. Distilled water was purified using a Milli-Q system (Millipore, USA-Bedford, M.D.). All other reagents were of analytical grade and used without further purification.Methods
Nanoparticle preparation
Nanoparticles were formed according to the method de- scribed previously [-6]. PC and water were mutually saturated for 1 min before use in order to ensure the initial thermodynamic equilibrium of both the liquids. Typically, 200 mg of PLA were dissolved in 10 ml of water-saturated PC and this organic solution (internal phase) was emulsi- fied with 20 ml of a 5% w/v PVAL PC-saturated aqueous
solution (dispersion medium) using a high-speed homog- enizer (Ultra-Turrax T 25, IKA Lab-Technik, Germany) at 8000 rpm for 10 min. After formation of an oil-in-water emulsion, 80 ml of water (dilution medium) were added to the system in order to allow diffusion of PC into the continuous phase, thus causing the aggregation of the polymer in nanoparticles (Fig. 1). Some batches were pre- pared according to the emulsification solvent-evaporation technique using dichloromethane as the polymer solvent [7]. These experiments were conducted in order to com- pare the particle size obtained by this technique with that from the emulsification diffusion method.
Viscosity measurement
The viscosity of the PVAL solutions was determined using a rotating viscosimeter (Rheomat 15 T-F Contraves, Ziirich, Switzerland) at room temperature.
Globule size and nanoparticle size measurement
The mean globule size and polydispersity (index expressed from 0 to 9) of PC-in-water emulsions prepared before the diffusion step were measured using a Coulter Nano- Sizer| TM (Coulter Electronics Harpenden, Herford- shire, UK) previously checked with polystyrene latex mean diameter 197_+ 10nm (Duke Scientific Co,, Palo Alto USA). To perform the measurements, the emulsions were diluted using the dispersion medium and the size was rapidly measured for 2 min. Nanoparticle size analysis was carried out by dilution of 250/~l of the nanoparticle sus- pension ( ~ 0 . 2 % w/v) to 5 ml with filtered distilled water, also using the Nano-Sizer. Measurements were made in triplicate for all the batches.
Scanning electron microscopy (SEM)
Some batches were purified and concentrated by cross- flow filtration (Minitan| Millipore, USA-Bedford, MD), as previously described [6]. A sample of the dispersion was finely spread over a slab and dried under vacuum. The sample was shadowed in a cathodic evaporator with a layer of gold ( ~ 2 0 nm thick). Then, the surface was observed using a JSM-6400 scanning electron microscope (JEOL, Tokyo, Japan).
Turbidimetric analysis
The turbidity measurements were carried out to evaluate globule size changes and polymer aggregation during the
642 Colloid & Polymer Science, Vol. 275, No. 7 (1997) 9 SteinkopffVerlag 1997
Fig. 1
Schematic representation of the nanoparticle preparation process Saturation 9 Dispersion medium Internal ) phaseI
I
;
L - ' - ' J
PLA nanoparticles wate rf stabilizer
PC Pt.A9
tO
g (~
%1
Y o O O o - ) Emulsification (O/W emulsion)~
l = _ Dilution medium (water) Solvent diffusiondiffusion step. T h e PC-in-water emulsion was prepared according to the m e t h o d described above. Typically, the diffusion step was followed by the gradual addition of 5 ml of water at 10 min intervals, the system being kept at 25 ___ 2 ~ by a water recirculation system. Samples of 50, 100 or 200/A were taken from the system depending on the P L A c o n c e n t r a t i o n and diluted to 5 ml with fresh disper- sion medium. T h e turbidity (z) of the samples was assayed s p e c t r o p h o t o m e t r i c a l l y (Beckman 35, Irvine CA, USA) 10 min after dilution, at 2 = 360 nm. T h e a b s o r b a n c e (A) o b t a i n e d was related to T by the relationship T = 2.303
All,
where I represents the optical p a t h l e n g t h [8]. T h e dilution effect due to the added water was taken into a c c o u n t for the calculations. Evaluations were m a d e by changing the P L A c o n c e n t r a t i o n of the inner phase as well as the stir- ring speed using a variable speed stirrer with a stainless- steel propeller (IKA R 1382, J a n k e & K u n k e , Staufen, G e r m a n y ) .
Results and discussion
It is well k n o w n that the emulsification of oil in water by mechanical shear produces, in most cases, an emulsion droplet whose size is 2 - 3 / t m and down to 1/~m in excep- tional circumstances [9]. However, if it is assumed that one polymer particle is formed in each emulsion droplet when the solvent is removed, then one can expect a good correlation between the initial droplet size and the final particle size. This supposition seems to be valid in the case of the d i c h l o r o m e t h a n e - i n - w a t e r emulsion solvent evapor- ation system. The particle size obtained, under the emul- sification conditions used, indicated a diameter larger than 1/~m, ranging from 1 1 1 0 + 9 1 n m for 2% w/v P L A to 1 4 2 0 + 8 2 nm for 5 % w/v PLA. In contrast, when the particles were formed by the process of emulsifica- tion-diffusion, the m e a n diameter of the resultant spheres
D. Quintanar-Guerrero et al. 643 Mechanistic study of formation of polymer nanoparticles
(Fig. 2) decreased drastically in relation to the initial glob- ule diameter, from 2620 _+ 243 nm to the submicron range, even at high P L A / P V A L ratios and low internal phase/ dispersion m e d i u m ratios (Fig. 3). F u r t h e r m o r e , no cor- relation was f o u n d between the calculated particle volume and the droplet volume, assuming one spherical, solid
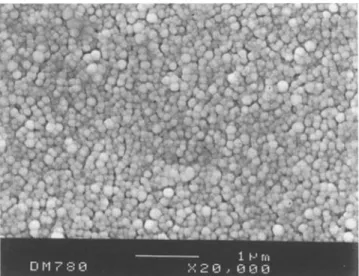
Fig. 2 Scanning electron micrography of PLA nanoparticles pre- pared by the emulsification-diffusion method
Fig. 3 Effect of PLA/PVAL ratio and internal phase/dispersion medium ratio on the mean size of the nanoparticles. Zone A: nano- particle formation; Zone B: nanoparticle formation with high polydispersity and presence of polymer flocs; Zone C: microparticle formation with polymer flocs; Zone D: Polymer aggregates or PLA insolubility (30 experimental points)
particle per emulsion droplet. Thus, a gradual solvent diffusion of the original globule to the continuous phase and subsequent aggregation of the contained p o l y m e r c a n n o t be invoked in the emulsification~liffusion technique.
It is possible that interfacial p h e n o m e n a d u r i n g diffu- sion c o n t r i b u t e to the subdivision of the globule into n a n o d r o p l e t s before nanoparticle formation. Previously [6], it has been suggested that the n a n o d r o p l e t s were formed by interfacial turbulence [10] generated d u r i n g diffusion. This mechanism has been used to explain the formation of n a n o s p h e r e s [11] and nanocapsules [12] by the solvent diffusion technique patented by Fessi et al. [13]. When a p o l y m e r solution in acetone is p o u r e d or injected into a a q u e o u s stabilizer solution (i.e. poly(vinyl alcohol)), a violent spreading is observed due to the mutual diffusion between the solvents. Droplets of oil, p r o b a b l y of n a n o m e t r i c size, are torn from the interface. These droplets are rapidly stabilized by the stabilizing agent, until diffu- sion of the solvent is complete and p o l y m e r aggregation has occurred. Davies and Rideal [14] have suggested that the interfaeial turbulence is caused by localized lowering of the interfacial tension, where the oil phase undergoes rapid and erratic pulsations or "kicks", each of which is quickly d a m p e d out by viscous drag. The energy necessary for these j e r k y m o v e m e n t s comes from the free energy released as the solvent is redistributed to its equilibrium state. T h e molecular m e c h a n i s m of interfacial turbulence could be explained by the c o n t i n u o u s formation of eddies of solvent (e.g. acetone) at the interface. Such eddies m a y originate either during d r o p formation, in thermal inequalities in the system, or, once the process has started, in m o v e m e n t s associated with previous kicks, that change the pressure inside the solvent either by increasing the surface pressure or decreasing the interfacial tension. Thus, if the solvent droplets formed contain polymer, this will tend to aggre- gate and form nanopartieles due to the c o n t i n u o u s diffu- sion of solvent and because of the presence of a n o n s o l v e n t m e d i u m [12]. A schematic representation of these phe- n o m e n a is s h o w n in Fig. 4. The term n a n o p r e c i p i t a t i o n [11, 15] is frequently used to define this process; however, it is i m p o r t a n t to point out that according to the m e c h a - nism described, the f o r m a t i o n of nanoparticles is due to p o l y m e r aggregation in stabilized emulsion droplets, a n d the nucleation and growth steps are not a p p a r e n t l y involved.
In contrast to the solvent diffusion technique, the par- tially water miscible solvents used for the emulsifica- tion-diffusion technique (PC, benzyl alcohol, etc.) do not emulsify spontaneously, as does acetone. D i m i t r o v a et al. [16] have established that superficial interfacial instabili- ties can turn out to be only a necessary, but n o t a sufficient condition for s p o n t a n e o u s emulsification. In o t h e r words, even when the interface is unstable, no emulsification will
644 Colloid & Polymer Science, Vol. 275, No. 7 (1997) 9 SteinkopffVerlag 1997
be o b s e r v e d if the coalescence rate of the droplets is high enough. Therefore, a great interracial area (emulsification) m u s t be generated before the diffusion step, to ensure n a n o p a r t i c l e formation. A l t h o u g h the diffusion p a t t e r n is different from t h a t o b s e r v e d d u r i n g the diffusion tech- nique, the interfacial turbulence could explain the subdivi-
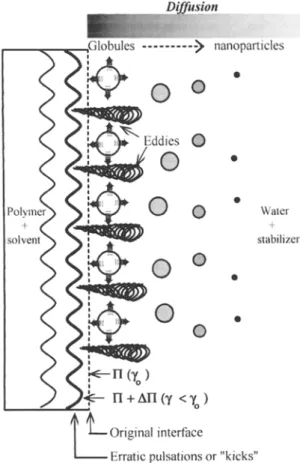
Fig. 4 Schematic representation of nanoparticle formation by the solvent diffusion process based on the interfacial turbulence mecha- nism, where/7 and 7 represent the surface pressure and the interfacial tension, respectively
sion of the original globules d u r i n g the diffusion step. In order to better u n d e r s t a n d the role of the interfacial turbu- lence in the emulsification-diffusion technique, some ex- periments were p e r f o r m e d in which the viscosity and the rate of d i l u t i o n - m e d i u m a d d i t i o n were modified. The con- centration of P L A in the P C solution (2% w/v), and the ratio of oil/water phases (1 : 2) were k e p t constant. Ideally, if interfacial turbulence is involved, changes of these p a r a - meters will affect the diffusion rate of P C and, conse- quently, the particle size. T a b l e s 1 a n d 2 show, s o m e w h a t unexpectedly, that this seems n o t to be the case. The division of the original globule f r o m 2600--3200 to < 300 n m particles, was o b s e r v e d in all cases. A difference of ~ 100 n m was o b t a i n e d between the lowest and the highest viscosity of 175 (r/-- 1 m P a s ) to 274 n m ( t / = 277 m P a s). This b e h a v i o r can be attributed to a p r o b a b l e c o n c e n t r a t i o n of the n a n o d r o p l e t s in the interfacial region, where they are able to coalesce a n d to p r o d u c e bigger particles. T h e increase in the polydispersity index seems to s u p p o r t this hypothesis.
Davies a n d Rideal [14] s h o w e d that the interfacial turbulence can be suppressed, at least partially, by dissolv- ing a salt (e.g. 1 N N a C I ) in the dilution medium. T h e presence of an electrolyte reduces the solubility of the a d d e d solvent, thus a v o i d i n g the violent interfacial spread- ing. A further series of e x p e r i m e n t s was therefore per- formed by p r e p a r i n g n a n o p a r t i c l e s with 1 N N a C I in the dispersion a n d dilution m e d i a (100 ml of the dilution me- d i u m were used to ensure the total diffusion of PC). In the interpretation of these experiments, the presence of elec- trolyte was considered to increase the a d s o r p t i o n p r o p e r - ties of P V A L [17].
N o difference in particle size was o b s e r v e d between the batches p r e p a r e d with or w i t h o u t NaC1. These results suggest that interfacial turbulence does not entirely ex- plain nanoparticle f o r m a t i o n b y the emulsification-diffu- sion method. O n the c o n t r a r y , b a t c h e s p r e p a r e d according to the solvent diffusion technique, by injecting 2 % w/v P L A solutions in acetone, into 1 0 0 m l of an a q u e o u s
Table 1 Influence of the dilution medium viscosity on the mean size of the nanoparticles
Batch External diffusion phase Mean size _ SD a Polydispersity b Aggregates c No. viscosity [m Pa s] [nm] 1 1.0 175 + 6 0 2 6.2 212 _+ 11 1 3 11.7 248 _+ 3 1 4 23.8 242 _+ 7 1 5 101.5 288 + 16 2 6 163.0 263 + 15 3 7 276.9 274 ___ 13 6 m m a n ~ 3 .
bindex expressed from 0 to 9.
D. Quintanar-Guerrero et al. 645 Mechanistic study of formation of polymer nanoparticles
Table 2 Influence of the dilution medium addition rate on the mean size of the nanoparticles
Batch Water addition Mean size __+ SD" Polydispersity b Aggregates c
No. rate [ml/s] Into]
8 0.31 188 + 3 0 9 1.01 187 _+ 6 1 l0 2.20 185 + 3 1 11 6.61 167 + 4 0 12 13.32 171 + 3 0 13 19.22 165 • 3 1 14 26.08 169 + 3 0
a.b.r the abbreviations are the same in Table 1.
solution of 5% w/v P V A L in the presence and absence of 1 N NaC1, revealed the clear influence of partial sup- pression of the interfacial turbulence. The size increased from 265 _+ 23 n m (polydispersity index = 2) to 782 _+ 93 nm (polydispersity index = 6) due to the presence of the electrolyte; this means that turbulence plays an i m p o r t a n t role in the f o r m a t i o n of nanoparticles by the solvent diffu- sion technique.
Therefore, the f o r m a t i o n of nanoparticles with the emulsification diffusion m e t h o d is attributed principally to solvent transport. In o r d e r to explain these p h e n o m e n a for the s p o n t a n e o u s emulsification processes of ternary systems with mass transfer, Davies and Rideal [14] used a mechanism called "diffusion and stranding", which in- volves a chemical instability and is entirely different from the interfacial turbulence mechanism which involves a mechanical instability. According to these authors, when an oil-solvent solution, is placed gently in contact with water, the solvent diffuses from the solution into the water carrying with it some oil (forming a three-component phase in the immediate vicinity of the interface). As the solvent diffuses further into the water, the associated oil is t h r o w n out of solution, and is "stranded" in the water in the form of fine emulsion drops. Ruschak and Miller [-18] have established that this type of spontaneous emulsifica- tion originates in the f o r m a t i o n of regions of local super- saturation in one or b o t h phases during the diffusion process, even though, b o t h initial mixtures are unsatu- rated. It is postulated that such supersaturation c a n n o t persist, and that emulsion droplets form due to phase transformation in these regions. Supersaturation near an interface m a y also p r o m o t e its breakup by a distinct but closely related chemical instability mechanism. This "diffusion and stranding" mechanism can be sufficiently extensive to explain the f o r m a t i o n of nanoparticles by the emulsification-diffusion technique. The basic idea is that diffusion of P C from the globules carries polymer mole- cules into the a q u e o u s phase, forming local regions of supersaturation from which, new globules or polymer ag- gregates (not totally desolvated) are formed; the stabiliz- ation of these " p r o t o n a n o p a r t i c l e s " by the presence of
P V A L is very important in order to avoid their coalescence a n d the f o r m a t i o n of agglomerates. T h e i m p o r t a n c e of this stabilization mechanism has been discussed previously [6]. Finally, complete diffusion of P C from the droplet will p r o v o k e p o l y m e r consolidation in the shape of n a n o p a r - ticles (Fig. 5). The results of the t u r b i d i m e t r y d e t e r m i n a - tions are consistent with this mechanism. Figure 6 shows the change in r during diffusion at different P L A concen- trations. T h e increase in r can be a t t r i b u t e d to the forma- tion of " p r o t o n a n o p a r t i c l e s " (increase of the n u m b e r of particles) and the aggregation of P L A (particles m o r e opaque). F o r all the P L A concentrations, a gradual in- crease in r is observed, until a limit is reached which is indicative of the final step of " p r o t o n a n o p a r t i c l e " forma- tion; then, the decrease in turbidity is explained by the consolidation of the " p r o t o n a n o p a r t i c l e s " , that is the total diffusion of P C and overlap of P L A chains, and by n o n c o r - rected dilution effects. It is i m p o r t a n t to m e n t i o n t h a t the point at which r begins to increase and the time at which the m a x i m u m value is reached, are P L A c o n c e n t r a t i o n - dependent. F o r P L A c o n c e n t r a t i o n s of 5 % and 7.5% w/v, nanoparticle f o r m a t i o n begins as the first v o l u m e of the diffusion m e d i u m is added, while for P L A c o n c e n t r a t i o n s of 2 % w/v and less, r decreases until a certain limit is reached; this fact could suggest that P C diffuses w i t h o u t carrying PLA, until a p o l y m e r c o n c e n t r a t i o n in the glob- ules is reached where p r o t o n a n o p a r t i c l e s begin to appear. This b e h a v i o r could explain the similarity in particle size (Fig. 3) found with P L A c o n c e n t r a t i o n s lower t h a n 5 % w/v. O n the o t h e r hand, with P L A c o n c e n t r a t i o n s greater than 5 % w/v, the size gradually increases with the consequence that the " p r o t o n a n o p a r t i c l e s " generated during diffusion are m o r e a b u n d a n t and are highly con- centrated at the interface. T h e p r o b a b i l i t y that they can collide and coalesce a m o n g themselves, or with the inter- face, is thus greater than at low concentrations. F i g u r e 7 shows the influence of stirring on ~ for batches p r e p a r e d at the same P L A c o n c e n t r a t i o n (2% w/v). The peak inten- sity increased with stirring rate and was inversely p r o p o r - tional to the particle size o b t a i n e d (Table 3), so we can say that the globule size of the original emulsion determines, to
646 Colloid & Polymer Science, Vol. 275, No. 7 (1997) 9 SteinkopffVerlag 1997
Fig. 5 Schematic description of the proposed formation mecha- nism of PLA nanoparticles by the emulsification-diffusion method based in the diffusion stranding mechanism
Fig. 7 Evaluation of the turbidimetric changes during the addition of dilution medium for batches prepared at different stirring rates: (A) 500 rpm; (m) 1500 rpm; cl 2000 rpm; (9 9000 rpm (samples of 200/~1)
Fig. 6 Evaluation of the turbidimetric changes during the addition of dilution medium for batches prepared with different concentra- tions of PLA: (m) 0.5 % w/v (samples of 200 ~1); (A) 1.0 % w/v (samples of 200/A); (D) 2.0% w/v (samples of 200/11); (e) 5.0% w/v (samples of 100/~1); ( + ) 7.5% w/v (samples of 50/~1)
a certain extent, the particle size. This fact could be ex- plained by the difference in thickness of the s u p e r s a t u r a t e d region a n d the r a p i d f o r m a t i o n a n d stabilization of p r o t o - n a n o p a r t i c l e s at high stirring rates. F o r coarse emulsions
(500 rpm), the curve s h o w e d a very different behavior; the large globule size a n d the p r o b a b l e coalescence could p r o v o k e the f o r m a t i o n of a p o l y m e r film a r o u n d the glob- ules, but the p o l y m e r c o n c e n t r a t i o n is insufficient to ob- tain s u p e r s a t u r a t e d regions a n d therefore n a n o p a r t i c l e f o r m a t i o n is not observed. E x p e r i m e n t s with droplets of 50/A of P L A 2 % w / v solution, injected into P V A L solu- tions, showed this behavior. These results suggest t h a t the exposed superficial a r e a g e n e r a t e d during emulsification is an i m p o r t a n t factor for the superficial p h e n o m e n a
D. Quintanar-Guerrero et al. 647 Mechanistic study of formation of polymer nanoparticles
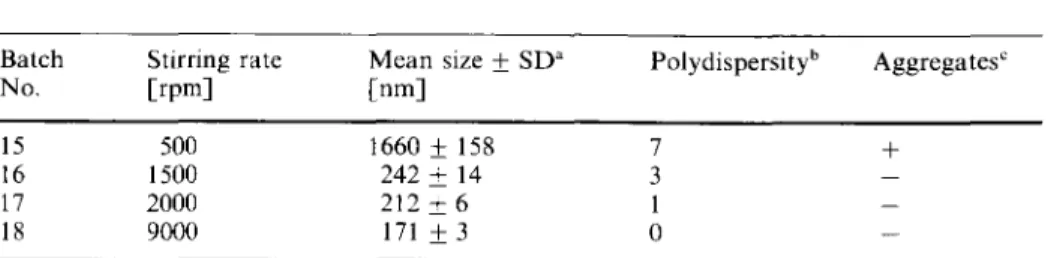
Table 3 Influence of the stirring rate on the mean size of the nanoparticles
Batch Stirring rate Mean size • SD a
No. [rpm] [rim] Polydispersity b 15 500 1660 • 158 16 1500 242 • 14 17 2000 212 • 6 18 9000 171 • 3 7 + 3 1 0
a.b.," the abbreviations are the same as in Table 1.
Aggregates c
i n v o l v e d in t h e d i f f u s i o n step a n d for c o n t r o l i n g the p a r t i c l e size o b t a i n e d .
Conclusions
T h e p r e s e n t w o r k has s h o w n t h a t n a n o p a r t i c l e f o r m a t i o n by the e m u l s i f i c a t i o n - d i f f u s i o n process i n v o l v e s interfacial p h e n o m e n a d u r i n g the d i f f u s i o n step t h a t c o n t r i b u t e to the g e n e r a t i o n of c o l l o i d a l particles. T h e c o n v e c t i o n d u e to i n t e r f a c i a l t u r b u l e n c e a p p a r e n t l y does n o t i n f l u e n c e n a n o p a r t i c l e f o r m a t i o n . T h e results s u p p o r t the h y p o t h e - sis t h a t s o l v e n t d i f f u s i o n p r o d u c e s r e g i o n s of local super- s a t u r a t i o n f r o m which, n a n o p a r t i c l e s are f o r m e d , due to p h a s e t r a n s f o r m a t i o n in these regions. P r e p a r a t i v e vari-
ables s u c h as p o l y m e r c o n c e n t r a t i o n a n d s t i r r i n g r a t e c o u l d be i m p o r t a n t f a c t o r s for t h e f o r m a t i o n a n d t h i c k n e s s of the s u p e r s a t u r a t e d r e g i o n , a n d m i g h t c h a n g e t h e m e a n n a n o p a r t i c l e size. W e p o i n t o u t t h a t the m e c h a n i s m of n a n o p a r t i c l e f o r m a t i o n w i t h the e m u l s i f i c a t i o n - d i f f u s i o n p r o c e s s p r o p o s e d i n this p a p e r is n o t a t t r i b u t e d t o a m e c h a n i c a l i n s t a b i l i t y , b u t to a c h e m i c a l i n s t a b i l i t y a n d is closely r e l a t e d to t h a t for i s o l a t e d p r e c i p i t a t i o n i n solid systems,
Acknowledgments D.Q.G. acknowledges a grant from CONACYT and FES-Cuautitlan, UNAM, Mexico. The authors are grateful to Mrs. Danielle Massuelle for technical assistance with the scanning electron microscope and Dr. Yogeshvar N. Kalia for critically re- viewing the manuscript.
References
1. All6mann E, Gurny R, Doelker E (1993) Eur J Pharm Biopharm 39:173 191 2. Couvreur P, Dubernet C, Puisieux F
(1995) Eur J Pharm Biopharm 41:2 13 3. Gurny R (1983) In: Breimer DD, Speiser
P (eds) Topics in Pharmaceutical Sci- ences. Elsevier, Amsterdam, pp 277 288 4. Gallardo MM, Roblot-Treupel L, Mahu- teau J, Genin I, Couvreur P, Plat M, Puisieux F (1989) Nanocapsules et nanosphbres d'alkyl-cyanoacrylate, in- teractions principe actif/polym6re. Proc APG1, 5th Int Conf on Pharmaceutical Technology, Paris (France) pp 36-45 5. Leroux JC, Allemann E, Doelker E,
Gurny R (1995) Eur J Pharm Biopharm 41:14-18
6. Quintanar-Guerrero D, All6mann E, Fessi H, Doelker E (1996) Int J Pharm 143:133-141
7. Sfinchez A, Vila-Jato JL, Alonso MJ (1993) Int J Pharm 99:263 273
8. Elving PJ, Winefordner JO (1982) In: Chemical Analysis. Wiley, New York, pp 255 257
9. E1-Aasser M (1979) In: Advances in Emulsion Polymerization and Latex Technology. Vol 2. Lehigh University, Bethleem, Pennsylvania, No. 19 10. Sterning CV, Scriven LE (1959) AIChEJ
5:514~523
11. Molpeceres J, Guzman M, Aberturas MR, Chacon M, Berges L (1996) J Pharm Sci 85:206 213
12. Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S (1989) Int J Pharm 55:R1-R4
13. Fessi H, Devissaguet JP, Puisieux F, Thies C (1988) French Pat 2 608 988 14. Davies JT, Rideal EK (1961) In: Inter-
facial Phenomena. Academic Press, New York, pp 319 326, pp 359 366
15. Stainmesse S, Orecchioni AM, Nakache E, Puisieux F, Fessi H (1995) Colloid Polym Sci 273:505-511
16. Dimitrova B, Ivanov YB, Nakache E (1988) Disp Sci Tech 9:321-341
17. Tadros TF (1979) J Colloid Interface Sci 72:505 514
18. Ruschak KJ, Miller CA (1972) Ind Eng Chem Fundam 11:534~540