Journal of Molecular Neuroscience 41 Volume 30, 2006 *Author to whom all correspondence and reprint requests should be addressed. E-mail: mcordeiro@bio.ua.pt
Vesicular Calcium Transport Shapes Rapid
Acetylcholine Secretion
J. Miguel Cordeiro,*
,1,2Yves Dunant,
2and Paula P. Gonçalves
1 1CESAM, Departmento Biologia, Universidade de Aveiro, 3810-193 Aveiro, Portugal;and2Départment de Neurosciences, CMU, 1211 Geneva 4, Switzerland
Introduction
Rapid secretion relies on the occurrence of spike-like Ca2+transients in active zones (Llinás et al., 1992; Yazejian et al., 2000; Dunant and Bloc, 2003). Presynaptic Ca2+nanodomains are to be restricted both in time and in space as to assure rapid onset and termination of transmitter release (Llinás et al., 1992; Pozzan et al., 1994; Yazejian et al., 2000; Dunant and Bloc, 2003). A very fast Ca2+-buffering mecha-nism should allow Ca2+rise above ~100 RM for less than ~250 Rs and then rapid reduction of Ca2+to subthreshold levels of release (Llinás et al., 1992; Pozzan et al., 1994; Yazejian et al., 2000; Dunant and Bloc, 2003). Swift Ca2+ clearance by vesicular Ca2+/H+ antiport as a low-affinity, high-capacity extrusion mechanism was postulated in the past (Pozzan et al., 1994; Dunant and Bloc, 2003). We demonstrated pH gradient ()pH)-dependent Ca2+ uptake by mammalian brain synaptic vesicles (Gonçalves et al., 1998, 2000). Moreover, this antiport activity is effective at [Ca2+] ranging from ~100 to 800 RM (max. at ~500 RM) (Gonçalves et al., 1998, 2000). We now show that the time course of acetylcholine (ACh) secretion in Torpedo neuro-electrocytic synapse is modified by bafilomycin A1 (baf.), which compromises antiport activity. Along with this mechanism, synaptic vesicles also have a P-type Ca2+ATPase, exhibiting half-maximal activa-tion for 0.6 RM Ca2+(Gonçalves et al., 2000). Here, we demonstrate the role of P-type Ca2+ATPase in
Journal of Molecular Neuroscience Copyright © 2006 Humana Press Inc.
All rights of any nature whatsoever are reserved. ISSN0895-8696/06/30:41–44/$30.00
JMN (Online)ISSN 1559-1166 DOI 10.1385/JMN/30:1-2:41
O
RIGINALA
RTICLEpreventing desensitization of the release mechanism by inhibiting it with orthovanadate.
Results and Discussion
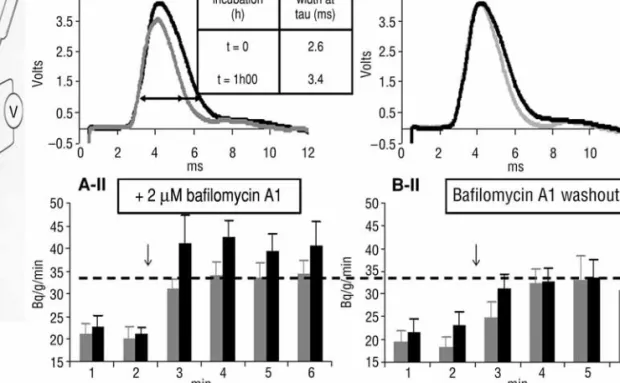
Rapid Ca2+sequestration operated by vesicular Ca2+/H+antiport was disrupted by using the specific V-type H+-ATPase inhibitor baf. This abolishes the )pH of synaptic vesicles that energizes the antiport and finally results in prolongation of the postsynaptic current from 2–4 ms up to >10 ms, by persistence of ACh release (Fig.1A-I,A-II). Simultaneous measure-ment of [14C]ACh release confirmed the presynaptic
nature of this phenomenon. Moreover, drug washout returned values to control levels (Fig.1B-I,B-II). Accordingly, baf. increased ACh release from electric organ synaptosomes, elicited by either veratridine or KCl depolarization, followed by Ca2+ addition. Conversely, when synaptosomes were exposed longer than 10 min to baf. in the presence of [Ca2+]out
= 3.5 mM prior to depolarization, desensitization of release occurred (not illustrated). Disrupting Ca2+ ATPase with 10 RM orthovanadate resulted in slow (hours) failure of transmission (Fig. 2) by a Ca2+ -dependent, presynaptic effect. 50 RM of BAPTA-AM, perfused in the presence of orthovanadate, quickly recuperated transmission (Fig. 2A), whereas ortho-vanadate washout was less efficient (Fig. 2B).
Phasic Ca2+ sequestration by vesicular Ca2+/H+ antiport activity shapes the time course of secretion at microsecond range. The antiport also participates
42 Cordeiro et al.
Journal of Molecular Neuroscience Volume 30, 2006
Fig. 1. Torpedo electric organ stacks of electrocytes (prisms) were labeled overnight with 14C-labeled acetate (Dunant
et al., 1980). Prisms were perfused with 2 RM baf. for 1 h prior to stimulation (A, black bars and trace). Control prisms are shown in gray. [14C]ACh diffused from tissue was collected before and after stimulus (r, A-II). Electrocyte responses
were measured (A-I) at the same time. The same parameters were assessed after 1 h drug washout (B-I,B-II). The black trace in B-I shows baf. effect as reference vs washout in gray.
Fig. 2. Torpedo prisms were perfused continuously with marine solution containing 10 RM Na+-orthovandate (black bars). Evoked electrophysiological responses were recorded for several hours. Complete desensitization of release mecha-nism (after 5 h and 55 min) is shown in traces, followed by rapid recuperation (A), after adding 50 RM BAPTA-AM (55 min) to medium or slow recuperation (B), by washing out orthovanadate. Bars show discharge amplitude.
Ca2+Transport Shapes Rapid ACh Secretion 43
Journal of Molecular Neuroscience Volume 30, 2006
in late Ca2+homeostasis and extrusion (Dunant, this issue), working with Ca2+ATPase in housekeeping tasks.
Acknowledgments
This work was supported by grants SFRH-BD-6403-2001 and EU lipidiet project qlk1-ct-2002.
References
Dunant Y. and Bloc A. (2003) Low- and high-affinity re-actions in rapid neurotransmission. Neurochem. Res. 28, 659–665.
Dunant Y., Eder L., and Servetiadis-Hirt L. (1980) Acetylcholine release evoked by single or a few nerve impulses in the electric organ of Torpedo. J. Physiol.
298,185–203.
Gonçalves P. P., Meireles S. M., Gravato C., and Vale M. G. (1998) Ca2+-H+antiport activity in synaptic vesicles isolated from sheep brain cortex. Neurosci. Lett. 247, 87–90.
Gonçalves P. P., Meireles S. M., Neves P., and Vale M. G. (2000) Distinction between Ca(2+) pump and Ca(2+)/H(+)antiport activities in synaptic vesicles of sheep brain cortex. Neurochem. Int. 37, 387–396. Llinás R., Sugimori M., and Silver R. B. (1992)
Microdomains of high calcium concentration in a pre-synaptic terminal. Science 256, 677–679.
Pozzan T., Rizzuto R., Volpe P., and Meldolesi J. (1994) Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 74, 595–636.
Yazejian B., Sun X. P., and Grinnell A. D. (2000) Tracking presynaptic Ca2+dynamics during neurotransmitter release with Ca2+-activated K+channels. Nat. Neurosci.
3,566.