Ab Initio Screening of Lithium Diffusion Rates
in Transition Metal Oxide Cathodes
for Lithium Ion Batteries
by Charles J. Moore
B. S. Materials Science and Engineering Northwestern University, 2006
ARCHVES
MASSACHUSETTS INSTMfrE OF TECHNOLOGYMAYS2113
L B A R IE
SUBMITTED TO THE DEPARTMENT OF MATERIALS SCIENCE AND ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR
THE DEGREE OF
MASTERS OF SCIENCE IN MATERIALS SCIENCE AND ENGINEERING AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY SEPTEMBER 2012
@2012 Massachusetts Institute of Technology. All rights reserved.
Signature of Author:
Certified by:
Accepted by:
Departmdnt of Materials Science and Engineering August 9, 2012 Gerbrand Ceder Professor of Materials Science and Engineering Thesis Supervisor
Gerbrand Ceder Professor of Materials Science and Engineering Chairman, Committee for Graduate Students
-Ab Initio Screening of Lithium Diffusion Rates in Transition Metal Oxide Cathodes for Lithium Ion Batteries
by
Charles J. Moore
Submitted to the Department of Materials Science and Engineering on August 9th, 2012 in Partial Fulfillment
of the Requirements for the Degree of Master of Science in Materials Science and Engineering
ABSTRACT
A screening metric for diffusion limitations in lithium ion battery cathodes is derived using transition state theory and common materials properties. The metric relies on net activation barrier for lithium diffusion. Several cath-ode materials are screened using this approach: 3'-LiFePO4, hexagonal
LiMnBO3, monoclinic LiMnBO3, Li3Mn(CO3)(PO4), and Li9V3 (P207)3(PO4)2.
The activation barriers for the materials are determined using a combined approach. First, an empirical potential model is used to identify the lithium diffusion topology. Second, density functional theory is used to determine
migration barriers.
The accuracy of the empirical potential diffusion topologies, the density functional theory migration barriers, and the overall screening metric are compared against experimental evidence to validate the methodology. The accuracy of the empirical potential model is also evaluated against the density functional theory migration barriers.
Thesis Supervisor: Gerbrand Ceder
Contents
1 Introduction 2 Background2.1 Transport theory . . . . 2.2 Experimentally measuring rate 2.3 Atomistic methods for diffusion 2.4 Similar studies . . . . 2.4.1 Layered materials . . . . 2.4.2 Olivine materials . . . . 2.4.3 Other materials . . . . . 3 Methodology 3.1 Topology Determination . . . . 3.2 Barrier Evaluation . . . . 4 Screening Results 4.1 )'-LiFePO4 . . . . 4.2 Hexagonal LiMnBO3 4.3 Monoclinic LiMnBO3 4.4 LiMn(CO3)(PO4) .. . 4.5 LixV3(P20 7)3(PO4)2
4.5.1 Evaluation of lithiated structure, x = 9 4.5.2
4.5.3
Evaluation of partially lithiated structure, x = 8 . . . . Comparison to experiments . . . . 5 Discussion
5.1 Accuracy of Topology Determination . . . . 5.2 Acuracy of Barrier Evaluation . . . .
5.3 Factors affecting Li diffusivity . . . . A Empirical Potential Specification
References 6 8 8 10 12 16 16 18 19 22 23 24 26 26 29 31 34 37 37 39 41 46 46 48 52 55 57 . . . . . . . . . . . . . . . . . . . . . .
1
Introduction
Cathode choice for Li-ion batteries is an extremely active area of research. While most currently commercialized cells use one of three classes of materials: lay-ered compounds, Spinel, or olivine, interest in potential new cathode materials spans a much wider range of materials. Some of the recently discovered potential cathodes include new phosphates [1], borates [2], and oxy-fluorides [3].
One effort at cathode discovery particularly worthy of mention is the high-throughput computational cathode design project at MIT [4] of which this thesis was a part. This design project sought to accelerate cathode discovery with com-putational methods. All known inorganic crystals structures from the Inorganic Crystal Structure Database were modeled using Density Functional Theory [5].
Novel materials were predicted using structure prediction methods [6]. Potential Li-ion cathode materials were then screened using a variety of computational pre-dictions: Li-voltage, phase stability, thermal stability, and rate prediction. This thesis contains the rate prediction aspect.
Rate limitations play an important role in determining the viability of a cath-ode material and high rate cathcath-odes have received much interest [7]. Indeed, one of the main factors preventing the widespread commercialization of electric vehi-cles is the limited range-between charges; doubtless, the range-between charges would be much less of an issue if charging times were under 5 minutes. Realis-tically, the choice of cathode material for commercial batteries is partially
deter-mined by matching the rate-capability of the cathode with the required rate for the application. High power applications, such as power tools and hybrid electric vehicles tend to skew towards higher power cathodes such as olivine and spinel cathodes while low power applications such as portable electronics and fully elec-tric vehicles tend to skew towards layered cathode materials due to the higher energy density.
Rate limitations in cathode materials may stem from a variety of factors in-cluding polaron-based electrical conductivity [8], surface transport [7] and bulk, solid-state diffusion. This thesis focused soley only on bulk, solid-state Li-diffusion. Since cycling an intercalation cathode involves inserting (and remov-ing) Li ions directly into (from) the crystal unitcell, the Li ions must be able to migrate to (egress from) each active Li site. Short-circuit diffusion processes such as grain boundary and surface diffusion do not access the entire active range of the material and are not sufficient for cycling. Ample solid-state bulk lithium dif-fusion may be considered a necessary, but not sufficient criteria for a high rate cathode.
There has recently been a surge in the use of atomistic modeling to try and evaluate Li-diffusion in electrodes for Li-ion batteries (Section 2.4). This the-sis further develops an automated methodology for performing such screenings by using emirical potentials to first identify the Li+ diffusion topology and then refining migration barriers with ab initio calculations. The method is applied to several novel cathode materials and the validity of the the results is used to
evalu-ated the validity of the underlying screening methodology.
2 Background
2.1
Transport theory
The kinetic theory of transport helps to link the inherent diffusivity of Li in a cath-ode to the rate at which it may be cycled. The solution to the diffusion equation in one dimension for the diffusivity of Li into a completely delithiated cathode reveals the following relation:
c(x, t) = co erfc j...
where c(x, t) is the concentration of Li at a depth of x at a time t, co is the concen-tration of Li at the surface, and D is the diffusivity of Lithium in the cathode [9]. Given that primary particle size may be as small as 100nm, significant solid state diffusion is required across at least 50nm during charging and discharging. In or-der for the concentration at 50nm to reach 80% of the concentration at the surface within 2 hours, a diffusivity of 2.7 -10-14cm2 is required. This is a reasonable
Sec
benchmark for the required diffusivity.
related to the hops by the relation:
F (r2)f
DLu = fd 2d
where F is the total hop frequency, (r2) is the mean squared distance Li travels per
hop, d is the dimensionality of the system (ID, 2D or 3D), and
f
is a correlation factor [9]. The correlation factorf
is between 0 and 1 and serves to suppress the diffusivity when the atoms tend to hop back and forth with no net motion. To first order, the hop frequency for any given hop F' is= -AEm/kT
where v is a characteristic frequency, and AEm is the migration barrier [9]. Vari-ance in DLu is dominated by variations in the activation barrier. The exponential dependance produces a ten fold reduction diffusivity for every 59 meV increase in migration barrier. As migration barriers may vary by over 500 meV between different materials, differences in migration barriers may account for nine orders of magnitude of differences in diffusivities!
With some typical values, it is possible to estimate an upper limit on activation barrier. A typical value for the attempt frequency is v = 10-1 s-1 [10-12]. Hop
distance is on the order of atomic lengths, take 3A as a typical value. To obtain a
diffusivity of 2.7. 10-4 c sec with these typical values requires a migration barrier of less than 635 meV. Due to the steep dependance of DLi on activation barrier, it
is possible to broadly classify materials with migration barriers less than 450 meV as facile bulk Li diffusers and materials with migration barriers above 700 meV as insufficient Li diffusers for Li-ion battery cathodes. Materials with intermediate activation barriers have indeterminate diffusivity.
2.2
Experimentally measuring rate
A variety of experimental techniques are used to evaluate the rate capabilities of cathode materials. The majority of these techniques test the material as an elec-trode in a electrochemical cell, generally a half-cell with a Li-metal anode. These techniques benefit from both their ease and technological relevance. Galvanos-tatic cycling at a variety of rates is arguably the most technologically relevant test, because it directly measures the performance of a cathode as it is to be used. It is also the most ubiquitous. However, it provides little to distinguish between differ-ent potdiffer-ential rate limiting mechanisms. Electrochemical impedance spectroscopy (EIS) provides time domain separation of rate-limiting processes, but still requires an equivalent circuit. The physical origins of different elements in this equivalent circuit are not always entirely clear. Similarly, other techniques such as galvanos-tatic intermittent titration technique (GITT) [13] and cyclic voltametry (CV) [14] suffer from the need for knowledge about underlying mechanisms and physical parameters such as electrode area, and diffusion isotropy/anisotropy before a dif-fusion constant can be backed out.
The various methods and assumptions can produce a wide range of measured diffusivities for the same system. Within a single study of a single sample, mea-surements of the diffusivity of Li.CoO2 differed by over 2 orders of magnitude depending on the technique used [15]. Comparing different studies of LiCoO2,
produced diffusion constants ranging from 10-8c sec to 10-15 CMsec2 [16]. Perhaps not surprisingly, it is extremely difficult to obtain reliable quantitative measure-ments on the fundamental property of bulk lithium diffusivity from experimental techniques evaluating a system as complex as a complete electrochemical cell.
Measurements of activation barriers from Arrhenius type plots are perhaps more quantitatively reliable than diffusivities, although identification of the rate-limiting process (surface vs. bulk and electronic vs. ionic) continues to be prob-lematic.
By using the material as a membrane or solid electrolyte through which Li must diffuse rather than as an electrode in an electrochemical system allows isola-tion of Li diffusivity. Unfortunately, to date only a few systems have been studied in this manner. Single crystal samples of LiFePO4 with LiI electronically blocking electrodes have been tested [17], as have highly oriented graphitic carbon mem-branes [18]. While these experiments provide valuable results, sample prepara-tion may be difficult or impossible to perform similar studies on other systems, especially if one wants to distinguish between diffusivity along different crystal-lographic orientations or evaluate bulk diffusion as opposed to grain boundary diffusion.
2.3 Atomistic methods for diffusion
In contrast to the experimental methods, atomistic methods are extremely
spe-cific. The knowledge of the kinetic mechanism being measured is inherent to the
simulation. They do however sometimes lack relevance. It is entirely possible to evaluate the bulk diffusivity of a material which in practice is limited by elec-tronic conduction, or surface transport. This is both a virtue and a drawback. As a drawback, it makes the total evaluation of a potential cathode incomplete; high Li+diffusivity is not sufficient for hight rate capability. As a virtue, it is possible to study mechanisms which are not rate limiting, allowing study of bulk diffusivity even when other processes limit the material. It also allows direct investigation of high energy pathways when lower energy pathways exist. Such information is extremely difficult to obtain from experimental results.
At the heart of atomistic modeling is the energy evaluation of a given structural configuration via a Hamiltonian. The optimization of this total energy with respect to the structural degrees of freedom, and determining the shape of the energy landscape through which ions must traverse, is the main goal of these atomistic methods. Two broad groups of Hamiltonians are relevant to this work: those based on empirical inter-atomic potentials, and those based on density functional theory. Also relevant is the optimization method of nudged elastic band for determining the energy at activated sites.
potential energy function between oppositely charged ions combined with a Coulom-bic sum over interactions between formal charges assigned to each ion [19].
To-gether, they serve as a widely used Hamiltonian for ionic systems. The main bene-fit of such potentials, is the computational ease with which they may be evaluated. The two main drawbacks are: (1) lack of accuracy (2) lack of generality.
Many empirical-potentials are able to accurately reproduce equilibrium and near-equilibrium properties such as lattice parameters, phonon modes, and elas-tic properties. However, their accuracy for activated states relevant to Li migra-tion is less clear. Migramigra-tion studies in LiFeSO4 have shown a large discrepancy between empirical-potential methods and density functional theory predictions. Results from DFT predict barriers of 208meV for 1D diffusion and 976meV for 3D diffusion [3]. Two studies with different empirical potentials have returned very different activation barriers. One study [20] show a migration barrier of 360 meV for 1D diffusion, and 440 meV for 3D diffusion, while another [21] obtained 220 meV and 1101 meV respectively. Similar discrepancies have been shown in olivine-type materials (Table 1).
Furthermore, established libraries of empirical potentials for inorganic mate-rials tend to include only a few dozen elements at most. The library due to Bush contains only 19 metals + oxygen [19]. The similar Bond-Valence derived Morse potential library due to Adams et al. [21,24] is much broader based, and was de-rived explicitly for determining conduction topologies. However, it still remains dependent on the existence of BondValence parameters, and formal valences.
Table 1: Comparison of published DFT and empirical-potential migration barriers in olivine type materials LiMPO4. All migration barriers are for [010] direction
and expressed in meV.
Cation M DFT [22] Empirical [23]
Mn 250 620
Fe 270 550
Co 360 490
Ni 130 440
Despite these shortcomings, empirical potential methods have proven very ef-fective at determining the topology for lithium diffusion. In the case of LiFeSO4 [3,20,21] and the Olivine materials [22,23], the empirical potential methods and density functional theory are in concurrence on the migration topology, and at identifying the lowest energy path. Expanded validation of empirical potentials to predict Li+ diffusion topology is one of the goals of this study. The results are discussed in Section 5.1.
Density Functional Theory (DFT) methods are more computationally expen-sive. However, pseudopotentials are available for the majority of the the periodic table through commercial software packages. By tracking the spin polarized den-sity throughout the material, rather than assigning formal charges to ions, the method can handle materials with non-integer valence states and different mag-netic configurations. The success of DFT at explaining Li diffusion in the stud-ied materials is best understood by considering materials on a case-by-case basis (Section 2.4). One of the primary aims of this study is to further validate DFT for
evaluating Li+ diffusion. The results are discussed in Section 5.2.
One common extension to DFT is the +U extension. In addition to the stan-dard DFT Hamiltonion, the +U method adds a penalty for delocalized electronic states [25]. This method is frequently used to force electrons to localize on tran-sition metal ions in oxide calculations. In structures with mixed valence ions, standard DFT (no +U) typically delocalizes the electrons, resulting in many ions with identical (or similar) non-integer valences. With the +U self interaction cor-rection, charge ordering at integer valences may be induced.
Given any of these Hamiltonians it is possible to calculate the energy of a given atomic configuration. Identification of the minimum energy path between two locally stable atomic configurations can be found using the Nudged Elastic Band method (NEB) [26, 27]. In the NEB method, a series of images of the structure are evaluated. Taken in order, the images represent a pathway from one local minimum to the other. Rather than optimizing the energy of a single image, the total set of structures is optimized together. The atomic positions of the intermediate images are constrained such that the images are equally spaced along the reaction pathway. As the number of images increases, the images converge to the minimum energy pathway [28]. The NEB method is amongst the most efficient algorithms available to find the minimum energy path [29]. It's principle limitation is that both the initial and final states must be known before calculation can commence.
2.4 Similar studies
This thesis is not the first attempt at computationally screening cathode materials for lithium diffusion. Two classes of cathodes: layered (Section 2.4.1) and olivine (Section 2.4.2) have been studied in depth. While not studied as in depth as the first two classes, a few other materials have been screened for diffusivity (Section 2.4.3). The prior studies on Li diffusion have been piecemeal, considering only a single structural prototype at a time. Most of them, simply calculate activation barriers, with no attempt at understanding why the barriers are such and little at-tempt is made at validating the underlying methodology. There is an obvious need to unify calculations across structural prototypes and validate the computational methodology.
2.4.1 Layered materials
The first cathode material to be computationally screened for Li diffusion is lay-ered LiCoO2 [30,31]. The in-plane Li migration barriers were calculated using
DFT and the Li migration barriers were parameterized as a function of neighbor-ing Li-occupation. A kinetic Monte Carlo (KMC) algorithm was used to simulate diffusion of Li throughout the material and extract the Li diffusivity. Two im-portant results are noteworthy: (1) Li diffusion was dominated by a divacancy mechanism at high Li-content. (2) the relevant migration barrier varied by nearly 400 meV depending on Li concentration. These two factors combined to cause the
overall diffusivity to vary between 10-10 and 10-6 £*. The maximum diffusivity
sec
occurs near the composition Lio.7CoO2. Similar results were found for layered
LiTiS2 [32].
While these two factors are very important for understanding the diffusivity in layered compounds, they are also fairly unique. The importance of the divacancy mechanism can be explained by the unusually close proximity of Li pathways to adjacent Li sites. Adjacent sites must all be vacant, or the Li-Li interaction effec-tively blocks the pathway. The necessity of a divacancy suppresses the diffusivity at high Li concentrations. The change in migration barriers with Li concentration can be traced to the large change in c-lattice constant associated with removing Li. Layered structures are unique in that delithiation results in removal of an entire plane of atoms, causing a drastic contraction in the layer spacing.
Kang et al. [33] studied a variety of layered compounds and identified four fac-tors which influence the migration barriers. First, the Li slab distance, defined as the distance between oxygen layers surrounding the lithium layer, has a large ef-fect on the migration barriers. This distance is known to vary as occupancy in the Li layer changes. The same effect has been found in layered TiS2 [32]. Second,
the migration barriers increase as the valence on the transition metal increases due to stronger Coulombic interaction. As Li is extracted, the average valence of the transition metal species increases. The third factor is the choice of transition metal
(LiCoO2 vs LiTiO2, etc.). It is argued that late transition metals allow more elec-tron density to sit on the oxygen atoms, and therefore provide better Coulombic
screening between the Li and the transition metal, lowering the activation barrier. Although the effect is small compared to the other effects. Finally, anion choice has a large impact. Comparisons between layered oxides and sulfides indicate that the sulfides have significantly lower ( 200meV) migration barriers. They attribute this to the larger size of Sulfide ions and the opening of the Li slab spacing. And improved Coulomb screening by Sulfur ions.
2.4.2 Olivine materials
As mentioned in section 2.3, the olivine class of materials, principally LiFePO4,
has been studied with both DFT [22, 34, 35] and empirical potentials [21, 36]. While significant differences were found with different transition metals, no study has identified the root cause of these differences.
Most of the work has focused on the commercialized LiFePO4. As in the
layered cases, migration barriers are higher at lower Li content. Initially, experi-mental results showing 3D diffusion conflicted with predictions of extremely fast ID Li diffusion. This can be explained by the presence of anti-site defects block-ing the one dimensional diffusion channels leadblock-ing to a size-dependant diffusivity and cross links between the chanenls [37]. This is in agreement with the experi-mentally observed ultrafast charging at small length scales [7]. Although, ultrafast rates require a surface coating, presumably to aid in transport of Li into and out of the diffusion channels. This theory has been supported by recent atomistic
simulations [21]. In addition, the material is also known have limited electrical conductivity [8] which in some cases may limit the rate.
Ultimately, the olivine materials are a good demonstration that atomistic dif-fusion prediction adds value by helping to understand the root cause of limited diffusivity. The importance of channel blocking defects, surface coating, and elec-trical conductivity to the overall rate of the material are crucial to designing a high rate olivine. Without atomistic modeling, the material may have been dismissed as inherently low-rate. It remains unclear how frequently other cathode materi-als are limited by anti-site blocking, surface diffusivity, and/or limited electrical conductivity.
2.4.3 Other materials
Recently, interest has been shown in the Tavorite-like compounds (e.g. LiFeSO4)
[3, 20, 21, 34]. As detailed in section 2.3 there is a discrepancy between some empirical potential studies and those using DFT. The DFT NEB results predict very low activation barriers in one dimension. This has yet to be demonstrated experimentally. More work is necessary to fully understand this material.
The novel cathode Li2NiO2 [7] has been studied in a similar manner. A
low-barrier pathway was identified with DFT. This agrees well with the ability of the material to be electrochemically cycled.
have been studied. First, evident from the Ti cases is the importance of Li site shifting. Li occupies different sites at different compositions. When x in the formula Lii+xTi204 is positive, Li occupies octahedral sites. When x is negative,
Li occupies tetrahedra sites. In Li4+xTi5012, some of the Ti sites are filled with Li.
This has a profound impact on the diffusion pathway and the migration barriers. Site shifting did not show up in the Mn study, because of the limited composition considered. It is also worth noting that true activation barriers were not calculated for the Mn case, rather only the difference in site energies of the two potential Li sites. There is a strong dependance with charge ordering on the Mn sites [38], but it is unclear how closely the site energy differences calculated represent true migration barriers.
A study on anatase TiO2 [41] offers a rare opportunity to compare GGA and
GGA+U functionals and charge ordering effects on migration barriers. The pri-mary expected effect of changing functionals on migration barriers is that the +U addition localizes charges on the transition metal sites. Most of the relevant NEB calculations are defect calculations on either a Li+ vacancy, or a single Li+ ion. To balance the charge, a corresponding electron or hole is also present. In GGA, this electron/hole tends to be distributed across the material. In GGA+U it tends to localize on a single transition metal ion.
In TiO2 [41], activation barriers were calculated with GGA, several charge
orderings with GGA+U, and a charged computational cell with GGA+U (so no charge ordering is necessary). The spread between different GGA+U charge
or-derings was 98 meV, with the lowest and most relevant barrier at 588 meV. The GGA result was 511 meV. An amazingly similar comparison can be found in the studies of LiFePO4 olivine. With GGA [22], the vacancy and Li barriers are
200meV and 250meV respectively. With GGA+U [35], the minimum vacancy and Li barriers are 190meV and 290meV respectively. Charge ordering effects were again on the order of 100meV.
At first glance, it may be surprising that while the +U method introduces charge ordering effects on the order of 100meV, but that the difference in the net activation barrier was at most 77 meV, and as small as 10 meV. Upon fur-ther inspection it is less surprising. Migration barriers are determined by the difference in energy between the endpoint configuration and the transition state configuration. Finding the net activation barrier requires a global optimization of the endpoint over both Li position and charge ordering configurations. Similarly, finding the net migration barrier requires finding the lowest energy transition state which allows net Li+ migration. Due to the increase proximity of the diffusing Li+ and nearby transition metals, the energetic effects are stronger for transition states than for the endpoints. As long as the energy barrier for charge reordering is less than the energy barrier for Li+ migration, finding the net migration
bar-rier requires optimizing both the endpoint Li configuration and the Li+ migration transition state across all charge orderings. Running in pure GGA (without +U) to some degree allows the electronic configuration to perform this optimization automatically, whereas the localized charges in GGA+U can easily find the wrong
configuration.
Lithium diffusion in Wurtzite-type Li2FeSiO4 cathodes has been studied via DFT [42]. Many potential pathways were examined, no pathway with a migra-tion barrier of less than 700meV was found. This may explain why experimental performance has been somewhat sluggish.
Li diffusion in graphitic anodes has been studied [18]. A careful experimental diffusivity measurement was performed, treating the graphite as a Li+ conducting membrane in a in a Devanathan-Stachurski-type cell. A diffusivity of 4.4.10-6 cm2 sec
was measured. Simultaneously,. diffusivity was predicted with DFT activation
barriers and KMC to be in the range of 10~7 to 10-8m. This is good agreementsec
considering the ambiguity of the vibrational prefactor needed in KMC.
3 Methodology
Screening of cathode materials was performed in two steps. First, an empirical po-tential was used to calculate the diffusion topology. Detection of diffusion topolo-gies is coupled with a symmetry analysis of the structure so that symmetrically distinct calculations are only run once. Once the diffusion topology was known, potential Li diffusion pathways were formed. A diffusion pathway is a series of links between Li sites (Li hops) which allows migration of either a Li+ vacancy or Li+ interstitial to traverse the entire unit-cell. The pathways were sorted based on
the empirical-potential barrier energy. The second step was an accurate evaluation of the barrier energies with DFT and the NEB method.
3.1
Topology Determination
In early works [22,30] pathways for Li diffusion were found through some com-bination of manual examination and exhaustive testing with DFT. When studying a large number of structural prototypes and large unitcell structures, this process is not only wasteful of computational time, but may quickly become unreliable. Initial studies benefited from structures with high symmetry and simpler topolo-gies than some of the structures of interest. As discussed in Section 4.5, some diffusion pathways are non-intuitive.
The diffusion topology was detected using an empirical potential model. The empirical potential model consists of a repulsive Li-Oxygen pair potential and screened Coulombic electrostatics. All atomic positions are fixed and any Li in the structure is removed. A potential energy surface is then calculated for Li by laying a grid across the the entire unitcell and calculating the total energy of the cell with a Li+ ion at each grid point. After obtaining the potential energy surface, it is searched for Li sites (local minima) and hops between sites (minimum energy paths between minima). The resulting network represents a diffusion topology. This method is similar to a method recently published [21]. The specifics of the empirical potential are specified in Appendix A.
3.2
Barrier Evaluation
To evaluate the migration barrier energies, NEB inputs were generated from the diffusion topology. The optimal setting for running the calculation was identi-fied by enumerating superlattices and selecting the smallest and most orthogonal unitcell where the distance between periodic images was above 9 Angstroms. In practice, this results in supercell of 60 to 110 atoms.
The first step in an NEB calculation is relaxing the endpoints with DFT. The initial atomic positions for the intermediate images were obtained by taking the minimum energy pathway from the empirical potential topology and shifting the atomic positions by an interpolation of the atomic shifts which occurred during the endpoint relaxation. These smart initial conditions greatly reduced the com-putational burden of the NEB runs.
Great effort was taken in finding optimal DFT parameters for running these jobs. The goal was to minimize the computational cost while maximizing ro-bustness, accuracy, and precision. Several complications existed: (1) NEB calcu-lations must be much more strictly converged than calcucalcu-lations for stability and voltage screening [5]. To obtain the precision within one order of magnitude of the diffusivity, the NEB calculations must be converged to within 59 meV per unitcell. This is much stricter than for voltage calculations where an error of 50 meV per
Li is only 0.050 volts. (2) All calculations were run ferromagnetic without the +U
but is not expected to dramatically effect the results as discussed in Section 2.4.3. All ab initio calculations were performed with the Vienna Ab Initio Simulation Package (VASP) [43,44]. The NEB relaxations were optimized with the transition state tools extension to VASP [45]. The PBE GGA functional was used [46]. To obtain the precision of 50meV per unitcell, the break criteria for the ionic relax-ation of endpoints was very strict at 5 - 10IeV. The break criteria for electronic optimizations was 5 - 10-6eV. The NEB calculations were considered converged
when the barrier energy had stabilized and the calculated atomic forces were con-sistent with the image energies in the NEB profile. The atomic forces were nomi-nally converged to around 0.05eV/Angs. If the atomic forces were not consistent with the NEB profile, the number of intermediate images was increased and the relaxation continued. To expedite the NEB calculation and increase reliability, the total spin of intermediate images was fixed at the value of the converged total spin obtained during the endpoint relaxations. The final magnetic states were checked to ensure that similar magnetic states were obtained for each image. The lattice parameters for both the endpoint and intermediate images were fixed at values obtained by relaxing the undefected bulk with the GGA+U method. The bulk relaxation was performed with the "accurate parameters" as specified by Jain et
4 Screening Results
The screening methodology was applied to a series of structural prototypes. Lithium vacancy migration barriers were calculated for lithium containing compositions and lithium interstitial migration barriers were calculated for compositions not containing Li. The net migration barrier for all structures was compared against the metric developed for diffusion Cathode materials in Section 2.1, namely that structures with net barriers greater than 700meV are diffusion limited, structures with barriers less than 450meV have facile diffusion, and structures with interme-diate barriers are indeterminate.
4.1
3'-LixFePO
4In addition to the olivine form (discussed in Section 2.4.2), LiFePO4 also exhibits
a high pressure phase, 3'-LiFePO4. The crystal structure of 3'-LiFePO4 is shown
in figure 1. The structure consists of edge sharing columns of FeO6 octahedra
extending along the b-axis. The empirical potentials indicated a Li+ diffusion topology consisting of two types of hops. The first hop is primarily in the c-b plane. These hops connect the central ion in figure 1 (labeled c) to four of the neighboring Li ions (labeled a). These hops form a 2D network in the c-b plane. The second hop is primarily in the a-b plane. Each Li ion is connected to two of it's neighbors (labeled b) by these types of hops. The second type of hop forms a 1D zig-zag diffusion network between and parallel to the FeO6 octahedra.
Both hops taken together form a 3D diffusion network. Both types of hops pass through a distorted oxygen quadrilateral where two sides are formed by edges of the FeO octahedra and two sides are formed by the edges of P04 polyhedra. Sides consisting of FeO edges oppose one another, as do the remaining P04 edges.
The DFT refined migration barriers are shown in Table 2. In all cases, the migration barriers were over 950 meV indicating severe diffusion limitations. The calculated barriers are in agreement with electrochemical results which show no electrochemical activity [47].
FeO*
4
Figure 1: Crystal structure of 3'-LiFePO4. Only the structure local to a single Li ion (labeled c) is shown. The six neighboring Li ions are labeled a and b according to their relationship to the central Li ion.
Table 2: Calculated DFT migration barrier energies in I'-LixFePO4[meV].
2D network ID channel
#'-LiFePO4 1004 1239
4.2 Hexagonal LiMnB03
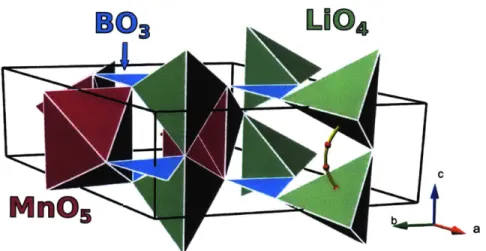
Two known phases exist for the compound LiMnB03: hexagonal and monoclinic. The diffusion pathway detected from empirical potentials for the heaxagonal form was primarily one dimensional, parallel to the c-axis. The pathway consists of a single hop between adjacent Li sites (Figure 2). This hop occurs when Li+ migrates across one of the faces of the equilibrium LiO4 tetrahedral site into an
adjacent empty square pyramidal void. The hop concludes with another face hop into the final tetrahedral site.
The calculated DFT migration barrier energies are given in (Table 3). With a net ID migration barrier of 724meV, the lithiated form may be classified as a diffusion limited material. While the delithiated form may be classifies as inde-terminate with a barrier of 529meV, the material is typically synthesized at the lithiated composition. Therefore, the material is expected to be severely diffusion limited and not practical as a battery cathode. Diffusion within the a-b plane was also investigated, but the migration barrier was over 1.5eV indicating no substan-tial diffusion.
Hexagonal LiMnBO3 has been electrochemically tested as a potential battery
cathode but no substantial capacity was found [48,49]. The experimental results are consistent with the prediction that this material will be diffusion limited.
L10
4
Figure 2: Crystal structure of hexagonal LiMnBO3 and low energy Li migration
pathway (gold). Red spheres on the diffusion pathway indicate Li location from intermediate NEB images.
Table 3: Calculated DFT migration barrier [meVI. LiMnBO3 MnBO3
I
ID channel 724 529energies in Hexagonal LiMnBO3
inter channel 1569
4.3 Monoclinic LiMnBO3
In the calculated stable structure, the Li ions reside on the same side of LiO5
trigonal-bipyramidal sites. These Li bipyramids form edge sharing columns as depicted in Figure 3. The diffusion pathway detected from the empirical poten-tials was one dimensional, parallel to the c-axis. Traversing the unitcell requires passing through four Li sites. Due to symmetry, only half of the pathway is dis-tinct. The two symmetrically irreducible hops are depicted in Figure 3. In hop A, the ion first migrates from the bottom to the top of the starting bipyramidal site. It then jumps directly across the shared edge to the adjacent bipyramid. In hop B, again, the ion first migrates from the bottom to the top of the starting bipyramid. It then jumps across one face of the bipyramid into an adjacent tetrahedral site before hoping across the face of the final bipyramid.
The calculated DFT migration barrier energies are given in (Table 4). The lithiated and delithiated 1D net migration barriers are 510 meV and 396 meV re-spectively. In both cases, the limiting step is the edge jump in hop A. The partially lithiated structure shows a shifting of Li location. The trigonal bipyramids are va-cated, and the Li instead resides in the tetrahedral site in the middle of hop B. The net migration barrier is only 311 meV, the limiting step remains hop A.
This material is predicted to be a facile diffuser at lithium concentrations con-centrations below one half. Above one half lithiation, the diffusivity is indetermi-nate. Inter channel diffusion was also investigated, but the migration barrier were
C
Ja
b)
c)
B
Figure 3: Calculated lithium locations and diffusion pathway in monoclinic LiMnBO3. The edge sharing LiO5 bipyramids are shown as (a) opaque, (b)
translucent, and (c) omitted. The calculated Li+ migration pathway is shown in gold. The four short arrows in the first subplot denote the tetrahedral Li site through which hop B passes.
Table 4: Calculated DFT migration barrier energies in Hexagonal Li.MnBO3 [meV].
hop A hop B inter channel
LiMnBO3 510 317 1367
LiO.5MnBO3 311 50
over 1.3eV indicating no substantial inter channel diffusion.
Jae Chul Kim et al. [50] have electrochemically cycled monoclinic LiMnBO3 with up to 1 lOmAh/g capacity (50% theoretical capacity). When the material was cycled at elevated temperatures, the accessible capacity increased markably. While it is not entirely clear if this is due to thermal activation of diffusion, the intermediate barrier energy of the lithiated state is consistent with this interpreta-tion. A subsequent study [12] suggest anti-site blockages of the Li channels may also be present to further hinder Li diffusion similar to the olivine LiFePO4
(dis-cussed in Section 2.4.2). It is unclear if the capacity limitation is due to anti-site defects, general diffusion limitation, or other factors.
4.4
LiMn(CO3)(PO
4)An new class of carbonophosphate cathode materias of the composition Li3M(CO3)(PO4),
M=Fe,Mn,Ni,Co. [51] has recently been synthesized. Diffusion screening of the triclinic Mn form is presented here.
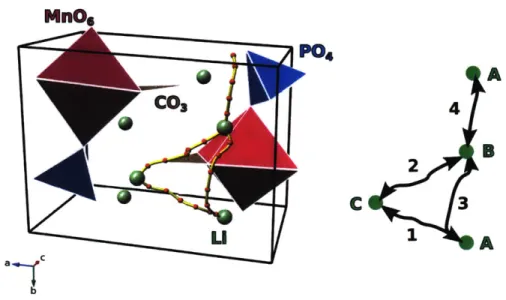
The empirical potential diffusion topology has low-energy one dimensional channels and intermediate barrier energy crosslinks between these channels. The low energy channels were investigated in depth. One such channel is shown in fig-ure 4. The channel encompases four distinct hops. However, in Li2Mn(CO3)(PO4),
and LiMn(CO3)(PO4), one of the Li sites (labeled C in figure 4) is vacant and hops
1 and 2 combine into a single hop.
The calculated activation energies are tabulated in Table 5. The net activation energy was determined by allowing the Li vacancy to choose the lower of either hop #1 and hop #2 or hop #3 to transverse the cell. In all cases, hop #4 was re-quired. With net migration barriers of 380 meV and 396 meV respectively, the Li3 and Lii lithiations are expected to have facile Li diffusion. The
intermedi-ate lithiation, with a net migration barrier of 478 meV, is on the low end of the indeterminate range.
Electrochemical cycling of the material has been shown [51]. The achieved experimental discharge capacity was 58% of the theoretical capacity indicating
Li+ diffusion across the composition range 2 < x < 3 for x in LiMn(CO3)(PO4).
MnOa P04 Cos A 4 2 B C 1 a-- C b A
Figure 4: Low energy diffusion pathway in LiMn(CO3)(PO4). The diffusion
pathway is shown within the unitcell (left) and in schematic form (right). The numbering of hops and lettering of Li sites, indicates symmetrically distinct por-tions of the pathway. The three Li sites through which the illustrated diffusion pathway does not pass are part of a symmetrically identical pathway.
Table 5: Calculated DFT migration barrier energies in LiMn(CO3)4SO4. The
"net" column lists the net activation energy required for bulk Li-diffusion. All values are in meV.
Formula Hop 1 Hop 2 Hop 3 Hop 4 Net Li3Mn(CO3)(PO4) 396 275 721 277 396
Li2Mn(CO3)(PO4) 657 478 173 478
cycling was kept relatively low at C/100 and ball milling was required to achieve this capacity. It is unclear if diffusion is fast enough in this material to allow higher rates of cycling. Furthermore, the tested samples had a large degree of off-stoicheometry. Approximately 19% of the Li was replaced with Na, due to incomplete ion exchange. Regardless, the prediction of facile to indeterminate diffusion agrees well with the ability of the material to electrochemically cycle at a low rate.
4.5 LixV3(P20 7)3(PO4)2
4.5.1 Evaluation of lithiated structure, x = 9
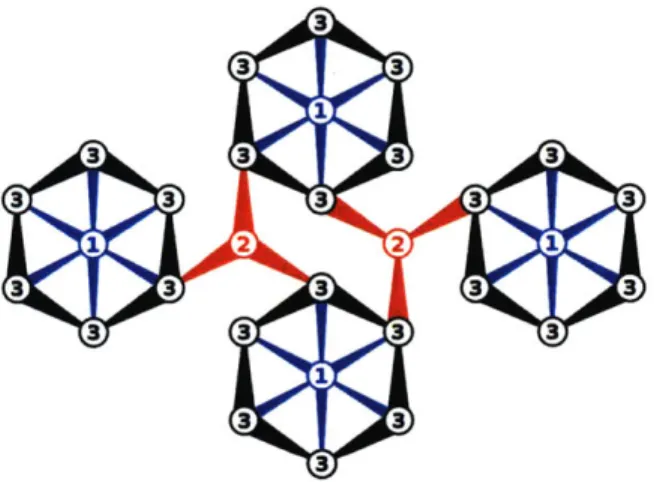
The crystal structure of Li9V3(P207)3(PO4)2 contains Li-layers and large c-axis
channels that contain only Li [52]. Three distinct Li sites exist: Li(l), Li(2), and Li(3). The empirical potentials predict 2D Li diffusion within the Li-layers.
A topological depiction of the predicted diffusion network is shown in Figure 5. The main features are rings of Li(3) sites with an Li(1) site in the center. The rings are connected to one another via Li(2) sites. In addition to the predicted diffusion network, we also investigated diffusion of Li down the large c-axis channels which connect the Li(1) sites to each other along the c-axis. The migration barrier down the c-axis channel was 740 meV indicating diffusion limitations. The calculated migration barriers are sumized in Table 6.
An alternate to the c-axis channels, is diffusion of Li across the Li layer. How-ever, endpoint relaxations indicate that Li vacancies are not stable at the Li(3) sites. The Li in the adjacent Li(1) relaxes into empty Li(3) with no activation bar-rier. This restricts the number of potential diffusion paths. One plausible diffusion path within the Li layer is a simultaneous Li(l) -+ Li(3) and Li(3) -+ Li(2)
mi-gration of the vacancy so that the vacancy does not rest on an Li(3) site. The total activation energy of this transition was extremely large at nearly 1.3 eV. Much of this net barrier can be traced to the fact that a vacancy in the Li(2) site is thermody-namically much higher in energy than a vacancy in the Li(1) site. The difference
Figure 5: Schematic of in-layer Li diffusion topology for Li9V3(P207)3(PO4)2.
The c-axis is normal to the plane of the diagram. The numbers 1, 2, and 3 represent the Li(1), Li(2), and Li(3) sites, respectively. Connections between sites are drawn with tapered lines to indicate relative displacement of the Li sites above/below the Li layer. Sites and transitions are colored according to symmetry
Table 6: Calculated migration barriers for Li vacancy diffusion in
LixV3(P20 7)3(PO4)2. The net activation energy is the energy difference between
the activated state and the lowest energy Li-vacany configuration at the given com-position.
Nominal Transition Migration Net activation
composition barrier (meV) energy (meV)
Li(l) -+ Li(l) between layers 740 740
x = 9 Li(l) -+ Li(3) i . 320 (forward) 1291
Li(3) -> Li(2)
5
simultaneous 1291 (reverse)361 (forward) 518
Li(2) -+ Li(3) 518 (reverse)
between layers 1534 1691
x = 8 inside 308 465
Li(3) -> Li(3) outside 588 745
in site energies was calculated to be 978meV.
A third potential path for Li(1) vacancy diffusion is a simultaneous hop
in-volving 4 Li ions: Li(l) -> Li(3), Li(3) -+ Li(2), Li(2) -> Li(3), and Li(3)
--Li(1). While it appears unlikely that four Li ions are migrating simultaneously, the balance between entering and departing Li from the high-energy Li(1) site may produce a lower net migration barrier. This complex transition has not been investigated.
4.5.2 Evaluation of partially lithiated structure, x = 8
Migration barriers in Li8V3(P20 7)3(PO4)2 were also calculated. Due to the large
site energy preference, the structure of Li8V3(P207)3(PO4)2 was assumed to be
the same as Li9V3(P207)3(PO4)2, but with all Li(1) sites vacant.
Several diffusion paths were investigated for the migration of an Li(2) vacancy first to the closest Li(3) site, and then to an adjacent Li(3) site. This series of hops is sufficient to form a percolating diffusion network through the Li layer (see Figure 5). For the first portion of the diffusion path, Li(2) -+ Li(3) vacancy migration, we calculated an activation barrier of 518 meV.
For the second portion of the diffusion path, Li(3) -+ Li(3), migration, we evaluated four different hops. The first hop tested is through the Li channel and crosses Li layers, similar to Li(1) diffusion in the channel. The remaining three hops are illustrated in Figure 6. Each Li(3) site is closely coordinated to three
oxygen ions which form a ring around the large c-axis channels. The first Li(3) -+ Li(3) migration pathway we tested consists of a Li-ion entering into the channel by passing between its neighboring oxygen ions, then exiting the channel via an analogous path (inside path). The second pathway consists of traveling around the exterior of the channel (outside path). The third pathway follows a S-shape which is interior to the channel near one Li(3) site and exterior near the other Li(3) site, transitioning between the two by passing through the oxygen triangle between the Li(3) site-coordinated oxygen triangles.
Figure 6: Diagram of the calculated Li(2) -> Li(3) hop and the three calculated
Li(3) -+ Li(3) hops.
For the Li(3) --> Li(3) migration across Li layers, the migration barrier of 1534
meV and net activation energy of 1691 meV are extremely high (Table 6). The high migration barrier is not surprising because it essentially involves
promot-ing a Li(3) vacancy to the Li(1) site and then Li(1) vacancy in-channel diffusion. Comparing to the fully lithiated case, it is possible to estimate the first part of this migration to be approximately 442 meV and the latter to be 740 meV (Table 6) for a total estimate of 1182 meV.
For the three remaining Li(3) -> Li(3) paths, our calculations indicate that the most favorable transition occurs via the inside path with a low net activation energy of 465 meV (Figure 7). The S-route and outside migration paths have higher activation energies of 646 meV and 745 meV, respectively. The energy profile of the migration path from Li(2) to Li(3) is plotted in Figure 7. Our results indicate that the rate-limiting step should be the first portion of Li(2) -+ Li(3) diffusion with an activation barrier of 518 meV.
4.5.3 Comparison to experiments
The calculations on Li9V3(P207)3(PO4)2 indicate negligible diffusion within the
Li-layer (1.3 eV barrier) and faster but still diffusion limited inter-layer diffusion
(740 meV). In contrast, our calculations on LisV3(P207)3(PO4)2 indicate indeter-minate diffusion within the Li-layer (518 meV) and negligible inter-layer diffusion (1.7 eV). The reversal of preferred diffusion orientations can be understood sim-ply from the large difference in site energies. When more than 8 Li are present per formula unit, vacancies are only expected on the Li(1) sites, and in-layer diffusion is sluggish because it requires traversing an Li(2) site. When fewer than 8 Li are
600- 5-Path
400-w
200-518 Inside +465
Li(2) Li(3) Li(3)
Li-vacancy Location
Figure 7: Calculated NEB profiles for Li(2) -+ Li(3) -+ Li(3) migration in the Li layer of LisV3(P207)3(PO4)2. The circles denote each intermediate image. The
connected lines are cubic spines; the slopes at each image were set determined
by the forces calculated with DFT. Three different paths were tested for Li(3)
present per formula unit, all Li(1) sites are vacant and additional vacancies will occupy either Li(2) or Li(3) sites. These additional vacancies may move within the layer, but inter-layer diffusion is sluggish because it first requires promoting a Li from a Li(3) site into a vacant high energy Li(1) site.
Our calculations are in reasonable agreement with experimental diffusivity
measurements by Poisson et al. [53] on the isostructural LigA13(P207)3(PO4)2
and LigFe3(P207)3(PO4)2 where the inter-layer activation barriers were measured
at 1.22 eV and 1.20 eV, respectively. The in-layer activation barriers were de-termined to be 660 meV and 690 meV, respectively. Although it is not known whether samples from Poisson et al might have been slightly Li deficient, the
acti-vation barriers they report fall between our calculated values for Li8V3(P20 7)3(PO4)2
and LigV3(P207)3(PO4)2
-Given the large calculated migration barrier for Li(1) diffusion, it is somewhat surprising that the material can be electrochemically cycled over 100mAh/g (56% Theoretical capacity) at a moderate rate of C/10 with a small overpotential [1]. Jain et al. found similar results [52]. However, Jain et al also found an unusually large discrepancy between the voltage profile calculated with DFT and the the experimental results, especially in the Lig to Li8 range of lithiation.
Several theories may explain the discrepancy between the predicted Li+ dif-fusion limitation and the ability of the material to cycle. Four are presented here. (1) If the experimentally tested materials were Li deficient, then the predicted be-havior of indeterminate diffusion would not conflict with the material's ability to
electrochemically cycle. (2) An uneven concentration gradient within the mate-rial could allow the majority of Li+ diffusion to occur at concentrations below Li8
where the material is not predicted to be diffusion limited. Taken to the extreme, Li will be first extracted from the surface states via diffusion over very short dis-tances. This produces a surface region nominally at and slightly below Li8 and
a core region with a composition of Lig. As more lithium is extracted, the core region shrinks and the surface region grows. If the interface between these two regions remains sharp, then Li+ diffusion in regions above a composition of Li8
need only occur over very short distances. However, upon insertion of Li+, a sur-face region of Lig composition forms around a core region of Li8. The surface
region would shield the core region, and insertion would stop with the bulk of the material at a nominal composition of Li8. (3) DFT may simply fail to model this
material, especially at the Li9 composition. (4) The actual diffusion mechanism
differs from what was considered here.
Corroborating or dismissing these four theories with the available information is difficult. Neither Jain nor Kuang reported precise measurement of atomic com-position in their experimental samples. Nor is such information trivial to obtain, making the theory of Li deficiency difficult to test. The sharp gradient model predicts incomplete Li insertion upon discharge. Taken together, Kuang and Jain report three electrochemical voltage profiles. Two of the three show larger capac-ity upon charge than discharge, but the third shows little discernible difference. Furthermore, It is unclear from the voltage profiles if the lost capacity was at high
Li concentration or low Li concentration, partially due to the discrepancy between observed voltages and Jain's predicted voltages. Of course, this discrepancy lends credence to the theory that DFT fails to correctly model the system. But, it re-mains unclear why this would be. Finally, the theory that something has been overlooked shall be ever-present.
In summary, it is unclear if the theoretical prediction of limited diffusivity in the compositional range 8 < x < 9 is in conflict with experiments. However, the good numerical agreement between the calculated values for the V system and the experimental measurements on the Fe and Al systems, suggests that the DFT migration barriers have predictive validity.
5
Discussion
5.1 Accuracy of Topology Determination
While prior studies have shown that empirical potentials can be effective at iden-tifying diffusion topologies (Section 2.3), the empirical potential applied here is novel and requires some degree of validation. It has already been shown that such empirical potential results may differ frequently from expectations based on
sim-ple geometric analysis [21]. This was also true in the case of Li9V3(P207)3(P20 4)2,
(Section 4.5). Experimental measurements showing Li+ diffusion primarily in the a-b plane differed greatly from the expected topology. The presence of large, open c-axis channels, suggested diffusion primarily along the c-axis. The empirical po-tentials correctly predicted in-plane diffusion, also consistent with DFT activation barriers.
Experimental topology information is also available for olivine LiFePO4. The
experimental topology obtained from neutron diffraction data [54] bears remark-able resemblance to to the empirical potential derived topology (Figure 8).
Unfortunately, experimental data on diffusion topology is extremely sparse. However, the predicted diffusion topologies and the the DFT derived minimum energy pathway may also be compared. Of the 32 NEB calculations presented in this study, none of them differed presented a significantly different topology than was predicted from the empirical potentials. The predicted 1D diffusion channels
Empirical Potentials Neutron Scattering S. Nishimura, Nature materials. 2008.
Figure 8: Comparison between Li+ diffusion topology by neutron diffraction data
[54] and an empirical potential model for LiFePO4. An isosurface for Li+ energy
in both monoclinic borates were verified with DFT refinement of both intra and inter channel NEB calculations. The empirical potential predicted aversion of Li+
to the large c-axis channels in the Li3V3(P207)3(P204)2 was also validated with
DFT.
The relationship between the DFT calculated barrier energies and the empir-ical potential barrier energies is shown in figure 9. The obvious correlation pro-vides the empirical potential models the ability to accurately determine diffusion topology. This data suggests that if a migration barrier of less than 700 meV is required, it is safe to exclude all diffusion pathways with an empirical potential migration barrier of greater than 1.5eV. At the same time, the data conclusively demonstrates that the empirical potential model is not sufficient to screen materi-als without DFT refinement. For example, the low energy diffusion pathways in the hexagonal LiMnBO3 are underestimated by nearly 500 meV.
5.2
Acuracy of Barrier Evaluation
This study is the first to apply of DFT and NEB Li+ diffusivity screening to mul-tiple oxide structural prototypes simultaneously. As such, it offers a valuable opportunity to examine how reliable DFT derived Li+ migration barrier energies in for oxide materials. As outlined in Section 2.2, experimental determination of activation barrier is very difficult, making direct quantitative comparison diffi-cult. However, electrochemical testing has been performed on all of the materials
4
S AA r7
--1000 2000 3000 Empirical Potential Barrier [meV]
* B-LiFePO4 m Hexagonal A Monoclinic X CarbonoPhosphate Diphosphate Olivine
Figure 9: Comparison between DFT calculated migration barriers, and migration barriers due to the empirical potential model.
3000 2500 2000 1500 -1000 500 0
discussed allowing qualitative comparison.
For each of the materials evaluated, the net activation barrier for Li+ diffu-sion is listed in Table 7. Also listed, is the expected behavior according to the metric derived in Section 2.1. Materials with net barriers less than 450 meV are expected to be facile Li diffusers. Materials with net barriers more than 700 meV are expected to be diffusion limited and materials with intermediate barriers are indeterminate. Finally, a summary of the observed experimental behavior is also listed. For added comparison data from the olivine and layered compounds are also Included in Table 7.
Most of the experimental observations are in agreement with the DFT diffu-sion predictions. The layered and olivine cathodes both cycle well. Neither of the
#'-LiFePO
4 and hexagonal LiMnB0 3 materials cycle. The remaining three materials all show electrochemical activity of around half of the theoretical ca-pacity. For the monoclinic LiMnBO3 and the carbonophosphate, it is not clearwhy only half of the capacity cycles. However, since many factors besides Li+ diffusion can limit a cathode material, the experimental observations are not in conflict with the diffusion prediction. The only remaining possible conflict be-tween the theoretical diffusivity prediction and experimental observations is the
diphosphate material. Although DFT predicted that Li9V3(P207)3(PO4)2 would
be diffusion limited, the material exhibits electrochemical activity. As discussed in Section 4.5.3 several possible explanations may resolve this apparent conflict. Without any obvious failures, the data strongly suggests that GGA DFT migration
Table 7: Summary of DFT Li+ diffusion screening and comparison to observed experimental behavior.
Material Barrier DFT predicted Experimental
[meV] Li+ diffusivity behavior
LiCoO2 225* indeterminatet
layered LiO.,5CoO2 400* facile Ample diffusion. Commercial
cath-CoO2 615* indeterminate ode matenal.
olivine LiFePO4 270t facile Ample diffusion. Commercial high
FePO4 200T facile rate cathode material.
LiFePO4 1004 limited
FePO4 961 limited No substantial Li+ extraction.
LiMnBO3 724 limited
hexagonal MnBO3 529 indeterminate No substantial Li+ extraction.
LiMnBO3 510 indeterminate Electrochemically cycles between
monoclinic LiO.5MnBO3 311 facile LiMnBO3 and LiO.5MnBO3 at
MnBO3 396 facile elevated temperatures.
Li3Mn(CO3)(PO4) 396 facile Electrochemically cycles
be-carbonophosphate Li2Mn(CO3)(PO4) 478 indeterminate tween Li3Mn(CO3)(PO4) and
LiMn(CO3)(P0 4) 380 facile Li2Mn(CO3)(PO4) at low rate.
Li9V3(P207)3(PO4)2 740 limited Electrochemically cycles at 56% of
Li8V3(P207)3(PO4)2 518 indeterminate theoretical capacity.
* The barriers for the layered compound were taken from
t The expected behavior for LiCoO2is indeterminate due
VanDerVen et al. [31]
to the required divacancy mechanism (Section 2.4.1) I The barriers for the olivine compound were taken from Morgan et al. [22]
barriers are an effective screening criteria for Li+ diffusivity in oxides.
In fact, careful parsing of the data suggests that DFT may be able to produce a more precise prediction of Li+ diffusivity than the simple facile vs. indetermi-nate vs. limited classification applied here. Notice that the commercial high-rate cathode material LiFePO4 has the lowest migration barriers of any material.
Fur-thermore, the monoclinic LiMnBO3 drifts into the indeterminate range and cycles better at elevated temperatures. However, more experimental data on Li+ diffu-sion is needed to quantitatively refine the accuracy of this method.
5.3
Factors affecting Li diffusivity
On of the common methods used to estimate the Li+ diffusivity of a material is an open space metric. The larger the bottleneck through which the Li+ ion must pass, the lower the migration barrier is presumed to be. It has already been show that this fails to describe the limited interlayer diffusion in LigV3(P207)3(PO4)2
(Section 4.5). The screening methodology applied in this thesis offers a unique opportunity to examine the general relationship between bottleneck size and mi-gration barrier.
The bottleneck size for each NEB hop was defined as follows. At the transition state of the NEB calculation, the three closest oxygen ions which formed a triangle through which the Li ion passed were identified. The corresponding positions of these oxygen ions in the bulk were taken to represent the constraining bottleneck.





![Figure 8: Comparison between Li+ diffusion topology by neutron diffraction data [54] and an empirical potential model for LiFePO 4](https://thumb-eu.123doks.com/thumbv2/123doknet/13811927.441862/47.918.173.716.391.755/figure-comparison-diffusion-topology-neutron-diffraction-empirical-potential.webp)
