Publisher’s version / Version de l'éditeur:
Canadian Journal of Chemistry, 44, 22, pp. 2617-2622, 1966-11
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1139/v66-394
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Phase transitions of adsorbates. I. Specific heat and dimensional
changes of the porous glass-water system
Litvan, G. G.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=55da3942-898b-4c04-b902-23d8d2ccb8df https://publications-cnrc.canada.ca/fra/voir/objet/?id=55da3942-898b-4c04-b902-23d8d2ccb8df
Ser
THl
N21r2 no. 293 c . 2BLDG
N A T I O N A L R E S E A R C H C O U N C I L C A N A D A C O N S E I L N A T I O N A L D E R E C H E R C H E SPHASE TRANSITIONS OF ADSORBATES I. Specific Heat and qimensional Changes of the
~ o r o ~ ~ ~ d 1 $ B ' ~ - W a t e r System &y,:8 .p- BY G. G. LITVAN Reprinted from CANADIAS JOURNAL O F C H E M I S T R Y VOL. 44 NO. 22 NOVEMBEIi 1966, P. 2617
RESEARCH PAPER No. 293 O F T H E
DIVISION O F BUILDING RESEARCH
T R A N S I T I O N S D E P H A S E D E S A D S O R E A T S I. Chaleur spCcihque e t changements dimensionnels du systbme
mixte verre de silice poreux e t eau SOMMAIRE
ON a mesurC sirnullan6ment la chaleur spCcifique e t les changements dimensionnels dl1 systhme mixte verre poreux de silice 96y0 e t eau B des tempCratures se situant entre -35 "C et +2 O C B
des teneurs Cquivalentes B 1.6, 4.0, 5.3 et 7.7 couches monomolCcul;lires. La chaleur spkcifique de I'adsorbat en-dessous de la rCgion d e transition de pilase est supCrieure B celle de la glace. On n'a pu dCtecter de zone d e transition lors de la fusion pour les concentrations les plus faibles. Aux teneurs plus ClevCes, la transition se produisait ioujours i une tempCrature approchant -9.5" et elle Ctait du type anormal de premier ordre. Ida chaleur latente spCcilique de fusion, calculCe en supposant que deux couches monomol6culaires ne ghlent pas, Ctait d e 49.4, 54.4, 55.5 cal. g.?, et on a constate qu'elle variait en fonction de la concentration. On a coils+CrC que la correlation existant entre les mesures calorimCtriques e t dimensionnelles Ctait satisfaisante.
PHASE TRANSITIONS OF ADSORBATES
1. S P E C I F I C HEAT AND DIMENSIONAL CHANGES O F T H E POROUS GLASS
-
WATERSYSTEM1
C . C . L I , ~ V ~ I N
Division of B z ~ i l d i z g Reseaiclz, National Researcl~ Cozr?~cil, Ottawa, Canada
Received March 24, 1966
'The specific heat and di~nensional changes of the porous 96% silica glass -water system were measured simultaneously between -35 "C and +2 "C with coverages of 1.6, 4.0, 5.3, a ~ ~ d 7.7 molecular layers. The specific heat of the adsorbate below the tmnsltion range was found to be higher than that of ice. No phase c h a ~ ~ g e mas detected with the lowest concentra- tion. A t the higher coverages, transition always occurred near -9.5" and was of the anomalous first order type. T h e values of the specific latent heat of fusion, calculated by assuming that t\vo monolayers do not freeze, are 49.4, 54.4, a!id 55.5 cal g-I, depending on the co~~centration. The correlation bet\vee~i the results of calor~metric and dimensional change measurements was considered satisfactory.
IS'TRODUCTIOS
Phase trailsitions of water adsorbed on porous glass differ froin those of other adsorbates in a t least tu7o aspects: (a) water has several freezing ranges (1, 2) u7hile benzene (3) and xenon ( I ) have one, and (b) its melting point is not concentration dependent as is t h a t of helium on jeweller's rouge (4), argon (5), nitrogen ( G ) , and methane (7) on rutilc. This anoinalous behavior and the importance of the freezing pheno~nenon from a practical point of view seemed to warrant further studies.
The tlzerinal properties and the dimensional changes of the partially and fully saturated systems \\-ere simultaneously measured to facilitate the correlation of the results.
E X P E R I M E N T A L
A pparatz~s
The adiabatic calorimeter i r ~ c o r p o r a t i ~ ~ g a Tuclcerman-type optical extellsometer (S), m a ~ ~ u f a c t ~ ~ r e d bq- American Instrument Co., Silver Spring, Maryland, was similar to that described in ref. 2 but with several ~ n o d i h c a t i o ~ ~ s . Instead of usir~g the phototube co~ltrollers, the te~nperature of the envelope and the t ~ ~ b c was regulated by a ~uicroswitch in the heating circuit a c t ~ ~ a t e d by a disc mou~lted on the slidc\vire shaft of a 0.1 mV Speedo~uax recorder and governed by the amplified e.m.f. of a seven-junction thermopile. The apparent temperature difference between the calorimeter and envelope was fO.OO1 "C. In addition, the temperature of the reference block and the bath was made to follo\v automatically that of the calorimeter without the need of manual adjustments. Adiabatic conditions were thus better ~nairltained durir~g tem- perature changes.
The reported (2) 0.1" temperature lowering of the calorimeter that occurred overnight was eliminated. The temperature remained either constant or increased by 0.01" overnight so t h a t the state of the adsorbate was not altered fro111 one of desorption to one of adsorption.
Precision and Acctiracy
The degree of precision increased a s the tests progressed; the final precision varied bet\\~een f27; and f0.02% depeltdi~lg on the working temperature range. T h e accuracy \vas determined by measuring the heat capacity of sodium chloride and it was found t o be f 0.2%.
Materials
T h e porous 96% silica glass had a nitrogen surface area of 112.5 m2g-1. T h e pore-size distribution, calc~llated from the nitrogen isotherm (9) showed that 66% of the total pore volu~ne consisted of pores with radii between 28 and 20
a.
T h e distribution curve had a maxi~num a t 21A.
Prior t o each series of ~neasurernents the glass was heated to 400 OC in air to burn off organic impurities and s u b s e q ~ ~ e n t l y evacuated a t 10-5 Inn1 pressure for a t least a day.'The water used was purified by several vacuum distillations. T h e amount adsorbed was determined with the aid of n burette (3), instead of gravimetrically (2).
'Issued as N.R. C. No. 9150.
2618 C A S A D I A N JOURNAI, O F CHEMISTRY. VOL. -14, 1966
CALCULATIONS
The heat capacity of the adsorbate was calculated by taking the difference between the heat capacity of the calorimeter systein with and without the adsorbate. The total heat capacity of the system is given by the formula due to AlIorrison et al. (10).
where
Q = heat supplied
c,,,, = heat capacity a t constant pressure of the caloriineter and adsorbent
c,, = heat capacity a t ccnstant pressure of the gaseous phase N, = number of moles sorbed
cN, = heat capacity of the adsorbed phase a t constant amount sorbed
q,, = isosteric heat of sorption p = vapor pressure
T = temperature
Vg = volume of gaseous phase in the calorimeter
Because of the experimental difficulties due to the small water vapor pressures a t low temperatures, no data are available for q,,, N,, and p. The maximum value, however, of dN,/dl' was < I X 10-5 mole deg-l and that of dp/dT was 0.14 min deg-l calculated with the saturation vapor pressure data (Vg = 310 1111). Consequently, the second, fourth, and fifth terms on the right-hand side of eq. [I] were considered negligible. The validity of the assumption that the adsorbent is inert, i.e. its heat capacity is not altered by the presence or the state of the adsorbate, was accepted due to necessity.
RESULTS
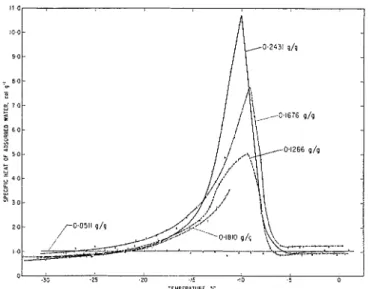
The heat capacity of the adsorbed water in fixed concentrations of 0.0511, 0.1266, 0.1676, and 0.2431 g/g were determined between -35 and +2 "C while heating the system. The systein with the highest concentration mas saturated. These concentrations are equivalent to 1.6, 4.0, 5.3, and 7.7 layers, respectively. hIeasurements of the fifth series with 0.1510 g/g (5.7 layers) were interrupted due to malfunctioning of the coin- pressor. The specific heat vs. temperature plot is shown in Fig. 1.
The specific heat a t temperatures below the heat anonlaly is, in all cases, higher than that of bulk ice (0.45 cal deg-') but a t temperatures above the anomaly it closely approaches the value of bull< water. For reasons yet unexplained the curve for 0.1676 g/g concentration lies above the others.
With the loxvest amount of water present, equivalent to 1.G layers, no anomaly taltes place. At higher concentrations anomalously large heat absorption occurs, beginning a fern degrees below -20 "C and extending over a temperature range of -12 deg. The curve ascends gradually but descends sharply after the lnaxin~un~ which is near -9.5". Norinal behavior, except for a small minimum, is resumed a t about -7".
The entropy changes,
L I T V A N : PHASE T R A N S I T I O N S O F .ADSORB.ATHS
"
I.Z5 -20 l5 .I0 5 0
TEMPERATURE. 'C
FIG. 1. Specitic heat of adsorbed water vs. temperature. T h e numerical values indicate the water content of the ;tdsorbent.
T E M P E R A T U R E , 'C
FIG. 2. Entropy chn~lge, S T - S 2 1 3 , of adsorbed water vs. temperature.
The latent heats of fusion (column 3, Table I ) \\-ere deterillined by graphical integration of the area between the c, vs. T curve and a straight line connecting the points a t -30" and -7 "C.
Figure 3 shows the expansion isosteres (fractional length changes plotted against temperature). At the lowest concentration no anomaly can be detected. In the case of higher concentrations, two anoinalies occurred on cooling, one around -20" and the other around -7".
CANADIAN JOURNAL O F CI-IEMISTRY. VOL. .I&, lSGG
T A B L E I
Specific latent heat and entropy of fusiorl of adsorbed water
Water adsorbed No. of Heat of fusiorl Entropy of fusion
( g / d layers (cal g-l) (cal mole-' deg-l)
0.0511 1 . 6 O.O* 0 . 0 t 0 1266 4 . 0 26.32* 49.40t 1.74 0.1676 0.2431 Bulk - ~ ~ - - p -
-"Values calculated by assunlirlg that all the water present Ins transformed.
tvalues calculated by assuming that a n amount of water equivalent to two monola).ers h a s not transformed.
FIG. 3. Expnnsion isosteres. Individ~!al points omitted. 1 2 1 ~ . 4. Derivative of the espansioll Isosteres.
From the tenlperature derivative of the fractional length changes (Fig. 4) it is apparent that the expansion occurring a t high temperature is concentration dependent: it shifted to higher temperature and decreased in magnitude with increasing water concentration, and it vanished a t saturation. This finding is in line with one previous study (I) but is only in partial agreement with another (2) where on the saturated system anomaly was found at -7". The other anomaly, ~vhich alxvays occurs around - 20°, seems to have not one but two inaxiina a t approximately -19" and -21' as indicated by Figs. 3 and 4.
On n-arming, only one expansion \\-as observed around -So and it became broader with increasing concentration. Pronounced hysteresis is exhibited with all except the lowest concentrations.
DISCUSSION
Absence of Transition
T h e finding that no transition occurs a t a coverage of 1.6 layers is in accordance wit11 findings of previous calorimetric (4-7) and dimensional change (1) measurements.
LITVAN : PI-IASE TRANSlTIOSS O F ADSORBATES 2621
Presumably, the strong adsorbent-adsorbate interaction prevents the water from re- arrangement necessary for transition. I t is remarkable t h a t the water-glass system behaves similarly in this respect to the rutile-nitrogen ( 5 ) and rutile-argon (6) s)istems \\-here the onset of freezing occurs after the coverage exceeds two layers.
Heat of Fusion
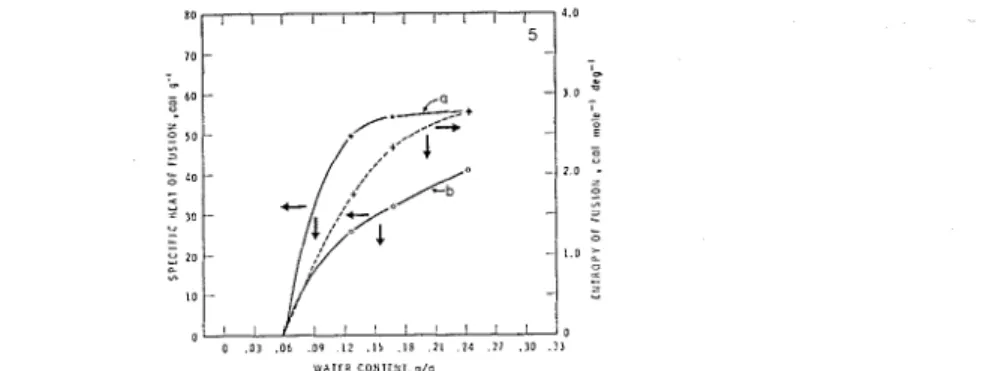
When calculating AH the question arises whether the first two layers still d o not suffer phase change with coverages exceeding this value. I t is conceivable t h a t the attrac- tive forces between the solid and the first layers, a t higher concentrations, are lvealcened by tlie ~vater-water interaction and consequently the adsorbate achieves the mobility required for ice formation. T h e latent heat of fusion was, therefore, calculated in two ~ v a y s : by assuming t h a t (a) all the water froze and (b) only the water in the third and
subsequent layers froze.2 T h e results are given in colun~ns 3 and 4 of Table I and the mean latent heat of fusion is plotted a s the function of the water concentration in Fig. 5. For thc present, assurnptio~l (b) is accepted n-itllout direct evidence. There are indications, ho\\-evcr, t h a t support this vie~v.
e A l l P C"lll[:ll, g / q
FIG. 5. Specihc heat of fusion and entropy of fusion vs. Ivater content (see test for esplanatioli).
If the attractive force holding the adsorbate in the surface is higher than a first order function of the distance, the heat of fusioil vs. concentration curve is expected t o be of the saturation type a s is tlie case with assuinption (b) b u t not \\-ith (a) (Fig. 5). 111 addition, consideriiig t h a t in the bull; state the specific heat of water is approximatcly twice t h a t of ice the high specific heat of thc adsorbate a t temperatures belour the melting point may be vie\\-ed as an indication of the presence of unfrozc~l water.
Antoniou ( 2 ) , when calculating the results for the saturated system, assuined t h e heat of fusion of the adsorbed water to be the same a s t h a t of the bulk and t h a t the observed net heat change was therefore low because not t\vo b u t three water layers remained un- frozen. Ibrlier extensometric measurements (I) sllo\ved, ho\\rever, t h a t the oilset of freezing occurs a t a coverage bet\veen 1.3 and 2.3 layers. Furthermore, application of this concept to the present case results in the improbable finding t h a t 2.7, 3.1, and 3.7 layers, respectively, remained unfrozen, i.e. the amount of unfrozen water increased with increasing concentr a t ' 1011.
T h e values for the heat of fusion are less than t h a t for bullc ice ; even a t saturation they reach only -70%. In other systems, such a s argon-rutile (5), methane-rutile (7), and nitrogen trifluoride - anatase (11), A H is equal t o the value for the bull< substance, presumably because of \\realter adsorbent-adsorbate interaction.
' f i r the spccijic hcut of tlre z~rLfi.oze?t ulutcr in the f i ~ s t lz ~ o l a y c i s tlze espeli?rlc?~tal aalires detei.i~ri?zed i r z the .sei.ies zaitl~ the lozncst c o i z r c ~ ~ t i a t l o i ~ Toere rised.
2622 CANADIAN JOURNAL O F CHEMISTRY. VOL. 41. 1SGO
T h e small entropy of fusion values show t h a t less disordering occurs in the adsorbed t h a n in t h e bulk state. Since the absolute values are not known i t cannot be decided whether t h e state of the solid or liquid or both phases are responsible for t h e smaller difference.
T h e transition is of the anonlalous first order type (12) a s indicated b y the shape of the
cp vs. T curve. A t least two conditions can account for such phase changes: (a) gradual
disordering of a honlogeneous phase a n d (b) t h e existence of dolllains with different properties.
Expansion Isosteres
T h e results of the length change measurements are in general accord 1%-ith those obtained when cooling t o -180 "C (I). Because in t h e present s t u d y the system was cooled only t o -35 "C, the agreement implies t h a t t h e portion of the water t h a t froze below -35" also melted in t h e same region a n d had no detectable effect on the melting process a t high temperatures.
T h e shape of the expansion isosteres (Figs. 3 a n d 4) changes gradually with concen- tration. T h e curve for t h e saturated system shows one special feature, namely, a con- traction occurring on cooling near
-
16". I t is noteworthy t h a t t h e disappearance of t h e menisci a t saturation does not cause inore significant changes.I n contrast t o Antoniou's finding (2) t h e correlation between t h e expansion isostcres a n d heat capacity curves can be considered a s satisfactory. A t lorn teniperatures the slope of the expansion isostere changes gradually corresponding t o the slow increase of cp. T h e expansion due t o melting begins a t t h e temperature of t h e maximum heat capacity (-9.5") and takes place in t h e region where the c, curve shows a minimum which is characteristic of anoinalous transitions.
AICKNOLVLEDGMENTS
T h e author is indebted t o i\lIr. P. J . Sereda for helpful discussions, t o Mr. B. I-Iurley for carrying o u t most of the measurenlents, a n d t o M r . H. F. Slade for technical assistance. T h i s contribution is published with t h e approval of t h e Director, Division of Building Research. National Research Council of Canada.
REFERESCES
I. G. G. L I ~ V A N and It. MCINTOSH. Can. J. Chem. 41, 3095 (1963). 2. A. A. .ANTONIOU. J. Phys. Che~n. 68, 2754 (1964).
3. C. HODGSON and I<. MCINTOSH. Can. J. Che~n. 38, 958 (1960). 4. H. P. R. FREDEICIKSE. Physica, 15, 860 (1949).
5. J. A. MORRISON and L. E. DRAIN. J . Chem. Phys. 19, 1063 (1951).
6. J. A. MORRISON, L. E. DRAIN, and J. S. DUGDALE. Carl. J. Chem. 30, 890 (1952). 7. I<. S. DENSIS, E. L. PACE, and CH. S. BAUGHMAN. J. Am. Chem. Soc. 75, 3'267 (1953). S. L. B. TUCKERMAX. Proc. ASTM, 23, 11, 602 (1923).
9. C. P ~ ~ i t c e . J . Phys. Chem. 57, 149 (1053).
10. J. A. MORRISOX, J. M. LOS, a~id I-. E. DRAIN. 'Trans. Faraday Soc. 47, 1023 (1051). 11. A. R. SIEBERT and E. L. PACE. J. Phps. Chein. 60, 828 (1956).