Characterizing cell-cycle as a global regulator of stochastic
transcription and noisy gene expression in S. cerevisiae
by Katie J. Quinn
B.Engineering (Chemical & Biological) & B.Science (Molecular Biology) University of Queensland, 2008
Submitted to the Department of Chemical Engineering in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Chemical Engineering at the
Massachusetts Institute of Technology June 2014
@ 2014 Massachusetts Institute of Technology. All rights reserved.
Signature of Author:
Certified by:
Accepted by:
Signature redacted
Department of Chemical Engineering May 20, 2014
Signature redacted
Narendra Maheshri Assistant Professor of Chemical Engineering Thesis cupervisor
Signature redacted
Vatlick S. Doyle Professor of Chemical Engineering Chairman, Committee for Graduate Students
MASSACHUSETT$ MN1TTUTE. OF TECHNOLOGY
JUN
3
0
2014
LIBRARIES
Characterizing cell-cycle as a global regulator of stochastic transcription and noisy gene expression in S cerevisiae
by Katie J. Quinn
Submitted to the Department of Chemical Engineering on May 20, 2014 in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Chemical Engineering
Abstract
Even in the same environment, genetically identical cells can exhibit remarkable variability, or noise, in gene expression. This expression noise impacts the function of gene regulatory networks, depending on its origins. Hence, a prerequisite for understanding or designing gene regulatory networks is characterizing the origins and statistics of the noise. Variability has been largely attributed to the inherently stochastic nature of transcription. Expression statistics from multiple organisms are consistent with an influential model of "bursty" expression, where promoters are generally inactive but infrequently produce multiple mRNA. But fluctuations in the cell environment can also contribute, leaving the origins of noise unclear.
We sought to determine the origins of noise in gene expression from the synthetic tetO promoter in S cerevisiae. We use single-molecule mRNA FISH to quantify nuclear and cytoplasmic mRNA in a population expression distribution, and models of stochastic mRNA production and degradation to infer underlying transcriptional dynamics. Rather than transcriptional bursting, we find that noise is driven by large differences in transcriptional activity between the G1 and S/G2/M stage of the cell cycle. Furthermore, we quantitatively characterize these dynamics of transcription by measuring expression in cells arrested at the G1/S and G2/M transition. Promoters activate in S/G2 with probability determined by activator level. mRNA statistics from an active promoter with a single operator are Poisson; expression with multiple operators is more variable. Promoters appear to inactivate at the M/G1 transition, with lower activator levels leading to increased probability of inactivation. Thus below a certain activator threshold, all cells are inactive in G1. mRNA processing and export introduces further variability. Similar analysis of the native, chromatin-regulated PHO5 promoter yields the same results.
Hence cell-cycle driven transcription dynamics may be prevalent among regulated yeast genes. The timing of S/G2 activation suggests DNA replication and chromatin maturation may be linked to repressed transcription. Cell-cycle-linked fluctuations in expression are likely to affect gene behavior in regulatory networks. This thesis advocates the importance of cellular context in gene regulation and reveals a novel role of cell-cycle as a driver of eukaryotic transcription, advancing our understanding of stochastic transcription and noise in gene expression.
Thesis Supervisor: Narendra Maheshri
Acknowledgements
I first thank my advisor Narendra Maheshri for his unfailing support and enthusiasm, boundless ideas and knowledge, and inspiring approach to science. On top of this, Narendra is also a natural and generous teacher, making for a wonderful mentoring experience.
I thank my thesis committee members, Arup Chakraborty and Christopher Love, for their involvement and insights, and all the ChemE staff and faculty for support during my time here at MIT. My thanks also go to my undergraduate advisor, Lars Nielson, who encouraged me to pursue a PhD in the USA and gave me my first taste of independent research at the University of Queensland in Australia. The General John Monash Awards provided generous financial support and welcomed me into a wonderful community of Australians abroad.
I owe my labmates, T.L., Tek-Hyung, C.J., Bradley, Shawn & Nick, for teaching me in the lab, for valuable feedback in group meetings, and for sharing in the everyday struggles of a PhD student. I also thank my ChemE friends, especially my housemates at Speridakis, for many good times in and out of Building 66. I whole-heartedly thank the past and present members of the MIT Cycling Club for being a highlight of my time at MIT. The friendships and shared experiences have been true pleasure, and I hope that's so for many more MIT students to come.
To Adam, thank you for sharing and supporting me in every step of the past few years. Finally, I thank my family, who helped me find my way to MIT in the first place. To my brothers and sister, Simon, Jess and Andrew, thanks for the listening and counselling
-it's been a great help. And to my parents, Greg and Julie, thank you for teaching me to love learning and for your selfless, endless support of all of my endeavors, even when they're on the other side of the world.
Contents
A b stra ct ... 3
A cknow ledgem ents ... 4
CHAPTER 1. Introduction...9
1.1 Genotype to phenotype: Regulation of transcriptional dynamics...9
1.2 Intrinsic expression noise from stochastic transcription dynamics...10
1.2.1 An introduction to stochastic transcription dynamics ... 10
1.2.2 A model for understanding stochastic transcription dynamics...11
1.2.3 Conflicting evidence for cis and trans regulators' modes of controlling transcription dynamics...15
1.3 Extrinsic noise in gene expression ... 19
1.4 Consequences of noise from stochastic transcription dynamics...20
1.5 T hesis aim and sum m ary ... 21
1.6 R eferen ces...24
CHAPTER 2. Mode of transcriptional regulation can qualitatively affect gene behavior in positive feedback ... 32
2 .1 A b stra ct ... 3 2 2.2 Introduction: Modes of regulating transcriptional bursting ... 33
2 .3 T h eo ry :...3 6 2.3.1 Steady-state expression of frequency- or size- regulated stochastic transcriptional bursting in feedback control...36
2.3.2 The regime of bimodal expression ... 38
2.3.3 Two modes of regulating burst size are equivalent in the bursting limit...45 2 .4 R esu lts:...4 6
2.4.1 Bimodal expression patterns associated with positive feedback loops are enhanced with burst frequency regulation but reduced with burst size
reg u la tio n ... 4 6
2.4.2 Mode of regulation affects mean expression... 49
2 .5 D iscu ssion ... 50
2 .6 R eferen ces...5 1 CHAPTER 3. The cell-cycle dependence of transcription is a dominant source of noise in gene expression...56
3 .1 A b stra ct ... 56
3 .2 In trod u ction ... 56
3 .3 R esu lts:...5 8 3.3.1 Multiple transcription patterns result in expression distributions consistent with transcriptional bursting...58
3.3.2 Static mRNA FISH reveals cell-cycle dependent expression may create extrinsic noise in expression ... 60
3.3.3 A stochastic model to infer cell-cycle dependent transcription from mRNA expression distributions...64
3.3.4 Regulated transcription at low activator levels is restricted to S/G2; Constitutive expression varies with gene dosage...65
3.3.5 Real-time fluctuations in protein levels corroborate mRNA measurements and reveal globally correlated activation...72
3 .4 D iscu ssion :...75
3.4.1 Implications for understanding stochastic gene expression...75
3.4.2 Gene activation kinetics are also cell-cycle dependent ... 75
CHAPTER 4. Characterization of the tetO gene regulatory function using cell-cycle arrested mRNA expression...82 4 .1 A b stra ct ... 8 2 4 .2 In trod u ction ... 83 4 .3 R esu lts:...8 4
4.3.1 Analysis of single-molecule nuclear and cytoplasmic mRNA FISH in arrested cells reveals instantaneous cell-cycle dependent transcription... 84 4.3.2 mRNA expression under cell-cycle arrest reveals that activator regulates
probabilistic activation of a long-lived transcribing state in S/G2...90
4.3.3 Conditional variances quantify the origins of gene expression noise...92 4.3.4 A model of transcription underlying arrested expression distributions reveals
that activator only regulates the probability and stability of activity, whereas the promoter determines active transcription dynamics ... 97
4.3.5 Reproducing cell-cycle kinetics with a model of S/G2/M and GI stationary transcription dynam ics ... 105 4.3.6 Cell-cycle dependent transcription at the yeast PHO5gene suggests
generality among regulated genes in yeast... 108 4 .4 D iscu ssion :... 109
4.4.1 Naive interpretation of expression distributions with the bursting
tran scription m odel ... 109
4.4.2 Reconsidering cis and trans modes of regulating transcriptional dynamics in y e a st ... 1 1 2 4.4.3 Predictions about cell-cycle as a global transcriptional regulator in other
org an ism s ... 115 4 .5 C on clu sion s... 116
CHAPTER 5. Future directions ... 119
5 .1 S y n o p sis ... 1 19 5.2 The origin of the S/G2 window for transcriptional activation... 119
5.3 The com plete m echanism of cell-cycle regulation of transcription ... 122
5.4 Prevalence of cell-cycle driven transcription... 125
5.5 Towards a generalized, predictive model for stochastic transcription dynamics... 126
5.6 Consequences of cell-cycle driven transcription in gene networks... 128
5.7 Future techniques to observe transcription with single-molecule precision in real-tim e ... 1 3 0 5 .8 R eferen ces ... 13 1 CHAPTER 6. Appendix ... 133
6.1 Yeast strains and plasmids ... 133
6 .2 P rotocols:... 134
6.2.1 Growth & arrest protocols ... 134
6.2.2 m RNA FISH ... 135
6.2.3 m RNA FISH im age analysis... 135
6.2.4 Num erical solutions to stochastic models ... 137
6.3 Quantification of m RNA dynamics ... 138
6.3.1 Nuclear and cytoplasm ic m RNA degradation half-life ... 138
6.3.2 Rate of nuclear mRNA export... 139
6.4 m RNA FISH error analysis... 140
CHAPTER 1. Introduction
1.1 Genotype to phenotype: Regulation of transcriptional dynamics An overarching goal of biology is to predict and explain cell and organism behavior in response to a set of environmental conditions. Whole genome sequencing has become commonplace, mapping not only genes but also the genetic regulatory elements that control their expression. A new challenge is to decipher how genetic regulatory networks integrate internal and external signals to actuate gene expression. A first-pass understanding of gene regulation relates regulatory conditions, such as the regulatory DNA sequence and the level of regulatory proteins, to the rate of mRNA or protein production. As such, a gene regulatory networks can in principle be modeled by ordinary differential equations that explicitly enumerate these regulatory relations. But transcription does not appear to occur in a continuous, deterministic manner , but instead as a random, intermittent process where a gene fluctuates between periods of activity and inactivity. Transcriptional dynamics include a gene's fluctuations between states of varying transcriptional activity, despite unchanging regulatory conditions. The resulting variability, or noise, in expression can affect the behavior of gene regulatory networks, and so the origins of variability are important to understand and predict gene function.
Noise in gene expression has been conceptually divided based on its origination from two general sources: Intrinsic noise describes variability that originates from molecular noise in the reactions inherent to transcription; extrinsic noise originates from variability in factors that influence transcription (Elowitz et a., 2002). The next two sections discuss each of these in turn, towards the thesis' central goal of characterizing how regulators and regulatory elements modulate transcriptional dynamics and the resulting variability in gene expression.
1.2 Intrinsic expression noise from stochastic transcription dynamics
1.2.1 An introduction to stochastic transcription dynamics
Transcription occurs through a series of molecular interactions at a gene's promoter leading to successful production of an mRNA. Like all chemistry, the interactions are inherently stochastic. Most systems of chemical reactions involve large pools of each molecular species (on the order of Avogadro's number) such that, while each molecular transformation is stochastic, the macroscopic behavior is just the average behavior of all of these molecules and can be described deterministically. However, a cell usually has just one or two copies of a gene in its nucleus and the average number of mRNA produced can be anywhere from 100-105, depending on the transcriptional activity and the organism. Thus stochastic fluctuations in transcriptional activity can contribute to variability in mRNA and protein expression between cells, or in a single-cell over time. Variability in expression at steady-state is quantified by noise, which is the coefficient of variance (the standard deviation divided by mean) of expression levels between cells in a population, or by the Fano factor, which is the variance divided by the mean of expression in the population, with intuitive units of mRNA or protein.
That stochastic chemistry could create biological variability was long-ago predicted from physical principles (e.g. Schr6dinger, 1944). It was first suspected as the cause of heterogeneous induction of the lac operon inherited over generations in isogenic cells (Novick & Weiner, 1957). Interest was more recently revived when stochastic expression appeared to explain the previously observed (DelbrOck, 1945) lysis/lysogeny decision of the phage lambda (McAdams & Arkin, 1999; Arkin, Ross & McAdams, 1998). Genome-wide studies of expression noise in budding yeast have since revealed that genes with highly regulated expression (such as stress-response genes) tend to be noisier than those that are constitutively expressed (Bar-Even et a]., 2006; Newman et a]., 2006), suggesting a qualitative difference in the dynamics of constitutive and regulated transcription.
often un-occluded by nucleosomes, existing in a state permissive to transcription, which is limited by diffusion and assembly of the general transcription factors and machinery at fairly regular time intervals. On the other hand, noisy genes regulated by the binding of one or more activators to operators in their promoter are enriched in nucleosome-occluded promoters, which rest in an inactive state where transcription is limited by chromatin remodeling. Noisy genes are also more likely to have TATA boxes, which ensure strong active transcription. Each of these aspects contribute to stabilize the "reinitiation complex" containing Mediator and other transcription cofactors (Yudkovsky, Ranish & Hahn, 2000), enabling several rounds of productive transcription after each slow-step of initiation (Figure 1-1). This fluctuation between promoter activity states is a potential origin of noise in gene expression and informs models of transcription.
Cofacto§
Mediator
Gene
Nucleosome binding sites
Figure 1-1: Components of the reinitiation complex in yeast transcription. Cis activator binding sites (red) stabilize trans activators (yellow) at the gene promoter providing a, foundation f)r assembly of the complete initiation complex.
1.2.2 A model for understanding stochastic transcription dynamics
A useful abstraction of this underlying molecular biology is the "two-state promoter" model. It was conceived with the earliest studies of gene regulation at the lac operon (Jacob & Monod, 1961) then developed by Peccoud & Ycart (1995). The model, depicted by Figure 1-2 and outlined in Equation 1-1, supposes a gene exists in either an inactive OFF (I) or active ON (A) state. When ON, it produces mRNA (M), which degrades by first-order kinetics.
I
A
M
Figure 1-2: Thw-state promoter model of gene expression. A promoter can exist in an inactive (1) or active (A) state and transitions between the states with rate A and y. An active promoter produces mRNA with rate p, which are degraded by first-order kinetics with rate 6.
A '> I 1-1
A > A+M
M
40>
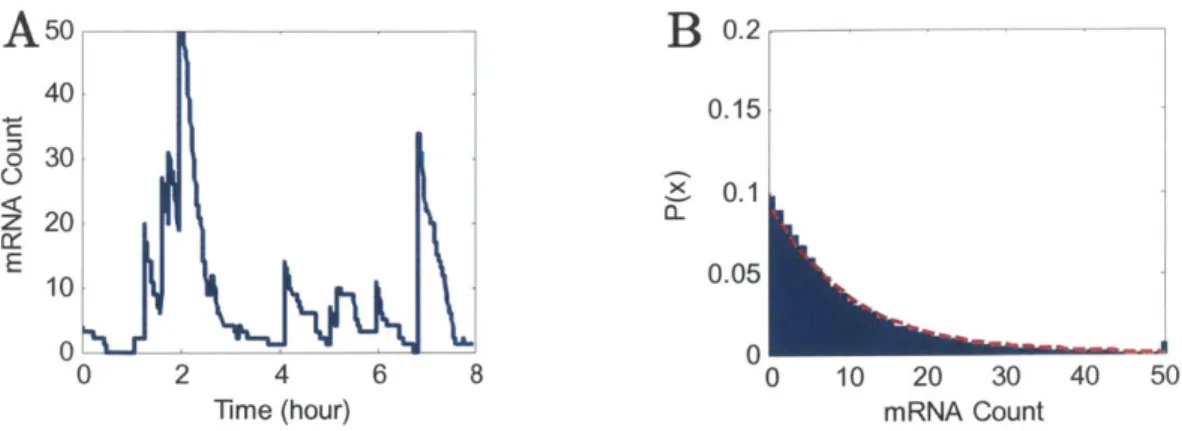
For low-noise constitutive genes, the model reduces to a single state, where the promoter lives in the active state and produces mRNA with rate p. At steady-state, constant stochastic production yields a Poisson distribution of mRNA. As such, Poisson mRNA statistics represents a "null" case of minimal expression noise. Figure 1-3 shows an example trajectory of production and degradation and the resulting steady-state mRNA expression distribution. Poisson statistics of mRNA have been observed for multiple constitutive genes in yeast (Larson et aL, 2011; Zenklusen, Larson & Singer, 2008).
A50
B
0.2
40 0.15 o 30 X5 0.1 0<~ z 20 E 0.05 0 2 4 6 8 0 10 20 30 40 50Time (hour) mRNA Count
Figure 1-3: The trajectozy and stationmiy expression distribution of a Poisson production process. (A) mRAA count fluctuates over time due to stochastic single-molecule production and degradation events (Burst frequency: A/6 = 10, Burst size: p/y = I mRNA). (B) Sampled over long times or across a laige population, expression is a Poisson distribution with mean and variance of 10 nRNA.
On the other hand, single-cell studies of population distributions and real-time activity suggest that expression of regulated eukaryotic genes can occur in bursts of transcription consistent with a model where the promoter switches randomly and rarely from a stable inactive state to a short-lived, actively-transcribing state (Larson et a]., 2011, Raj et a].,
2006). Rare transcriptional initiation and then rapid reinitiation (discussed above) may represent the molecular basis for this dynamic behavior (Hahn, 1998; Struhl, 1996; Yudkovsky, Ranish, & Hahn, 2000). In this case, the two-state promoter operates in the
"bursting" regime, with rare activation compared to inactivation (<<7) and promoter fluctuations are faster than the mRNA lifetime (f >
1
), where tilde denotes that a parameter is normalized by the degradation rate. In this regime, f is the burst frequency, and ;i /f
is the burst size and the distribution can be described by a two-parameter Gamma distribution:1-2 P X1
This is equivalent to the result of Friedman, Cai & Xie (2006). The discrete equivalent, representing integer mRNA counts, is the negative binomial distribution. The two parameters specify the burst frequency and burst size respectively and their product is the expression mean. While the model says nothing about the actual molecular events leading to transcription, the burst frequency is thought to correspond to the transcriptional initiation rate and the burst size may correspond to the transcriptional reinitiation and/or elongation rate. Figure 1-4 shows an example trajectory of transcriptional bursting and the resulting steady-state expression distribution, parameterized by a burst frequency and burst size, and fit with the negative binomial distribution of those parameters. (While Master equations that describe the two-state model at steady-state have an analytical solution, more complicated stochastic models that lack analytical solutions must be solved numerically, with kinetic Monte Carlo simulations or a Finite Markov Chain method
2 4 6 8 Time (hour)
BO.2
0.15 2 0.1 0-0.05 0 0 10 20 30 40 50 mRNA CountFig-ure 1-4: The tra jectory and stationaiy expression distribution of a bursting process. (A) mRNA
count fluctuates widely over time due to "bursts" of transcription (Burst frequency: A16 = 1. Burst
size: p/y = 10 mRNA). (B) Sampled over long times or across a large population. expression is a ne'g-ative binomial distribution with a mean of 10 mRNA and parameters corresponding to the burst frequency and burst size. This bursting and the previous Poisson example (Figure 1-3) have tMe same mean expression level but very different distributions.
A regulatory element's mode of affecting transcriptional dynamics could be inferred from how the expression distribution changes with mean expression. For the case of the two-state promoter model, the product of the burst frequency and burst size gives the mean expression. Transcriptional regulators could affect expression via the frequency or size of bursts. Regulation of burst frequency will increase sampling as the mean increases, thereby decreasing the noise; regulation of burst size will not (Figure 1-5).
A
Cg SignalB
0 ZiBurst frequency regulation Burst size regulation
Mean (log)
Figure 1-5: Regulation via burst frequency or burst size will affect expression noise. For two hypothetical genes with equivalent mean expression in response to an activating signal (A), the gene regulated via burst frequency will decrease expression noise as activation increases whereas the gene
under burst size control will not (B).
A50
C 0 z E 40 30 20 10 0L 01.2.3 Conflicting evidence for cis and trans regulators' modes of controlling transcription dynamics
Many studies have sought to observe and characterize transcriptional dynamics. These studies use single-cell techniques that fall in two classes: measuring mRNA dynamics in real-time and back-calculating transcription events (corresponding to the trajectories of Figure 1-3 & 1-4 A); and measuring mRNA expression across a static cell population and inferring steady-state transcriptional dynamics (as in the distributions of Figure 1-3 & 1-4 B).
Golding et a]. (2005) were the first to visualize transcription in real-time, introducing repeats of secondary structure in mRNA that bound an MS2 phage coat protein fused to GFP. This enabled counting the production of individual MS2-GFP-labeled mRNA transcripts using in growing F. coli.. mRNA appeared to be produced in "bursts" of transcription. Studies in mammalian cells (Suter et a]. (2011) and Harper et aL. (2011)) using luciferase as a readout suggest mammalian promoters are activated in bursts, followed by an inactive refractory periods.
Results of earlier studies which inferred transcriptional dynamics from protein noise in static populations are also consistent with bursting dynamics. Consistent with expectations, when Ozbudak et a]. (2002) modulated bursting at the translational level in the prokaryote
B. subtilis (via point mutations that affect ribosome binding and thus translational
efficiency, p), noise remained high with increasing expression level (Kaern et a]., 2005). Blake et a]. (2003) demonstrated the same in S. cerevisiae. Raser & O'Shea (2004) used a yeast strain with two homologous reporters of PHO5 expression to measure intrinsic noise at the protein level (2004). A mutant of the TATA-binding site, expected to decrease transcription rate, p, decreased noise. A mutation of the activator binding site, expected to decrease promoter activation, A, increased noise, both consistent with the noise-mean trends of the bursting model.
Multiple studies have examined noise in protein expression genome-wide in both S.
cerevisiae (Bar-Even et a]., 2006; Newman et a]., 2006; Hornung et aL, 2012) and E coli
noisy gene. They also found that, in general, noise decreased with the inverse square-root of mean expression, suggesting that gene activity is predominantly controlled via modulating the frequency of activation events. Similar global regulation of burst frequency was seen in bacteria (Taniguichi, 2010). However regulated genes are often repressed in standard growth conditions and thus all such genes may not be captured in these trends. Also, the inverse square root scaling of noise with protein abundance was not seen clearly at high levels of expression, where extrinsic noise is expected to dominate.
Additional studies have focused on measuring expression statistics from several different promoters to infer how changes in cis regulatory elements can affect noise. Zenklusen et a].
(2008) found Poisson statistics at three constitutive genes, as expected. But expression of the regulated PDR5 gene had higher noise, and an expression distribution well-fit by the negative binomial distribution solution to stationary bursting. Carey et a]. (2013) conditioned on activator-specific effects by measuring expression from multiple genes activated by the same transcription factor, and saw that the level of noise, or "burstiness", was a function of the promoter sequence. They also saw that the degree of noise depended on whether the transcription factor acted as an activator or repressor (repression lowered the apparent burst size). So et a]. (2011) measured expression from multiple bacterial genes and concluded that both burst frequency (at lower levels) and burst size (at higher levels) increased expression level. Dar et a]. (2012) and Skupsky et a]. (2010) integrated a single promoter at many locations in a human genome and reported evidence of bursting across all locations with burst frequency and then burst size increasing with mean expression. However the latter study did not measure stationary expression, a requirement for the applicability of the negative binomial noise-mean trends. A further cause-for-thought is that this trend is consistent with the expected dominance of extrinsic noise at high expression levels (discussed in the next section).
More detailed studies systematically varying regulatory elements within a single gene's promoter has proven particularly effective for identifying regulatory trends. In yeast, Murphy et a]. (2007) saw that placing transcription factor binding sites closer to the
mammalian cells (Raj et al. 2006, Suter et al. 2011) compared expression from promoters identical except for the number of activator binding sites and showed noise increases with binding site number. Dadiani et al. (2013) performed an interesting study in yeast, engineering increased expression of a single gene via either increased binding site strength or nucleosome-disfavoring sequence. The former substantially increased expression noise ("burst size"), the latter did not. This suggests the nucleosome disfavoring sequence transitioned the dynamics out of the "bursting" regime and into continuous activity. Raj et
al.
(2006) measured mRNA expression distributions from two identical copies of a gene in single diploid mammalian cells and saw that fluctuations between the two loci were largely uncorrelated, suggesting intrinsic origins. The shape of the distributions was consistent with bursting.Table 1-1 summarizes the conclusions of these studies according to whether they found
cis or trans regulators to affect burst size, frequency, or both. There is strong evidence for cis elements, such as promoter architecture (binding site number, chromatin structure) and
local chromatin environment, dictating the "size" of transcriptional bursts. How both gene-specific and global trans regulators affect bursting is less clear.
While the paradigm of "bursty" transcriptional dynamics seems prevalent, its veracity and ubiquity is not conclusive. Muramoto et al. (2012) detected periodic, long-lived pulses of transcription in real-time in Dictyostelum, rather than bursts. And particularly troubling is the fact that models for transcriptional bursting describe the variability in mRNA levels from purely intrinsic sources, whereas appreciable amounts of extrinsic noise have been measured in a wide number of studies, although generally at the protein level. Using FACS to measure protein noise does allows for gating by cell shape, but removes no other extrinsic variability. Several studies (including Blake et al., 2003; Raser & O'Shea, 2004; So et al., 2011; To & Maheshri, 2010) used scaling arguments to justify that noise in their data was intrinsic: It was claimed that i2 decreasing monotonically with mean, a/p approaching one at very low expression levels and q2 decreasing sharply with mean at high expression levels were all indicative of intrinsic rather than extrinsic noise. Our noise data also has these scaling properties, but its origins have proven to be predominantly extrinsic. Exceptions
that do explicitly account for extrinsic variability are few, including the original study with two mRNA reporters (Raj et a]., 2006) and a theoretical study (Shahrezaei, Ollivier & Swain,
2008).
Table 1-1: Summarv of studies identifying cis- and trans- regulation of bursting dynamics
Burst size regulation
Binding site number or strength:
S. cerevisiae:
Raser & O'Shea 2004 Blake et a]. 2006 Murphy et aL. 2007 To & Maheshri 2010 Dadiani et aL 2013 Mammalian: Raj et al. 2006 Suter et a]. 2011 TATA strength: S. cerevisiae:
Raser & O'Shea 2004 Mogno et a]. 2010 Hornung et al. 2012 Nucleosome occupancy/remodeling: S. cerevisiae-Bai et a]. 2010 Dadiani et a]. 2013 Genomic location: Mammalian: Skupsky et aL. 2010 Dar et a]. 2012
Multiple genes (promoters):
E. col: So et aL. 2011 S. cerevisiae: Hornung et a]. 2012 Activator levels: E. col: Choi et a]. 2008 S. cerevisiae: Mao et a]. 2010 Carey et aL. 2013
Burst frequency regulation
Activator levels or activity:
E. coli:
Pedraza & van Oudenaarden 2005 Golding et aL 2005
S. cerevisiae:
Raser & O'Shea 2004 Mao et a]. 2010
Global protein noise:
E. col: Taniguchi et a]. 2010 S. cerevisiae-Bar-Even et al. 2006 Newman et a]. 2006 Cis variation Trans variation
1.3 Extrinsic noise in gene expression
Extrinsic noise originates due to fluctuations in upstream factors that impact gene expression. Upstream fluctuations can occur in global factors (affecting all genes) or gene-specific pathway components. Upstream fluctuations may also derive from intrinsic noise in their expression, but it need not be the case. For example, cell size seems an important determinant of global transcriptional activity (Raj, unpublished). The cell-cycle, including the doubled DNA copy number of S/G2/M, may also be a potential source of transcription variability (Elliott & McLaughlin, 1978, Volfson et a]., 2006). Stochastic partitioning of
mRNA and protein upon cell division is another source of extrinsic noise, and appeared as intrinsic noise in a popular experimental method to measure contributions from the two sources (Huh & Paulsson, 2011). The early experimental example of consequential stochastic gene expression, the lysis/lysogeny decision of phase lambda, was later attributed to extrinsic sources (St-Pierre & Endy, 2008). Hilfinger & Paulsson (2011) noted that even intrinsic noise parameters will depend on extrinsic factors in the history of the cell. Studies of global protein expression (Bar-Even et a]., 2006) showed evidence of a baseline of extrinsic noise, with noise never measured below a coefficient of variation of 0.2. Yet these important sources of extrinsic variability are suggested to have small, non-qualitative effects on variability of mRNA and transcription, because of the large wealth of experimental results consistent with the transcriptional bursting model (Table 1-1).
1.4 Consequences of noise from stochastic transcription dynamics
Stochastic transcription and expression variability is of particular interest because it can have qualitative consequences for phenotype. In synthetic gene circuits, noise has stabilized toggle switches and oscillators (Becskei, Seraphin & Serrano, 2001; Elowitz & Leibier, 2000; Gardner, Cantor & Collins, 2000). Our lab previously demonstrated that noise can cause bimodal expression in a transcriptional positive feedback loop even when deterministic models predict no bistability (To & Maheshri, 2010). Stochastic expression also has consequences in evolution and development of organisms. Heterogeneous expression among a population of isogenic unicellular organisms may lead to variability that assists with responses to changes in nutrients (e.g. lactose utilization, Ozbudak et a]., 2004), to stress (e.g. competence in B. subtilis, Maamar, Raj & Dubnau, 2007; Suel et a]., 2006; Suel et al,
2007) or to pathogens (e.g. bacterial persistence against antibiotics, Blake et aL., 2006) and may play a role in development (e.g. variability in a stem cell marker correlated strongly with choice of lineage, Chang et a]., 2008).
But consistent with intuition about control systems, noise in gene expression can also be detrimental, limiting information transfer (Bialek & Setayeshgar, 2005; Lestas, Vinnicombe & Paulsson, 2010), such that gene regulatory networks have evolved to suppress noise effects (McAdams & Arkin, 1999; Raj
et
a]., 2010). Noise from transcriptional dynamics may be anunavoidable biophysical limitation of achieving a highly-regulable range of transcription (Bremer & Ehrenberg, 1995; Guptasarma, 1996; Salman et a]., 2012). (One counter-argument is that ribosomal genes, which have high dynamic range with little noise. But these genes fundamentally differ, transcribed by their own polymerase (RNA Pol I) with ON/OFF, rather than graded, control.) In either case, the fact that organisms are known to both exploit and evolve to minimize noise is evidence of its significance for gene regulatory network performance.
1.5 Thesis aim and summary
One summary of the current understanding of transcriptional dynamics is: Transcription occurs in continuous, stochastic burst events, with a frequency regulated by activator level and a size dependent on promoter architecture and constraints of the molecular biology of transcription in the particular organism. But studies have emerged suggesting that extrinsic, global regulators of gene expression have gone un-appreciated, suggesting a direction for the field to advance. Another question remaining answered is how transcriptional dynamics control the kinetics of a response to changing regulatory signals. This thesis is a case of the former, exploring a novel case of global transcriptional regulation. While kinetics are not covered here, characterization of stationary dynamics is a key step towards predicting kinetic behavior.
This work aims to characterize transcriptional dynamics, and thus the origin of noise in gene expression, at the yeast tetO gene in response to cis and trans regulators and the cell-cycle, which we reveal as a global regulator of transcription. In contrast to current understanding of single-cell dynamics and noise, we establish that large difference in transcription between cell-cycle stages drives noisy expression at the tetO promoter, and suggest this may be prevalent at other regulated yeast genes. Specifically, we ask: What are the dynamics of transcription in response to the level of activator proteins, number of activator binding sites at the gene promoter, and global process of the cell-division cycle?
In Chapter 2 we present a case study demonstrating how the mode of regulating transcriptional dynamics can affect phenotype. We consider a hypothetical pair of promoters transcribed with bursting dynamics: one whose expression is modulated by the frequency of bursts, and the other by the size of bursts. Hence they differ in how their intrinsic noise varies with changes in expression level. We analyze expression in positive feedback and see that regulation via burst frequency can create bimodal expression, or stabilize deterministic bistability; whereas regulation of burst size never creates bimodality, and instead destabilizes bistability. Hence regulators' mode of controlling dynamics is important for gene function.
Previous studies' attempts to infer transcription dynamics have been quick to attribute all noise in expression to the model of bursting transcription. But a strain with two copies of our "noisy" gene of interest revealed that much of the noise we observed was extrinsic, deriving from sources other than transcription itself. When probing the origin of this extrinsic noise, we uncovered a strong dependence of transcription on the cell-cycle.
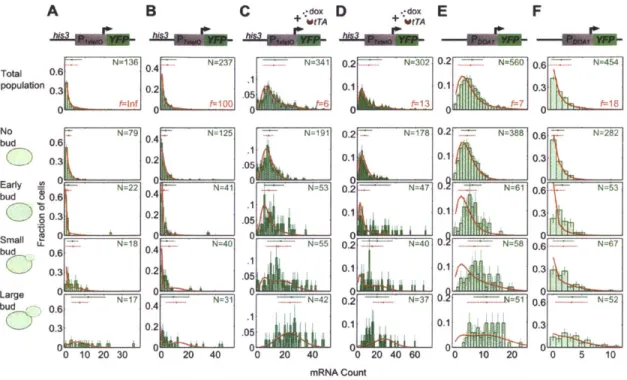
In Chapter 3, we establish the nature of this cell-cycle driven transcription. We measure transcription rates with two single-cell methods: by tracking protein levels in real-time we back-calculate time courses of transcription rates, albeit with relatively low molecular and temporal resolution; by counting single mRNA molecules in a static population segregated by cell-cycle stage, we obtain a higher resolution readout of recent transcription but with no temporal information. An immediate result is that transcription increases 2-fold from GI to S/G2/M for constitutive or highly-expressed, regulated genes. Though previously underappreciated, this is expected because the copy number of each gene doubles during S-phase DNA replication between G1 and G2. We show that this dominates super-Poissonian noise in growing populations' constitutive gene expression. Of greater interest, we find that transcription under repressed conditions only occurs in S/G2/M. Clearly, something is at play alleviating transcriptional repression in early S/G2. We hypothesize that chromatin maturation following DNA replication creates a permissive window for transcription from otherwise inactive genes.
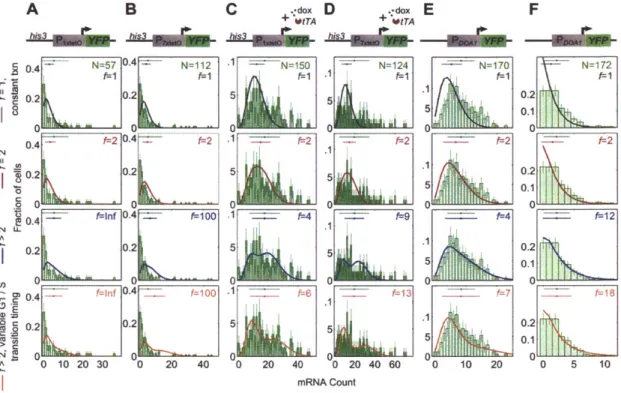
But the resolution afforded by these techniques limited further characterization of transcriptional dynamics. mRNA expression blurs fluctuations in transcription on the timescale of its turnover. In Chapter 4, we seek a new approach to quantify both the cell-cycle dependence of transcription and transcriptional dynamics within a single cell-cell-cycle stage. We find that cell-cycle arrest obtains mRNA expression that reflects the pseudo-steady-state transcription dynamics of each cell-cycle stage. Levels of mRNA at the site of transcription in the nucleus provides a more immediate readout of transcriptional activity than the cytoplasmic mRNA, which have undergone processing and export.
We find that cells with or without nuclear mRNA have very different levels of cytoplasmic mRNA, indicating an active state lifetime on the timescale of a cell-cycle stage.
modulates the stability of the active state across the M/G1 transition, such that there is a distinct activator threshold below which there is no GI expression. But expression from an active loci is essentially activator-independent. An active loci with a single operator has minimally-variable Poisson expression statistics; multiple operators result in higher expression noise, perhaps accessing multiple activity states that correspond to discrete promoter occupancy states. The growing population's normalized variance (i.e. the Fano factor) peaks at an activator level that is finally revealed to derive from noise peaking when 50% of cells activate in S/G2. This is a new picture of transcriptional dynamics that challenges previous expectations of transcriptional bursting dynamics.
This thesis answers some questions but creates many more. We hypothesize that S/G2 alleviation of repression is due to the delay in maturation and assembly of new chromatin following DNA replication. Several studies link chromatin maturation to transcription, but is this at play here, and if so what is the molecular mechanism? Is this phenomena of cell-cycle dependent de-repression of transcription universal, or shared among a smaller class of genes, and why? Can it affect protein-level behavior within a signaling network? What developments in experimental techniques are necessary to complete our understanding of transcriptional dynamics? We discuss these open questions in Chapter 5.
Together, this thesis reveals a novel pattern of transcriptional regulation and provides an example of the importance of considering cell context when studying gene expression and regulation. The role of cell cycle as a regulator is remarkable and previously unappreciated in the context of noisy gene expression. It has implications for network design in synthetic biology, and for understanding gene expression perturbed by disease, particularly in the fast-growing cells of cancers and developing organisms. This work to characterize the transcriptional dynamics of a single gene is one very small step towards developing generalized and predictive models of gene regulation, which may eventually enable us to understand and engineer biology as we would any other physical system.
1.6 References
Arkin, A., Ross, J., & McAdams, H. H. (1998). Stochastic kinetic analysis of
developmental pathway bifurcation in phage-lambda infected Escherichia coli cells.
Genetics, 149(4), 1633-1648.
Bai, L., Charvin, G., Siggia, E. D., & Cross, F. R. (2010). Nucleosome-depleted regions in cell-cycle-regulated promoters ensure reliable gene expression in every cell cycle.
Developmental Cell, 18(4), 544-555.
Bar-Even, A., Paulsson, J., Maheshri, N., Carmi, M., O'Shea, E., Pilpel, Y., & Barkai, N. (2006). Noise in protein expression scales with natural protein abundance. Nature
Genetics, 38(6), 636-643.
Becskei, A., Seraphin, B., & Serrano, L. (2001). Positive feedback in eukaryotic gene networks: Cell differentiation by graded to binary response conversion. EMBO
Journal, 20(10), 2528-2535.
Bialek, W., & Setayeshgar, S. (2005). Physical limits to biochemical signaling. Proceedings
of the National Academy of Sciences of the United States of America, 102(29),
10040-10045.
Blake, W. J., Balazsi, G., Kohanski, M. A., Isaacs, F. J., Murphy, K. F., Kuang, Y., Collins, J. J. (2006). Phenotypic consequences of promoter-mediated transcriptional
noise. Molecular Cell, 24(6), 853-865.
Blake, W. J., Kaern, M., Cantor, C. R., & Collins, J. J. (2003). Noise in eukaryotic gene expression. Nature, 422(6932), 633-637.
Bremer, H., & Ehrenberg, M. (1995). Guanosine tetraphosphate as a global regulator of bacterial RNA synthesis: A model involving RNA polymerase pausing and queuing.
Carey, L. B., van Dijk, D., Sloot, P. M. A., Kaandorp, J. A., & Segal, E. (2013). Promoter sequence determines the relationship between expression level and noise. PLoS Biol,
11(4), e1001528.
Chang, H. H., Hemberg, M., Barahona, M., Ingber, D. E., & Huang, S. (2008). Transcriptome-wide noise controls lineage choice in mammalian progenitor cells.
Nature, 453(7194), 544-547.
Cheung, A. M., & Cramer, P. (2012). A movie of RNA polymerase II transcription. Cell,
149(7), 1431-1437.
Choi, P. J., Cai, L., Frieda, K., & Xie, X. S. (2008). A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science, 322(5900), 442-446.
Dadiani, M., van Dijk, D., Segal, B., Field, Y., Ben-Artzi, G., Raveh-Sadka, T., Segal, E. (2013). Two DNA-encoded strategies for increasing expression with opposing effects on promoter dynamics and transcriptional noise. Genome Research, 23(6), 966-976.
Dar, R. D., Razooky, B. S., Singh, A., Trimeloni, T. V., McCollum, J. M., Cox, C. D., ...
Weinberger, L. S. (2012). Transcriptional burst frequency and burst size are equally modulated across the human genome. Proceedings of the National Academy of
Sciences, 109(43), 17454-17459.
Delbruck, M. (1945). The burst size distribution in the growth of bacterial viruses (bacteriophages). The Journal of Bacteriology, 50(2), 131-135.
Elliott, S. G., & McLaughlin, C. S. (1978). Rate of macromolecular synthesis through the cell cycle of the yeast saccharomyces cerevisiae. Proceedings of the National Academy
of Sciences, 75(9), 4384-4388.
Elowitz, M. B., & Leibier, S. (2000). A synthetic oscillatory network of transcriptional regulators. Nature, 403(6767), 335-338.
Elowitz, M. B., Levine, A. J., Siggia, E. D., & Swain, P. S. (2002). Stochastic gene expression in a single cell. Science, 2945584), 1183-1186.
Friedman, N., Cai, L., & Xie, X.
S.
(2006). Linking stochastic dynamics to population distribution: An analytical framework of gene expression. Physical Review Letters, 97(16), 168302.Gardner, T. S., Cantor, C. R., & Collins, J. J. (2000). Construction of a genetic toggle switch in escherichia coli. Nature, 403(6767), 339-342.
Guptasarma, P. (1996). Cooperative relaxation of supercoils and periodic transcriptional initiation within polymerase batteries. Bioessays, 18(4), 325-332.
Hahn, S. (1998). Activation and the role of reinitiation in the control of transcription by RNA polymerase II. Cold Spring Harbor Symposia on Quantitative Biology, 63, 181-188.
Harper, C., Finkenst~dt, B., Woodcock, D., Friedrichsen, S., Semprini, S., Ashall, L., White, M. (2011). Dynamic analysis of stochastic transcription cycles. PLoS Biology.
9(4), e1000607.
Hilfinger, A., & Paulsson, J. (2011). Separating intrinsic from extrinsic fluctuations in dynamic biological systems. Proceedings of the National Academy of Sciences,
108(29), 12167-12172.
Hornung, G., Bar-Ziv, R., Rosin, D., Tokuriki, N., Tawfik, D. S., Oren, M., & Barkai, N. (2012). Noise-mean relationship in mutated promoters. Genome Research, 22(12), 2409-2417.
Huh, D., & Paulsson, J. (2011). Non-genetic heterogeneity from stochastic partitioning at cell division. Nature Genetics, 43(2), 95-100.
Huisinga, K. L., & Pugh, B. F. (2004). A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in saccharomyces cerevisiae. Molecular
Cell, 13(4), 573-585.
Jacob, F., & Monod, J. (1961). On the regulation of gene activity. Cold Spring Harbor
Symposa on Quantitative Biology, 26, 193-211.
Jacob, F., & Monod, J. (1961). Genetic regulatory mechanisms in the synthesis of proteins. Journal of Molecular Biology, 3(3), 318-356.
Kaern, M., Elston, T. C., Blake, W. J., & Collins, J. J. (2005). Stochasticity in gene expression: From theories to phenotypes. Nature Reviews. Genetics, 6(6), 451-464.
Larson, D. R.., Zenklusen, D., Wu, B., Chao, J. A., & Singer, R. H. (2011). Real-time observation of transcription initiation and elongation on an endogenous yeast gene.
Science, 332(6028), 475-478.
Lestas, I., Vinnicombe, G., & Paulsson, J. (2010). Fundamental limits on the suppression of molecular fluctuations. Nature, 467(7312), 174-178.
Maamar, H., Raj, A., & Dubnau, D. (2007). Noise in gene expression determines cell fate in bacillus subtilis. Science, 317(5837), 526-529. doi:10.1126/science.1140818
Mao, C., Brown, C. R., Falkovskaia, E., Dong, S., Hrabeta-Robinson, E., Wenger, L., & Boeger, H. (2010). Quantitative analysis of the transcription control mechanism. Mol
Syst Bid, 6(1), -.
McAdams, H. H., & Arkin, A. (1999). It's a noisy business! Genetic regulation at the nanomolar scale. Trends in Genetics, 15(2), 65-69.
Mogno, I., Vallania, F., Mitra, R. D., & Cohen, B. A. (2010). TATA is a modular component of synthetic promoters. Genome Research, 20(10), 1391-1397.
Munsky, B., & Khammash, M. (2006). The finite state projection algorithm for the solution of the chemical master equation. The Journal of Chemical Physics, 124(4), -.
Murakami, K. S., & Darst, S. A. (2003). Bacterial RNA polymerases: The whole story.
Current Opinion in Structural Biology, 13(1), 31-39.
Muramoto, T., Cannon, D., Gierliaski, M., Corrigan, A., Barton, G. J., & Chubb, J. R. (2012). Live imaging of nascent RNA dynamics reveals distinct types of
transcriptional pulse regulation. Proceedings of the National Academy of Sciences, 109(19), 7350-7355.
Murphy, K. F., Balszsi, G., & Collins, J. J. (2007). Combinatorial promoter design for engineering noisy gene expression. Proceedings of the National Academy of Sciences,
104(31), 12726-12731.
Newman, J. R. S., Ghaemmaghami, S., Ihmels, J., Breslow, D. K., Noble, M., DeRisi, J. L., & Weissman, J. S. (2006). Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature, 441(7095), 840-846.
Novick, A., & Weiner, M. (1957). Enzyme induction as an all-or-none phenomenon.
Proceedings of the National Academy of Sciences of the United States of America, 43(7), 553-566.
Ozbudak, E. M., Thattai, M., Lim, H. N., Shraiman, B. I., & Van Oudenaarden, A. (2004). Multistability in the lactose utilization network of escherichia coli. Nature, 427(6976), 737-740.
Ozbudak, E. M., Thattai, M., Kurtser, I., Grossman, A. D., & van Oudenaarden, A. (2002). Regulation of noise in the expression of a single gene. Nature Genetics, 31(1), 69-73.
Pedraza, J. M., & van Oudenaarden, A. (2005). Noise propagation in gene networks.
Science, 307(5717), 1965-1969.
Raj, A., Rifkin, S. A., Andersen, E., & van Oudenaarden, A. (2010). Variability in gene expression underlies incomplete penetrance. Nature, 463(7283), 913-918.
Raj, A., Peskin, C., Tranchina, D., Vargas, D., & Tyagi, S. (2006). Stochastic mRNA synthesis in mammalian cells. PLoSBio, 4(10), e309-e309.
Raj, A., van, d. B., Rifkin, S. A., van Oudenaarden, A., & Tyagi, S. (2008). Imaging individual mRNA molecules using multiple singly labeled probes. Nat Meth, 5(10), 877-879.
Raser, J. M., & O'Shea, E. K. (2004). Control of stochasticity in eukaryotic gene expression. Science, 304(5678), 1811-1814. doi:10.1126/science.1098641
Salman, H., Brenner, N., Tung, C., Elyahu, N., Stolovicki, E., Moore, L., Braun, E. (2012). Universal protein fluctuations in populations of microorganisms. Physical
Review Letters, 108(23), 238105.
Sanchez, A., & Golding, I. (2013). Genetic determinants and cellular constraints in noisy gene expression. Science, 342(6163), 1188-1193.
Schr6dinger, E. (1944). What is life? Cambridge University Press.
Skupsky, R., Burnett, J. C., Foley, J. E., Schaffer, D. V., & Arkin, A. P. (2010). HIV promoter integration site primarily modulates transcriptional burst size rather than frequency. PLoS Computational Biology, 6(9), e1000952.
So, L., Ghosh, A., Zong, C., Sepulveda, L. A., Segev, R., & Golding, I. (2011). General properties of transcriptional time series in Escherichia coli. Nature Genetics, 43(6), 554-560.
St-Pierre, F., & Endy, D. (2008). Determination of cell fate selection during phage lambda infection. Proceedings of the National Academy of Sciences, 105(52), 20705-20710.
Struhl, K. (1996). Chromatin structure and RNA polymerase II connection: Implications for transcription. Cell, 84(2), 179-182.
Suel, G. M., Garcia-Ojalvo, J., Liberman, L. M., & Elowitz, M. B. (2006). An excitable gene regulatory circuit induces transient cellular differentiation. Nature, 440(7083), 545-550.
Suel, G. M., Kulkarni, R. P., Dworkin, J., Garcia-Ojalvo, J., & Elowitz, M. B. (2007). Tunability and noise dependence in differentiation dynamics. Science, 315(5819),
1716-1719.
Suter, D. M., Molina, N., Gatfield, D., Schneider, K., Schibler, U., & Naef, F. (2011). Mammalian genes are transcribed with widely different bursting kinetics. Science, 332(6028), 472-474.
Taniguchi, Y., Choi, P. J., Li, G., Chen, H., Babu, M., Hearn, J., Xie, X. S. (2010). Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science, 329(5991), 533-538.
Tirosh, I., Barkai, N., & Verstrepen, K. J. (2009). Promoter architecture and the evolvability of gene expression. Journal of Biology, 8(95).
Tirosh, I., & Barkai, N. (2008). Two strategies for gene regulation by promoter nucleosomes. Genome Research, 18(7), 1084-1091.
To, T., & Maheshri, N. (2010). Noise can induce bimodality in positive transcriptional feedback loops without bistability. Science, 327(5969), 1142-1145.
Wang, Z., Gerstein, M., & Snyder, M. (2009). RNA-seq: A revolutionary tool for transcriptomics. Nature Reviews. Genetics, 10(1), 57-63.
Yudkovsky, N., Ranish, J. A., & Hahn, S. (2000). A transcription reinitiation intermediate that is stabilized by activator. Nature, 408(6809), 225-229.
Zenklusen, D., Larson, D. R., & Singer, R. H. (2008). Single-RNA counting reveals
alternative modes of gene expression in yeast. Nat Struct Mol Biol, 15(12), 1263-1271.
Zhang, Z., Revyakin, A., Grimm, J. B., Lavis, L. D., Tjian, R., & Kadonaga, J. T. (2014). Single-molecule tracking of the transcription cycle by sub-second RNA detection.
CHAPTER 2. Mode of transcriptional regulation can
qualitatively affect gene behavior in positive feedback
2.1
Abstract
Cellular information processing often employs multi-stability for decision-making, memory and bet-hedging. Within gene networks, multi-stability is accomplished via positive feedback loops. We demonstrate with a theoretical case study that gene expression noise in these networks either stabilize/create or destabilize/eliminate bimodal gene expression patterns when transcriptional activators modulate burst frequency or burst size, respectively. This illustrates how the mode by which regulatory elements actuate transcription can have profound implications for network and cell behavior. Hence correct characterization of stochastic transcription dynamics are important for design and analysis of genes networks.
2.2 Introduction: Modes of regulating transcriptional bursting
Myriad studies have demonstrated that gene expression is stochastic, and a stochastic description of even simple regulatory networks can lead to unintuitive and even qualitatively different behavior as compared to a deterministic description (Acar, Becskei & van Oudenaarden, 2005; Blake et a]., 2006; Cagatay et a]., 2009; Elowitz et a]., 2002; Kaern et
a]., 2005; Maamar, Raj & Dubnau, 2007; Maheshri & O'Shea, 2007; McAdams & Arkin,
1997; Raser & O'Shea, 2004; Suel et al., 2006; To & Maheshri, 2010; Turcotte, Garcia-Ojalvo
& Suel, 2008). For example, stochastic noise propagated through networks can either increase (Rosenfeld et al., 2005) or decrease (Paulsson & Ehrenburg, 2000; Thattai & van Oudenaarden, 2001) variability of downstream gene expression. It has been debated whether noise in gene expression could have evolutionary benefits, such as in creating diversity in response to a change in environmental conditions (e.g. the decision between the lytic or lysogenic response in phage infected bacteria), (Raj & van Oudenaarden, 2008) or perhaps noise is an unavoidable consequences of molecular events and evolution has uniformly selected against noise (McAdams & Arkin, 1999; Raj et al., 2010).
Recent single molecule approaches suggest that noisy gene expression largely arises from random and intermittent "bursts" of transcription (Cai, Friedman & Xie, 2006; Chubb et al., 2006; Golding et al., 2005; Raj & van Oudenaarden, 2009; Taniguchi et al., 2010). A two-state promoter model has been employed to interpret these results (Peccoud & Ycart, 1995; Raj et al., 2006; Shahrezaei, Ollivier & Swain, 2008; introduced in Chapter 1). It models promoters transitioning between inactive and active states, and produces transcript when active (Figure 1-2). This model has three kinetic parameters -- the promoter activation rate, the promoter deactivation rate, and the transcription rate when active. The observed "bursty" transcription is consistent with rare, transitions of the promoter from the inactive to a short-lived, but highly productive active state, resulting in a burst of mRNA expression. The statistics of bursting can be succinctly described by two parameters: the burst frequency (the promoter activation rate) which is the number of bursts over the lifetime of mRNA or protein and the burst size (the ratio of the transcription rate to the deactivation rate), which is the number of mRNA or proteins produced per burst.
For a "bursty 7 gene, the mean level of gene expression is simply the product of burst size and burst frequency. Transcription factors that modulate this mean level may do so by either affecting burst frequency, burst size, or a combination of both (Hahn, 1998). Which mode of regulation is being employed can be inferred by examining how the intrinsic noise in gene expression scales with the mean expression level (Friedman, Cai & Xie, 2006; Raser & O'Shea, 2004). When activators regulate burst frequency, gene expression noise decreases with increased expression (with a square root dependence on protein abundance); in contrast, when activators regulate burst size, gene expression noise is constant (Figure 2-1). Several experimental studies have examined the dependence of gene expression noise on
cis and trans factors. Strong TATA boxes (Mogno et a]., 2010; Raser & O'Shea, 2004) and
higher number and affinity of activator binding sites (Raj et a]., 2006; Suter et a]., 2011; To & Maheshri, 2010) appears to increase burst size. In cases where intrinsic noise in protein expression was directly measured in response to changing a transcriptional activator, such as at the PHO5 gene in budding yeast (Raser & O'Shea, 2004) or a lac promoter variant in E. coli (Pedraza & van Oudenaarden, 2005), the intrinsic noise appears to scale with the inverse square root of protein abundance, characteristic of burst frequency regulation.
Still, a wealth of biochemical evidence exists for activators (and repressors) to influence transcriptional reinitiation and thereby potentially burst size (Hahn, 2004). In eukaryotes,
burst sizes have been measured in the range of 10O-102 mRNA (reviewed in Sanchez &
Golding, 2013). Generally, regulable genes exhibit low basal expression, including a basal burst size in the absence (or presence) of activators (or repressors). A typical average mRNA copy number of such repressed genes in yeast is -0.1-1 mRNA per cell (Holland, 2002). If these genes were subject to pure burst frequency regulation, then the basal burst size would remain 10-100 mRNAs at very low levels of expression. That would mean a small but measurable (-0.1%) fraction of cells would appear strongly ON for gene expression, yet this has not been reported. Therefore, it would seem that burst size must be regulated at some point in the transition from basal to regulated expression. Single molecule studies examining the synthetic Tet OFF system in HeLa cells (Raj et a., 2006) and the lac repressor (Choi
Newman et a]., 2006) show that noise of the majority of these genes scale with the inverse square root of abundance at low to intermediate levels of expression when extrinsic noise does not dominate. One interpretation consistent with the data is that the higher expression level of stronger promoters is due burst frequency. While this may be true for many "constitutive", housekeeping genes that tend to be less noisy, highly regulable genes tended to deviate from this dependence (Bar-Even et a]., 2006; Zenklusen, Larsen & Singer, 2008). Close examination of the E. coli data set indicates that low expressing promoters (corresponding to <20 proteins) span a 10-fold range of both burst size and frequency (Taniguchi et aL., 2010). This is consistent with recent examination of burst statistics in several different E. coli promoters (So et a]., 2011) that suggests that differences in expression level are largely due to changes in burst size. Taken together, the notion that burst size and frequency can both be regulated by trans factors for many genes seems a reasonable one.
Here, we investigate the key differences burst size and frequency regulation can have on the outcome of simple gene circuits involving feedback loops. Positive feedback with burst frequency regulation has previously been shown to stabilize deterministically bistable states or create a bimodal expression distribution when a bistability is not predicted (Friedman, Cai & Xie, 2006; Karmakar & Bose, 2007; Samoilov, Plyasunov & Arkin, 2005; To & Maheshri, 2010). Using both analytical and computational methods, we demonstrate that burst size regulation can have the opposite effect, destabilizing deterministically bistable states and eliminating bimodal expression.