HAL Id: hal-02331617
https://hal.archives-ouvertes.fr/hal-02331617
Submitted on 19 Nov 2020HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Selective Synthesis of THF-Derived Amines from
Biomass-Derived Carbonyl Compounds
Shi Jiang, Changru Ma, Eric Müller, Marc Pera-Titus, François Jérôme,
Karine de Oliveira Vigier
To cite this version:
Shi Jiang, Changru Ma, Eric Müller, Marc Pera-Titus, François Jérôme, et al.. Selective Synthesis of THF-Derived Amines from Biomass-Derived Carbonyl Compounds. ACS Catalysis, American Chemical Society, 2019, 9 (10), pp.8893-8902. �10.1021/acscatal.9b03413�. �hal-02331617�
Selective synthesis of THF-derived amines from
biomass-derived carbonyl compounds
Shi Jiang,† ‡ Changru Ma,‡ Eric Muller,§ Marc Pera-Titus,*,‡ François Jerôme† and Karine De
Oliveira Vigier*,†
†
Institut de Chimie des Milieux et Matériaux de Poitiers, CNRS_Université de Poitiers, 1 rue Marcel Doré, ENSIP, TSA 41195, 86073 Poitiers Cedex 9, France
‡Eco-Efficient Products and Processes Laboratory (E2P2L), UMI 3464 CNRS-Solvay, 3966 Jin
Du Road, Xin Zhuang Ind. Zone, 201108 Shanghai, China
§SOLVAY-Advanced Organic Chemistry & Molecule Design Laboratory, Recherche &
ABSTRACT: Selective transformation of biomass or biomass-based feedstocks into value-added
amines is highly desirable and constitutes one of the most important challenges in Catalysis. As of today, only few amination studies have been reported on bio-based substrates, targeting mainly the reductive amination of small platform molecules issued directly from biomass. Here we present a simple and highly efficient system using NH3 or amines as a nitrogen source and molecular hydrogen as reducing agent for the transformation of furan-derived ketones into THF-derived amines over Pd/Al2O3 (up to 98% yield). Detailed analysis of the reaction system provided insight into the reaction mechanism. To further realize the production of THF-derived amines, a one-pot two-step strategy combining C-C and C-N condensation reactions was attempted. A high yield (85%) towards 5-methyl-1-(tetrahydrofuran-2-yl)hexan-3-amine could be successfully achieved starting directly from furfural.
Introduction
Amines represent a valuable class of compounds of wide interest in the chemical industry due to their nucleophilic properties, conferring them high reactivity.1-7 Among the different types of amines, primary amines constitute valuable fine and bulk chemicals, which serve as versatile feedstocks and key intermediates for the synthesis of advanced chemicals, life science molecules and polymers.8-11 Industrially relevant aliphatic and aromatic amines, as well as aminoalcohols, are currently manufactured from fossil resources.12 In contrast, transformation of biomass into valuable nitrogen-containing chemicals is still very limited owing to the lack of efficient amination methods for biomass-derived molecules.7,8,13,14 So far, the conversion of carbohydrates into valuable nitrogen compounds is a good opportunity and starts to be an important goal of research programs.15-19
The promotion of catalytic system for the selective and sustainable synthesis of amines from available and inexpensive starting materials using green reagents and mild reaction condition is highly demanded and challenging.19-22 As a rule, four mechanisms can be exploited for amine alkylation: (1) nucleophilic substitution of alkyl halides with amines;12 (2) N-alkylation of amines with alcohols under catalytic conditions;23,24 (3) reductive amination of carbonyl compounds with amines.25 (4) hydroamination of olefins with amino source.26 The first method is not recommended from a green synthesis point of view, since a massive amount of useless inorganic salts is generated. Alkylation of amines with alcohols normally requires harsh conditions, especially high temperature or well-designed catalyst, as well as in some cases the use of additives. Hydroamination of olefins is acknowledged as a typical way for synthesis of aliphatic amines, combining alkenes and simple N−H functional groups in a direct and atom-economical fashion. At the same time it is one of 10 challenges for catalysis and limited examples are reported, which
promotes the potential to create a significant synthetic benefit. Direct reductive amination, involving the reaction of carbonyl compounds, represents one of the most versatile, and waste-free process to access a variety of amines.6,10,20 However, overalkylation, reduction to the corresponding alcohols and poor catalyst stability are common drawbacks in catalytic processes for the direct synthesis of amines, especially in the presence of NH3.27-35 Hence, from an atom-economy and green standpoint, there is an urgent need to develop a selective and efficient system for producing bio-based primary amines, which is the prime task of this investigation.
Recently, we reported an efficient method for the synthesis of furfural from highly concentrated xylose in biphasic media, where furfural was extracted by methyl isobutyl ketone (MIBK).36 As a top chemical produced from biomass, furfural is furanic derivative that is available in large scale (>200 kT/year), making it an attractive raw material for the production of value-added chemicals.37,38 For instance, furfural can be selectively converted into furfurylamine by reductive amination with NH3 in presence of H2,39,40 which is used in the synthesis of pesticides, herbicides and pharmaceuticals (for diuretic furosemide synthesis). Besides, furan-based compounds are highly versatile and key derivatives used in the manufacture of a wide range of chemicals, and can serve as precursors for the synthesis of jet fuels substitutes or additives such as alkanes.41-52
Herein, we present a simple and efficient catalytic system combining C-C and C-N condensation reactions for producing 5-methyl-1-(tetrahydrofuran-2-yl)hexan-3-amine, and extensively a library of THF-derived biobased amines with variable molecular complexity and diversity, starting directly from furfural (Scheme 1). In particular, Pd/Al2O3 was found as an active catalyst for the highly selective formation of THF-derived primary amines using NH3 and H2 in an efficient one-step process starting from bio-derived ketone (methylisobutylketone, MIBK which
is obtained from aceton a bulk chemical). This approach was successfully extended to the selective synthesis of a new family of THF-derived amines. The presence of the THF moiety is of prime importance for solvent properties and the manufacture of polymers.53-55
Scheme 1. Synthesis of THF-derived amines starting directly from furfural.
Results and Discussion
Catalyst Characterization. X-ray diffraction of all noble metal catalysts were performed and are
shown in Figure 1.
Figure 1. XRD patterns of all noble metal catalysts (peak indication: *=γ-Al2O3)
30 40 50 60 70 80 Ru/Al2O3 Pt/Al2O3 Rh/Al2O3
*
*
*
*
*
Inten si ty (a.u. ) 2 Theta (degree)*
(006) Pd/C Pd/Al2O3The XRD patterns only contain features of the supports (Al2O3/C), implying the absence of large metal particles. The textural properties of the catalysts are listed in Table 1.
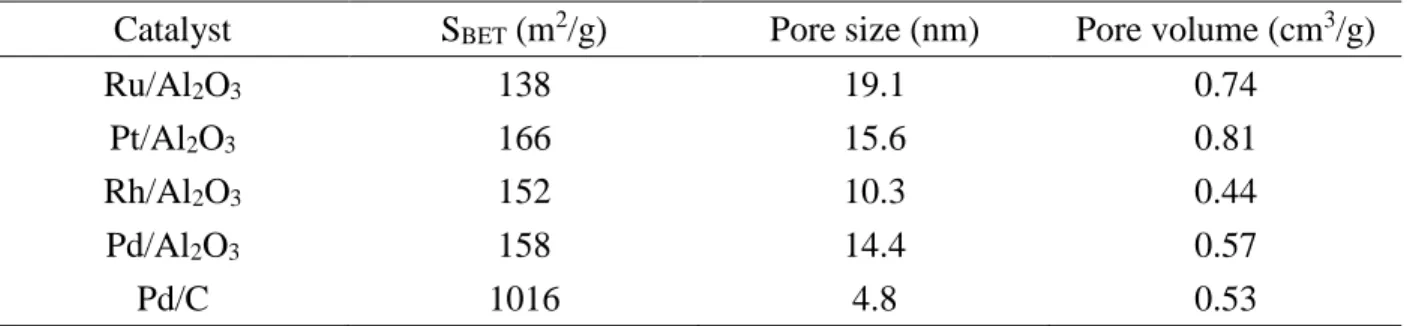
Table 1. Physical-chemical properties of all noble metal catalysts.
Catalyst SBET (m2/g) Pore size (nm) Pore volume (cm3/g)
Ru/Al2O3 138 19.1 0.74
Pt/Al2O3 166 15.6 0.81
Rh/Al2O3 152 10.3 0.44
Pd/Al2O3 158 14.4 0.57
Pd/C 1016 4.8 0.53
The highest surface area is obtained for the carbon support (1016 m2/g) and the other four alumina-supported catalysts show quite similar surface area (between 138 and 166 m2/g). One can mention that the pore size is on the opposite, the lowest for Pd supported on carbon (4.6 nm) and for the other catalysts it in in the range of 10.3 to 19.1 nm. The pore volume of all the catalysts are comprise between 0.44 to 0.81 cm3/g.
XPS analysis was performed in order to identify the probable species of noble metals on the catalysts surfaces. The identification of the probable metal species is based on XPS patterns obtained from the National Institute of Standards and Technology (NIST). To correct binding energies (BEs), the Al 2p peak with BE 74.4 eV for Al-containing samples and the adventitious carbon C 1s peak with BE 284.8 eV are used as reference. Figure S1 presents the XPS spectra of selected Ru, Pt, Rh and Pd catalysts and binding energies of Ru 3d5/2, Pt 4d5/2, Rh 3d5/2 and Pd 3d5/2 for the analyzed solids. It is clearly noted that all noble metal species contained oxidized species due to passivation under air due to experimental work up. However, reduced species are the main species for all the catalysts. Hence, Pt, Rh, Pd catalysts could be sufficiently reduced into metallic species at room temperature whereas H2-temperature-programmed reduction (Figure S2) reveals that Ru on Al2O3 can be reduced into metallic Ru at the reaction temperature 90 °C. These
results indicate that all the noble metal catalysts contain mostly metallic species under the reaction conditions.
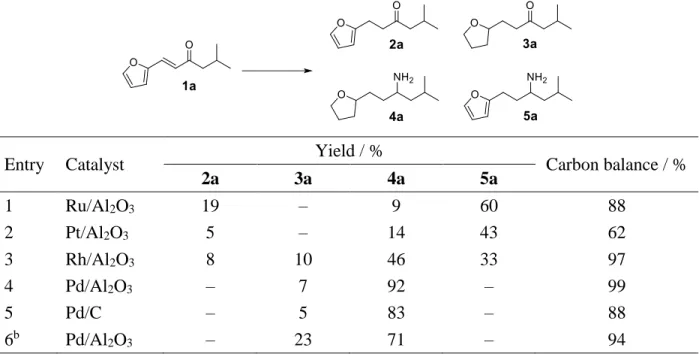
Catalytic Testing. On the guidance of a previous study,56 a bio-based carbonyl-conjugated vinylfuran (1a) was selected as model platform, which can be selectively synthesized by the aldol condensation reaction of furfural with methyl isobutyl ketone (MIBK). The reductive amination of 1a with NH3 to the primary amine using H2 was chosen as benchmark reaction. Due to the polyfunctionnality of 1a (C=C, C=O bonds and furanic cycle), various products can be in principle generated, including furanic ketone (2a), THF-derived ketone (3a), THF-derived amine (4a), or furanic amine (5a). Targeting the selective synthesis of amine products and more specifically 4a, a series of commercially available noble metal catalysts based on Ru, Pt, Rh and Pd (5 wt% metal) supported over alumina (Al2O3) or active carbon (C) were screened, and the results are shown in Table 2.
In a first set of experiments, 0.2 g of 1a (10 wt% in EtOH) was reacted in the presence of 10 mg of the alumina-supported Ru, Pt, Rh and Pd catalysts at 120 °C for 12 h with 5 bar NH3 and 20 bar H2. Except for Pd/Al2O3, a complex mixture of 2a-5a products was obtained. In particular, Ru and Pt favored the formation of the furanic amine 5a with ~50% yield, but the latter exhibited a lower carbon balance which is probably due to condensation reaction. Rh afforded the formation of all the products with a yield of 8%, 10%, 46% and 33% of products 2a, 3a, 4a and 5a, respectively. In the presence of Pd/Al2O3, the yield of product 4a was around 90% with a carbon mass balance over 95%. We further investigated the effect of the support on the catalytic properties. Pd/C afforded 83% and 5% yield of products 4a and 3a, respectively, with a carbon mass balance of 88%. When the Pd/Al2O3 catalyst loading was decreased to 5 mg, a drop of the
4a yield (from 92% to 71%) was observed along with an increase of the 3a yield from 7% to 23%.
In light of these results, we further optimized the process conditions for the Pd/Al2O3 catalyst.
Table 2. Reductive amination of 1a over supported metal catalystsa
Entry Catalyst Yield / % Carbon balance / %
2a 3a 4a 5a 1 Ru/Al2O3 19 – 9 60 88 2 Pt/Al2O3 5 – 14 43 62 3 Rh/Al2O3 8 10 46 33 97 4 Pd/Al2O3 – 7 92 – 99 5 Pd/C – 5 83 – 88 6b Pd/Al2O3 – 23 71 – 94
aReaction conditions: 1a (0.2 g), catalyst (0.01 g), EtOH (2 g), NH
3 (5 bar), H2 (20 bar), 120 °C, 12 h. bPd/Al2O3 (5
mg). The 1a conversion was over 99% for all catalysts
Effect of the Reaction Temperature. To attain high selectivity to product 4a at mild conditions
and also to understand the reaction mechanism, the effect of the reaction temperature on the catalytic properties was first investigated (Figure 2a). No effect was observed on the 1a conversion, but the product distribution differed substantially. Indeed, a decrease of the temperature from 120 °C to 80 °C led to a drastic decrease of the yield of THF-derived amine 4a from 90% to 33% to the benefit of the furanic ketone (2a) and furanic amine (5a). A further decrease of the temperature down to 50 °C resulted in few amine products at the expense of a mixture the hydrogenated products 2a and 3a with a yield of 44% and 39%, respectively, at full
1a conversion. These results suggest that a temperature below 80 °C is insufficient for generating
hydrogenation of the ketone compared to the intermediate imine. In light of these results, a reaction temperature of 120 °C was selected to ensure the formation of THF-derived amine 4a.
Figure 2. a) Effect of temperature; b) Effect of H2 pressure on the catalytic performance of Pd/Al2O3 in the reductive amination of 1a. Reaction Conditions: a) Pd/Al2O3 (10 mg, 0.42 mol%),
1a (0.2 g), EtOH (2 g), NH3 (5 bar), H2 (20 bar), 12 h; b) Pd/Al2O3 (10 mg), 1a (0.2 g), EtOH (2 g), NH3 (5 bar), 120 °C, 12 h. The 1a conversion was over 99 % in all the tests.
Effect of the Hydrogen Pressure. H2 as the reducing agent plays an important role in the reaction
(1 mol of reagent 1a could consume 4 mol of H2, Scheme 2). Thus, we investigated the effect of the H2 pressure keeping the other parameters constant (Figure 2b). When the H2 pressure was decreased from 20 bar to 10 bar, the yield of the THF-derived amine 4a decreased from 92% to 67% to the benefit of 2a, 3a and 5a with a yield of 5%, 13% and 4%, respectively. In contrast, an increase of the H2 pressure from 20 bar to 40 bar did not result in an appreciable change of the catalytic performance (94% yield of 4a and 4% yield of 3a). These results point out two possible pathways for generating the THF-derived primary amine (Scheme 2): (i) hydrogenation of the furanic amine, or (ii) reductive amination of the THF-derived ketone. One can mention that the
50 80 120 0 20 40 60 80 100 Yield / % T / oC 2a 3a 4a 5a a) 10 20 40 0 20 40 60 80 100 Yield / % p / bar 2a 3a 4a 5a b)
formation of the Schiff base is likely to occur in the presence of amine and ketone in organic solvent. However, due to the electronic hindrance of the ketone or amine groups, we did not detect its existence in our work.
Scheme 2. Possible reaction pathways for generating THF-derived amines: (i) reductive amination
of THF-derived ketone; and (ii) hydrogenation of the furanic amine.
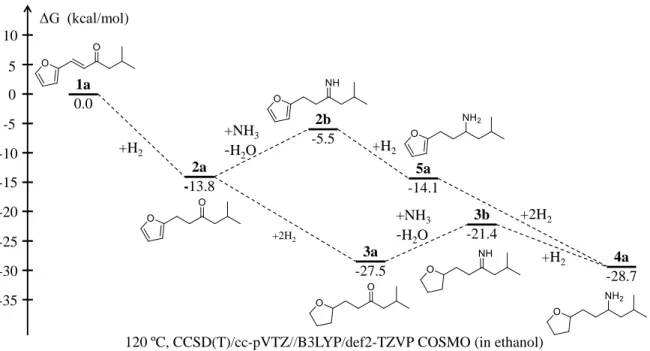
To sort out the contribution of both pathways, DFT calculations at the CCSD(T)/cc-pVTZ level were conducted to unveil the thermodynamic stability of the different products (Figure 3, see calculation details in the SI). In the presence of H2, carbonyl-conjugated vinylfuran (1a) was first converted into the furanic ketone (2a) with a free energy of reaction [ΔGo (1a→2a)] of -13.8 kcal mol-1 (120 °C, in ethanol). In the presence of NH3, a narrowly positive free energy of reaction compared to 2a [ΔGo (2a→2b) = +8.3 kcal mol-1] pushed the formation of the furan imine 2b, followed by the hydrogenation to the furanic amine 5a [ΔGo (2b→5a) = -8.6 kcal mol-1]. Finally, by deep hydrogenation, the THF-derived amine can be generated with a further stabilization [ΔGo (2a→4a) = -14.9 kcal mol−1
] compared to 2a. In the meantime, in the presence of H2, a thermodynamically more favorable reaction can occur driven by the hydrogenation of the furan ring [ΔGo (2a→3a) = -13.7 kcal mol−1 compared to 2a], resulting in the formation of the THF-derived ketone (3a). Then the THF-THF-derived amine can be accessed by the reductive amination of
feasible for generating THF-derived amines. Here we only calculated the free energies of the reactions, not the barriers. Thus the reaction kinetics was not considered in the calculations.
Figure 3. Free energy diagrams for the reductive amination of 1a and related reactions; Values in
parentheses are the computed free energies referred to 1a.
Reaction Kinetics. We further measured the kinetics of the formation of the different products
over Pd/Al2O3 by varying the reaction time from 1 min to 18 h (Figure 4). The results show a complete transformation of the product mixture into 4a from the very beginning of the reaction. Indeed, after 1 min, 50% of reactant 1a was converted into 2a and 3a with 28% and 8% yield, respectively, with little formation of the amine and imine products. This observation suggests a faster hydrogenation rate of the vinyl group after 1 min of reaction (2a, 0.315 mmol min-1) compared to the furan ring (3a, 0.090 mmol min-1). After 2 min, 1a was fully converted into a mixture of products 2a and 3a, whereas when the reaction was prolonged to 20 min, the target
0.0 1a -13.8 2a -5.5 2b -14.1 5a -28.7 4a -27.5 3a +H2 +H2 +NH3 -H2O +2H2 +2H2 +NH3 -H2O -21.4 3b +H2 0 5 10 -5 -10 -15 -20 -25 -30 -35 ΔG (kcal/mol)
product 4a started to be generated, reaching a maximum yield of 98% after 18 h. These results point out that, on a kinetic perspective, pathway (ii) is favored for the formation of the THF-derived amine 4a from the carbonyl-conjugated vinylfuran 1a over Pd/Al2O3 (Scheme 2). First, the hydrogenation of 1a gives the furanic ketone 2a, followed by the deep hydrogenation to the THF-derived ketone 3a. The subsequent reversible reaction of 3a and NH3 leads to the formation of the imine intermediate with concomitant H2O formation. Meanwhile, 4a reaches a maximum as the reaction proceeds. In light of these results, Pd/Al2O3 appears as a very active and chemo-selective catalyst for promoting the transformation of 1a into 4a without the formation of undesirable by-products.
Figure 4. Time-evolution of the reaction products in the reductive amination of 1a over Pd/Al2O3. Reaction conditions: Pd/Al2O3 (10 mg), 1a (0.2 g), EtOH (2 g), NH3 (5 bar), H2 (20 bar), 120 °C.
Catalyst Recyclability. The recyclability of the Pd/Al2O3 catalyst was evaluated in the reductive amination of 1a over a series of consecutive runs (Figure 5). After each run, the catalyst was
0 2 4 6 8 10 12 14 16 18 0 20 40 60 80 100 2a 3a Yield / % Time / h 4a
recovered by centrifugation and reused without further reactivation. After the 2nd run, the yield of
4a decreased slightly from 97% to 91%, while product 3a appeared with 5% yield. After the 3rd
and 4th runs, the product distribution fully changed, since the yield of 4a decreased to <50% at the expense of 5a. One can mention that the recycled Pd/Al2O3 catalyst behave like Ru, Pt and Rh/Al2O3 catalysts but with the formation of less by-products. After the 4th run, the catalyst was recovered by filtration and washed with ethanol three times, dried overnight, calcined at 500 °C for 2 h and reduced under H2. The refreshed catalyst exhibited a yield of product 4a of 78% along with 13% yield of 5a. Interestingly, no Pd loss was detected during the reaction, as inferred from ICP measurements in the reaction solution after the 4th run. These results point out that Pd leaching is not at the origin of the observed catalyst deactivation. To explain this change in the selectivity, the spent catalyst after the 4th run was subjected to characterization using different techniques.
Figure 5. Product distribution in consecutive catalytic runs in the reductive amination of 1a.
Reaction conditions: Pd/Al2O3 (20 mg), 1a (0.4 g), EtOH (4 g), NH3 (5 bar), H2 (20 bar), 120 °C, 20 h. 1st 2nd 3rd 4th Refresh 0 20 40 60 80 100 Yield / % 2a 3a 4a 5a
Characterization of Recycled Pd/Al2O3. Figure S3 plots the N2 adsorption/desorption curves at 77 K of the fresh and recycled Pd/Al2O3 catalyst after the 4th run, whereas Table 3 lists the textural properties. Although, both samples exhibited the presence of mesopores, the spent sample showed a slight decline of the specific surface area, pore volume and average pore size. This observation might be linked to the modification of the active sites leading to a different pathway driven by a change of the Pd particle, as well as by pore blockage due to coking or due to the adsorption of oligomers.
Table 3. Textural properties of the Pd/Al2O3 catalysts.
Pd/Al2O3 SBET (m2/g) Pore volume (cm3/g) Pore size (nm)
Fresh 158 0.57 14.4
Recycled 147 0.51 13.9
TG analysis was then performed on the spent catalyst (Figure 6). The thermal profile of the fresh Pd/Al2O3 exhibited three main regions i) at around 100 °C (water desorption; (ii) at about 200 °C (decomposition or vaporization of small organic molecules); and (iii) in the range of 250-600 °C (possible dehydration of the alumina support). The TG curve of the recycled Pd/Al2O3 under air exhibited a quite different thermal profile. For the spent catalyst, three regions were observed in the temperature range 250-600 °C reflecting the possible formation of carbon deposits. Besides, the total weight loss increased from 9.7 wt% for the fresh catalyst to 12.3 wt% for the recycled catalyst. Thus the catalyst can be regenerated by calcination at 500 °C.
Figure 6. TG profiles of Pd/Al2O3 under air: (a) fresh and (b) recycled catalyst after the 4th run.
To further understand the nature of adsorbed species on the recycled Pd/Al2O3, the catalyst was analyzed by FT-IR. Figure 7 shows the FT-IR spectra of the fresh, spent and regenerated Pd/Al2O3. Two characteristic bands centered at 1157 cm-1 and 1230 cm-1 belonging to C-O bonds in the furan ring were preserved after regeneration.57 Furthermore, by calcination at 500 °C for 1 h, the carbon deposits could be almost completely removed.
To achieve more information on the catalyst surface, the recycled Pd/Al2O3 was analyzed by XPS after 4th run (Figure 8) and it has two doublets with BE (Pd 3d
5/2) 335.1 and 336.7 eV attributed to metallic Pd0 and Pd2+ (PdO), respectively.58,59 Both of the states coexisting on the surface almost had no shift in comparison to that of fresh Pd/Al2O3 (335.1 and 336.9 eV). At the same time most of the Pd (82 atom%) still kept in metallic form, suggesting that the apparent change of selectivity is not attributed to Pd oxidation.
800 600 400 200 0 86 88 90 92 94 96 98 100 3% 0.9% 2.4% 2.7% 3.3% Wei ght f rac tio n (% ) Fresh Pd/Al2O3 Recycled Pd/Al2O3 a b 4.7% 2% 3% Temperature (oC)
Figure 7. FT-IR spectra of (a) fresh, (b) recycled and (c) regenerated Pd/Al2O3.
Figure 8. XPS Pd 3d spectra of fresh Pd/Al2O3 and recycled Pd/Al2O3.
Finally TEM was used to measure the size distribution and average size of Pd nanoparticles in the Pd/Al2O3 catalyst before and after reaction (Figure 9). The recycled catalyst exhibits an obvious increase of the average size of the Pd nanoparticles from 2.3 nm to 4.7 nm. In the meantime, a clear agglomeration of Pd nanoparticles is observed (Figure S4). Moreover, EDS analysis on the fresh and spent catalysts (Figure S5 and S6) confirmed the formation of carbon
2500 2000 1500 1000 0 20 40 60 80 100 Tran smita nc e (% ) Wavenumber (cm-1) Fresh Pd/Al2O3 Recycled Pd/Al2O3
Recycled Pd/Al2O3 (calcinated)
a b 1157 1230 c C-O 345 340 335 330 Pd2+ 3d5/2 Pd0 3d5/2 Pd 3d XP inten sity
Binding Energy (eV) Fresh Pd/Al2O3 335.1 336.9 345 340 335 330 Pd2+ 3d5/2 Pd0 3d5/2 Pd 3d XP inten sity
Binding Energy (eV)
Recycled Pd/Al2O3
335.1
deposits during the reaction. Overall, catalyst deactivation during the reductive amination of 1a occurred mainly by two different pathways: (1) sintering/agglomeration of Pd nanoparticles, and (2) formation of carbon deposits on Pd. It seems that in the presence of used catalyst, the reaction pathway changed and the major route is reaction pathway (i) (Scheme 2).
Figure 9. TEM micrographs of the catalyst and distribution of the Pd nanoparticle size. (a) Fresh
Pd/Al2O3; (b) Recycled Pd/Al2O3.
Substrate Scope. The scope of the reaction was further extended to the reductive amination of a
series of furan-derived ketones over Pd/Al2O3 (Table 4). The detailed reaction conditions are summarized in the SI. For all substrates, the corresponding amines were efficiently obtained with 91-98% yield. These results clearly show that Pd/Al2O3 can enable the efficient and selective synthesis of a large variety various THF-derived amines from the corresponding biomass-derived carbonyl vinylfurans. 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 0 10 20 30 40 50 60 <d> = 2.3 nm Freque ncy (%) Diameter (nm)
2.3 nm
(a)
2 3 4 5 6 7 8 9 0 10 20 30 40 50 60 <d> = 4.7 nm Frequency (% ) Diameter (nm)4.7 nm
(b)
Table 4. THF-derived amines produced from furan-derived ketones over Pd/Al2O3.
Entry Substrate Product Yield / %
1 94 2 96 3 96 4 98 5 97 6 91 7 92
One pot two step reaction. It is highly desired to design intensified reactions in a single reactor
avoiding the separation and purification of intermediates. In this view, first the aldol condensation reaction was optimized using Amberlyst-26 (A26) as catalyst to achieve high yield towards the carbonyl-conjugated vinylfuran 1a at an equimolar MIBK/furfural ratio in ethanol. When the reaction was conducted at 80 °C with an A26/furfural weight ratio of 40 wt%, a maximum yield
of 4a of 90% was achieved after 6 h with 99% furfural conversion (Figure S8). Furthermore, when the MIBK/furfural molar ratio increased to 1.2, 96% yield of 1a was achieved after 6 h at 80 °C.
Table 5. Catalytic conversion of furfural to THF-derived amine (4a) with Amberlyst-26 and
Pd/Al2O3 solid catalysts in a one-reactor process.a
Entry Substrate Catalyst (wt%) Time (h) P H2 (bar) Yield (%) A-26 Pd/Al2O3 1 st 2nd 2a 3a 4a 5a 1b 1a – 5 – 20 20 n.d. n.d. 98 n.d. 2c Furfural 40 5 6 20 20 10 29 36 6 3 Furfural 40 5 6 20 20 n.d. 23 60 n.d. 4 Furfural 40 5 6 40 20 n.d. 11 74 n.d. 5 Furfural 40 5 6 20 40 n.d. 10 75 n.d. 6 Furfural 40 10 6 20 20 n.d. 11 74 n.d. 7d Furfural 40 10 6 20 20 n.d. 9 85 n.d.
aFurfural conversion was 100% for all the reactions. (1st) All runs were carried out at 80 ºC in 2 g EtOH with 108 mg
furfural while keeping an equimolar MIBK/furfural ratio and an Amberlyst-26/furfural weight ratio of 40 wt%, (2nd)
the reductive amination reactions were all following the first step of aldol condensation, the mass ratio of Pd/Al2O3 –
theoretical 1a 5 wt%, 5 bar NH3, H2. bComparing Reaction: Pd/Al
2O3 (10 mg), 1a (0.2 g), EtOH (2 g), NH3 (5 bar), H2 (20 bar), 120 °C, 20h. cPd/Al
2O3 was added with Amberlyst-26 at the beginning of the reaction. dThe MIBK/furfural molar ratio was 1.2/1.
The one-reactor catalytic tests were further carried out using a physical mixture of A26 and Pd/Al2O3 (Table 5). A yield of 36% to product 4a was obtained together with a complex mixture of hydrogenated products and amines (entry 2). A much higher yield (60%) of 4a was obtained when Pd/Al2O3 was added during the reductive amination step (entry 3). The reaction conditions of the second step were further optimized to increase further the yield of product 4a (entries 4-6). A yield of product 4a up to 75% with 10% yield of product 3a could be achieved by increasing the reaction time from 20 h to 40 h, the H2 pressure from 20 bar to 40 bar, or the Pd/Al2O3 amount from 5 wt% to 10 wt%. By increasing the MIBK/furfural molar ratio in the first step to 1.2 to
promote the formation of the aldolization product 1a, a maximum yield of 85% of product 4a could be achieved in the presence of 10 wt% Pd/Al2O3 (entry 7).
Conclusion
In summary, a new family of THF-derived amines was selectively synthesized with ~98% yield starting from bio-derived ketones using NH3 or amines as a nitrogen source, molecular hydrogen as a reducing agent and Pd/Al2O3 as catalyst. This system efficiently catalyzed the hydrogenation of the unsaturated C=C bond and the reductive amination of the carbonyl group at mild reaction conditions in a single reactor. Although no Pd leaching from the catalyst was evidenced during the reaction, a change of the catalyst selectivity was observed after the 3rd catalytic run mostly due to the Pd sintering and the deposit of carbon species. Besides, furfural could be converted into functionalized THF-amine in a one-pot two steps process without any purification between the two steps and with a yield up to 85%. Thus, our present work opens a new avenue for producing biobased amines with high potential for the synthesis of biosolvents or biosurfactants. Besides, this study provides a toolbox that enables the sustainable conversion of furfural into target THF-derived amines. The deposition of Pd over a multifunctional supports and the design of a continuous flow processes are current lines of investigation to optimize the performance of the one-reactor process.
ASSOCIATED CONTENT
Supporting Information. A listing of the contents of each file supplied as Supporting
Information should be included. For instructions on what should be included in the Supporting Information as well as how to prepare this material for publications, refer to the journal’s
Instructions for Authors. Corresponding Authors *marc.pera-titus-ext@solvay.com *karine.vigier@univ-poitiers.fr Author Contributions Funding Sources
ANR agency for the funding of FurCab Project ANR-15-CE07-0016. Région Nouvelle Aquitaine for the funding of this project through the FR CNRS INCREASE 3707, the chaire TECHNOGREEN and FEDER, the University of Poitiers and the CNRS for their financial support.
Notes
Any additional relevant notes should be placed here.
ACKNOWLEDGMENT
The authors would like to thank the ANR agency for the funding of FurCab Project ANR-15-CE07-0016. Authors are also grateful to the Région Nouvelle Aquitaine for the funding of this project through the FR CNRS INCREASE 3707, the chaire TECHNOGREEN and FEDER. CNRS and University of Poitiers are also acknowledged.
ABBREVIATIONS
THF, tetrahydrofuran; MIBK, methyl isobutyl ketone.
(1) Lawrence, S. A. Amines: Synthesis, Properties and Applications; Cambridge University Press, 2004.
(2) Koutinas, A. A.; Du, C.; Wang, R. H.; Webb, C. Introduction to Chemicals from Biomass, Wiley, Hoboken, 2008, pp. 77.
(3) Gandini, A.; Lacerda, T. M.; Carvalho, A. J. F.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637-1669.
(4) Delidovich, I.; Hausoul, P. J. C.; Deng, L.; Pfützenreuter, R.; Rose, M.; Palkovits, R. Alternative Monomers Based on Lignocellulose and Their Use for Polymer Production.
Chem. Rev. 2016, 116, 1540-1599.
(5) Willcox, D.; Chappell, B. G. N.; Hogg, K. F.; Calleja, J.; Smalley, A. P.; Gaunt, M. J. A General Catalytic β-C-H Carbonylation of Aliphatic Amines to β-Lactams. Science 2016,
354, 851-857.
(6) Jagadeesh, R. V.; Murugesan, K.; Alshammari, A. S.; Neumann, H.; Pohl, M.-M.; Radnik, J.; Beller, M. MOF-derived Cobalt Nanoparticles Catalyze a General Synthesis of Amines.
Science 2017, 358, 326-332.
(7) Pelckmans, M.; Renders, T.; Van de Vyver S.; Sels, B. F. Bio-Based Amines through Sustainable Heterogeneous Catalysis. Green Chem. 2017, 19, 5303-5331.
(8) Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181-14224.
(9) Talwar, D.; Salguero, N. P.; Robertson, C. M.; Xiao, J. Primary Amines by Transfer Hydrogenative Reductive Amination of Ketones by Using Cyclometalated IrIII Catalysts.
(10) Senthamarai T.; Murugesan, K.; Schneidewind, J.; Kalevaru, N. V.; Baumann, W.; Neumann, H.; Kamer, P. C. J.; Beller, M.; Jagadeesh, R. V. Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines. Nat. Commun.
2018, 9, 4123.
(11) Tan, X.; Gao, S.; Zeng, W.; Xin, S.; Yin, Q.; Zhang, X. Asymmetric Synthesis of Chiral Primary Amines by Ruthenium Catalyzed Direct Reductive Amination of Alkyl Aryl Ketones with Ammonium Salts and Molecular H2. J. Am. Chem. Soc. 2018, 140, 2024-2027. (12) Roose, P.; Eller, K.; Henkes, E.; Rossbacher, R.; Höke, H. Amines, Aliphatic, Ullmann’s
Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 2015; pp 1−55.
(13) Deng, W.; Wang, Y.; Zhang, S.; Gupta, K. M.; Hülsey, M. J.; Asakura, H.; Liu, L.; Han, Y.; Karp, E. M.; Beckham, G. T.; Dyson, P. J.; Jiang, J.; Tanaka, T.; Wang, Y.; Yan, N. Catalytic amino acid production from biomass-derived intermediates. PNAS, 2018, 115(20), 5093-5098.
(14) Li, H.; Guo, H.; Su, Y.; Hiraga, Y.; Fang, Z.; Hensen, E. J. M.; Watanabe, M.; Smith, R. L. N-formyl-stabilizing quasi-catalytic species afford rapid and selective solvent-free amination of biomass-derived feedstocks. Nat. Commun. 2019, 10, 699.
(15) Yan, N.; Chen, X. Don’t Waste Seafood Waste. Nature, 2015, 524, 155-157
(16) Brun, N.; Hesemann, P.; Esposito, D. Expanding the Biomass Derived Chemical Space.
Chem. Sci. 2017, 8, 4724-4738
(17) Imm, S.; Bahn, S.; Zhang, M.; Neubert, L.; Neumann, H.; Klasovsky, F.; Pfeffer, J.; Haas, T.; Beller, M. Improved Ruthenium-Catalyzed Amination of Alcohols with Ammonia: Synthesis of Diamines and Amino Esters. Angew. Chem. Int. Ed. 2011, 50, 7599-7603.
(18) Chatterjee, M.; Ishizaka, T.; Kawanami, H. Reductive Amination of Furfural to Furfurylamine Using Aqueous Ammonia Solution and Molecular Hydrogen: an Environmentally Friendly Approach. Green Chem. 2016, 18, 487-496.
(19) Pelckmans, M.; Vermandel, W.; Waes, F. V.; Mooen, K.; Sels, B. F. Low-Temperature Reductive Aminolysis of Carbohydrates to Diamines and Aminoalcohols by Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2017, 56, 14540-14544.
(20) Liang, G.; Wang, A.; Li, L.; Xu, G.; Yan, N.; Zhang, T. Production of Primary Amines by Reductive Amination of BiomassDerived Aldehydes/Ketones. Angew. Chem. Int. Ed. 2017,
56, 3050-3054.
(21) Niemeier, J.; Engel, R. V.; Rose, M. Is Water a Suitable Solvent for the Catalytic Amination of Alcohols? Green chem. 2017, 19, 2839-2845.
(22) Knaus, T.; Böhmer, W.; Mutti, F. G. Amine Dehydrogenases: Efficient Biocatalysts for the Reductive Amination of Carbonyl Compounds. Green Chem. 2017, 19, 453-463.
(23) Yan, T.; Feringa, B. L.; Barta, K. Iron Catalysed Direct Alkylation of Amines with Alcohols.
Nat. Commun. 2014, 5, 5602-5609.
(24) Elangovan, S.; Neumann, J.; Sortais, J.-B.; Junge, K.; Darcel, C.; Beller, M. Efficient and Selective N-Alkylation of Amines with Alcohols Catalysed by Manganese Pincer Complexes. Nat. Commun. 2016, 7, 12641-12649.
(25) Gomez, S.; Peters, J. A.; Maschmeyer, T. The Reductive Amination of Aldehydes and Ketones and the Hydrogenation of Nitriles Mechanistic Aspects and Selectivity Control.
Adv. Synth. Catal. 2002, 344, 1037-1057.
(26) Utusunomiya, M.; Kuwano, R.; Kawatsura, M.; Hartwig, J. F. Rhodium-Catalyzed Anti-Markovniko Hydroamination of Vinylarenes. J. Am. Chem. Soc. 2003, 125, 5608-5609.
(27) Ogo, S.; Uehara, K.; Abura, T.; Fukuzumi, S. pH-Dependent Chemoselective Synthesis of α-Amino Acids. Reductive Amination of α-Keto Acido Acids with Ammonia Catalyzed by Acid-Stable Iridium Hydride Complexes in Water. J. Am. Chem. Soc. 2004, 126, 3020-3021. (28) Maya, R. J.; Poulose, S.; John, J.; Varma, R. L. Direct Reductive Amination of Aldehydes
via Environmentally Benign Bentonite-Gold Nanohybrid Catalysis. Adv. Synth. Catal. 2017,
359, 1177-1184.
(29) Gallardo-Donaire, J.; Ernst, M.; Trapp, O.; Schaub, T. Direct Synthesis of Primary Amines
via Ruthenium-Catalysed Amination of Ketones with Ammonia and Hydrogen. Adv. Synth.
Catal. 2016, 358, 358-363.
(30) Gross, T.; Seayad, A. M.; Ahmad, M.; Beller, M. Synthesis of Primary Amines: First Homogeneously Catalyzed Reductive Amination with Ammonia. Org. Lett. 2002, 41, 2055-2058.
(31) Yuan, H.; Li, J.; Su, F.; Yan, Z.; Kusema, B. T.; Streiff, S.; Huang, Y.; Pera-Titus, M.; Shi, F. Reductive Amination of Furanic Aldehydes in Aqueous Solution over Versatile NiyAlOx Catalysts. ACS Omega 2019, 4, 2510-2516.
(32) Deng, D.; Kita, Y.; Kamata, K.; Hara, M. Low-Temperature Reductive Amination of Carbonyl Compounds over Ru Deposited on Nb2O5·nH2O. ACS Sustainable Chem. Eng.
2019, 7, 4692-4698.
(33) Manzoli, M.; Gaudino, E. C.; Cravotto, G.; Tabasso, S.; Baig, R. B. N.; Colacino, E.; Varma, R. S. Microwave-Assisted Reductive Amination with Aqueous Ammonia: Sustainable Pathway Using Recyclable Magnetic Nickel-Based Nanocatalyst. ACS Sustainable Chem.
(34) Murugesan, K.; Beller, M.; Jagadeesh R. V. Reusable Nickel Nanoparticles-catalyzed Reductive Amination for Selective Synthesis of Primary Amines. Angew. Chem. 2019, 131, 5118-5122.
(35) Zhang, Y.; Yang, H.; Chi, Q.; Zhang, Z. Nitrogen-doped carbon supported nickel nanoparticles: a robust catalyst to bridge the hydrogenation of nitriles and the reductive amination of carbonyl compounds for the synthesis of primary amines. ChemSusChem 2019,
12, 1246-1255.
(36) Jiang, S.; Verrier, C.; Ahmar, M.; Lai, J.; Ma, C.; Muller, E.; Queneau, Y.; Pera-Titus, M.; Jérôme, F.; De Oliveira Vigier, K. Unveiling the Role of Choline Chloride in Furfural Synthesis from Highly Concentrated Feeds of Xylose. Green Chem. 2018, 20, 5104-5110. (37) Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A
Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels.
Energy Environ. Sci. 2016, 9, 1144-1189.
(38) Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621-7640.
(39) Komanoya, T.; Kinemura, T.; Kita, Y.; Kamata, K.; Hara, M. Electronic Effect of Ruthenium Nanoparticles on Efficient Reductive Amination of Carbonyl Compounds. J. Am. Chem. Soc.
2017, 139, 11493-11499.
(40) Chandra, D.; Inoue, Y.; Sasase, M.; Kitano, M.; Bhaumik, A.; Kamata, K.; Hosono, H.; Hara, M. A High Performance Catalyst of Shape-Specific Ruthenium Nanoparticles for Production of Primary Amines by Reductive Amination of Carbonyl Compounds. Chem. Sci. 2018, 9, 5949-5956
(41) Huber, G. W.; Chheda, J. N.; Barrett, C. J.; Dumesic, J. A. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446-1450.
(42) Sutton, A. D.; Waldie, F. D.; Wu, R.; Schlaf, M.; Silks, L. A. P., III; Gordon, J. C. The Hydrodeoxygenation of Bioderived Furans into Alkanes. Nat. Chem. 2013, 5, 428-432. (43) Julis, J.; Leitner, W. Synthesis of 1-Octanol and 1,1-Dioctyl Ether from Biomass-Derived
Platform Chemicals. Angew. Chem. Int. Ed. 2012, 51, 8615-8619.
(44) Xia, Q.; Xia, Y.; Xi, J.; Liu, X.; Wang, Y. Energy-Efficient Production of 1-Octanol from Biomass-Derived Furfural-Acetone in Water. Green Chem. 2015, 17, 4411-4417.
(45) Shao, Y.; Xia, Q.; Liu, X.; Lu, G.; Wang, Y. Pd/Nb2O5/SiO2 Catalyst for the Direct Hydrodeoxygenation of Biomass-Related Compounds to Liquid Alkanes under Mild Conditions. ChemSusChem 2015, 8, 1761-1767.
(46) Li, M.; Xu, X.; Gong, Y.; Wei, Z.; Hou, Z.; Li, H.; Wang, Y. Ultrafinely Dispersed Pd Nanoparticles on a CN@MgO Hybrid as a Bifunctional Catalyst for Upgrading Bioderived Compounds. Green Chem. 2014, 16, 4371-4377.
(47) Luska, K. L.; Bordet, A.; Tricard, S.; Sinev, I.; Grünert, W.; Chaudret, B.; Leitner, W. Enhancing the Catalytic Properties of Ruthenium Nanoparticle-SILP Catalysts by Dilution with Iron. ACS Catal. 2016, 6, 3719-3726.
(48) Xu, J.; Li, N.; Yang, X.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of Diesel and Jet Fuel Range Alkanes with Furfural and Angelica Lactone. ACS Catal. 2017,
(49) Xu, J.; Li, L.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Li, N. Synthesis of Renewable C8-C10 Alkanes with Angelica Lactone and Furfural from Carbohydrates. ACS Sustainable Chem.
Eng. 2018, 6, 6126-6134.
(50) Rojas-Buzo, S.; García-García, P.; Corma, A. Hf-Based Metal-Organic Frameworks as Acid-Base Catalysts for the Transformation of Biomass-Derived Furanic Compounds into Chemicals. Green Chem. 2018, 20, 3081-3091.
(51) Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Solvent-Free Synthesis of C10 and C11 Branched Alkanes from Furfural and Methyl Isobutyl Ketone.
ChemSusChem 2013, 6, 1149-1152.
(52) Lee, R.; Vanderveen, J. R.; Champagne, P.; Jessop, P. G.; CO2-Catalysed aldol condensation of 5-hydroxymethylfurfural and acetone to a jet fuel precursor. Green Chem. 2016, 18, 5118-5121.
(53) He, X.; Conner, A. H.; Koutsky, J. A. Evaluation of furfurylamines as curing agents for epoxy resins. J. Polym. Sci., Part A: Polym. Chem. 1992, 30, 533–542
(54) Zhu, Y.; Batchelor, R.; Lowe, A. B.; Roth, P. J. Design of Thermoresponsive Polymers with Aqueous LCST, UCST, or Both: Modification of a Reactive Poly(2-vinyl-4,4-dimethylazlactone) Scaffold. Macromolecules 2016, 49, 672-680.
(55) Liu, Y.; Zhou, K.; Shu, H.; Liu, H.; Lou, J.; Guo, D.; Wei, Z.; Li, X. Switchable synthesis of furfurylamine and tetrahydrofurfurylamine from furfuryl alcohol over RANEY® nickel.
Catal. Sci. Technol. 2017, 7, 4129-4135.
(56) Jiang, S.; Muller, E.; Pera-Titus, M.; Jerôme, F.; De Oliveira Vigier, K. Direct catalytic conversion of furfural to furan-derived amines, Green Chem. 2019, submitted.
(57) Tian, Q.; Yuan, Y. C.; Rong, M. Z.; Zhang, M. Q. A Thermally Remendable Epoxy Resin.
J. Mater. Chem. 2009, 19, 1289-1296.
(58) Wang, Y.; Yao, J.; Li, H.; Su, D.; Antonietti, M. Highly Selective Hydrogenation of Phenol and Derivatives over a Pd@Carbon Nitride Catalyst in Aqueous Media. J. Am. Chem. Soc.
2011, 133, 2362-2365.
(59) Ainembabazi, D.; An, N.; Manayil, J. C.; Wilson, K.; Lee, A. F.; Voutchkova-Kostal, A. M. Acceptorless Amine Dehydrogenation and Transamination Using Pd-Doped Hydrotalcites.
BRIEFS (Word Style “BH_Briefs”). If you are submitting your paper to a journal that requires a brief, provide a one-sentence synopsis for inclusion in the Table of Contents.