Publisher’s version / Version de l'éditeur:
Neuroscience letters, 492, pp. 76-79, 2011-01-21
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1016/j.neulet.2011.01.055
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Baicalin prevents the production of hydrogen peroxide and oxidative
stress induced by Abeta aggregation in SH-SY5Y cells
Yin, Fei; Liu, Jianhui; Ji, Xiuhong; Wang, Yanwen; Zidichouski, Jeffrey;
Zhang, Junzeng
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=f0955e6b-d4f3-439b-b385-ff7423ac411c
https://publications-cnrc.canada.ca/fra/voir/objet/?id=f0955e6b-d4f3-439b-b385-ff7423ac411c
Neuroscience Letters
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / n e u l e t
Baicalin prevents the production of hydrogen peroxide and oxidative stress
induced by A aggregation in SH-SY5Y cells
Fei Yin
a,b, Jianhui Liu
a,b,∗, Xiuhong Ji
b, Yanwen Wang
b, Jeffrey Zidichouski
b, Junzeng Zhang
b,∗∗aResearch Center of Medicinal Chemistry and Chemical Biology, Chongqing Technology and Business University, Chongqing 400067, PR China bInstitute for Nutrisciences and Health, National Research Council of Canada, Charlottetown, PE C1A 4P3, Canada
a r t i c l e
i n f o
Article history:
Received 14 December 2010
Received in revised form 19 January 2011 Accepted 21 January 2011 Keywords: Baicalin Copper A Aggregation Hydrogen peroxide
a b s t r a c t
Alzheimer’s disease (AD) is a common form of neurodegenerative disease. Mounting evidence suggests that metal ions play a key role in the aggregation of amyloid peptide (A), which acts as a factor or cofactor in the etiopathogenesis of AD. Therefore, inhibition of A aggregation emerges as a potential approach for the treatment of AD. We have found that baicalin can interact with copper directly and inhibits A1–42 aggregation. In addition, baicalin protects SH-SY5Y cells from oxidative injuries induced by A1–42 aggregation through decreasing H2O2production that is normally formed as a deleterious by-product of beta amyloid aggregation and the formation of plaques. Taken together, these data indicate that baicalin may be a potential agent to inhibit A aggregation and thereby delay, mitigate or modify the progression of neurodegenerative diseases such as AD.
Crown Copyright © 2011 Published by Elsevier Ireland Ltd. All rights reserved.
Alzheimer’s disease (AD) is a devastating neurodegenerative dis-order and leading cause of senile dementia[2]. One of the major histopathological features of AD is the observation of extracel-lularly located amyloid plaques that are composed principally of fibrillar-amyloid (A) peptide, with small amounts of other pro-teins[20]and transition metal ions[5,23]. It is reported in the year 2000 that 25 million people were afflicted with AD and this number is predicted to increase to 114 million by 2050 if new preventive and therapeutic solutions do not emerge[22].
Complexes formed between A and copper ions have been pro-posed to be an aberrant interaction which has been implicated in the development of AD, where copper ion is known to be involved in A aggregation and reported to lead to the increased produc-tion of reactive oxygen species (ROS)[1,15]. Moreover, copper ion can potentiate A cytotoxicity because Cu-A complexes produce ROS catalytically in the presence of a physiological reductant (e.g., ascorbate, Vitamin E) [9,14,26]. Hydrogen peroxide (H2O2) and superoxide anion (O2•−) are the major ROS produced in tissues. H2O2 is viewed as being more important contributor to cellular damage and pathological events than O2•−as H2O2can easily cross
∗ Corresponding author at: Research Center of Medicinal Chemistry and Chem-ical Biology, Chongqing Technology and Business University, 19 Xuefu Rd., Nan’an District, Chongqing 400067, China. Tel.: +86 23 6276 9652; fax: +86 23 6276 9652. ∗∗ Corresponding author at: Institute for Nutrisciences and Health, National Research Council of Canada, 550 University Avenue, Charlottetown, PE C1A 4P3, Canada. Tel.: +1 902 566 8129; fax: +1 902 566 7445.
E-mail addresses:jhliu@ctbu.edu.cn(J. Liu),junzeng.zhang@nrc.gc.ca(J. Zhang).
biological membranes inflicting damage both intra and extracel-lularly depending the availability of substrate to react with[25]. A large body of evidence shows that A-induced cytotoxicity is caused by intracellular accumulation of H2O2, ultimately leading to the peroxidation of membrane lipids and if unchecked can proceed to cell death[4]. Thus, inhibition of A aggregation is viewed as an important intervention strategy to slow or halt the progression of AD.
Baicalin is the predominant flavonoid isolated from the roots of Scutellaria lateriflora Georgi (Huang Qin). It has been reported that this compound exhibits many different pharmacological activ-ities, including that of an antioxidant[10], anti-inflammatory[28], anti-tumor agent[16]and as an anti-viral[32]. Recently, the neuro-protective effect of baicalin against A-induced neurotoxicity has been reported, and this effect is thought to be associated with reduction of oxidative stress [12]. However, the mechanism by which baicalin reduces A-induced neurotoxicity remains to be clarified.
A1–42 was purchased from Anaspec (San Jose, CA, USA) and prepared according to the previous reference[17]. Baicalin was supplied by Active Ingredients Group, Inc. (Changsha, Hunan, China), with a purity of 95% as verified using NMR and HPLC. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), Thioflavin T (ThT), CuCl2, Glycine, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT), and phosphate buffered saline (PBS) were obtained from Sigma–Aldrich (St. Louis, MO, USA).
ThT dye was used to determine the presence of amyloid-like aggregates. The extent of A aggregation in the samples prepared as described above was followed up by periodically taking 10L of
0304-3940/$ – see front matter. Crown Copyright © 2011 Published by Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.neulet.2011.01.055
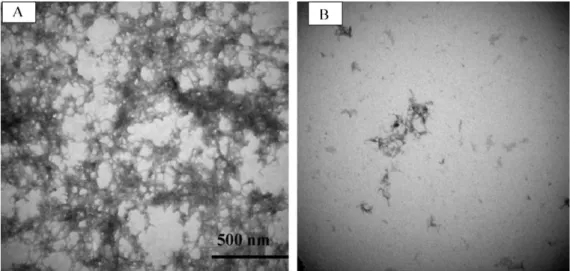
Fig. 1. Electron microscopy images of A1–42 aggregation without (A) or with baicalin (B). After incubation with 50 M A1–42 for 72 h in the presence or absence of 50 M baicalin, 2l of each sample was collected for electromicroscopic imaging. Images were acquired using Hitachi 7500 transmission electron microscopy at 80 kV, with the magnification of 100,000× and scale bar of 500 nm.
A1–42 from incubated samples and transferring this volume to a single well of a 96-well plate. To each sample, 200L of 10 M ThT in 0.1 M glycine buffer (pH 8.9) was added and the plate was read on a microplate reader (Varioskan, Thermo, USA) for fluorescence intensity at excitation of 450 nm and emission of 482 nm. All ThT fluorescence experiments were performed in triplicate.
To prepare specimens for TEM imaging, 100M A1–42 was incubated for 3 days in the presence or absence of 50M baicalin at room temperature, and then a 5L aliquot of each sample was spot-ted onto a glow-discharged, carbon-coaspot-ted formvar grid. The grid was stained with 5L uranyl acetate for 1 minute. Samples were examined using a Hitachi H7500 transmission electron microscopy (Hitachi, Japan). All images were captured at a voltage of 80 kV.
Since baicalin has low solubility in water, a concentrated stock solution of baicalin (10 mM) was first prepared in DMSO and kept at 4◦C. Prior to use, baicalin was diluted in PBS to achieve the final concentrations used. To assess the interaction between baicalin and Cu2+, 100L baicalin at 100 M (diluted from stock solution) was mixed with 100L Cu(Gly)Cl2(prepared with equal molar amount of glycine in water, as Cu2+tends to precipitate in water solution) and the change in UV absorbance of baicalin was recorded over a spectral range from 200 to 420 nm using a Varioskan plate reader (Thermo, USA).
SH-SY5Y human neuroblastoma cell line was purchased from American Type Tissue Culture (Manassas, VA, USA), and cultured in DMEM supplemented with 12% heat-inactivated FBS, 100 U/mL penicillin and 100g/mL streptomycin in a humidified incubator with 5% CO2at 37◦C. Cells were trypsinized and seeded onto 12-well plates with 2 × 105cells/well. After the cells attached, they were rinsed once with PBS and the media was replaced with phe-nol red-free and serum-free DMEM. To evaluate the protection of baicalin on SH-SY5Y cells from toxicity and death induced by A1–42 aggregation, the cells were preincubated with various doses of baicalin for 2 h.
SH-SY5Y cells were seeded onto 6-well plates at 3 × 105 cells/well and incubated overnight. The cells were washed once with PBS and cultured in phenol red-free and serum-free DMEM. Cells were incubated with baicalin at various concentrations for 2 h, to each well A1–42 was then added to achieve a final concentra-tion of 2.5M and followed with another 24 h of incubation time. The medium was collected and measured for H2O2concentration using a commercially available H2O2Assay kit (Biovision, USA).
Cell viability was determined using the MTT assay[24]. MTT was dissolved in PBS at a concentration of 5 mg/mL. At the end of
treatment, the MTT solution was added to the cell medium at a final concentration of 0.5 mg/mL. After 2 h of incubation at 37◦C, absorbance was read on a Varioskan plate reader (Thermo, USA) at 570 nm with the reference set to 630 nm.
To determine whether baicalin affects A1–42 aggregation, we used a ThT assay to measure A1–42 aggregation after a 3-day incubation period in the absence or presence of different doses of baicalin. The results demonstrated that baicalin dose-dependently inhibited the aggregation of A1–42 with a calculated IC50 of 1.77M (R2= 0.99834, data not shown).
To visually confirm the effect of baicalin on A1–42 aggrega-tion, we used electromicroscopic imaging technique to evaluate the fibril formation of A1–42 in the absence (Fig. 1, panel A) or presence of baicalin (Fig. 1, panel B). We incubated 50M solution of A1–42 in 20 M PBS (pH 7.4) for 3 days at room temperature in the absence or presence of 50M baicalin. Under these conditions, the micrographs show that baicalin markedly inhibited the aggre-gation of A1–42 as much smaller and fewer fibrils were observed in baicalin’s presence compared to its absence.
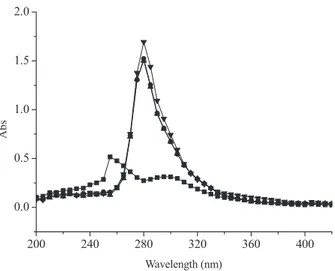
As shown inFig. 2, baicalin has a maximum absorbance at a wavelength of 275 nm, and the inclusion of copper caused the max-imum absorbance to shift to a longer wavelength (300 nm) and also significantly increased the intensity of absorbance. We also assessed the influence of time (post mixing) on the interaction between baicalin and copper. The results showed that there was no significant change observed in the intensity of absorbance mea-sured after 30 min, after 90 min or after a full 24 h (Fig. 2) and is consistent with the notion that the binding between baicalin and Cu2+is a rapid process and appears to be irreversible.
As baicalin inhibited the aggregation of A1–42 in both a time-and dose dependent-manner, we have further assessed the pro-tective effect of baicalin against neuron death/damage caused by A aggregates using SH-SY5Y cells. After 48 hours of incubation in A1–42, cell viability was determined via an MTT assay. We found that baicalin increased cell viability in a dose-dependent manner, and pretreatment with 10M baicalin for 2 h prior to the addition of A1–42 increased cell viability from 57% to 78% as compared to cells exposed to A1–42 alone (Fig. 3).
Hydrogen peroxide is generated during the very early stages of A aggregation and plays a critical role in the development of neu-rodegenerative diseases, including AD[29]. To further investigate the potential neuroprotective actions of baicalin, we measured the level of H2O2in A1–42-treated SH-SY5Y cells. The results demon-strated that baicalin reduced the production of H2O2and this effect
200 240 280 320 360 400 0.0 0.5 1.0 Abs Wavelength (nm)
Fig. 2. Absorbance spectra of 50M baicalin in pH 7.4 phosphate buffer mixed with 50M Cu2+. Spectra were obtained at 3 different time points: (1) 30-min
incubation (indicated by diamonds), (2) 90-min incubation (triangle), and (3) 24-h incubation (indicated by inverted triangles). These data were compared to the spec-trum obtained for baicalin alone (indicated by squares). The absorbance specspec-trum (range of 200–420 nm) for each sample was obtained using a Varioskan plate reader (Thermo, USA).
occurred in a dose-dependent manner (Fig. 4). These results sug-gest that baicalin prevents SH-SY5Y cells against damage induced by A1–42 through inhibition of A aggregation as a known neu-rotoxic by-product of its aggregation, namely H2O2, was found to be reduced in the presence of baicalin.
Scutellaria baicalensis Georgi (Huang Qin), an important medic-inal herb, has been widely used in China to treat stroke and inflammatory diseases for thousands of years. Baicalin, a flavonoid compound isolated from the root of S. baicalensis, is believed to be the major bioactive component. Previous studies showed that baicalin attenuated oxygen-glucose deprivation-induced injury in rat cortical neurons via inhibiting NMDA receptor-mediated 5-lipoxygenase activation[11]and reduced the cytotoxicity of A peptides in PC12 cells[12]. In the present study, we have found that baicalin is a novel inhibitor of A aggregation. It also prevented
SH-Ctrl 0 0.1 1 10 0 20 40 60 80 100 * ce ll via b ility (% of contro l) Baicalin (μΜ) *
Fig. 3. Effect of baicalin on the cell viability of SH-SY5Y in the presence or absence of A1–42. After cells were pre-treated with baicalin at indicated concentrations for 2 h in phenol red- and serum-free media, 2.5M A1–42 was added and the cells were incubated for another 48 h. Cell viability was determined with the MTT assay. Data are means ± SD from independent experiments (n = 3). *P < 0.05 compared to A1–42 alone. 2.5 0 C 5 10 0 20 40 60 80 100 120 140 160 ** H2 O2 (% of control) Baicalin (µ * **
Fig. 4. Baicalin decreases H2O2production in A-stressed SY5Y cells. After
SH-SY5Y cells were pre-incubated with baicalin for 2 h, A1–42 was added to attain a final concentration of 2.5M, and incubated for additional 24 h. The concentration of H2O2in media was determined with the commercial H2O2assay kit. Data are
means ± SD from two independent experiments, with two wells per experiment. *P < 0.05, **P < 0.01 compared to A1–42 alone.
SY5Y cells against injuries through inhibiting A1–42 aggregation and reducing H2O2-mediated oxidative stress and damage.
Mounting evidence indicates that metal ions, such as copper, zinc and iron, are involved in the etiology AD[1,27]. In the case of copper, evidence suggests that when Cu complexes with A, the product might be directly involved in the increased production of ROS[14]. Moreover, it is thought that metal binding to A is aber-rant, as Cu-A was detected in AD but not under healthy conditions [1]. There are two key steps involved in the process through which Cu possibly contributes to the development of AD: (1) copper is able to bind A directly and modulate its aggregation[6]; (2) redox active metal ions like copper is crucial for the production of reac-tive oxygen species and oxidareac-tive stress[8]. In present study, we found that baicalin inhibited the aggregation of A1–42 peptide in the presence and absence of copper. In the presence of copper, the UV–VIS spectra of baicalin changed significantly, suggesting that baicalin may interact with copper directly. Further, baicalin also decreased the production of H2O2 in A-treated SH-SY5Y cells. Therefore, the neuroprotective effects of baicalin in A-stressed SH-SY5Y cells may be due to the inhibition of baicalin on the A aggregation process and subsequently reducing the production of H2O2and thus damage due to oxidative stress.
Together with the neuroprotective effects of baicalin reported by others [7,11,12,19,21,30,31], this study provides further sup-portive evidence for the potential use of baicalin as a therapeutic agent to reduce A-induced neuronal cytotoxicity. It is possible that baicalin may be beneficial to prevent or treat AD acting through the aforementioned mechanisms, however, further in vivo studies are needed to determine whether baicalin or its metabolites can indeed be transported across the blood brain barrier to reach brain tissue and accumulate up to a level for performing such functions, as the existing evidence of baicalin absorption and tissue distribution data published to date for baicalin are inconclusive[3,13,18,30,33].
Acknowledgments
This work was supported financially by NRC-INH (to Drs. Zhang, Wang, and Zidichouski), Program for New Century Excellent Tal-ents in University (NCET-07-0913 to Dr. Liu), and China Scholarship Council (with a visiting scholar fellowship to Dr. Liu). The authors
are grateful to Ms. Dorota Wadowska from the Atlantic Veterinary College, University of Prince Edward Island for her expert technical assistance in electron microscopy.
References
[1] P.A. Adlard, A.I. Bush, Metals and Alzheimer’s disease, J. Alzheimers Dis. 10 (2006) 145–163.
[2] A. Aguzzi, T. O’Connor, Protein aggregation diseases: pathogenicity and thera-peutic perspectives, Nat. Rev. Drug Discov. 9 (2010) 237–248.
[3] T. Akao, K. Kawabata, E. Yanagisawa, K. Ishihara, Y. Mizuhara, Y. Wakui, Y. Sakashita, K. Kobashi, Baicalin, the predominant flavone glucuronide of scutel-lariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form, J. Pharm. Pharmacol. 52 (2000) 1563–1568. [4] C Behl, J.B. Davis, R. Lesley, D. Schubert, Hydrogen peroxide mediates amyloid
beta protein toxicity, Cell 77 (1994) 817–827.
[5] A.I. Bush, Metal complexing agents as therapies for Alzheimer’s disease, Neu-robiol. Aging 23 (2002) 1031–1038.
[6] A.I. Bush, R.E. Tanzi, Therapeutics for Alzheimer’s disease based on the metal hypothesis, Neurotherapeutics 5 (2008) 421–432.
[7] Y. Cao, G. Li, Y.F. Wang, Z.K. Fan, D.S. Yu, Z.D. Wang, Y.L. Bi, Neuroprotective effect of baicalin on compression spinal cord injury in rats, Brain Res. 1357 (2010) 115–123.
[8] M.P. Cuajungco, C.J. Frederickson, A.I. Bush, Amyloid-beta metal interaction and metal chelation, Subcell Biochem. 38 (2005) 235–254.
[9] S.I. Dikalov, M.P. Vitek, R.P. Mason, Cupric-amyloid beta peptide complex stim-ulates oxidation of ascorbate and generation of hydroxyl radical, Free Radic. Biol. Med. 36 (2004) 340–347.
[10] Z. Gao, K. Huang, H. Xu, Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells, Pharmacol. Res. 43 (2001) 173–178.
[11] Q.F. Ge, X. Hu, Z.Q. Ma, J.R. Liu, W.P. Zhang, Z. Chen, E.Q. Wei, Baicalin attenuates oxygen-glucose deprivation-induced injury via inhibiting NMDA receptor-mediated 5-lipoxygenase activation in rat cortical neurons, Pharmacol. Res. 55 (2007) 148–157.
[12] H.J. Heo, D.O. Kim, S.J. Choi, D.H. Shin, C.Y. Lee, Potent Inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid beta protein-induced neuro-toxicity, J. Agric. Food Chem. 52 (2004) 4128–4132.
[13] Y.C. Hou, S.P. Lin, S.Y. Tsai, M.H. Ko, Y.C. Chang, P.D. Chao, Flavonoid pharmacoki-netics and tissue distribution after repeated dosing of the roots of Scutellaria baicalensis in rats, Planta Med. (2010), doi:10.1055/s-0030-1250433. [14] X. Huang, C.S. Atwood, M.A. Hartshorn, G. Multhaup, L.E. Goldstein, R.C. Scarpa,
M.P. Cuajungco, D.N. Gray, J. Lim, R.D. Moir, R.E. Tanzi, A.I. Bush, The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction, Biochemistry 38 (1999) 7609–7616.
[15] Y.H. Hung, A.I. Bush, R.A. Cherny, Copper in the brain and Alzheimer’s disease, J. Biol. Inorg. Chem. 15 (2010) 61–76.
[16] T. Ikezoe, S.S. Chen, D. Heber, H. Taguchi, H.P. Koeffler, Baicalin is a major com-ponent of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest, Prostate 49 (2001) 285–292.
[17] M. Janusz, M. Woszczyna, M. Lisowski, A. Kubis, J. Macala, T. Gotszalk, J. Lisowski, Ovine colostrum nanopeptide affects amyloid beta aggregation, FEBS Lett. 583 (2009) 190–196.
[18] M.Y. Lai, S.L. Hsiu, S.Y. Tsai, Y.C. Hou, P.D. Chao, Comparison of metabolic phar-macokinetics of baicalin and baicalein in rats, J. Pharm. Pharmacol. 55 (2003) 205–209.
[19] C.T. Li, W.P. Zhang, S.H. Fang, Y.B. Lu, L.H. Zhang, L.L. Qi, X.Q. Huang, X.J. Huang, E.Q. Wei, Baicalin attenuates oxygen–glucose deprivation-induced injury by inhibiting oxidative stress-mediated 5-lipoxygenase activation in PC12 cells, Acta Pharmacol. Sin. 31 (2010) 137–144.
[20] L. Liao, D. Cheng, J. Wang, D.M. Duong, T.G. Losik, M. Gearing, H.D. Rees, J.J. Lah, A.I. Levey, J. Peng, Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection, J. Biol. Chem. 279 (2004) 37061–37068.
[21] L.Y. Liu, E.Q. Wei, Y.M. Zhao, F.X. Chen, M.L. Wang, W.P. Zhang, Z. Chen, Pro-tective effects of baicalin on oxygen/glucose deprivation- and NMDA-induced injuries in rat hippocampal slices, J. Pharm. Pharmacol. 57 (2005) 1019–1026. [22] A. Lleo, S.M. Greenberg, J.H. Growdon, Current pharmacotherapy for
Alzheimer’s disease, Annu. Rev. Med. 57 (2006) 513–533.
[23] M.A. Lovell, J.D. Robertson, W.J. Teesdale, J.L. Campbell, W.R. Markesbery, Cop-per, iron and zinc in Alzheimer’s disease senile plaques, J. Neurol. Sci. 158 (1998) 47–52.
[24] T Mosmann, Rapid colorimetric assay for cellular growth and survival: appli-cation to proliferation and cytotoxicity assays, J. Immunol. Methods 65 (1983) 55–63.
[25] G. Multhaup, T. Ruppert, A. Schlicksupp, L. Hesse, D. Beher, C.L. Masters, K. Beyreuther, Reactive oxygen species and Alzheimer’s disease, Biochem. Phar-macol. 54 (1997) 533–539.
[26] C. Opazo, X. Huang, R.A. Cherny, R.D. Moir, A.E. Roher, A.R. White, R. Cappai, C.L. Masters, R.E. Tanzi, N.C. Inestrosa, A.I. Bush, Metalloenzyme-like activ-ity of Alzheimer’s disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2), J. Biol. Chem. 277 (2002) 40302–40308.
[27] J.T. Rogers, A.I. Bush, H.H. Cho, D.H. Smith, A.M. Thomson, A.L. Friedlich, D.K. Lahiri, P.J. Leedman, X. Huang, C.M. Cahill, Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer’s disease, Biochem. Soc. Trans. 36 (2008) 1282–1287.
[28] Y.C. Shen, W.F. Chiou, Y.C. Chou, C.F. Chen, Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes, Eur. J. Pharmacol. 465 (2003) 171–181.
[29] B.J. Tabner, O.M. El-Agnaf, S. Turnbull, M.J. German, K.E. Paleologou, Y. Hayashi, L.J. Cooper, N.J. Fullwood, D. Allsop, Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia, J. Biol. Chem. 280 (2005) 35789–35792.
[30] T. Tarrago, N. Kichik, B. Claasen, R. Prades, M. Teixido, E. Giralt, Baicalin, a pro-drug able to reach the CNS, is a prolyl oligopeptidase inhibitor, Bioorg. Med. Chem. 16 (2008) 7516–7524.
[31] X.K Tu, W.Z. Yang, S.S. Shi, C.H. Wang, C.M. Chen, Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia, Neurochem. Res. 34 (2009) 1626–1634.
[32] G. Xu, J. Dou, L. Zhang, Q. Guo, C. Zhou, Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum, Biol. Pharm. Bull. 33 (2010) 238–243.
[33] L. Zhang, D. Xing, W. Wang, R. Wang, L. Du, Kinetic difference of baicalin in rat blood and cerebral nuclei after intravenous administration of Scutellariae Radix extract, J. Ethnopharmacol. 103 (2006) 120–125.