Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
219th meeting of the Electrochemical Society, Canadian Section [Proceedings],
2011-05-06
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=c1395584-2254-466b-b735-43d3bf90049d https://publications-cnrc.canada.ca/fra/voir/objet/?id=c1395584-2254-466b-b735-43d3bf90049d
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
ZnMn2O4 as Anode Material for Li-ion Batteries
Courtel, Fabrice M.; Duncan, Hugues; Abu-Lebdeh, Yaser; Davidson, Isobel
J.
ZnMn2O4 as Anode Material for Li-ion Batteries
Fabrice M. Courtel, Hugues Duncan, Yaser Abu-Lebdeh* and Isobel J. Davidson
Institute for Chemical Process and Environmental Technology,
National Research Council of Canada 1200 Montreal Road, Ottawa, ON, Canada K1A 0R6
*E-mail: Yaser.Abu-Lebdeh@nrc-cnrc.gc.ca
Carbon graphite is commonly used as an anode material in lithium-ion batteries because of its long cycle life and moderate cost. However, it has a theoretical capacity that is limited to 372 mAh g-1. In 2000, transition-metal oxides were proposed as alternative anode materials for lithium-ion batteries. Poizot et al. have shown that these oxides react with lithium via a reversible conversion reaction where the metal oxide is reduced into metal nanoparticles (1-2 nm) embedded into a matrix of lithium oxide upon discharge (1). The best materials were found to be CoO and Co3O4 with reversible capacities of 700 mAh g-1 and
900 mAh g-1, respectively (1). The same reaction occurs with other transition-metal oxides (Cr, Mn, Fe, etc…). Recently, we reported on the synthesis and the electrochemical characterization of ZnMn2O4 (2), a high
capacity anode material for lithium-ion batteries (784 mAh g-1). ZnMn2O4 was synthesized through a simple
co-precipitation route using oxalic acid. The precipitate was filtered, dried and calcined at temperatures ranging from 400 °C to 1000 °C. A capacity of 690 mAh g-1 was obtained at C/10, along with good rate capability and excellent capacity retention (88%) with ZnMn2O4
calcined at 800 °C, and using lithium carboxymethlycellulose (LiCMC) as a binder (hereafter referred to as ‘optimized ZnMn2O4’).
In this paper, we present a study of the effect of the electrolyte and the cycling temperature on the performance of ZnMn2O4. In addition, a study of the
composition of the solid electrolyte interphase (SEI) of ZnMn2O4 electrodes by XPS will be presented.
As illustrated in Figure 1, ZnMn2O4 adopts the I41/amd
tetragonal structure for all calcination temperatures with crystallite sizes ranging from 11.4(2) nm at 400 °C to 324(14) nm at 1000 °C. The effect of the electrolyte on the performance of optimized ZnMn2O4 is shown in
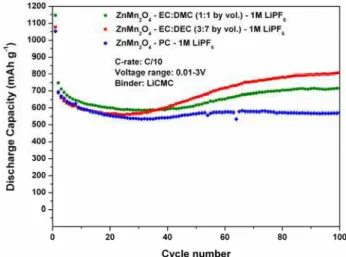
Figure 2. Three different electrolytes were tested using 1M LiPF6 as salt: EC:DMC (1:1), EC:DEC (3:7) and PC.
A discharge capacity of 800 mAh g-1 after 100 cycles was obtained for ZnMn2O4 in EC:DEC electrolyte while the
use of PC provided a capacity of 600 mAh g-1 after 100 cycles. A good rate capability and capacity retention were obtained for all electrolytes. The influence of the cycling time, the cycling temperature and the electrolyte on the composition of the SEI, studied by XPS, will be discussed. Figure 3 shows the C 1s, O 1s, and Li 1s spectra of a ZnMn2O4 pristine cast, and the ZnMn2O4
electrodes from batteries opened after being cycled 100 times in EC:DMC and EC:DEC. Different species such as LiF, polyethers and carbonates were identified as components of the SEI.
Figure 1. XRD patterns of ZnMn2O4 powders sintered at
400 °C, 600 °C, 800 °C, and 1000 °C.
Figure 2. Discharge capacities of ZnMn2O4 with using
three different electrolytes using 1M LiPF6 as salt:
EC:DMC (1:1), EC:DEC (3:7) and PC.
Figure 3. C 1s, O 1s, and Li 1s spectra of ZnMn2O4 a)
Pristine cast, b) 100 cycles, EC:DMC 1:1 + 1M LiPF6, c)
100 cycles, EC:DEC 3:7 1M LiPF6.
References
1. P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 407, 496 (2000).
2. F. M. Courtel, H. Duncan, K. Yanez, M. Nasirpour, Y. Abu-Lebdeh and I. J. Davidson, AMn2O4 (A = Co, Ni,
Cu and Zn) as Anode Material for Li-Ion Batteries, in
The 15th International Meeting on Lithium Batteries - IMLB 2010, ECS, June 27 - July 2, 2010 , Montreal,
Quebec, Canada (2010). Abstract #277, 219th ECS Meeting, © 2011 The Electrochemical Society