Effects of progesterone at the enteric level in a mouse
model of Parkinson’s disease
Mémoire
Hend Jarras
Maîtrise en sciences pharmaceutiques - avec mémoire
Maître ès sciences (M. Sc.)
Effects of progesterone at the enteric level
in a mouse model of Parkinson’s disease
Mémoire
Hend Jarras
Sous la direction de :
Denis Soulet, directeur de recherche
Thérèse Di Paolo, codirectrice de recherche
Résumé
La maladie de Parkinson (MP) est la deuxième maladie neurodégénérative la plus répandue dans le monde. Elle se caractérise par des symptômes moteurs causés par une perte des neurones dopaminergiques de la substance noire du cerveau. Les patients souffrent aussi de symptômes non-moteurs pouvant apparaître jusqu’à plusieurs années avant les déficits moteurs. Parmi ceux-ci se retrouvent des troubles gastro-intestinaux, suggérant l’implication du système nerveux entérique (SNE) dans la pathologie. En effet, des neurones dopaminergiques sont retrouvés dans le plexus myentérique (PM) du SNE, dont un des rôles est la régulation de la motilité du tube digestif. De plus, une augmentation de l’inflammation est observée chez les patients. L’incidence de la MP est plus élevée chez les hommes que chez les femmes, ce qui suggère un effet bénéfique des hormones féminines. La progestérone a été montrée neuroprotectrice pour les traumatismes crâniens, ainsi que dans des modèles animaux de la MP au niveau du système nerveux central. N’ayant pas été étudiée au niveau entérique dans les modèles de la MP, l’objectif de ce projet visait donc à évaluer les effets de la progestérone dans le PM de souris lésées au 1-méthyl-4-phényl-1,2,3,6-tétrahydropyridine (MPTP), une neurotoxine qui modélise la maladie. Différentes doses de progestérone (0.4, 8, 16 mg/kg) ont également été administrées et les résultats ont été obtenus suite à des immunohistochimies et immunofluorescences sur le PM de l’iléon. Nos résultats ont montré des effets neuroprotecteur et anti-inflammatoire de la progestérone dans le SNE des souris. Puisqu’il n’existe encore que des traitements symptomatiques de la MP, cette étude s’avère pertinente dans l’optique du développement de thérapies neuroprotectrices.
Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease in the world. It is characterized by motor symptoms caused by the loss of dopaminergic neurons in the brain’s substantia nigra. Patients also suffer from non-motor symptoms that can appear many years before non-motor symptoms. Among them are gastrointestinal problems, which suggest the implication of the enteric nervous system (ENS) in the pathology. Indeed, dopaminergic neurons are found in the myenteric plexus (MP) of the SNE, of which one role is the regulation of the gut’s motility. Moreover, increased inflammation can be observed in patients. The incidence of PD is higher in men than in women, suggesting a beneficial effect from female hormones. Progesterone was shown to be neuroprotective in traumatic brain injuries, as well as in the central nervous system of PD animal models. Having not been studied at the enteric level in PD models, the objective of this project was thus to evaluate progesterone’s effects in the MP of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mice, a neurotoxin that model the disease. Different doses of progesterone (0.4, 8, 16 mg/kg) were also administered and results were obtained by immunohistochemistry and immunofluorescence experiments on the MP of the ileum. Our results showed neuroprotective and anti-inflammatory effects of progesterone in the mice’s ENS. Since only symptomatic treatments are currently available for PD, this study proves relevant for the eventual development of neuroprotective therapies.
Table of contents
Résumé ... iii
Abstract ... iv
Table of contents ... v
List of figures ... vii
List of tables ... viii
List of abbreviations ... ix Acknowledgements ... xii Foreword ... xiii Introduction ... 1 Parkinson’s disease ... 1 Epidemiology ... 1 Etiology ... 2 Nigrostriatal system ... 6 Motor symptoms ... 8 Treatments ... 10 Non-motor symptoms ... 14
Enteric nervous system ... 16
Inflammation ... 18
Animal models ... 20
Sex differences ... 23
Progesterone ... 25
Problematic ... 27
Hypotheses and objectives ... 28
Chapter 1: Neuroprotection and immunomodulation of progesterone in the gut of a mouse model of Parkinson’s disease ... 29
1.1 Résumé ... 29
1.2 Abstract ... 32
1.4 Materials and Methods ... 35 1.4.1 Animals ... 35 1.4.2 Treatments ... 35 1.4.3 Tissue preparation ... 36 1.4.4 Immunohistochemistry ... 37 1.4.5 Immunofluorescence ... 37 1.4.6 Image analysis ... 38 1.4.7 Statistical analysis ... 38 1.5 Results ... 39 1.6 Discussion ... 40 1.7 Acknowledgements ... 46 1.8 Conflict of interest ... 46 1.9 Author contributions ... 46 Chapter 2: Discussion ... 56 Perspectives ... 60 Conclusion ... 62 References ... 63
List of figures
Figure 1 Comparison of the nigrostriatal pathway in healthy people and diseased
patients
Figure 2 Immunohistochemical marking of LBs in a SN dopaminergic neuron
containing a-syn and ubiquitin
Figure 3 Example of micrographia
Figure 4 Example of the spiral test in a normal person and a PD patient Figure 5 Representation of the non-motor symptoms of PD
Figure 6 The different layers of the intestines Figure 7 Metabolism of MPTP in the brain
Figure 8 Representation of neuroprotective processes
Figure 1.1 Progesterone treatment before and immediately after MPTP prevents the
MPTP-induced decrease in TH+ neuron density in the myenteric plexus
Figure 1.2 Effects on TH+ neuron density when progesterone treatment is started 5
days post MPTP lesion
Figure 1.3 Progesterone treatment prevents inflammation in the myenteric plexus Figure 1.4 MPTP and progesterone treatments do not affect the relative expression
of myenteric GFAP
Figure 1.5 Progesterone treatment increases the expression of BDNF in myenteric
List of tables
Table 1 Classification of some pharmacological dopaminergic treatments Table 2 PD animal models and their effects
Table 1.1 Summary of the effects of progesterone treatments in MPTP mice
List of abbreviations
6-OHDA: 6-hydroxydopamine
ALS: amyotrophic lateral sclerosis ANS: autonomic nervous system a-syn: alpha-synuclein
BBB: blood-brain barrier
BDNF: brain-derived neurotrophic factor BSSG: b-sitosterol b-d-glucoside
COMT: catechol-O-methyltransferase CNS: central nervous system
CSF: cerebrospinal fluid DA: dopamine
DAB: 3,3’-diaminobenzidine DAT: dopamine transporter DBS: deep brain stimulation ENS: enteric nervous system ERa: estrogen receptor alpha GABA: gamma-Aminobutyric acid GBA1: glucocerebrosidase 1
GDNF: glial-derived neurotrophic factor GFAP: glial fibrillary acidic protein GI: gastrointestinal
GPER1: G-protein coupled estrogen receptor GPi: globus pallidus interna
IBD: inflammatory bowel disease IL-1b: interleukin 1 beta
ip: intraperitoneal KO: knockout
L-AAAD: L-aromatic amino acid decarboxylase LBs: Lewy bodies
L-dopa: levodopa
LRRK2: leucine-rich repeat kinase 2 MAO-B: monoamine oxidase B
MHC II: major histocompatibility complex II min: minutes
MP: myenteric plexus
MPDP+: 1-methyl-4-phenyl-2,3-dihydropyridinium MPP+: 1-methyl-4-phenylpyridinium
mPR: membrane progesterone receptor
MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine NF: nuclear factor
nPR: nuclear progesterone receptor NSAIDs: nonsteroidal anti-inflammatory drugs PARK2: gene encoding parkin
PBS: phosphate buffered saline PD: Parkinson’s disease
PDC: Parkinsonism dementia complex PINK1: PTEN-induced kinase 1
Prog: progesterone
RBD: rapid eye movement sleep behaviour disorder ROS: reactive oxygen species
s.c.: subcutaneous
SERM: selective estrogen receptor modulator SN: substantia nigra
SNCA: gene encoding alpha-synuclein STN: subthalamic nucleus
TBI: traumatic brain injury TH: tyrosine hydroxylase TNF: tumor necrosis factor
UPDRS: Unified Parkinson’s Disease Rating Scale VMAT2: vesicular monoamine transporter 2
Acquire knowledge and teach it to people. Omar ibn al Khattab
Acknowledgements
First of all, I would like to offer my sincerest appreciation to my director Dr. Denis Soulet and my co-director Dr. Thérèse Di Paolo for accepting me in their laboratories during my internship and my master studies. This project was very stimulating and allowed me to learn a lot.
I also want to thank my colleagues in the team, especially Andrée-Anne for having trained me in the laboratory. I truly appreciate all the time and effort you put in to help me. I want to thank Katherine for her help as well during my studies. Thank you to everyone who contributed to this project.
I would also like to thank the members of my master’s jury for reviewing my thesis.
I am grateful for my friends Sara, Marie-Kim, Dajana, Laura, Razan, Lucie, Sarah and Mitra for the good moments spent inside and outside the laboratory. I really appreciate your friendship and all the moments spent together. A very special thanks to Emina and Heikel as well. You have been there through so many good and bad times. Thank you for coping with me all these years and for the numerous advices you gave me.
Finally, I am thankful to my family for the support given during my studies. A special thanks also to my cousins for the great moments we spend together in summer playing and laughing.
Foreword
The research done during my master’s degree led to the writing of an article, of which I am the first author, entitled “Neuroprotection and immunomodulation of progesterone in the gut of a mouse model of Parkinson’s disease”. This paper is found in chapter 1. It was submitted to the Journal of Neuroendocrinology on March 29th, 2019. It was recommended for publication on April 19th, 2019 following some
modifications and was accepted for publication on August 16th, 2019. For this paper,
I performed the microdissections on the gut tissues, the histological experiments and analyzed all the results. I also prepared the graphs and took part in the writing of the manuscript. Mélanie Bourque designed the progesterone administration protocol and performed the progesterone in vivo experiments. Andrée-Anne Poirier collected the gut samples. Marc Morissette treated the animals. Katherine Coulombe processed the gut tissues. Dr. Thérèse Di Paolo designed the progesterone in vivo experiments and participated to the writing and correction of the manuscript. Dr. Denis Soulet designed the myenteric plexus experiments and participated to the writing and correction of the manuscript.
Introduction
Parkinson’s disease
Parkinson’s disease (PD) has first been characterized as a neurological disorder in 1817 by James Parkinson in his work “An Essay on the Shaking Palsy” (Parkinson 2002, Przedborski 2017). He described in detail the progressive appearance of symptoms based on six clinical cases, stating that it is a rare disease with very slow development (Parkinson 2002). Other descriptions of the motor symptoms have also been found in ancient texts of Eastern and Western civilizations (Li and Le 2017). It is only at the end of the 19th century and early 20th century that
the substantia nigra (SN) was discovered as being involved in the pathogenesis and that further studies led to better understanding of the process (Li and Le 2017, Przedborski 2017). PD’s characteristic is a triad of motor symptoms: bradykinesia, muscular rigidity and resting tremor, with postural instability usually coming later in the progression of the pathology (Xia and Mao 2012). These symptoms are caused mainly by the progressive degeneration of dopaminergic neurons in the brain’s SN (Sveinbjornsdottir 2016).
Epidemiology
PD is the second most common neurodegenerative disorder in the world and the first cause of motor disorder, which affected more than six million people worldwide in 2015 (Stojkovska et al. 2015, Sveinbjornsdottir 2016, Group 2017). Its prevalence in the general population is about 0.3%, but this number rises to 1% in people over 60 years old and 4% in people over 80, making age the greatest risk factor for developing the disease (Dexter and Jenner 2013, Gillies et al. 2014, Sveinbjornsdottir 2016). Its prevalence is thus expected to increase in coming years due to the global aging of the population (Kalia and Lang 2015). Moreover, men are
more affected than women with a ratio of 1.5:1 (Shulman 2007, Bourque et al. 2011, Elbaz et al. 2016). Except for one (Camacho-Soto et al. 2018), studies have also shown that prevalence of PD is higher in patients of inflammatory bowel disease (IBD) (Lin et al. 2016, Peter et al. 2018, Villumsen et al. 2019, Zhu et al. 2019).
Etiology
The origin of most PD cases is still poorly understood. 5 to 10% are due to genetic mutations in familial PD (Lesage and Brice 2009, Tysnes and Storstein 2017). However, the other 90-95% are sporadic cases and their causes are unknown. Many environmental risk factors have been found in studies, although the implication of most of them in PD is still controversial and not clearly understood (Delamarre and Meissner 2017, Tysnes and Storstein 2017).
Genetic factors
As mentioned earlier, a small proportion of PD cases are due to genetic mutations. One of the important genes involved in the genetic cases is that of alpha-synuclein (a-syn): SNCA. a-syn is a pre-synaptic protein and is the main component of Lewy bodies (LBs), the histopathological hallmark of PD (Lanciego et al. 2012, Koros et al. 2017). It is subject to a few mutations, the major one being the A53T (Kim and Alcalay 2017). This genetic mutation was the first to be discovered as involved in the development of PD (Polymeropoulos et al. 1997). It has been associated with early onset, causes an accelerated progression and is a more aggressive form of the disease (Kim and Alcalay 2017). In fact, this mutation increases the aggregation potential of the protein, thus favoring the formation of amyloid-like structures (Polymeropoulos et al. 1997, Koros et al. 2017).
The SNCA gene is also subject to multiplications. Duplications are more common, but triplications are more severe (Kasten and Klein 2013). Triplications
cause an early age of onset and dementia is one of its characteristics, which starts soon after onset (Aarsland and Kurz 2010). Duplications usually have a milder course of the disease and a lower penetrance (Koros et al. 2017).
Another gene linked to PD is leucine-rich repeat kinase 2 (LRRK2). It has been associated with familial cases as well as sporadic PD (Li et al. 2014, Ferreira and Massano 2017). Many mutations have been reported in the sequence, but the most common is by far G2019S (Bardien et al. 2011, Li et al. 2014). LRRK2 is inherited in an autosomal dominant way with incomplete penetrance (Kumari and Tan 2009). Genetic mutations in LRRK2 have been found to cause similar clinical phenotypes as idiopathic PD, thus being an interesting candidate to study in order to better understand the pathology. In most cases of LRRK2-associated PD, the disease seems to be less severe than sporadic PD (Martin et al. 2014). The interesting aspect of LRRK2 is that not many autopsies have confirmed synucleinopathies, rather they found nigral degeneration, tauopathy or ubiquitin-positive inclusions (Koros et al. 2017). The toxicity of LRRK2 mutants has long been linked to its kinase activity (West et al. 2005, Sheng et al. 2012). However, suggestions have been made that the level of expression of the mutant protein might also be at cause (Chan and Tan 2017).
Other genes have been linked to PD, some of them being GBA1, PARK2 and
PINK1 (Lees et al. 2009). GBA1 encodes for an enzyme, glucocerebrosidase, which
is a lysosomal hydrolase (Bae et al. 2014). Mutations in that gene cause a loss-of-function of the protein, leading eventually to a-syn aggregation and is also accompanied by lysosomal dysfunctions (Barkhuizen et al. 2016). In fact, synucleinopathies have been consistently found in GBA1-associated PD (Garcia-Sanz et al. 2017, Koros et al. 2017). Parkin, encoded by PARK2, and PINK1 (PTEN-induced kinase 1) are both involved in mitophagy (Poewe et al. 2017). The loss-of-function of either causes early-onset PD (Pickrell and Youle 2015). Indeed, PINK1 is a mitochondrial kinase, while Parkin is an E3 ubiquitin ligase which acts downstream of PINK1 (Kumar et al. 2017). PD patients with these mutations have
milder disease cases with slower progression and sustained response to levodopa (L-dopa) (Truban et al. 2017). Parkin is the first cause of early-onset PD and often shows no synucleinopathy with a restricted neurodegeneration to the SN and locus
coeruleus (Koros et al. 2017, Truban et al. 2017).
Environmental factors
As most PD cases are not familial, many environmental factors have been studied in order to better understand its cause. These can be classed into two categories: risk factors and protective factors.
Among the risk factors we can find the most prominent is age. Indeed, PD is almost non-existent under 40 years of age, and the incidence increases as the population gets older as mentioned previously (Kieburtz and Wunderle 2013, Pringsheim et al. 2014). Likewise, gender seems to be a factor to consider as men are about 50% more at risk of developing PD than women (Ascherio and Schwarzschild 2016).
Exposition to certain herbicides and pesticides is also thought to increase the risk of developing PD. In fact, some meta-analyses supported their exposure to significantly increase the risk of PD (van der Mark et al. 2012, Van Maele-Fabry et al. 2012, Pezzoli and Cereda 2013). Compounds such as paraquat and rotenone, which are herbicides and pesticides respectively are considered among the risk factors (de Lau and Breteler 2006, Pouchieu et al. 2018). They are both used also as animal models, with rotenone inhibiting the complex I in mitochondria and paraquat generating oxidative stress that lead to damage of lipid, proteins and nucleic acids (Jackson-Lewis et al. 2012). It is also the case of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a toxin derived from drug synthesis (Nandipati and Litvan 2016). Moreover, suffering from traumatic brain injury was shown to increase the risk of developing PD later in life (Bower et al. 2003, Goldman et al. 2006). Another environmental risk factor is the ingestion of cycad seeds. Indeed, it
was found last century that amyotrophic lateral sclerosis (ALS) and parkinsonism-dementia complex (PDC) had a high prevalence in the indigenous Chamorro people of Guam (Kurland 1988). The incidence increased after World War II and led scientists to explore environmental sources for these syndromes (Kurland 1988). The water insoluble phytosterol glucoside b-sitosterol b-d-glucoside (BSSG) was shown to induce loss of motor neurons and motor symptoms similar to PD (Tabata et al. 2008, Van Kampen et al. 2015).
Among protective factors there are smoking, caffeine consumption, other dietary factors and nonsteroidal anti-inflammatory drugs (NSAIDs) (Ascherio and Schwarzschild 2016). Regarding smoking, it has been suggested that it might have a neuroprotective effect (Kieburtz and Wunderle 2013). However, many biases can occur with this point. For example, earlier death of smokers from other causes may under-represent them in the population of PD patients (Bellou et al. 2016). Hence, more studies are needed to evaluate its effects. Coffee intake was also found to be protective against PD (Ross et al. 2000, Hernan et al. 2002). To determine the exact compound responsible for this effect, studies have been made and found caffeine to have a similar response to coffee (Costa et al. 2010). Other caffeinated sources also had positive effects, but not decaffeinated coffee, confirming that caffeine, an inhibitor of the adenosine A2 receptor, is the active compound (Ross et al. 2000,
Ascherio et al. 2001). The protective effects of smoking and coffee drinking have been suggested to be due to their influence on the gut microbiota (Derkinderen et al. 2014). Indeed, bacteria can impact peripheral inflammation, which can lead to different effects on a-syn, thus affecting the pathogenesis (Derkinderen et al. 2014). Some dietary factors are also thought to be protective. Antioxidants such as vitamins might have a beneficial effect, although no study showed a significant association (Logroscino et al. 1996, Morens et al. 1996, Zhang et al. 2002). More recently, unsaturated fatty acids were found to significantly reduce the risk of PD (Abbott et al. 2003, de Lau et al. 2005). Finally, the use of NSAIDs might also have a protective effect. Studies are still conflicting sometimes, but some drugs like ibuprofen and
aspirin might be beneficial (Chen et al. 2003, Bornebroek et al. 2007, Wahner et al. 2007, Gao et al. 2011).
Nigrostriatal system
PD is a disease that affects mostly, but not only, the dopaminergic system of the brain. Indeed, the progressive degeneration occurs in the SN pars compacta, where most of the dopaminergic neurons can be found (Dexter and Jenner 2013, Zhang et al. 2017). Those neurons project their axons towards the caudate and putamen nuclei of the striatum (figure 1) (Jellinger 2012, Lanciego et al. 2012). Hence, their degeneration induces a decrease in the amount of dopamine (DA) available in the striatum, causing the characteristic motor problems seen in PD (Chinta and Andersen 2005).
Figure 1 Comparison of the nigrostriatal pathway in healthy people and diseased
(putamen and caudate nuclei). When they are lost in PD, their innervation of the striatum decreases as well and causes a deficiency in DA (Saraiva et al. 2016).
The neurons that are still alive often present aggregates of proteins called Lewy bodies (LBs), including largely misfolded a-syn as can be seen in figure 2 (Braak et al. 2002). These aggregates are the histopathological hallmark of PD (Lanciego et al. 2012). The presence of LBs along with an important loss of neurons in the SN are a prerequisite for the diagnosis of the disease (Braak et al. 2002, Kalia and Lang 2015).
Figure 2 Immunohistochemical marking of LBs in a SN dopaminergic neuron,
containing a-syn and ubiquitin (Dauer and Przedborski 2003).
a-syn is a protein expressed in neural tissues and localizes mainly at presynaptic terminals (Maroteaux et al. 1988, Iwai et al. 1995). It is true of both the central and peripheral nervous systems, suggesting that it plays a role in synaptic transmission (Benskey et al. 2016). Its exact function though, is still not completely understood (Benskey et al. 2016). Its central domain is highly hydrophobic and is responsible for the aggregation potential of the protein (Benskey et al. 2016, Ottolini et al. 2017). Under pathological conditions such as PD, a-syn can be misfolded and aggregate. Initially, it was thought that these aggregations were harmful (Ottolini et al. 2017). However, it was discovered that the soluble oligomeric forms of the protein
were toxic, indicating that aggregation might be a protective mechanism to sequestrate the toxic forms (Karpinar et al. 2009, Winner et al. 2011).
The team of Dr Heiko Braak studied the evolution of a-syn pathology, which they separated into six stages (Braak et al. 2003). They studied brains of PD patients, people who showed no PD symptoms, but had LBs and people who had no symptoms nor LBs. They discovered that the Lewy pathology starts by affecting the olfactory bulb and anterior olfactory nucleus in what they named stage 1, which could explain the loss of smell seen in preclinical stages in many patients. Stage 2 is characterized by the progression of the lesions to the lower brainstem, which could be involved in non-motor symptoms such as sleep disorders and some autonomic characteristics. Motor symptoms are thought to appear at stages 3 and 4, when the synucleinopathy continues to spread to the substantia nigra and other deep nuclei of the midbrain and forebrain regions. In the final 5th and 6th stages, LBs are now
found in limbic structures and mature neocortex which could be involved in neuropsychiatric disorders (Braak et al. 2002, Chaudhuri et al. 2006).
Motor symptoms
As mentioned earlier, the principal symptoms of PD affect motility. In fact, it is at the appearance of motor symptoms that the disease is usually diagnosed (Kalia and Lang 2015, Sveinbjornsdottir 2016). By that time, there is an estimated loss of 30-80% of the dopaminergic neurons in the SN of patients (Fearnley and Lees 1991, Lang and Lozano 1998, Davie 2008, Grosch et al. 2016, Sveinbjornsdottir 2016). For PD to be diagnosed, bradykinesia needs to be present with at least one other of the prominent motor symptoms – muscular rigidity, resting tremor and postural instability (Sveinbjornsdottir 2016). Bradykinesia is defined as a slowness of movement and is the most characteristic clinical aspect of PD (Tarakad and Jankovic 2017).
Figure 3 Example of micrographia. A – Handwriting during the “off” period (without
L-dopa). B – Handwriting during the “on” period (1 hour after taking L-dopa). (Ling et al. 2012)
Apart from these four symptoms, patients suffer from other disabilities. One of the most debilitating is freezing gait, which is the inability to initiate voluntary movements seen for walking for example (Dauer and Przedborski 2003). Patients experience difficulty to walk and exhibit particular characteristics such as hesitation to initiate the walk and difficulty to move the feet when turning. It also commonly causes falls (Jankovic 2008). Furthermore, there is decreased function of the arms and hands. Typical diagnostic tests are based on this feature, as they were found to correlate with the score from the Unified Parkinson’s Disease Rating Scale (UPDRS) (Opara et al. 2017). Tremors for instance generally affect the hands (Jankovic 2008). A majority of patients will also display micrographia (figure 3), which is small handwriting (Moustafa et al. 2016). They also show other handwriting deficits that can be detected with the spiral test for example (figure 4), where patients draw a spiral that is usually smaller than for normal people (Alty and Kempster 2011).
Figure 4 Example of the spiral test in a normal person and a PD patient; adapted
from (Alty and Kempster 2011).
Treatments
Pharmacological treatments
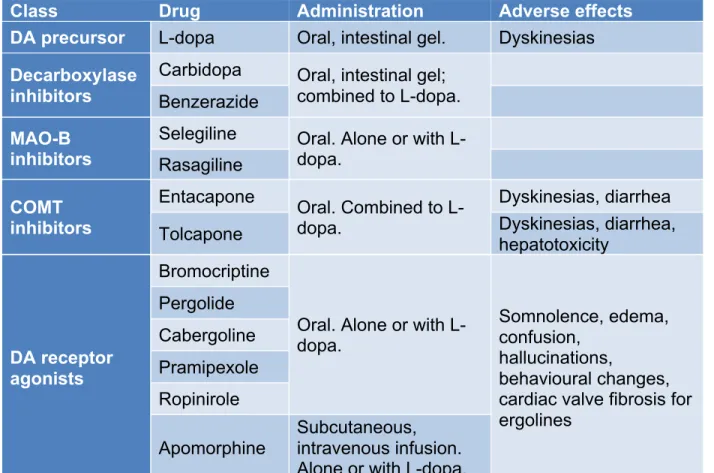
The treatments currently available for PD patients are only symptomatic, meaning that they aim at attenuating motor symptoms to allow patients to continue with their daily activities, but they do nothing to prevent or stop further progression of the disease (Connolly and Lang 2014, Sveinbjornsdottir 2016, Tarakad and Jankovic 2017). Therefore, the pathology continues to worsen over time. The gold-standard treatment currently used is L-dopa (Jankovic and Stacy 2007, LeWitt 2015). It is the endogenous precursor of DA and is an effective DA-replacement drug (LeWitt 2015, Tambasco et al. 2018). In fact, response to L-dopa is considered a criterion for the diagnosis of PD (Fahn 2006). It has the capacity to pass the blood brain barrier (BBB), where it is converted to DA by the enzyme L-aromatic amino acid decarboxylase (L-AAAD) (LeWitt 2015). As it can also be metabolized in the periphery, it is often administered with other decarboxylase inhibitors that act peripherally, such as carbidopa and benzerazide to get a maximum of the molecule in the brain (LeWitt 2015, Tarakad and Jankovic 2017). However, L-dopa is not a
perfect drug, as it comes with side effects when used in the long term. Indeed, around 50-80% of patients will develop dyskinesias (involuntary movements) and motor fluctuations after 5 to 10 years of use (Olanow et al. 2004, Jankovic and Stacy 2007).
Other categories of medications have also been developed. For example, monoamine oxidase B (MAO-B) inhibitors are available (Munchau and Bhatia 2000). This enzyme is responsible for the breakdown of DA, so its blocking allows for a longer effect of L-dopa, and increased DA levels in the striatum (Connolly and Lang 2014, LeWitt 2015). It can be used on its own as an initial treatment if symptoms are mild, or together with L-dopa to increase its efficacy (Jankovic and Stacy 2007, Tarakad and Jankovic 2017).
Another class of drugs are catechol-O-methyltransferase (COMT) inhibitors. COMT catabolizes L-dopa (Jankovic and Stacy 2007). Hence, by inhibiting it in the periphery, the half-life of L-dopa is increased, as well as its bioavailability (LeWitt 2015, Tarakad and Jankovic 2017). Some of these inhibitors can also act centrally to block DA metabolism, and their use was associated with the increased risk of developing dyskinesias (Bonifati and Meco 1999, Sampaio and Ferreira 2010, Muller 2015).
DA receptor agonists also exist (Munchau and Bhatia 2000). They directly activate DA receptors and can reduce the “off” time of the L-dopa treatment in patients (Connolly and Lang 2014). They are separated into 2 classes of drugs: ergolines, which derive from ergot alkaloids, and nonergolines. Ergolines include bromocriptine, pergolide and cabergolide, and nonergolides include pramipexole, ropinirole and apomorphine (Kim et al. 2017). They can be used as drugs on their own or added to another therapy such as L-dopa. An advantage of this class of drugs is that they cause less dyskinesias (Schapira et al. 2006, Dietrichs and Odin 2017). They do have other adverse effects though, such as somnolence, confusion,
hallucinations, edema, behavioural changes and cardiac valve fibrosis for ergolines (Schapira et al. 2006).
Class Drug Administration Adverse effects DA precursor L-dopa Oral, intestinal gel. Dyskinesias
Decarboxylase inhibitors
Carbidopa Oral, intestinal gel; combined to L-dopa. Benzerazide
MAO-B inhibitors
Selegiline Oral. Alone or with L-dopa. Rasagiline COMT inhibitors Entacapone Oral. Combined to L-dopa. Dyskinesias, diarrhea Tolcapone Dyskinesias, diarrhea, hepatotoxicity
DA receptor agonists
Bromocriptine
Oral. Alone or with L-dopa.
Somnolence, edema, confusion,
hallucinations,
behavioural changes, cardiac valve fibrosis for ergolines Pergolide Cabergoline Pramipexole Ropinirole Apomorphine Subcutaneous, intravenous infusion. Alone or with L-dopa.
Table 1 Classification of some pharmacological dopaminergic treatments (Schapira
et al. 2006, Fox 2013, Connolly and Lang 2014, Muller 2015, Dietrichs and Odin 2017, Kim et al. 2017, Finberg 2019).
Non-pharmacological treatments
Deep brain stimulation (DBS) is a therapy that is used in some PD patients at late stages, who do not benefit anymore from the pharmacological treatments (Smith et al. 2016). It is a therapy consisting in implanting an electrode in the globus pallidus
interna (GPi) or the subthalamic nucleus (STN) and sending high-frequency
electrical pulses (Benabid et al. 2009, Beudel and Brown 2016). It was shown to be very efficient in patients who suffer from dyskinesias (Sankar and Lozano 2011).
Nevertheless, this is not a perfect treatment and important risks are associated with the surgery. For example, it can cause hemorrhages, infections and seizures (Benabid et al. 2009, Aum and Tierney 2018). The effect of the current can also spread to nearby structures and cause diplopia, dyskinesias, dysarthria or paraesthesias, though these complications can diminish by lowering the intensity of the current or moving the electrode (Bjarkam and Sorensen 2004).
DBS is thus not recommended for everyone, nor does everyone want to go through such an invasive surgery. A possibility of a noninvasive treatment is transcranial magnetic stimulation. It is an intervention that sends repetitive magnetic pulses to a specific brain region through the skull (Chou et al. 2015, Wagle Shukla et al. 2016). The stimulation can be at low or high frequency, the former enhancing cortical excitability and the latter inhibiting it (Chung and Mak 2016). Clinical trials have shown improvement of motor symptoms with this technique, but more research is needed to better it (Chou et al. 2015, Chung and Mak 2016, Wagle Shukla et al. 2016).
Transplantation of embryonic dopaminergic cells is another method that can be used. Indeed, motor improvement was assessed due to long-term survival of grafted cells in the brain and restoration of DA levels in the striatum (Lindvall 2015). However, significant clinical improvement does not happen in all patients (Barker et al. 2013). An important aspect to note is that some studies showed the development of transplant-induced dyskinesias, mostly in patients who had prior levodopa-induced dyskinesias (Freed et al. 2001, Olanow et al. 2003). Apart from this, no important adverse effects of the transplants were seen (Lige and Zengmin 2016). Additionally, ethical considerations limit the use of this technique (Bjarkam and Sorensen 2004).
Finally, another treatment currently in clinical phase for PD patients is neurotrophic factor delivery in the brain. Neurotrophic factors are proteins that promote neuron survival and their good development, suggesting that their role
might be of importance in PD (Kelly et al. 2015). In fact, some neurotrophic factors have a reduced expression in patients (Staudt et al. 2016). The most promising one is glial-derived neurotrophic factor (GDNF). Studies of in vitro and in vivo models of PD have shown a protective effect of GDNF on dopaminergic neurons. (Deierborg et al. 2008). Clinical studies showed sometimes contrasting results, with some having no improvement of the patients’ condition and adverse effects, while others observed improvements (Gill et al. 2003, Nutt et al. 2003, Lang et al. 2006, Patel et al. 2013). More studies are needed to determine the efficacy of this therapy in PD patients.
Non-motor symptoms
Along with motor symptoms, PD patients also suffer from a variety of non-motor symptoms affecting many systems of the body (Rana et al. 2015). In fact, they are the main cause of the decreased quality of life of patients (Schapira et al. 2017). They tend to become more severe with the progression of the disease and with age (Chaudhuri et al. 2006, Kalia and Lang 2015). Some of them are caused by the disease process and might start up to a decade before the beginning of motor symptoms, while others may be due to treatments (Schrag et al. 2015, Cooney and Stacy 2016). Non-motor symptoms can be classified into four major classes: sensory, neuropsychiatric, sleep and autonomic disorders (Poewe 2008). Among them are anxiety, dementia, psychosis, insomnia, day-time sleepiness, cognitive deficits, bladder disturbances, sweating, cardiovascular complications, drooling, dysphagia, nausea, pain and hallucinations (Doty et al. 1988, Chaudhuri et al. 2006, Martinez-Martin 2011, Sung and Nicholas 2013, Cooney and Stacy 2016, Pfeiffer 2016, Schapira et al. 2017). Hyposmia, rapid eye movement sleep behavior disorder (RBD), constipation and depression often appear in the prodromal phase of the disease (Schapira et al. 2017). Gastrointestinal (GI) problems are especially common among PD patients and involve the whole length of the GI tract (Poirier et al. 2016a). In fact, a-syn depositions have been found throughout the enteric nervous system (ENS) (Fasano et al. 2015, Su et al. 2017). An issue with non-motor
symptoms is their lack of response to dopaminergic treatments, due to the fact that most of them are caused by alterations to other neurotransmitters than DA (Schapira et al. 2017). Therefore, patients need to take more medications, when it is available, but many do not receive them (Pfeiffer 2016). Better management is thus needed for the non-motor symptoms.
Enteric nervous system
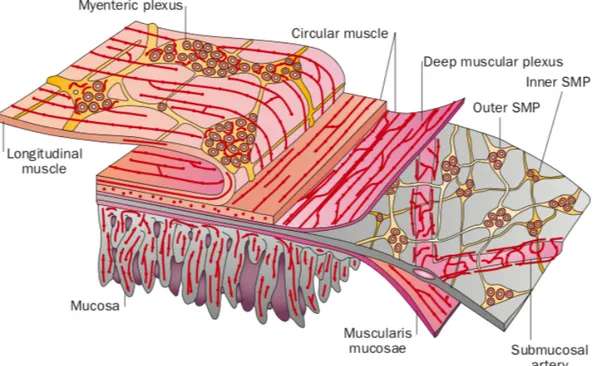
As stated earlier, patients suffer from a variety of GI problems, indicating the major involvement of the ENS in PD. The ENS is the branch of the autonomic nervous system (ANS) that innervates the whole length of the GI tract (Furness et al. 2014, Rao and Gershon 2016). Except from the proximal and distal ends, the ENS can function independently from central nervous system (CNS) input although it usually does not (Furness 2012, Rao and Gershon 2016). In fact, it contains many of the neurotransmitters that can be found in the brain (Li et al. 2004). Its functions are many, for example it controls secretions, it regulates movements of the GI tract, it also regulates the movement of fluids between the lumen and body fluid compartments, it controls the release of gut hormones and more (Furness et al. 2014). In the small intestine, it is separated into two plexi: the submucous plexus and the myenteric plexus, as can be seen in figure 6. The former is also called Meissner’s plexus and is found between the submucosa and the circular muscle layer, while the latter is also called Auerbach’s plexus and is found between both circular and longitudinal muscular layers (Furness and Costa 1980). It is the myenteric plexus that controls the motility of the gut. In fact, that is where most dopaminergic neurons, which are essential to gut motility, can be found (Rao and Gershon 2016). Dopaminergic neurons in the gut exert an inhibitory action on its motility (Hirst and Silinsky 1975). The submucous plexus on the other hand is the one controlling secretion (Porter et al. 1999).
Figure 6 The different layers of the intestines. The myenteric plexus is the one in
charge of motility (Furness 2012).
Connections exist between the GI tract and the brain, which are termed the gut-brain axis (Perez-Pardo et al. 2017). Indeed, the vagus nerve, for example, is a major mediator of this axis as it transfers a lot of sensory information from the gut to the brain, and sends efferent control pathways to affect the activity of enteric neurons (Furness et al. 2014). The gut-brain axis is also regulated by other pathways, for instance, it was shown recently to be influenced by the gut microbiota (Rhee et al. 2009, Grenham et al. 2011, Mayer et al. 2015). Moreover, this communication between both organs can be mediated by the immune and the endocrine systems (Grenham et al. 2011, Mulak and Bonaz 2015).
Braak hypothesis
The team of Dr. Heiko Braak proposed a hypothesis for the pathogenesis of PD after some discoveries from their studies on post-mortem brains (Braak et al. 2003). Indeed, they developed the six stages of Lewy pathology after analyzing
a-syn immunoreactivity in brains of PD patients, non-symptomatic people who displayed LBs and people with no symptoms nor LBs. Their six stages show a progression of the aggregates in the brain in an irreversible way in presymptomatic and symptomatic cases (Braak et al. 2003). Following this, they discovered the presence of LBs in the enteric nerve cells of both presymptomatic and symptomatic PD cases (Braak et al. 2006). They then suggested that the pathological process might start together at two different sites: the olfactory bulb and the anterior olfactory nucleus, as well as the enteric nerve cells (Hawkes et al. 2007). The pathology would then progress to the brain. PD motor symptoms are thought to begin when the pathology spreads to the SN. In more severe cases of the disease, LBs can be found in subcortical areas as well (Braak et al. 2003, Hawkes et al. 2007).
One of the ways suggested for the progression of the pathology is through the vagus nerve, which innervates the GI tract (Hawkes et al. 2007). Supporting the Braak hypothesis, interesting studies have shown that people who underwent a truncal vagotomy had less chance of developing PD, compared to superselective vagotomy and to the general population (Svensson et al. 2015, Liu et al. 2017). Furthermore, a recent study showed that appendectomy also reduced the risk of developing PD later in life, and that it delayed the onset of symptoms (Killinger et al. 2018). The team also observed high levels of a-syn aggregates, as well as truncated forms of a-syn, similar to those seen in LBs in the vermiform appendix (Killinger et al. 2018).
Inflammation
Clinical evidences show that there is increased inflammation in PD patients. Indeed, levels of activated microglia and pro-inflammatory cytokines in the brain and cerebro-spinal fluid (CSF) were detected to be statistically higher than controls (McGeer et al. 1988, Mogi et al. 1994, Mogi et al. 1996, Gerhard et al. 2006). Moreover, signs of inflammation have been found in the periphery, such as in the GI tract (Devos et al. 2013). An interesting fact is the involvement of LRRK2 in PD
pathogenesis, as it is a gene also mutated in Crohn’s disease, another inflammatory disorder, suggesting the important role of inflammation in PD (Hui et al. 2018). Not only that, but the use of anti-tumor necrosis factor (TNF) therapy in IBD patients was also shown to decrease PD risk (Peter et al. 2018). In fact, an increasing number of studies propose that neuroinflammation could play a role in the pathogenesis of PD (More et al. 2013, Stojkovska et al. 2015, Wang et al. 2015). However, it is still not clear what exactly is this role. The presence of activated microglia in the brain could be a protective mechanism, but it could also be detrimental and enhance the pathology. Indeed, a minor inflammatory stress could push the microglia to release anti-inflammatory cytokines and growth factors. However, under greater stress, they can secrete pro-inflammatory cytokines to recruit other immune cells, as well as toxic factors to kill pathogens that could also be detrimental to neurons (Antony et al. 2013).
Studies made in animal models of the disease have shown an important contribution of inflammation (Cebrian et al. 2015). For instance, the mouse model injected with MPTP was shown to cause inflammation, not only in the brain, but in the gut as well. At the enteric level, not only did it increase inflammation, but inflammation was found necessary for the damaging effects of MPTP to take place (Cote et al. 2011, Cote et al. 2015b). Indeed, by partially depleting the population of pro-inflammatory macrophages, the effects of MPTP on the dopaminergic neurons of the ENS were not seen anymore, indicating that the loss usually observed is mediated by the macrophages (Cote et al. 2015b). Furthermore, knockout (KO) of the MyD88 pathway, involved in inflammation, is also protective against the loss of TH+ neurons, the infiltration of monocytes from the blood and the switch to a
pro-inflammatory phenotype (Cote et al. 2011). Hence, inflammation plays a major role in PD and more research is needed to fully understand its role.
Animal models
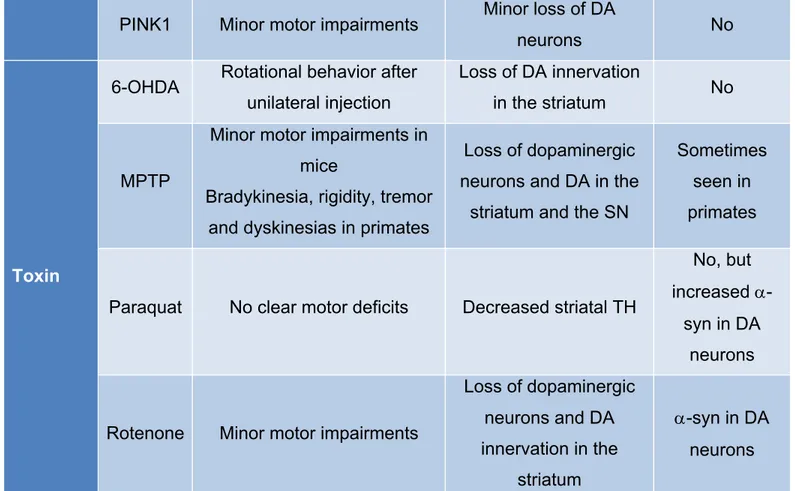
In order to study the disease in laboratories, animal models have been developed. Among them are transgenic models and neurotoxin-based models (Imbriani et al. 2018). No model is able to reproduce all characteristics of PD (Duty and Jenner 2011). It is thus important to choose the right model to study the aspect of interest. Transgenic models mostly use overexpression or knockout of some genes known to be involved in familial cases, such as a-syn, LRRK2, DJ-1, Parkin and PINK1 (Dawson et al. 2010). The major issue with these models is that most of them do not develop a PD phenotype. As described in table 2, most genetic models lack motor symptoms and LBs (Blesa et al. 2012). There is also little nigrostriatal degeneration, but they allow for a study of the pathogenesis and the function of the targeted proteins in situation of disease (Blandini and Armentero 2012). Toxin models on the other hand, are less relevant for the study of the pathogenesis, but can induce motor symptoms, making them good choices to try therapies (Le et al. 2014). They also show mild to severe DA depletion. However, some of these models do not show protein aggregation, a major hallmark of the disease. These models include 6-hydroxydopamine (6-OHDA), MPTP, paraquat and rotenone (Blesa and Przedborski 2014).
Category Model Symptoms Dopaminergic loss LBs
Genetic
a-syn Severe motor deficits in the A53T model Usually no DA neuron loss a-syn aggregation in DA neurons
LRRK2 Few behavioral deficits in Drosophila
Minor loss of DA
neurons No
DJ-1 Minor motor impairments Minor loss of DA
neurons No
Parkin No motor deficits Minor loss of DA
PINK1 Minor motor impairments Minor loss of DA
neurons No
Toxin
6-OHDA Rotational behavior after unilateral injection
Loss of DA innervation
in the striatum No
MPTP
Minor motor impairments in mice
Bradykinesia, rigidity, tremor and dyskinesias in primates
Loss of dopaminergic neurons and DA in the
striatum and the SN
Sometimes seen in primates
Paraquat No clear motor deficits Decreased striatal TH
No, but increased
a-syn in DA neurons
Rotenone Minor motor impairments
Loss of dopaminergic neurons and DA innervation in the striatum a-syn in DA neurons
Table 2 PD animal models and their effects (Blesa et al. 2012, Le et al. 2014).
The neurotoxin precursor MPTP
The neurotoxin MPTP was first discovered in humans, following impure heroin injections containing MPTP (Langston et al. 1983). The small group of people who used the drug developed PD-like symptoms within a few days, and responded to L-dopa treatment, suggesting that the MPTP had affected the nigrostriatal dopaminergic system (Langston et al. 1983). This led to further study of this toxin and its use in animals such as mice and primates to study PD (Burns et al. 1983, Markey et al. 1984, Le et al. 2014). The toxin MPTP being a lipophilic molecule, it can easily pass through the BBB (figure 7). Once in the brain, it is metabolized by the MAO-B enzyme mostly in astrocytes into 1-methyl-4-phenyl-2,3-dihydropyridinium (MPDP+) and then spontaneously oxidizes into
1-methyl-4-phenylpyridinium (MPP+) (Heikkila et al. 1984, Ransom et al. 1987, Gubellini and
Kachidian 2015). It is MPP+ that is actually toxic and can cause neuronal damage
(Langston et al. 1984). After its metabolism, it can enter dopaminergic neurons through the DA transporter (DAT) (Javitch et al. 1985, Shen et al. 1985). Once inside, it can either be transported into vesicles through the vesicular monoamine transporter 2 (VMAT2), or go to the mitochondria, where it can enter through an active process driven by the membrane gradient (Singer and Ramsay 1990, Smeyne and Jackson-Lewis 2005). There, it blocks the complex I of the electron transport chain, impairing respiration. This causes changes in energy metabolism and calcium homeostasis, as well as production of reactive oxygen species (ROS). Therefore, oxidative stress is increased, causing damage to neurons and eventually leading to apoptosis (Vila and Przedborski 2003).
Although there is a lack of LBs, the MPTP model of PD has been extensively used and has been constantly shown to be highly reproducible (Jackson-Lewis et al. 2012). The loss of dopaminergic neurons, however, does not happen in a progressive manner. MPTP induces an increase in inflammation in the brain, as well as in the gut (Nagatsu et al. 2000, Hirsch and Hunot 2009, Cote et al. 2015a, Poirier et al. 2016b). The loss of dopaminergic neurons happens also at the enteric level and was shown to depend on the inflammatory process (Anderson et al. 2007, Cote et al. 2011, Cote et al. 2015b). Toxic effects of MPTP in the ENS of mice are largely mediated by pro-inflammatory macrophages as mentioned previously (Cote et al. 2011, Cote et al. 2015b).
Sex differences
It was noted in many researches that PD affects more men than women (Shulman 2007, Miller and Cronin-Golomb 2010, Bourque et al. 2011, Gillies et al. 2014). Indeed, men are about 1.5 times more at risk of developing the disease than women, and women in average develop motor symptoms two years later than men (Bourque et al. 2019). This suggests that female hormones could have a protective effect in the development of this pathology. Many studies were done in order to find a link with the use of hormone therapy (mainly estrogens) and the risk of PD (Tsang et al. 2000, Benedetti et al. 2001, Currie et al. 2004, Ragonese et al. 2004, Popat et al. 2005, Noyce et al. 2012, Liu et al. 2014). Results were variable, which is not surprising seeing as the type of hormones taken were not necessarily the same, or the study designs were different (Lundin et al. 2014). Additionally, hormone therapy might represent a risk for the development of reproductive cancers in women (Bourque et al. 2019). Combined hormone therapy (estrogen with a progestin) was reported to increase breast cancer risk, whereas estrogen alone did not show this risk (Chlebowski and Anderson 2014). The progestin used is also very important, as it was found that medroxyprogesterone acetate, norethisterone and levonorgestrel increased the risk, but progesterone and dydrogesterone did not (Yang et al. 2017).
Neuroprotection studies were done with both estrogens and progesterone, which have shown that both hormones are neuroprotective compounds in many disorders (Grandbois et al. 2000, Kumon et al. 2000, Morali et al. 2005, Pettus et al. 2005, Bourque et al. 2009, Baraka et al. 2011). Several studies have been conducted to confirm the protective role of estrogens and other estrogenic compounds, but few have investigated progesterone’s effect (Bourque et al. 2009, Baraka et al. 2011, Cote et al. 2015a, Poirier et al. 2016b). The results are still controversial, with some studies finding positive outcomes for progesterone while others do not (Yu and Liao 2000, Chao et al. 2011, Bourque et al. 2016, Litim et al. 2017). Undoubtedly, more work is needed in this field to elucidate its role.
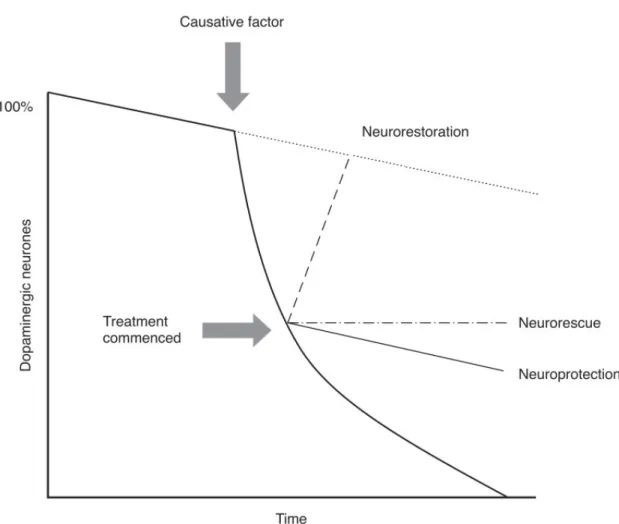
Figure 8 Representation of neuroprotective processes. Neuroprotection is defined
to the saving of dying neurons, preventing further loss. Neurorestoration aims at increasing the number of neurons. (The National Collaborating Centre for Chronic Conditions 2006)
Studies conducted with estrogens in mice models of PD showed its neuroprotective and immunomodulatory effects. Indeed, not only was it beneficial for dopaminergic neurons of the brain, it was also beneficial at the ENS level (Bourque et al. 2009, Bourque et al. 2012, Poirier et al. 2016b). An interesting finding is that the protective effect of such compounds passes mainly through the G-protein coupled estrogen receptor (GPER1) and the nuclear estrogen receptor alpha (ERa). Indeed, studies were made with activators of the different estrogen receptors known, and the specific activation of GPER1 by G1 resulted in similar effects as with estrogen (D'Astous et al. 2004, Bourque et al. 2013, Bourque et al. 2015). With these interesting results, it would be tempting to try estrogenic therapy with PD patients. However, estrogen is a feminizing hormone and therefore cannot be given to men for long term treatment (Bourque et al. 2019). The selective estrogen receptor modulator (SERM) raloxifene was also studied and showed protective effects in the CNS and in the ENS (Bourque et al. 2014, Poirier et al. 2016b). Similar to estrogen, raloxifene had neuroprotective and anti-inflammatory actions that were mediated through GPER1 (Bourque et al. 2014, Poirier et al. 2016b).
Progesterone
An alternative to estrogen is the other female hormone progesterone. Indeed, it is a hormone that has no feminizing effects, and could thus potentially be given to men as well as women (Bourque et al. 2019). Progesterone was also found to have neuroprotective effects. It was studied, among others, in animal models of traumatic brain injury (TBI) (Roof et al. 1996, Thomas et al. 1999, Wright et al. 2001, Si et al. 2014, Lopez-Rodriguez et al. 2015, Brotfain et al. 2016, Allitt et al. 2017). Following the positive results, it was afterwards continued to clinical studies, which were
promising at first, but failed at later stages (Wright et al. 2007, Xiao et al. 2008, Skolnick et al. 2014, Wright et al. 2014, Ma et al. 2016). This may be due, among others, to the complexity of TBI and the inappropriate doses and duration of treatments. (Wei and Xiao 2013, Stein 2015, Howard et al. 2017).
Progesterone was also studied in animal models of PD in the brain. Although the research is not as extensive as with estrogen, most studies suggest a beneficial effect from progesterone treatment, although some did not see a protective effect (Grandbois et al. 2000, Callier et al. 2001, Morissette et al. 2008, Casas et al. 2011, Chao et al. 2011, Casas et al. 2013, Bourque et al. 2016, Litim et al. 2017). In one model of rats lesioned with 6-OHDA, progesterone administered 24 hours after the lesion for 13 days was found to exacerbate the motor symptoms and was not neuroprotective in the brain (Chao et al. 2011). However, in other studies with 6-OHDA-treated rats, progesterone given 7 days after the lesion was found to exert a neuroprotective and neuromodulatory function on dopaminergic, glutamatergic and gamma-Aminobutyric acid (GABA)ergic systems, in addition to improving cognitive and motor functions (Casas et al. 2011, Casas et al. 2013). In the MPTP mouse model, progesterone was protective as a pre- and post-lesion treatment, as well as when it was administered an hour post-MPTP lesion (Bourque et al. 2016). Another study was made in order to see if progesterone was protective even when given later after the lesion (24 hours and 5 days). Beneficial effects were seen 24 hours after but not 5 days after the lesion (Litim et al. 2017).
Along with its neuroprotective effect, progesterone was discovered to have immunomodulatory effects (He et al. 2004, Pettus et al. 2005, Wei and Xiao 2013). It is particularly important in progesterone’s reproductive role. Indeed, inflammation is involved in the different stages of pregnancy, such as ovulation, implantation of the embryo and labor and delivery (Lu et al. 2015, Akison et al. 2018, Polikarpova et al. 2019). Dysregulation of inflammation in these processes can cause infertility and miscarriages (Lee et al. 2015). Progesterone can modulate the production of different cytokines, such as interleukin 1 beta (IL-1b) and TNFalpha (TNFa), and
inhibit immune cell activation and migration (Wei and Xiao 2013, Aryanpour et al. 2017, Preciado-Martinez et al. 2018). Results in this field are also conflicting, with some studies finding anti-inflammatory effects, and other finding pro-inflammatory ones (Garg et al. 2017). This diversity in progesterone’s effect on immune cells may be due to the type of cells, the type of receptors targeted and the biological microenvironment in which the immune cells are. Some studies suggest that progesterone exerts its effect on immune cells mostly through the membrane progesterone receptors (mPRa and b), rather than through the classical nuclear receptor (nPR) (Schust et al. 1996, Dosiou et al. 2008, Polikarpova et al. 2019). Most immune cells express no or very low nPRs, but monocytes express both mPRs and nPRs, potentially explaining the mixed results from monocytes (Polikarpova et al. 2019).
Problematic
Considering that no curative agent is yet available for the treatment of PD, it becomes imperative to continue the research in this sphere in order to present patients with better options. The search for neuroprotective compounds has thus been going on for many years, with no success still (Francardo et al. 2017, Kim 2017). One of the main issues in this domain is the fact that the diagnosis comes at a stage when more than half of the dopaminergic neurons are lost (Schapira et al. 2017). This raises the problematic of biomarkers for an early diagnosis (Lotankar et al. 2017). As stated previously, some non-motor symptoms arise in patients many years before the apparition of motor symptoms, such as GI problems, suggesting that the neurodegenerative process starts long before clinical symptoms appear (Schapira et al. 2017). If patients could be diagnosed in the preclinical phase of the pathology, neuroprotective medications could be more efficient and provide a better outcome. Therefore, for this project, we studied the effect the progesterone on the gut of MPTP-treated mice in order to determine if it could be a potential novel approach for neuroprotective therapies in PD.
Hypotheses and objectives
As mentioned earlier, the use of estrogenic compounds was found to have positive effects on dopaminergic neurons of the brain and in the ENS, as well as on the inflammatory response that ensued with the lesion in the MPTP mouse model. As we have seen that progesterone also has a neuroprotective effect in the brain of the PD mouse model, and that it was shown to be immunomodulatory in other conditions, we hypothesized that:
1. Progesterone will have a neuroprotective effect on the dopaminergic neurons of the myenteric plexus of MPTP-treated mice
2. Progesterone will have an immunomodulatory effect in the myenteric plexus of MPTP-treated mice
The objectives of this master’s thesis are thus to study the neuroprotective and immunomodulatory effects of progesterone at the enteric level of the MPTP mouse model of PD. We used the MPTP model of PD as it is reliable and reproducible. In our case, we investigated an acute model in order to reproduce the preclinical phase of the disease.
Chapter 1: Neuroprotection and immunomodulation
of progesterone in the gut of a mouse model of
Parkinson’s disease
1.1 Résumé
Les symptômes gastro-intestinaux apparaissent chez les patients de la maladie de Parkinson plusieurs années avant les symptômes moteurs suggérant l’implication des neurones dopaminergiques du plexus myentérique de l’intestin. L’inflammation est également augmentée dans la pathologie. Précédemment, nous avons rapporté une neuroprotection de la progestérone dans le cerveau des souris lésées avec le 1-méthyl-4-phényl-1,2,3,6-tétrahydropyridine (MPTP) et avons émis l’hypothèse qu’elle aurait également des actions neuroprotectrices et immunomodulatrices dans l’intestin. Pour tester cette hypothèse, nous avons administré la progestérone à des souris C57BL/6 mâles adultes pour 10 jours et traités au MPTP au cinquième jour. Dans une autre expérience, la progestérone a été administrée pendant 5 jours suivant le traitement au MPTP. Au dixième jour de traitement, les iléons ont été collectés et les plexus myentériques ont été isolés. Les neurones dopaminergiques étaient réduits d’environ 60% et les macrophages pro-inflammatoires étaient augmentés d’environ 50% chez les souris lésées au MPTP comparées aux contrôles. Ces changements ont été complètement prévenus par la progestérone administrée en pré- et post-MPTP traitement et ont été normalisés avec la progestérone administrée à une dose de 8 mg/kg post-MPTP. Dans le cerveau des souris MPTP, le facteur neurotrophique issu du cerveau (BDNF) et la protéine acide fibrillaire gliale (GFAP) étaient corrélés à la neuroprotection de la progestérone. Dans le plexus myentérique, les niveaux de BDNF étaient plus élevés chez les souris MPTP avec un post-traitement de progestérone de 8 mg/kg comparées aux contrôles, alors que les niveaux de GFAP sont restés constants. En conclusion, les résultats obtenus montrent des effets neuroprotecteurs et anti-inflammatoires de la progestérone dans le plexus myentérique des souris MPTP similaires aux
précédents résultats au cerveau. La progestérone est non-féminisante et pourrait être utilisée chez les hommes et chez les femmes à des stades pré-symptomatiques de la maladie.
Original article
Neuroprotection and immunomodulation of progesterone in the gut of a mouse model of Parkinson’s disease
Hend Jarras1,2, Mélanie Bourque1, Andrée-Anne Poirier1,2, Marc Morissette1, Katherine Coulombe1, Thérèse Di Paolo1,2* and Denis Soulet1,2*
1 Axe Neurosciences, Centre de Recherche du CHU de Québec (Pavillon CHUL), Quebec, Canada
2 Faculty of Pharmacy, Laval University, Quebec City, Quebec, Canada
*Co-senior authors
Correspondence to:
Denis Soulet, Ph.D.
Axe Neurosciences, T2-32 Centre de recherche du CHU de Québec-Université Laval 2705, Boulevard Laurier Québec, QC, G1V 4G2, Canada Tel: (418) 656-4141 ext. 46449 Fax: (418) 654-2298 E-mail: denis.soulet@crchul.ulaval.ca or Therese Di Paolo, PhD Axe Neurosciences, T2-40 Centre de recherche du CHU de Québec-Université. Laval 2705, Boulevard Laurier Québec, QC, G1V 4G2, Canada Tel: (418) 525-4444 ext. 46240 Fax: (418) 654-2298 E-mail: therese.dipaolo@crchul.ulaval.ca
1.2 Abstract
Gastrointestinal symptoms appear in Parkinson’s disease (PD) patients many years before motor symptoms suggesting the implication of dopaminergic neurons of the gut myenteric plexus (MP). Inflammation is also known to be increased in PD. We previously reported neuroprotection with progesterone in the brain of mice lesioned with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and hypothesized that it also has neuroprotective and immunomodulatory activities in the gut. To test this hypothesis, we investigated progesterone administered to adult male C57BL/6 mice for 10 days and treated with MPTP on day five. In an additional experiment progesterone was administered for five days following MPTP treatment. Ilea were collected on day ten of treatment and micro-dissected to isolate the MP. Dopaminergic neurons were reduced by approximately 60% and pro-inflammatory macrophages were increased by approximately 50% in MPTP mice compared with intact controls. These changes were completely prevented by progesterone administered before and after MPTP treatment and were normalized by 8 mg/kg progesterone administered after MPTP. In the brain of MPTP mice brain-derived neurotrophic peptide (BDNF) and glial fibrillary acidic protein (GFAP) were associated with progesterone neuroprotection. In the MP, increased BDNF levels compared to controls were measured in MPTP mice treated with 8 mg/kg
progesterone started post MPTP while GFAP levels remained unchanged. In
conclusion, the present results showed neuroprotective and anti-inflammatory effects of progesterone in the MP of MPTP mice similar to our previous findings in the brain. Progesterone is non-feminizing and could be used for both men and women in pre-symptomatic stages of the disease.
1.3 Introduction
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder (Olanow et al. 2009). While some patients develop the disease at a young
age, the majority have an older age of onset, hence the prevalence is expected to increase due to the expanding aging population. (Siderowf and Stern 2003). PD predominantly involves the death of dopamine (DA) neurons in the brain substantia
nigra, although other neurotransmitters and neuromodulators are also affected (Del
Rey et al. 2018). Moreover, gene mutations in familial PD are reported, yet the cause of the majority of cases is currently unknown (Olanow et al. 2009). There is still no cure for PD, but symptomatic treatments are available (Zeuner et al. 2019). Indeed, neuroprotection or disease modification, defined as an intervention that would protect or rescue vulnerable neurons, thereby slowing, stopping, or reversing disease progression, is not yet available for PD, but is the focus of intensive research (Olanow et al. 2009). Most PD patients experience autonomic dysfunctions (Poewe 2006, Reichmann 2010, Zeuner et al. 2019) involving the gastrointestinal system many years before the appearance of motor symptoms (Abbott et al. 2001, Savica et al. 2009), complicating management of the disease and lowering their quality of life (Natale et al. 2008). Accumulating evidence supports the bidirectional connection between brain and gut in both physiological and pathological conditions (Rhee et al. 2009, Mayer et al. 2014, Carabotti et al. 2015, Breen et al. 2019, Kowalski and Mulak 2019).
More specifically, there is now substantial support that PD might originate in the enteric nervous system (ENS) before spreading to the brainstem (Braak et al. 2006). In fact, alpha-synuclein (ASYN) aggregates known as Lewy bodies (LBs) are observed in tyrosine hydroxylase (TH) neurons in the ENS of PD patients (Phillips et al. 2008), and these may display prion-like behavioural characteristics (Hansen et al. 2011, Holmqvist et al. 2014).
The involvement of inflammation in the neurodegeneration of DA neurons in PD has also been well documented (More et al. 2013). An increase in pro-inflammatory cytokines has been measured in the brains of PD patients that is associated with damage to nigrostriatal DA neurons (Nagatsu et al. 2000, Nagatsu and Sawada 2005, Sawada et al. 2006). This has also been observed in the colon
of PD patients and has been correlated with progression of the disease (Devos et al. 2013).
Epidemiological and clinical studies have shown that men are more affected by PD than women (1.5:1 ratio) (Shulman 2007, Miller and Cronin-Golomb 2010, Bourque et al. 2011, Savica et al. 2013, Gillies et al. 2014) and various PD animal models have shown a neuroprotective role of estrogens and progesterone on brain DA neurons (reviewed in: (Cyr et al. 2000, Cyr et al. 2002, Morissette et al. 2008, Bourque et al. 2009, Al Sweidi et al. 2012, Bourque et al. 2012, Litim et al. 2016, Bourque et al. 2019)).
Interestingly, the estrogen compounds (17ß-estradiol (Cote et al. 2015a), raloxifene (Poirier et al. 2016b) and G1 (Cote et al. 2015a)) that we showed to be neuroprotective for brain DA neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD were also found to protect the myenteric DA neurons. Moreover, we have previously shown that progesterone administered both pre and post MPTP treatment protects striatal DA content and transporters against toxicity and that changes in brain-derived neurotrophic peptide (BDNF) and glial fibrillary acidic protein (GFAP) were associated with progesterone neuroprotection (Grandbois et al. 2000, Callier et al. 2001, Bourque et al. 2016, Litim et al. 2017). Hence, brain dopaminergic neuroprotection with the other ovarian steroid, namely progesterone, could also extend to the periphery. In addtition, studies supporting the close link between brain and gut, such as in traumatic brain injury (Chen et al. 2008) and subarachnoid hemorrhage (Zhao and Zhou 2011),were reported to affect the intestines of male rats. Furthermore, in both cases, beneficial effects of progesterone treatment were related to its anti-inflammatory activity in the intestines. Hence, based on the above evidence, the present study tested the hypothesis that progesterone has neuroprotective and immunomodulatory activities in the gut of MPTP parkinsonian mice.