Publisher’s version / Version de l'éditeur:
ASME 2006 International Mechanical Engineering Congress and Exposition, pp.
31-37, 2006
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1115/IMECE2006-14458
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
A numerical investigation of NOx formation in counterflow CH4/H2/air
diffusion flames
Guo, Hongsheng; Neill, W.; Smallwood, Gregory J.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=9c82ba3a-b09c-4d8e-918b-917133aab371 https://publications-cnrc.canada.ca/fra/voir/objet/?id=9c82ba3a-b09c-4d8e-918b-917133aab371Proceedings of IMECE2006 2006 ASME International Mechanical Engineering Congress & Exposition
November 5-10, 2006, Chicago, Illinois, USA
IMECE2006-14458
A NUMERICAL INVESTIGATION OF NO
XFORMATION IN COUNTERFLOW CH
4/H
2/AIR
DIFFUSION FLAMES
Hongsheng Guo
Institute for Chemical Process and Environmental Technology, National Research Council of Canada 1200 Montreal Road, Ottawa, Ontario, Canada K1A 0R6
Fax: (613)957-7869, phone: (613)991-0869 Email: hongsheng.guo@nrc-cnrc.gc.ca
Stuart W. Neill
Institute for Chemical Process and Environmental Technology, National Research Council of Canada 1200 Montreal Road, Ottawa, Ontario, Canada K1A 0R6
Fax: (613)957-7869, phone: (613)990-2408 Email: stuart.neill@nrc-cnrc.gc.ca
Gregory J. Smallwood
Institute for Chemical Process and Environmental Technology, National Research Council of Canada 1200 Montreal Road, Ottawa, Ontario, Canada K1A 0R6
Fax: (613)957-7869, phone: (613)993-1391 Email: greg.smallwood@nrc-cnrc.gc.ca
ABSTRACT
A detailed numerical study was carried out for the effect of hydrogen enrichment on flame structure and NOX formation in
counterflow CH4/air diffusion flames. Detailed chemistry and
complex thermal and transport properties were employed. The enrichment fraction was changed from 0 (pure CH4) to 1.0
(pure H2). The result indicates that for flames with low to
moderate stretch rates, with the increase of the enrichment fraction from 0 to 0.5~0.6, NO emission index keeps almost constant or only slightly increases. When the enrichment fraction is increased from 0.5~0.6 to about 0.9, NO emission index quickly increases, and finally NO formation decreases again when pure hydrogen flame condition is approached. However, for flames with higher stretch rates, with the increase of hydrogen enrichment fraction from 0 to 1.0, the formation of NO first quickly increases, then slightly decreases and finally increases again. Detailed analysis suggests that the variation of the characteristics in NO formation in stretched CH4/air
diffusion flames is caused by the change of flame structure and NO formation mechanism, when the enrichment fraction and stretch rate are changed.
Keywords: diffusion flame, NOX, fuel enrichment. INTRODUCTION
Fuel enrichment combustion is a promising concept for substantial reduction in fuel consumption and pollutant emission. Many studies have been conducted for some fundamental concepts of fuel enrichment combustion. For example, it has been shown that fuel enrichment can improve
flame stability and thus significantly reduce NOX formation by
allowing a combustor to operate at leaner condition [1-4] in premixed flames. For diffusion combustion, fuel enrichment can suppress the formation of soot particles [5,6] and shorten ignition delay [7,8].
Relatively, not enough attention has been paid to the effect of fuel enrichment on NOX formation in diffusion flames. In
general, NO, the dominant component of NOX, is mainly
formed by the prompt route in a hydrocarbon diffusion flame. When an enrichment component, such as hydrogen or carbon monoxide, is added to a hydrocarbon diffusion flame, it is expected that the formation of NO by the prompt route can be reduced because of the reduction in radical CH. On the other hand, the addition of an enrichment component may modify flame temperature, which in turn may change the formation of NO by the thermal route. Therefore, the net effect of fuel enrichment on NOX formation in a hydrocarbon diffusion flame
depends on the relative variations of the thermal and prompt routes. Naha and Aggarwal [9] investigated the effect of hydrogen addition on NOX formation in stretched nonpremixed
methane and n-heptane flames at a fixed stretch rate (100 s-1). They found that the addition of hydrogen has minor effect on NOX formation in methane flames and reduces the formation of
NOX in n-heptane flames.
In real applications, stretch rate significantly changes. The variation in stretch rate modifies the residence time of reactants in the reaction zone of a flame. The effect of fuel enrichment on NOX formation for flames at different stretch rates may differ.
enrichment on NOX formation in diffusion flames at various
stretch rates.
In this paper, a detailed numerical study on the effect of hydrogen addition on NOX formation in CH4/air diffusion
flames with various stretch rates was conducted. Hydrogen was selected because it has been shown to be an effective enrichment component that can suppress soot formation in diffusion flames [5,6] and shorten ignition delay [7,8]. The fraction of hydrogen changed from 0 to 1.0. The investigated stretch rate covers a wide range.
NUMERICAL MODEL
The flame configuration studied is an axisymmetric laminar counterflow diffusion flame, with fuel stream issuing from one nozzle and air from another, as shown in Fig. 1. The simulations assumed the stagnation point flow approximation. The governing equations can be found elsewhere [10]. The calculations were carried out with a code revised from that of Kee et al. [11]. Upwind and center difference schemes were, respectively, used for the convective and diffusion terms in all the governing equations. Adaptive refinement of meshes was done to obtain grid independent results. Radiation heat loss was accounted for by an optically thin model [12].
Stagnation plane Flame
CH4+H2
Air
Fig. 1 Flame configuration.
Two different free stream conditions – potential and plug flow – were alternately used in the literature for counterflow flame simulation. Based on the method by which the jets were produced, one of them may be closer to the experimental measurements than the other. However, both conditions produce similar qualitative results. As a pure numerical study, the potential boundary conditions were used in this paper.
The chemical reaction mechanism used is GRI-Mech 3.0 [13], which is an optimized mechanism for methane combustion. It has been validated over a wide range of flame conditions. The thermal and transport properties were obtained by using the database of GRI-Mech 3.0 and the algorithms given in [14, 15]. The pressure and the fresh mixture temperature were, respectively, 1 atm and 298 K.
RESULTS AND DISCUSSION
In all the studied flames, fuel stream consists of methane and hydrogen. The fraction of hydrogen is defined asαH2=VH2/(VH2+VCH4), with VH2 and VCH4 being, respectively, the volume flow rates of hydrogen and methane. The quantity a in all the figures represents stretch rate. Although we are interested in fuel enrichment combustion, which only requires a small amount of hydrogen addition, the studied fraction of hydrogen covers a range from 0.0 to 1.0 at several typical stretch rates for completeness.
a = 10 s-1
Distance from stagnation plane, cm
-3.0 -2.0 -1.0 0.0 1.0 T e mp e ra tu re , K 300 600 900 1200 1500 1800 2100 2400 2700 αH2 = 0.0 αH2 = 0.5 αH2 = 0.6 αH2 = 0.98 αH2 = 1.0 a = 100 s-1
Distance from stagnation plane, cm
-1.0 -0.5 0.0 0.5 1.0 T e mp e ra tu re , K 300 600 900 1200 1500 1800 2100 2400 2700 αH2 = 0.0 αH2 = 0.5 αH2 = 0.6 αH2 = 0.98 αH2 = 1.0 a = 300 s-1
Distance from stagnation plane, cm
-1.0 -0.5 0.0 0.5 1.0 T e m p e ra tu re , K 300 600 900 1200 1500 1800 2100 2400 2700 αH2 = 0.0 αH2 = 0.5 αH2 = 0.6 αH2 = 0.98 αH2 = 1.0
Fig. 2 Flame temperature distribution.
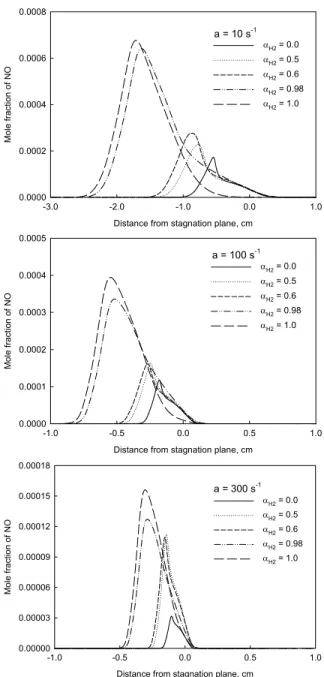
Figures 2 and 3 display the distributions of flame temperature and NO mole fraction in flames with stretch rates of 10 s-1, 100 s-1 and 300 s-1 and containing 0, 50, 60, 98 and 100% hydrogen. Fuel stream comes from the right side, and air from the left side. The three stretch rates were selected because they represent three typical values, a lower, a moderate and a higher one. The higher one (300 s-1) is close to the stretch extinction limit for CH4/air diffusion flame at atmosphere
pressure and room temperature condition. From Figs. 2 and 3, we first observe that with increasing the fraction of hydrogen addition, the primary reaction zone moves further away from stagnation plane to the air (left) side. This is because hydrogen combustion needs less oxygen than methane. Secondly, the increase in the fraction of hydrogen causes monotonic increase in flame temperature and thickness. It is caused by the higher adiabatic temperature and diffusion coefficient of hydrogen.
However, the variation of peak NO mole fraction shows some complex phenomena.
a = 10 s-1
Distance from stagnation plane, cm
-3.0 -2.0 -1.0 0.0 1.0 M o le f ra c ti o n o f N O 0.0000 0.0002 0.0004 0.0006 0.0008 αH2 = 0.0 αH2 = 0.5 αH2 = 0.6 αH2 = 0.98 αH2 = 1.0 a = 100 s-1
Distance from stagnation plane, cm
-1.0 -0.5 0.0 0.5 1.0 M o le f ra c ti o n o f N O 0.0000 0.0001 0.0002 0.0003 0.0004 0.0005 αH2 = 0.0 αH2 = 0.5 αH2 = 0.6 αH2 = 0.98 αH2 = 1.0 a = 300 s-1
Distance from stagnation plane, cm
-1.0 -0.5 0.0 0.5 1.0 M o le f ra ct io n o f N O 0.00000 0.00003 0.00006 0.00009 0.00012 0.00015 0.00018 αH2 = 0.0 αH2 = 0.5 αH2 = 0.6 αH2 = 0.98 αH2 = 1.0
Fig. 3 NO mole fraction distribution.
More detailed information can be found from Fig. 4, where the variation of NO emission index (NOEI), a more reasonable
quantity describing the characteristics of NOX formation, is
shown. Since the fuel stream is a mixture of methane and hydrogen, NO emission index is defined based on total heat release rather than fuel consumption, i.e. NO emission index equals the ratio of total formed NO to total heat release (g-NO/J-heat). For the sake of comparison and presentation below, the variations of peak NO mole fraction (peak NO) and temperature (Tmax) are also shown in Fig. 4. It is found that at a
given stretch rate, although the peak flame temperature monotonically increases, the behaviors of NO emission index and peak NO mole fraction are complex, as the fraction of hydrogen is increased. Moreover, the variation trends of NO
emission index and peak NO mole fraction change for different stretch rates. a = 10 s-1 Fraction of H2 0.0 0.2 0.4 0.6 0.8 1.0 N O e m issi o n i n d e x, g -N O /J-H e a t 2.0e-8 4.0e-8 6.0e-8 8.0e-8 1.0e-7 1.2e-7 1.4e-7 Ma xi mu m te m p e ra tu re , K 1900 2000 2100 2200 2300 2400 2500 P e a k N O mo le f ra ct io n 0.0001 0.0002 0.0003 0.0004 0.0005 0.0006 0.0007 0.0008 NOEI Tmax Peak NO a = 100 s-1 Fraction of H2 0.0 0.2 0.4 0.6 0.8 1.0 N O e m issi o n i n d ex, g -N O /J-h e at 3.0e-8 4.0e-8 5.0e-8 6.0e-8 7.0e-8 8.0e-8 9.0e-8 1.0e-7 1.1e-7 Ma x im u m te m p e ra tu re , K 1900 2000 2100 2200 2300 2400 2500 P e a k N O m o le f ra c ti o n 0.00010 0.00015 0.00020 0.00025 0.00030 0.00035 0.00040 0.00045 NOEI Tmax Peak NO a = 300 s-1 Fraction of H2 0.0 0.2 0.4 0.6 0.8 1.0 N O e m issi o n i n d e x , g -N O /J -h e a t 1.0e-8 1.5e-8 2.0e-8 2.5e-8 3.0e-8 3.5e-8 4.0e-8 4.5e-8 5.0e-8 M a x im u m te mp e ra tu re , K 1800 1900 2000 2100 2200 2300 2400 P e a k N O mo le f ra c ti o n 0.00002 0.00004 0.00006 0.00008 0.00010 0.00012 0.00014 0.00016 0.00018 NOEI Tmax Peak NO
Fig. 4 Variations of NO emission index, peak temperature and peak NO mole fraction.
Stretch rate, s-1 0 50 100 150 200 250 300 N O e m is si o n i n d e x , g -N O /J-h e a t re le a s e 1.0e-8 2.0e-8 3.0e-8 4.0e-8 5.0e-8 6.0e-8 αH2 = 0.0 αH2 = 0.2 αH2 = 0.5
Fig. 5 Variation of NO emission index with stretch rate at different hydrogen fractions.
At a lower or moderate stretch rate (a = 10 or 100 s-1), there is a critical hydrogen fraction between 0.0 and 1.0, at which NO emission index reaches its maximum value. The value of this critical hydrogen fraction is higher when stretch rate is 100 s-1 than when stretch rate is 10 s-1. However, such a critical hydrogen fraction does not exist and NO emission index reaches the maximum at gH2 = 1.0, when stretch rate is 300 s-1.
The peak NO mole fraction always reaches the maximum at gH2
= 1.0, regardless of the variation in stretch rate.
N
2HCN
HOCN
HNCO
NH
2NH
NCO
HNO
NO
N
H
2CN
HCNO
+CH +H,OH +OH +CH2 +HCCO +CH3 +H2O,OH +O +H,OH +O +H +H,OH +H +O +H +H +OH +CH3 ~1.0 ~4.0 ~8.0 Mole/(cm2⋅s) (a)N
2HCN
HOCN
HNCO
NH
2NH
NCO
HNO
NO
N
H
2CN
HCNO
NH
3 +H +OH +O +H2 +H,OH +H2 +H2 ~1.0 ~4.0 ~8.0 Mole/(cm2⋅s) (b)Fig. 6 NO formation pathway in CH4/air and H2/air flames at
stretch rate of 10 s-1. (a). CH4/air flame; (b). H2/air flame.
When stretch rate is lower (10 s-1), both NO emission index and the peak NO mole fraction keep almost constant as the fraction of hydrogen is increased from 0.0 to 0.4, and then quickly increase until the maximum NO emission index is reached at the critical hydrogen fraction of gH2 = 0.95. Finally,
with further increasing the hydrogen fraction from the critical value to 1.0, NO emission index decreases, while the peak NO mole fraction keeps increasing.
The situation changes for flames with a moderate (100s-1) or higher (300s-1) stretch rate. At the beginning, both peak NO mole fraction and NO emission index rise with the increase in
the fraction of hydrogen. The rise is more significant when stretch rate is 300 s-1 than when stretch rate is 100-1. Then the increase of hydrogen fraction causes a slight decrease in both the peak NO mole fraction and NO emission index. Finally with the further increase in the fraction of hydrogen, both the peak NO mole fraction and NO emission index quickly rise until the critical hydrogen fraction is reached when stretch rate is 100 s-1, or the pure hydrogen flame condition is reached when stretch rate is 300 s-1. When the fraction of hydrogen is greater than the critical value, the variations of peak NO mole fraction and NO emission index at stretch rate of 100 s-1 are qualitatively similar to those at stretch rate of 10 s-1.
Figure 5 demonstrates the variation of NO emission index at three different hydrogen fraction levels (0.0, 0.2 and 0.5), as stretch rate changes. It is further confirmed that at lower to moderate stretch rates, hydrogen enrichment has minor influence on NO formation. This qualitatively agrees with the conclusion obtained by Naha and Aggarwal [9] at a fixed stretch rate. However, the effect of hydrogen addition on NO formation becomes more significant at higher stretch rates. It should be pointed out that the lower NO formation at stretch rate of 10 s-1 than that at some higher stretch rates is because of radiation heat loss that increases with the decrease in stretch rate [12].
The above observed phenomena can be explained by the variations in the mechanisms of NO formation and flame structure, when hydrogen is added at different stretch rates. It is well known that NO generally can be formed by four routes in a hydrocarbon flame, i.e. the thermal, the prompt, the N2O and
the NNH intermediate routes. The thermal NO formation route is comprised of the three reactions: N2 + O = N + NO; N + O2 =
NO + O; and N + OH = NO + H; of which the first one is the initiation reaction that converts molecular nitrogen to NO and atomic nitrogen. The prompt NO in hydrocarbon flames is initiated by the rapid reactions of hydrocarbon radicals with molecular nitrogen, and then the formed atomic nitrogen and species containing elementary nitrogen are converted to NO. The N2O intermediate route is initiated by the reactions: N2O
(+M) = N2 + O (+M); N2O + H = N2 + OH; N2O + O = N2 +
O2; and N2O + OH = N2 + HO2; and then N2O formed is
partially converted to NO. In addition, NO formation can also be initiated by the reactions of molecular nitrogen with other hydrocarbon-free radicals, such as H, OH, H2, to form NNH,
and NNH is later convertedto NO. This last route to form NO is known as the NNH intermediate route. Figure 6 displays the pathways of NO formation in the pure CH4/air and H2/air
diffusion flames when stretch rate equals 10 s-1. The thickness of each line represents the magnitude of the rate and the arrow indicates the direction of the reaction. The paths with rates less than 1.0x10-8 mole/(cm2⋅s) have been neglected. The species not participating in any reaction in the H2/air flame are still kept
in Fig. 6b for comparison. It is observed that most NO is formed by the reactions HNO (+H, OH) → NO and N (+OH) → NO in the CH4/air flame, and by N (+OH) → NO and N2
(+O) → NO in the H2/air flame. Apparently the reactions HNO
(+H, OH) → NO in the CH4/air flame belong to the prompt
route, since species HNO is from the paths resulting from the reaction of molecular nitrogen with radical CH. Although both flames share the reaction N (+ OH) → NO, which was attributed to the thermal NO formation route in many references, it is noted that atomic nitrogen participating in this
reaction in the two flames comes from different paths. In the CH4/air flame, it is from the paths N2 (+CH)→ HCN → NCO
→ NH → N, N2 (+CH)→ HCN → NH → N and N2 (+CH)→
N. Therefore, the formation of atomic nitrogen in the CH4/air
flame is initiated by the reaction of molecular nitrogen with radical CH, which is the typical prompt route nitrogen conversion. On the other hand, in the H2/air diffusion flame, the
atomic nitrogen is from the path N2 (+O)→N that is the thermal
route. This tells us that the method to identify the mechanism of NO formation in a flame should not be based on how NO is finally formed, but on how molecular nitrogen is initially converted to atomic nitrogen or species containing element nitrogen. The paths of NO formation through the N2O and
NNH intermediate routes are not shown in Fig. 6, since their rates are less than 1.0x10-8 mole/(cm2⋅s). It is clear from Fig. 6 that the formation of NO in the CH4/air flame is mainly due to
the prompt route, while in the H2/air flame is due to the thermal
route. Based on above analysis and for simplification, we examine the mechanism of NO formation of other flames according to the consumption rates of molecular nitrogen by different routes, rather than the final formation of NO.
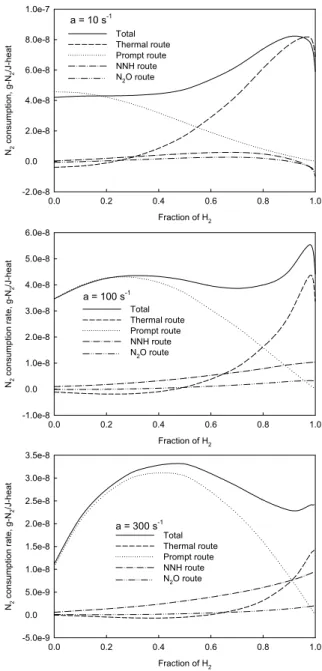
Figure 7 shows the variations of molecular nitrogen consumption rates by different routes in flames of three typical stretch rates, when the fraction of hydrogen is changed. The definition of nitrogen consumption rate is similar to that for NO emission index. Positive value means nitrogen is consumed (converted to NO or species containing elementary nitrogen), and negative value indicates that nitrogen is formed (NO or species containing elementary nitrogen is converted back to molecular nitrogen). For completeness, the nitrogen consumption rates by the N2O and NNH intermediate routes are
also shown, although their contributions are very small in all the studied flames. The identification method of the nitrogen consumption by different routes can be found elsewhere [3]. It is observed that for all the pure CH4/air flames, the prompt
route dominates the conversion of nitrogen. The consumption rate of nitrogen by the thermal route is actually slightly negative. It is because a large amount of atomic nitrogen is formed by the reaction N2 + CH = HCN + N in a CH4/air flame,
resulting in that the forward rate of the reaction NO + N = N2 +
O exceeds the reverse rate. Differently, the thermal route contributes most nitrogen conversion in all the H2/air diffusion
flame. The combination of the variations in the nitrogen consumption rates by the thermal and prompt routes can explain most of the phenomena observed in Fig. 4, when the fraction of hydrogen is increased from 0.0 to 1.0.
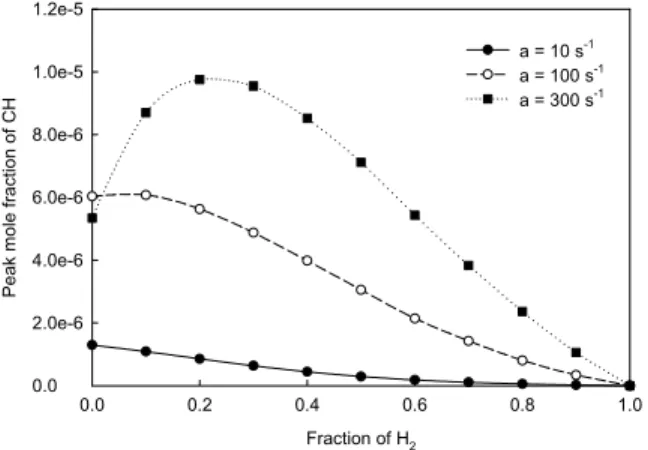
When stretch rate equals 10 s-1, the consumption rates of nitrogen by the thermal and prompt routes respectively increases and decreases, leading to that the net (or total) nitrogen consumption rate and NO emission index keep constant, as the fraction of hydrogen is increased from 0.0 to 0.4. With the further increase of hydrogen fraction from 0.4 to the critical value (0.95), the consumption rate of nitrogen by the thermal route quickly increases, while that by the prompt route gradually decreases, resulting in that the net nitrogen consumption rate and NO emission index rapidly increase. The monotonic decrease of nitrogen consumption rate by the prompt route is because of the decrease in the concentration of CH radical, as shown in Fig. 8, as hydrogen is added. For the thermal route, when the fraction of hydrogen is increased from 0.0 to the critical value, the increase of nitrogen consumption
rate is caused by the increase in flame temperature, as shown in Fig. 4. The slower increase rate of nitrogen consumption by the thermal route at lower hydrogen fraction is because the absolute temperature level is relatively lower. When the fraction of hydrogen is greater than the critical value, the decrease in nitrogen consumption rate and NO emission index is caused by the decrease in the consumption of nitrogen by the thermal route. This will be further explained later.
a = 10 s-1 Fraction of H2 0.0 0.2 0.4 0.6 0.8 1.0 N2 c o n su mp ti o n , g -N 2 /J-h e a t -2.0e-8 0.0 2.0e-8 4.0e-8 6.0e-8 8.0e-8 1.0e-7 Total Thermal route Prompt route NNH route N2O route a = 100 s-1 Fraction of H2 0.0 0.2 0.4 0.6 0.8 1.0 N2 c o n s u mp ti o n ra te, g -N 2 /J-h e a t -1.0e-8 0.0 1.0e-8 2.0e-8 3.0e-8 4.0e-8 5.0e-8 6.0e-8 Total Thermal route Prompt route NNH route N2O route a = 300 s-1 Fraction of H2 0.0 0.2 0.4 0.6 0.8 1.0 N2 co n s u mp ti o n ra te , g -N 2 /J -h e a t -5.0e-9 0.0 5.0e-9 1.0e-8 1.5e-8 2.0e-8 2.5e-8 3.0e-8 3.5e-8 Total Thermal route Prompt route NNH route N2O route
Fig. 7 Nitrogen consumption rates.
When stretch rate equals 100 or 300 s-1, as the fraction of hydrogen first increases from 0.0 to a certain value (0.3 and 0.5, respectively, for a = 100 and 300 s-1), the consumption rate of nitrogen by the prompt route increases, while that by the thermal route keeps almost constant, leading to that the net nitrogen consumption rate and NO emission index increase. At this stage, the almost constant nitrogen consumption rate by the thermal route is due to the net effect of the temperature increase, which tends to raise the nitrogen consumption rate by
the thermal route, and the rise in the nitrogen consumption by the prompt route that generates a large amount of atomic nitrogen and thus intensifies the forward rate of the reaction NO + N = N2 + O. The increase in the consumption rate of
nitrogen by the prompt route is caused by the fact that a small amount of hydrogen addition increases the concentration of radical CH, as shown in Fig. 8, when stretch rate is 100 or 300 s-1, because the addition of a small amount of hydrogen intensifies the combustion of a CH4/air diffusion flame if
stretch rate is not very low. This effect of a small amount hydrogen addition does not happen at a lower stretch rate, such as a = 10 s-1, since the residence time of reactants in the reaction zone of a lower stretch rate flame is long enough to complete the combustion for a CH4/air diffusion flame, and
thus the addition of hydrogen only increases flame temperature and reduces the concentration of radical CH. With the fraction of hydrogen being increased to over a certain value, the concentration of radical CH starts to decrease, resulting in the reduction in the consumption rate of nitrogen by the prompt route and NO emission index when stretch rate equals 100 and 300 s-1. Finally, with the further increase in the fraction of hydrogen to the critical value at a stretch rate of 100 s-1 or to 1.0 at a stretch rate of 300 s-1, the nitrogen consumption rate by the thermal route and NO emission index increases again due to the significantly increased temperature.
Fraction of H2 0.0 0.2 0.4 0.6 0.8 1.0 Pe a k m o le f ra c ti o n o f C H 0.0 2.0e-6 4.0e-6 6.0e-6 8.0e-6 1.0e-5 1.2e-5 a = 10 s-1 a = 100 s-1 a = 300 s-1
Fig. 8 Variation of peak CH mole fraction.
Now we explain the phenomena when the fraction of hydrogen is greater than the critical value at a lower or moderate stretch rate. When the fraction of hydrogen exceeds the critical value, the increase of hydrogen fraction reduces the nitrogen consumption rate and NO emission index, while increases the peak mole fraction of NO, Figs. 4 and 7. This is because when the fraction of hydrogen is increased to a higher level, the reaction zone is moved further away from the stagnation plane, as shown in Figs. 2 and 3. The formation of NO in the primary reaction zone keeps increasing because of the rise in flame temperature, as the fraction hydrogen is increased from the critical value to 1.0 at a given stretch rate. This results in that the peak NO mole fraction keeps increasing. However, when the formed NO is transported to the region close to stagnation plane, part of NO is converted back to molecular nitrogen by the reaction NO + N = N2 + O, leading to
the decrease in NO emission index for flames at a lower or moderate stretch rate. However, at a higher stretch rate, this
phenomenon does not happen, since the primary reaction zone is closer to stagnation plane for flames at all hydrogen addition levels.
In summary, the simulation results show that the addition of hydrogen in a CH4/air diffusion flame does not have
significant effect on NO formation for lower and moderate stretch rate flames, as long as the fraction of added hydrogen is not big enough to close to 1.0. Although the addition of a small amount of hydrogen increases the formation of NO in higher stretch rate flames, this increase is expected to be controlled by adding some other components, such as EGR. Giving the other advantages of hydrogen addition in diffusion flames, like the reduction in the formation of soot, we can say that we do benefit from hydrogen enrichment combustion technology for diffusion flames.
CONCLUSIONS
A detailed numerical study on the effect of hydrogen enrichment on flame structure and NOX formation in
counterflow CH4/air diffusion flames has been conducted. The
result indicates that for flames with low to moderate stretch rates, with the increase of the enrichment fraction from 0 to 0.5~0.6, NO emission index keeps almost constant or only slightly increases. When the enrichment fraction is increased from 0.5~0.6 to about 0.9, NO emission index quickly increases, and finally NO formation decreases again when pure hydrogen flame condition is approached. However, for flames with higher stretch rates, with the increase of hydrogen enrichment fraction from 0 to 1.0, the formation of NO first quickly increases, then slightly decreases and finally increases again. Detailed analysis suggests that the variation of the characteristics in NO formation in stretched CH4/air diffusion
flames is caused by the change of flame structure and NO formation mechanism, when the enrichment fraction and stretch rate are changed.
REFERENCES
1. Jackson, G.S., Sai, R., Plaia, J.M., Boggs, C.M., Kiger, K.T., 2003, “Influence of H2 on the response of
lean premixed CH4 flames to high strained flows”,
Combust. Flame, 132, pp.503-511.
2. Ren, J.Y., Qin, W., Egolfopoulos, F.N., Mak, H., Tsotsis, T.T., 2001, “Methane reforming and its potential effect on the efficiency and pollutant emissions of lean methane-air combustion”, Chemical Engineering Engineering Science, 56, pp.1541-1549. 3. Guo, H., Smallwood, G.J., Liu, F., Ju, Y., Gülder,
Ö.L., 2005, “The Effect of Hydrogen Addition on Flammability Limit and NOx Emission in Ultra Lean
Counterflow CH4/Air Premixed Flames”, Proc.
Combust. Inst., 30, pp.303-311.
4. Guo, H., Smallwood, G.J., Gülder, Ö.L., 2006, “The effect of reformate gas enrichment on extinction limits and NOX formation in counterflow CH4/air premixed
flames”, Proc. Combust. Inst., 31, in press.
5. Gülder, Ö.L., Snelling, D.R., and Sawchuk, R.A., 1996, “Influence of hydrogen addition to fuel on temperature field and soot formation in diffusion flames”, Proc. Combust. Inst., 26, pp.2351-2358. 6. Guo, H., Liu, F., Smallwood, G.J., and Gülder, Ö.L.,
hydrogen addition on soot formation in a laminar ethylene-air diffusion flame”, Combust. Flame, 145, pp.324-338.
7. Ju, Y., and Niioka, T., 1994, “Reduced kinetic mechanism of ignition for nonpremixed hydrogen/air in a supersonic mixing layer”, Combust. Flame, 99, pp.240-246.
8. Fotache, G.G., Kreutz, T.G., and Law, C.K., 1997, “Ignition of hydrogen-enriched methane by heated air”, Combust. Flame, 110, pp.429-440.
9. Naha, S., and Aggarwal, S.K., 2004, “Fuel effects on NOX emissions in partially premixed flames”,
Combus. Flame, 139, pp.90-105.
10. Giovangigli, V., and Smooke, M.D., 1987, “Extinction of strained premixed laminar flames with complex chemistry”, Combust. Sci. Tech., Vol. 53, pp.23-49. 11. Kee, R.J., Grcar, J.F., Smooke, M.D., and Miller, J.A.,
A Fortran Program for Modelling Steady Laminar One-Dimensional Premixed Flames, Report No. SAND85-8240, Sandia National Laboratories, 1985. 12. Guo, H., Ju, Y., Maruta, K., Niioka, T. and Liu, F.,
1997, “Radiation Extinction Limit of Counterflow Premixed Fuel-Lean Methane-Air Flame”, Combust. Flame, 109, pp.639-646.
13. Gregory P. Smith, David M. Golden, Michael Frenklach, Nigel W. Moriarty, Boris Eiteneer, Mikhail Goldenberg, C. Thomas Bowman, Ronald K. Hanson, Soonho Song, William C. Gardiner, Jr., Vitali V.
Lissianski, and Zhiwei Qin http://www.me.berkeley.edu/gri_mech/.
14. Kee., R. J., Warnatz, J., and Miller, J. A., A Fortran Computer Code Package for the Evaluation of Gas-Phase Viscosities, Conductivities, and Diffusion Coefficients, Report No. SAND 83-8209, Sandia National Laboratories, 1983.
15. Kee., R. J., Miller, J. A., and Jefferson, T. H., A
General-Purpose, Problem-Independent, Transportable, Fortran Chemical Kinetics Code
Package, Report No. SAND 80-8003, Sandia National Laboratories, 1980.