HAL Id: hal-02146490
https://hal.archives-ouvertes.fr/hal-02146490
Submitted on 28 Nov 2019HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Insights into the Headgroup and Chain Length
Dependence of Surface Characteristics of
Organic-Coated Sea Spray Aerosols
S. Cheng, S. Li, N. Tsona, C. George, L. Du
To cite this version:
S. Cheng, S. Li, N. Tsona, C. George, L. Du. Insights into the Headgroup and Chain Length De-pendence of Surface Characteristics of Organic-Coated Sea Spray Aerosols. ACS Earth and Space Chemistry, ACS, 2019, 3 (4), pp.571-580. �10.1021/acsearthspacechem.8b00212�. �hal-02146490�
Insights into the head-group and chain-length dependence of surface
1
characteristics of organic-coated sea spray aerosols
2 3
Shumin Cheng,† Siyang Li,† Narcisse T. Tsona,† Christian George,‡,§ and Lin Du*,†
4 5
†Environment Research Institute, Shandong University, Binhai Road 72, Qingdao 266237, China
6
‡School of Environmental Science and Engineering, Shandong University, Binhai Road 72, Qingdao
7
266237, China
8
§University of Lyon, Université Claude Bernard Lyon 1, CNRS, IRCELYON, F-69626 Villeurbanne,
9
France
10 11
Corresponding author: Email: lindu@sdu.edu.cn, Tel: +86-532-58631980
12 13 14
15 16
ABSTRACT
17
The structure of sea spray aerosols (SSAs) has been described as a saline core coated by organic
18
surfactants. The presence of surface-active compounds at the air-water interface can have a large
19
impact on physical, chemical and optical properties of SSAs. The surfactant molecules chosen for
20
this study, palmitic acid (PA), stearic acid (SA), arachidic acid (AA), methyl palmitate (MP), methyl
21
stearate (MS) and methyl arachidate (MA), were used to investigate the effect of alkyl chain-length,
22
head-groups and sea salts on the surface properties of these monolayers. A Langmuir trough was used
23
for measuring surface pressure−area (π−A) isotherms to reveal macroscopic phase behavior of the
24
surface films at the air-water interface. Infrared reflection absorption spectroscopy (IRRAS) was
25
employed to have a molecular-level understanding of the interfacial molecular organization. The π−A
26
isotherms indicated that sea salts, present in the subphase, exert a strong condensing effect on fatty
27
acid monolayers, while exerting expanding effect on fatty acid methyl ester monolayers, which was
28
confirmed by results from IRRAS experiments. IRRAS further revealed that the alkyl chains were in
29
an all-trans conformation, which can be evidenced by the relatively low νa(CH2) and νs(CH2)
30
stretching frequencies. The conformational order changes in the alkyl chains of different film-forming
31
species (C16 < C18 < C20) were directly revealed by analyzing the relative intensity of the νa(CH2)
32
and νs(CH2) peaks in the C-H stretching region.Thus, all the three factors alter the phase behavior
33
and molecular packing of the monolayers at the air-aqueous interface.
34 35
Keywords: Langmuir films; fatty acid; fatty acid methyl ester; sea spray aerosols; sea surface
36
microlayer
37 38
1. INTRODUCTION
39
Based on mass concentration, sea spray aerosol (SSA) is one of the largest sources of primary
40
atmospheric aerosol particles.1-2 SSAs formed at the sea surface microlayer through bubble-mediated
41
processes, are commonly composed of a sea salt core coated by a thin organic layer.3-6 This organic
42
layer is enriched with various organic species from both biological and anthropogenic sources, among
43
which, surface-active species account for a significant portion.5,7 Moreover, surface-active species in
44
the sea surface microlayer are expected to be more efficiently transferred into SSA, thus exhibiting
45
much higher concentration than that measured in the sea surface microlayer.7-9 Organic films present
46
in SSAs are reported to have inverse micelle structures with the hydrophilic head-groups toward the
47
aqueous phase and the hydrophobic tails to the air.10-11 The morphology and conformation of organic
48
films will affect aerosol growth and volatility,12 radiative absorption and scattering,6,13 reactivity with
49
atmospheric gases,10 and cloud condensation nuclei activity.3,8,14-15 Thus, understanding the surface
50
characteristics of surfactant molecules is important in atmospheric chemistry because of their
51
significant influence in physical, chemical and optical properties of SSAs.10,16
52
Previous measurements suggested that fatty acids make up a large fraction of the organic
53
materials residing at the interface of marine aerosols.4,7,17 Fatty acids present in sea surface microlayer
54
are released primarily during the lysis of phospholipid cellular membranes of marine organisms.11,14
55
Saturated fatty acids, particularly palmitic acid (PA) and stearic acid (SA), contribute significantly to
56
the organic coating of sea salt particles,11,18-19 with arachidic acid (AA) showing a relatively lower
57
abundance.20 On the other hand, a former analysis of marine aerosols collected over the
58
Mediterranean Sea using a five-stage cascade impactor, found the existence of fatty acid methyl esters
59
from C14 to C34 in SSAs, among which, methyl palmitate (MP) and methyl stearate (MS) were found
60
to be predominant.21-22 Natural sources of these fatty acid methyl esters from hydrolysis of various
61
biosynthesized esters such as triglycerides, glycolipids, phospholipids, or waxes, have been
62
reported.21 Degradation of particulate material suspended in seawater from marine organisms has
63
been found to be the source of saturated and unsaturated fatty acid methyl esters.23
64
Investigation of the surface properties of these organics at the air-water interface can provide a
65
better understanding of the chemical and physical processes taking place at the surfaces of SSAs in
66
the atmosphere. The film-forming species selected for this research are long chain fatty acids and
67
fatty acid methyl esters with alkyl chain-lengths of C16, C18 and C20. Langmuir trough has been
68
extensively used in atmospheric chemistry to understand the properties of surfaces and surfactant
69
molecules at the interface of aqueous aerosols.10,16 Surface pressure−area (π−A) isotherms of
Langmuir monolayers on aqueous surfaces are capable of revealing the underlying phase information
71
of the monolayers being subject to constant compression. However, to gain molecular-level insights
72
into the monolayers, spectroscopic techniques are needed. Infrared reflection absorption spectroscopy
73
(IRRAS) is a helpful technique in studying surface phenomena. Possessing the advantage of being a
74
fast and nondestructive technique,24 IRRAS is sensitive to Langmuir monolayers if sufficient scans
75
are taken to obtain spectra with good signal-to-noise ratios.25 The application of IRRAS enables us
76
to acquire information about molecular structure, conformation, and orientation of the film-forming
77
species.25-27 There are extensive studies elucidating the microscopic profile of Langmuir films of
78
saturated fatty acids at the air-water interface using IRRAS.4,28-30 In addition, fatty acid methyl esters
79
are also known to be able to form organized structures at the air-water interface.31-34 Previous studies
80
of saturated long chain fatty acid and fatty acid methyl ester monolayers were carried out at the
air-81
water interface with IRRAS technique,by which the conformational order of the monolayers were
82
shown to be increased with increasing alkyl chain-length.35-36 The response of SA and AA Langmuir
83
monolayers to atmospheric inorganic ions was explored by Langmuir and IRRAS methods, which
84
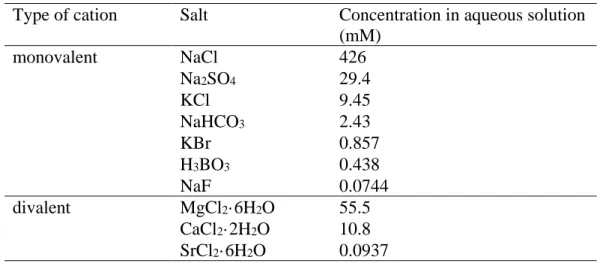
confirmed the existence of inorganic ions in the fatty acid monolayers and its impact on the surface
85
properties of aqueous-phase aerosols.15 Former investigations were mainly conducted on pure water
86
(PW) or ion-containing subphases. However, properties of sea surface relevant surfactant monolayers
87
on the artificial seawater (ASW) subphase have been rarely studied.37 The ASW used in this work is
88
pure water enriched with relevant sea salts, which is more representative of the environment that the
89
surfactant monolayers are exposed to.
90
In the present study, fatty acid/fatty acid methyl ester-ASW systems were chosen as proxies to
91
further understand the surface properties of marine aerosols. To investigate the influence of sea salts
92
on the studied monolayers, PW was also used as aqueous phase for comparison. Phase behavior and
93
molecular-level features of the fatty acid (PA, SA, AA) and fatty acid methyl ester (MP, MS, MA)
94
monolayers at the air-aqueous interface were examined using π−A isotherms and IRRAS spectra.
95
These compounds commonly have a carboxylic or methyl ester head-group connected to a saturated
96
hydrocarbon chain with different chain-lengths. The objective of the experiments outlined in this
97
paper is to determine the effect of alkyl chain-length, head-groups and sea salts on the properties of
98
the surfactant monolayers at the air-aqueous interface.
99 100
2. EXPERIMENTAL SECTION
101
2.1 Materials. PA (≥98%, Adamas-beta), SA (98%, Aladdin), AA (99%, Aladdin), MP (99%,
102
Aladdin), MS (99%, Aladdin) and MA (≥98%, Aladdin) were used without further purification. These
103
chemicals were dissolved in chloroform to a final concentration of 1 mM. Ultrapure water with a
104
resistivity of 18.2 MΩ was obtained from a Millipore Milli-Q purification system. ASW (see Table
105
1 for the detailed composition and concentrations) is a ten components mixture with a total
106
concentration of approximately 0.53 M.37-38 Specifically, it consists of: NaCl (≥99%, Acros Organics),
107
Na2SO4 (99%, Alfa Aesar), KCl (3 M, Alfa Aesar), NaHCO3 (≥99.7, Alfa Aesar), KBr (≥99%, Alfa
108
Aesar), H3BO3 (99.5%, Innochem), NaF (≥99%, Acros Organics), MgCl2·6H2O (≥99, Aladdin),
109
CaCl2·2H2O (99%, Adamas-beta), SrCl2·6H2O (≥99, Alfa Aesar). All these salts were used as
110
received. The pH of the ASW was measured in the range of 8.0 ± 0.2, a value representative of the
111
real seawater. The prepared ASW solution was allowed to equilibrate for several hours before
112
experiments were conducted.
113 114
Table 1. Composition of the artificial seawater
115
Type of cation Salt Concentration in aqueous solution (mM) monovalent NaCl 426 Na2SO4 29.4 KCl 9.45 NaHCO3 2.43 KBr 0.857 H3BO3 0.438 NaF 0.0744 divalent MgCl2·6H2O 55.5 CaCl2·2H2O 10.8 SrCl2·6H2O 0.0937 116
2.2 Monolayer Spreading and Isotherm Measurements.
117
The surface pressure π is a measurement of the difference between the surface tension when the
118
surface is covered in a surfactant and the surface tension of the bare surface (π = γ0 - γLangmuir, where
119
γ0 is the surface tension of pure water and γLangmuir is the surface tension of water with the Langmuir
120
film at the air-water interface).16,39 The surface tension value of ASW was calculated to be in the
121
range of 73.7-74.0 mN/m at 291 ± 1 K (details are given in the Supporting Information). Standard
122
deviations of the molecular area and surface pressure were ±1 Å2/molecule and ±0.5 mN/m,
123
respectively.
π−A isotherms of the monolayers at the air-aqueous interface were recorded using a
computer-125
controlled Langmuir trough with two movable barriers sitting on top of the aqueous surface. The
126
trough is made out of Teflon and has inside dimensions of 65 mm × 280 mm × 3 mm. It was placed
127
on a vibration isolation table and closed in aPlexiglas box. At the beginning of the experiments, the
128
two barriers were placed at the ends of the trough.Tens of microliters of chloroform solutions of fatty
129
acids or fatty acid methyl esters were spread dropwise onto PW or ASW subphase using a glass
130
microsyringe. After deposition, about 15 min was allowed before compression to permit the solvent
131
to evaporate and the film to spread spontaneously. A pressure sensor with a Wilhelmy plate made
132
from a piece of rectangular filter paper was used to monitor the surface pressure with high sensitivity.
133
The π−A isotherms were obtained with the pressure sensor while the surface area available for the
134
surfactant molecules was decreased between the barriers. The monolayer at the air-aqueous interface
135
was continuously compressed at a constant rate of 3 mm/min. All experiments were performed at
136
ambient temperature (291 ± 1 K). Each experiment was run at least three times to ensure
137
reproducibility.
138 139
2.3 IRRAS Measurements. IRRAS spectra of the monolayers were recorded on a Bruker Vertex 70
140
FTIR spectrometer equipped with an external variable angle reflectance accessory for monolayer
141
measurements. To have maximum signal strength, the incidence angle of the IR beam was set at 40°
142
with respect to the surface normal. IRRAS spectra were collected over the range of 4000-400 cm-1 by
143
using a liquid-nitrogen cooled HgCdTe (MCT) detector and averaged for 2000 scans at a resolution
144
of 8 cm-1. For IRRAS spectra collection, the monolayers were continuously compressed to a desired
145
surface pressure from ∼0 mN/m. When the barriers were stopped, IRRAS spectra were obtained after
146
a time delay of 60 s, allowed for film equilibrium between trough movement and data collection.
147
IRRAS spectra were obtained at the surface pressure of 28 mN/m, which corresponded to the untilted
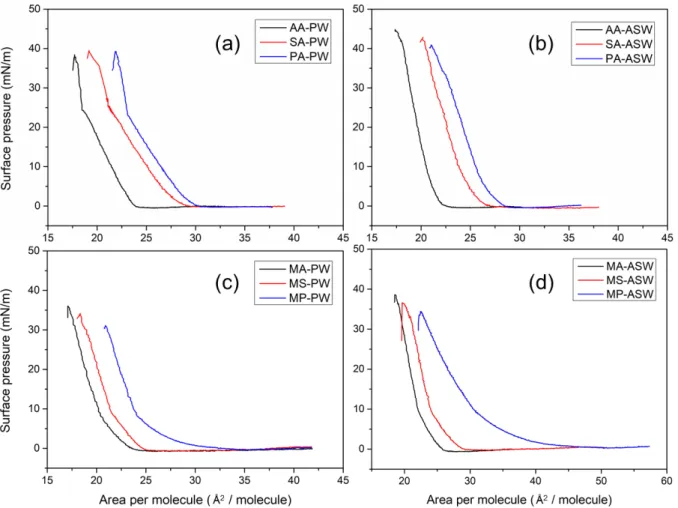
148
condensed phase of the π−A isotherms. During the IRRAS data collection, surface pressure changed
149
slightly for the monolayers (≤0.2 mN/m).
150 151
3. RESULTS AND DISCUSSION
152
3.1 Surface Pressure−Area Isotherm. π−A isotherms provide information about the phase behavior
153
of the monolayers at air-water interfaces. During the movement of the barriers, the phase of the
154
surface monolayer changes with the increase of the surface pressure. The phase changes can thus be
155
recognized from characteristics of the π−A isotherms. The surface pressure monitored as a function
of surface area of fatty acid and fatty acid methyl ester monolayers at room temperature are shown in
157
Figure 1. These π−A isotherms show remarkable changes along with alkyl chain-length, head-groups,
158
and aqueous subphases.
159 160
161
Figure 1. Surface pressure−area isotherms of fatty acid ((a), (b)) and fatty acid methyl ester ((c), (d))
162
monolayers on pure water (PW) and artificial sea water (ASW) subphases.
163 164
It can be seen from Figure 1(a) that the monolayers of fatty acids on pure water (PW) subphase
165
exhibit the following typical features in π−A isotherms upon compression. At low surface pressure
166
(π = 0 mN/m), the fatty acid monolayers show a gaseous-tilted condensed (G-TC) coexistence phase
167
before the lift-off area. In this phase, the alkyl chains are mostly free in space.40 And then, the
168
monolayers were enforced into a tilted condensed (TC) phase after subsequent compression. In this
169
phase, there are less spatial movements for the fatty acid molecules. Further compression results in a
170
kink indicates a second-order phase transition from TC to an untilted condensed (UC) phase. In the
171
UC phase the hydrocarbon chains of fatty acids are almost perpendicular to the water subphase.16 In
addition, the surface pressure of second-order phase transitions from TC to UC phase upon
173
compression occur at about 24 mN/m, which is consistent with previous studies.25,41-42 Finally,
174
compressing the monolayer even further leads to a collapse state where the monolayer forms
three-175
dimensional structures because the surface is no longer stable.16,43-44
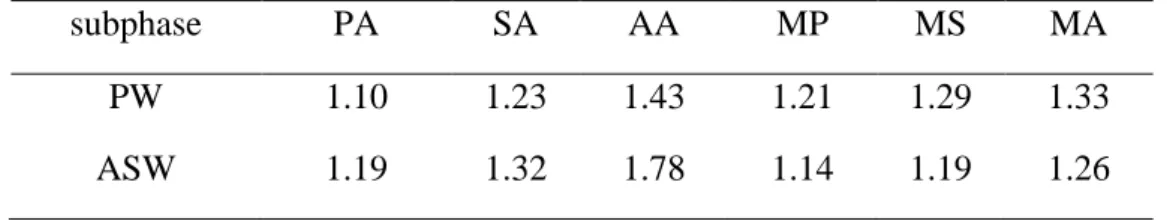
176
The π−A isotherms of fatty acids obtained on ASW subphase are presented in Figure 1(b). The
177
isotherm conducted solely with the ASW subphase is shown in Figure S1, from which can be seen
178
that the surface pressure fluctuates around 0 mN/m, indicating that no or negligible surface active
179
substance is present in the ASW subphase. Thus, the influence of organic contaminants present in sea
180
salts can be ruled out. In the presence of sea salts, some changes are found in comparison with the
181
isotherms obtained on the PW subphase. It can be clearly seen that the TC phase disappears, as well
182
as the second-order phase transition. Beyond the lift-off points, the surface pressure increases steeply
183
with a formation of UC phase until the collapse of the monolayers occurs. This is in agreement with
184
a previous report studying the π−A isotherms for PA monolayers formed on CaCl2 solution in.25 This
185
behavior was attributed to the condensing effect of metal cations as a result of forming fatty acid
186
salts.25,45 Thus, a more orderly packed structure can be speculated for fatty acid monolayers on ASW
187
subphase. The differences in properties of π−A isotherms between Figures 1(a) and 1(b) indicate that
188
sea salts present in the subphase alter the macroscopic phase behavior of the long chain fatty acid
189
monolayers.
190
A strong chain-length dependence of fatty acids is also observed, as the lift-off areas are
191
concerned, which becomes gradually smaller with increasing chain-length. The lift-off areas of the
192
isotherms on PW are 30.3, 29.3 and 24.2 Å2/molecule for PA, SA and AA, respectively. The sequence
193
and general shape of the interfacial isotherms herein are consistent with previous reports.37,46-47 The
194
chain-length dependence of lift-off areas indicates that the van der Waals energy increases with the
195
length of chain, and draws the molecules closer as the intermolecular attraction increases.47-48 On the
196
other hand, compared to PW, condensation of the fatty acid monolayers occurs when ASW is used
197
as subphase as illustrated in Figure 1(b). It is evident that with ASW as subphase, the lift-off areas
198
are decreased for individual fatty acids. The change in lift-off areas for PA, SA and AA are -1.4, -2.2,
199
-1.7 Å2/molecule, respectively. Consequently, all the studied fatty acid molecules become more
200
densely packed on ASW than on PW.46 At the air-ASW interface, the favorable electrostatic
201
interaction or complexation between fatty acids and sea salts leads to a denser chain packing relative
202
to that of air-PW interface.49
203
Being molecules with saturated single alkyl chains like fatty acids, fatty acid methyl ester
molecules are fairly compressible as well, and ultimately, can be packed in a highly ordered structure
205
at high surface pressures. As can be seen from Figures 1(c) and 1(d), the slight TC-UC transition
206
occurs at about 8 mN/m as the surface pressure rises for all the three fatty acid methyl ester
207
monolayers, irrespective of the subphase. Specifically, the π−A isotherm obtained for MP in this
208
study is consistent with a previous research about the temperature dependence of MP monolayers,
209
which found that they are fully condensed below 293 K and no plateau can be observed in the
210
isotherm.35,50 Unlike fatty acid monolayers, the presence of sea salts does not change significantly the
211
shape of fatty acid methyl ester isotherms, although a clear shift to larger mean molecular areas can
212
be observed. Thus, sea salts exert an opposite effect on fatty acid methyl esters compared to fatty
213
acids, resulting in an expansion of the fatty acid methyl ester monolayers. The lift-off areas are clearly
214
observed at about 34.6 and 25.4 and 24.4 Å2/molecule for MP, MS and MA monolayers on PW,
215
respectively. However, when ASW is used as subphase, the values shift to about 45.5, 29.3 and 26.3
216
Å2/molecule. Similarly, a former investigation of MP on PW and NaCl solutions with different ion
217
concentrations (1, 2 and 3 M), found the expanding effect of NaCl on the MP monolayer from the
218
increasing lift-off area with increasing NaCl concentration.50 The reason of this behavior was
219
explained to be the increased ionic strength, which influences interactions of the water molecules
220
with the carboxyl group.
221
To sum up, the presence of sea salts in the subphase has opposite effects on the fatty acid and
222
fatty acid methyl ester monolayers. In addition, similar with fatty acids, the π−A isotherms of fatty
223
acid methyl esters shift to smaller molecular areas with increasing chain-length. Hence, further
224
increase in the hydrocarbon chain-length results in more densely packing of the fatty acid methyl
225
ester molecules. Thus, the conformational order of the alkyl chains increases with increasing
chain-226
length can be concluded from the π−A isotherms: C16 < C18 < C20, irrespective of head-groups or
227
subphases.
228
When comparing Figures 1(b) and 1(d), the difference in π−A isotherms on ASW subphase
229
indicates that the phase sequence is changed because of the esterification of the carboxyl group, with
230
the appearance of TC phase and second-order phase transitions in fatty acid methyl ester monolayers.
231
When ASW is used as subphase, it can be seen from Figures 1(b) and 1(d) that the lift-off areas of
232
fatty acid methyl esters are much larger than corresponding fatty acids. The main difference between
233
the fatty acids and the fatty acid methyl esters is the head-group structure, where the acids are
234
sensitively influenced by the subphase pH. Empirical evidence suggests that under the conditions of
235
about pH=8.2, the carboxyl group is partially dissociated to be negatively charged.4,25,51 It is likely
that the stability and surface activity of these long chain fatty acids decrease upon dissociation of the
237
carboxylic acid proton.4 However, the stability of these monolayers can be greatly improved by
238
electrostatic attractions or complexation with various sea salts in the aqueous subphase.49,52 By means
239
of IRRAS, the surface propensity of PA molecules was found to be increased by adding NaCl into
240
the subphase,4 suggesting that deprotonated fatty acids may be found at the air-aqueous interface due
241
to the role of sea salts in surface stabilization. A study of SA monolayers was carried out on 1, 10 and
242
100 times diluted ASW.37 The π−A isotherms of the SA monolayers show an enhanced stability of
243
the film against fracture when the sea salt concentration of the subphase was higher. In case of fatty
244
acid methyl esters, no effect is expected due to ionization of head-groups, because the pH of the
245
subphase is too low to observe any measurable hydrolysis of the esters. The observed different trends
246
in the isotherms may in part be due to the different interaction mechanisms between the sea salts and
247
the head-groups.
248
It has been well documented that at low surface pressure, the methyl ester head-group is
E-249
configured for expanded fatty acid methyl ester monolayers, where substantial part of the oxo-methyl
250
group is pointing out of the water (Figure S2). The E isomer of fatty acid methyl esters allows the
251
hydration of the polar group and hinders the electrostatic interactions between hydrophilic head-group
252
and cation. However, at higher surface pressure, the head-group is Z-configured with the oxo-methyl
253
component directed into the water for more orderly packed compressed states.35,53 In addition, several
254
studies have described the expulsion of water molecules from the monolayer as surface pressure
255
increases.32-33,35 When the fatty acid methyl ester monolayers are in Z-configuration, the carbonyl
256
group is shielded by the oxo-methyl component and, thus, the carbonyl group is more or less
257
prevented from being hydrogen-bridged by water molecules. The expelled water from fatty acid
258
methyl ester films could affect the arrangement of Z isomer, allowing the cations to penetrate into the
259
film (Figure S3). In this way, the growth of three-dimensional structures could be favored because
260
the partial charge of the head-group of fatty acid methyl ester would be compensated.33 Therefore,
261
we can speculate that the main cause of the instability of the fatty acid methyl ester monolayers in the
262
presence of sea salts is a collapse process which involves the formation of three-dimensional nuclei
263
on the monolayer surface.
264
The above descriptions suggest that both interactions between adjacent amphiphiles and
265
interactions between amphiphiles and the subphase are important to the macroscopic phase behavior
266
of the monolayers. However, to get deep insights into microscopic molecular arrangement and
267
underlying mechanisms, advanced spectroscopic techniques are necessary. This consideration led us
to explore the molecular conformation of the monolayer films by means of the IRRAS technique.
269 270
3.2 IRRAS Spectra. The monolayers of fatty acids with different chain-lengths (PA, SA, and AA)
271
and corresponding fatty acid methyl esters (MP, MS, MA) on air-aqueous interface were examined
272
using IRRAS. This technique enables us to probe microscopic information such as the conformation
273
order and orientation of the monolayers at the molecular-level. The vibrational modes investigated
274
encompass both head and tail groups of the sample molecules. The stretching vibrations of C-H
275
(ν(CH2)) at 2820-2950 cm-1, the ν(COO) and scissoring band of C-H (δ(CH2)) at 1400-1500 cm-1,
276
and the ν(C=O) at 1700-1800 cm-1 were probed.
277 278
279
Figure 2. IRRAS spectra (2820-2950 cm-1) of the fatty acid ((a), (b)) and fatty acid methyl ester ((c),
280
(d))monolayers recorded at 28 mN/m on pure water (PW) and artificial sea water (ASW) subphases
281
at the incidence angle of 40°.
282 283
IRRAS spectra (2820-2950 cm-1) of the fatty acid and fatty acid methyl ester monolayers in the
284
UC phase on PW and ASW subphases are shown in Figures 2(a)-(d). In Figure 2(a), for fatty acid
285
monolayers on air-PW interface, the two bands at around 2916 cm-1 and 2850 cm-1 can be assigned
286
to the methylene antisymmetric (νa(CH2)) and methylene symmetric (νs(CH2)) stretching vibrations
of the hydrocarbon chains, respectively. At the incidence angle of 40°, these bands show negative
288
reflection absorbance. The νa(CH2) and νs(CH2) frequencies have been known to be sensitive to the
289
conformation order of hydrocarbon chains.45,54 Lower frequencies are characteristic of preferential
290
all-trans conformers in highly ordered chains, while the number of gauche conformers increases with 291
increasing frequency and width of the bands.17,29 For all-trans conformations of the fully extended
292
tail chains, the symmetric and asymmetric stretching vibrations of the methylene groups are usually
293
present in the narrow ranges of 2846-2850 and 2915-2918 cm-1, respectively, and in the distinctly
294
different ranges of 2854-2856 and 2924-2928 cm-1 for disordered chains characterized by a significant
295
presence of gauche conformations.55 The relatively low frequency positions of the νs(CH2) and
296
νa(CH2) stretching modes at about 2850 cm-1 and 2916 cm-1, indicate that the alkyl chains are mostly
297
in highly ordered all-trans conformations.56 This shows clearly that the alkyl chains are almost
298
perpendicular to the air-water interface. The all-trans conformation can also be found in other
299
compressed monolayers shown in Figures 2(b), 2(c) and 2(d), irrespective of the head-groups or the
300
subphases. The highly ordered structure of fatty acids directly correlates with the van der Waals
301
interaction between adjacent alkyl chains, the interactions between adjacent COOH head-groups and
302
those between head-groups and aqueous subphase. An orderly packed structure can maximize
303
interactions with an all-trans conformation between adjacent alkyl chains.25 For the fatty acid methyl
304
ester monolayers, the steric demand in the E-configuration is very high. This structure results in quite
305
strongly tilted molecules and a poor conformational order. When the monolayer is further compressed,
306
a reduction of the available area forces molecules to approach each other and pack more densely. As
307
a compromise, the methyl group is squeezed into the subphase, thus resulting in a Z-conformation of
308
amphiphiles in the condensed phase. With this conformation, the oxo-methyl group facilitates the
all-309
trans configuration of the alkyl chains. Consequently, the head-group can be forced into the Z-310
configuration with increasing surface pressure and result in the all-trans conformation of the alkyl
311
chains.35
312
All the fatty acids and corresponding methyl esters commonly possess saturated hydrocarbon
313
chains with different chain-lengths. IRRAS bands arising from these alkyl chains provide the clear
314
spectra and hence reliable information about molecular conformation in the monolayers.30 The peak
315
heights and areas of the νa(CH2) and νs(CH2) bands indicate the packing density of the alkyl chains.35
316
It can be evidenced from Figure 2 that the peak heights and areas for the methylene stretching
317
vibrations increase with increasing chain-length, irrespective of head-groups or subphases. As the
318
directions of νa(CH2) and νs(CH2) vibrational modes are orthogonal to the molecular axis, strong
intensities of the bands indicate that the molecule stands nearly perpendicular to the water subphase
320
when the hydrocarbon chain is in the all-trans conformation.57 Hence, the much smaller peak
321
intensity of C16 relative to the higher homologues is indicative of a substantially stronger tilt and less
322
ordered conformation of the molecules, reflecting more orderly packed structure of higher
323
homologues at the UC state. It is necessary to consider these data in relation to the π−A isotherms of
324
Figure 1, which shows that smaller areas were occupied by monolayers formed by surfactants with
325
longer alkyl chain-length.
326
Table 2. The peak-height intensity ratio between the antisymmetric and symmetric bands of the CH2
327
groups (Ias/Is) for the fatty acid and fatty acid methyl ester monolayers on pure water (PW) and
328
artificial sea water (ASW) subphases.
329 330
331
The conformational order changes in the alkyl chains introduced by sea salts, head-groups or
332
alkyl chain-length can be further revealed by analyzing the relative intensity of the νa(CH2) and the
333
νs(CH2) peaks in the C-H stretching region.15 The peak-height intensity ratios between the
334
antisymmetric and symmetric bands of the CH2 groups (Ias/Is) for the studied monolayers are
335
presented in Table 2 for direct comparison. In Figure 2, the intensities of νs(CH2) peaks are relatively
336
weaker than those of the νa(CH2) peaks, thus giving Ias/Is values greater than one. Qualitatively
337
speaking, larger Ias/Is ratio indicates more orderly packed alkyl chains with nearly all-trans
338
conformation.58-60 It can be seen from Table 2 that the ratio values are smaller for monolayers formed
339
by molecules with shorter alkyl chain-length, irrespective of head-groups or subphases, indicating the
340
existence of gauche defects in corresponding monolayers. Therefore, the pronounced chain order
341
increase with increasing chain-length can be concluded from IRRAS spectra: C16 < C18 < C20,
342
which is in line with the conclusion obtained from the π−A isotherms (Figure 1). As was shown in
343
the π−A isotherms, sea salts demonstrate a condensing effect on the fatty acid monolayers, which
344
consequently leads to the absence of the TC phase. The IRRAS spectra obtained on the ASW surface
345
confirm this effect, as can be seen from the larger intensity ratios of the νa(CH2) over the νs(CH2) than
346
those on the PW subphase in individual spectra, with values of Ias/Is increasing from 1.10, 1.23 and
347
1.43 to 1.19, 1.32 and 1.78, respectively. This result can be attributed to the decrease in the
348
subphase PA SA AA MP MS MA
PW 1.10 1.23 1.43 1.21 1.29 1.33 ASW 1.19 1.32 1.78 1.14 1.19 1.26
concentration of gauche defects and tilt angle of monolayers formed on ASW subphase. With respect
349
to fatty acid methyl esters, the intensity ratios of Ias/Is obtained on ASW (1.14, 1.19 and 1.26) surface
350
are smaller than those on PW (1.21, 1.29 and 1.33), which indicates that the fatty acid methyl ester
351
monolayers are disordered by sea salts. This can be evidenced by the expanding effect introduced by
352
sea salts on fatty acid methyl ester monolayers. Therefore, IRRAS experiments confirm the contrary
353
effects of sea salts on fatty acid and fatty acid methyl ester monolayers as can be observed from π−A
354
isotherms.
355
Evidences of monolayer orientation and structural changes along with alkyl chain-length,
head-356
groups, and subphases are provided mainly by details of the νa(CH2) and the νs(CH2) bands. Peak
357
position, height, area and intensity ratios were utilized to support the analysis. These characteristics
358
correlate well with those shown by the π−A isotherms. Information about the dependence of the chain
359
order on the alkyl chain-length and subphases, i.e., the overall effect of an increase in alkyl
chain-360
length leading to an increase in order, ASW acts to condense fatty acid films and expand fatty acid
361
methyl ester films, was inferred from both the IRRAS spectra and the π−A isotherms. The IRRAS
362
technique not only allows for the characterization of all the above chain conformation and orientation
363
details, but also provides valuable information about molecular interaction between the monolayers
364
and the aqueous subphase.
365 366
Figure 3. IRRAS spectra (1380-1800 cm-1) of the fatty acid ((a), (b)) and fatty acid methyl ester ((c),
368
(d)) monolayers recorded at 28 mN/m on pure water (PW) and artificial sea water (ASW) at the
369
incidence angle of 40°.
370 371
Figure 3 shows IRRAS spectra (1800-1380 cm-1) of the ν(C=O), δ(CH2) and ν(COO) regions of
372
the fatty acid and fatty acid methyl ester monolayers in the UC phase on the PW and ASW subphases.
373
Three peaks are observed at 1739, 1720, and 1704 cm-1, which can be attributed to the stretching
374
vibrations of the free C=O group, the C=O group involved in one and two hydrogen bonds,
375
respectively. A previous IRRAS investigation of SA monolayer at the air-water interface gave similar
376
results.36 The ability of the carbonyl group to form hydrogen bonds was explained to be a mixture
377
effect of hydration by the water subphase and by side-bridging hydrogen-bond formation between
378
adjacent fatty acid molecules.36,61 The sharp singlet observed at 1472 cm-1 is ascribed to the δ(CH2)
379
band of the methylene groups.29,54
380
IRRAS spectra of fatty acids on the ASW subphase (Figure 3 (b)) in the region of COO
381
stretching vibrations can provide insights into interaction mechanisms between carboxylic acid
head-382
groups and the subphase. In the presence of sea salts, additional peaks arising from the asymmetric
383
(νa(COO) and symmetric (νs(COO)) stretching modes of the COO group are observed. The three
384
peaks including 1558, 1542, 1523 cm-1 are resulted from the splitting of the νa(COO) stretching
385
vibration,15 while the peak at 1419 cm-1 is assigned to the νs(COO) stretching mode.38 Complexation
386
of ions to surface-active species has been known to alter their orientation, packing, and surface
387
morphology.17,62 The stability of the SA monolayer was found to be increased significantly at high
388
pH values due to ionization of the surfactant by Ca2+ and Mg2+ in the subphase.63 It has been reported
389
that metal cations can bind to the carboxylate group in several ways including ionic binding,
390
unidentate type, bidentate chelate type and bidentate bridging type.24 The bonding type of metal
391
cations to the carboxylate group in the UC state can be classified by the difference between the
392
antisymmetric and symmetric COO stretching frequencies. In this work, the differences in νa(COO)
393
and νs(COO) stretching frequencies give three values of about 139, 123 and 104 cm-1, respectively.
394
The difference in νa(COO) and νs(COO) stretching frequencies for dissociated acid was estimated to
395
be 138 cm-1.24 Typically, the values for bidentate bridging coordination are somewhat close to that
396
for a dissociated carboxylate ion, and values of bidentate chelate coordination are less than that of a
397
dissociated carboxylate ion.24 Thus, the main component at 1558 cm-1 belongs to a bidentate bridging
structure, while the components at 1542 cm-1 and 1523 cm-1 can be attributed to bidentate chelate
399
coordinations. Hence, in the presence of the ASW subphase, dissociated fatty acids form bidentate
400
bridged and bidentate chelate coordinations. As the ASW used herein is a complex mixture of sea
401
salts, it is hard to distinguish which cation the fatty acids are binding to. In this regard, sea salts are
402
treated as a whole to consider their interaction with the head-groups of fatty acid and fatty acid methyl
403
ester molecules.
404 405
3.3 Atmospheric Implications. The organic films that reside at the air-water interface exert a
406
significant impact on many properties of SSAs, such as its ability to exchange species including water
407
molecules and traces gases across the interface,64 its ability to absorb or scatter radiation,6,10,13 and its
408
reactivity towards oxidative gases.65 Long chain fatty acid and fatty acid methyl ester monolayers at
409
the air-aqueous interface were utilized as simplified model of organic-coated SSAs. The impact of
410
sea salts, head-groups and alkyl chain-length on phase behavior and molecular organization of the
411
monolayer films was fully characterized. The higher stability of monolayers formed by species with
412
longer alkyl chain-length is clear. The lifetime of the hydrophobic layer on SSA is dependent on many
413
variables, an important one being the carbon chain-length of the surfactants comprising the coating.
65-414
66 Properties of the aqueous core, including pH and composition, also affect the stability of the organic
415
surface films. Adding sea salts into the subphase improves the stability of the fatty acid films by
416
binding to the carboxylic acid groups through bidentate bridged and bidentate chelate coordinations.
417
Thus, deprotonated fatty acids may be found at the air-aqueous interface of aerosol particles partly
418
due to the role of sea salts in surface stabilization.
419
ASW caused condensation of the fatty acid surface films, leading to tightly packed molecules.
420
However, the expansion effect was introduced by ASW toward fatty acid methyl ester films, which
421
led to loosely packed molecules. Thus, we can speculate that fatty acid molecules reside at the
422
interface of SSAs with greater stability and higher packing density relative to fatty acid methyl esters.
423
This is in line with field measurements of marine aerosols utilizing time-of-flight secondary ion mass
424
spectrometry (TOF-SIMS) as a surface sensitive analysis technique. The aerosols collected in the
425
field exhibited surface layers dominated by fatty acids.67 One major effect of surface active organic
426
monolayer shown both by observations and modeling, is the lowering of the particle surface
427
tension.39,68 Surface active species present at the air-water interface have the potential to lower the
428
surface tension of a growing droplet relative to pure water at a given relative humidity.69 A lower
429
surface tension promotes small particle growth at lower relative humidity in accord with the Kelvin
effect69-70 and increasing particle cloud condensation nuclei activation efficiency,39,68 thereby causing
431
the droplets to grow larger than predicted. As can be seen from the surface pressure-area isotherms,
432
beyond the lift-off points, the surface pressure of the interface increases with decreasing mean
433
molecular areas, indicating the reduction of surface tension. The surface tension of ASW interface
434
covered by fatty acid reduces more rapidly along with deceasing mean molecular areas than in
435
corresponding methyl ester. Thus the effectiveness of the film on surface tension reduction will
436
depend on the species of film-forming molecules as well as chemical composition of the aqueous
437
core. In addition, the optical properties of aerosols are largely dependent upon their size and in this
438
regard, the alteration in aerosol size will affect their scattering efficiency.46
439 440
4. CONCLUSIONS
441
In this work, monolayers of long chain fatty acids and fatty acid methyl esters (C16, C18, C20)
442
at the air-aqueous interface were used as proxies for the organic-coated SSAs. Both π−A isotherms
443
and IRRAS spectra were applied to systematically investigate the effect of alkyl chain-length,
head-444
groups and sea salts on the surface properties of organic monolayers. It was shown by π−A isotherms
445
that sea salts have a condensing effect on fatty acid monolayers, meanwhile, obvious differences in
446
phase behavior were detected over the PW and ASW subphases. However, an expansion effect of sea
447
salts on fatty acid methyl ester monolayers was observed, without any distinct change of the phase
448
transitions between π−A isotherms detected over the PW and ASW subphases. The pronounced chain
449
order increase with increasing chain-length (C16 < C18 < C20) was revealed by π−A isotherms,
450
irrespective of head-groups or subphases. These findings were further confirmed by IRRAS spectra.
451
Substantial intensity ratio increases in the Ias/Is were observed on monolayers formed by species with
452
longer chain-length. From the differences between νa(COO) and νs(COO) stretching frequencies, the
453
dominant binding coordinations between deprotonated fatty acids and sea salts were found to be
454
bidentate bridging and bidentate chelate. These results indicate that the surface characteristics of
455
organic-coated SSAs are influenced by both the chemical composition of the aqueous core and
456
species of film-forming molecules.
457 458 ASSOCIATED CONTENT 459 Supporting Information 460
Calculation of surface tension of artificial seawater, surface pressure−area isotherms of artificial
461
seawater and isotherm of steric acid monolayer on artificial seawater subphase (Figure S1), schematic
representation of E- and Z-conformation of fatty acid methyl esters at the air-water interface (Figure
463
S2), and schematic representation of fatty acid methyl ester monolayers at the air-seawater interface
464 (Figure S3). 465 466 AUTHOR INFORMATION 467 Corresponding Author 468
*Email: lindu@sdu.edu.cn, Tel: +86-532-58631980
469
Notes
470
There are no conflicts of interest to declare.
471 472
ACKNOWLEDGMENTS
473
This work was supported by National Natural Science Foundation of China (91644214, 21876098),
474
Shandong Natural Science Fund for Distinguished Young Scholars (JQ201705) and the the Marie
475
Curie International Research Staff Exchange project MARSU (Grant 690958).
476 477
REFERENCE
478
(1) Braun, R. A.; Dadashazar, H.; MacDonald, A. B.; Aldhaif, A. M.; Maudlin, L. C.; Crosbie, E.;
479
Aghdam, M. A.; Mardi, A. H.; Sorooshian, A. Impact of wildfire emissions on chloride and bromide
480
depletion in marine aerosol particles. Environ. Sci. Technol. 2017, 51 (16), 9013-9021.
481
(2) Quinn, P. K.; Coffman, D. J.; Johnson, J. E.; Upchurch, L. M.; Bates, T. S. Small fraction of
482
marine cloud condensation nuclei made up of sea spray aerosol. Nat. Geosci. 2017, 10 (9), 674-679.
483
(3) Jayarathne, T.; Sultana, C. M.; Lee, C.; Malfatti, F.; Cox, J. L.; Pendergraft, M. A.; Moore, K. A.;
484
Azam, F.; Tivanski, A. V.; Cappa, C. D.; Bertram, T. H.; Grassian, V. H.; Prather, K. A.; Stone, E.
485
A. Enrichment of saccharides and divalent cations in sea spray aerosol during two phytoplankton
486
blooms. Environ. Sci. Technol. 2016, 50 (21), 11511-11520.
487
(4) Adams, E. M.; Wellen, B. A.; Thiraux, R.; Reddy, S. K.; Vidalis, A. S.; Paesani, F.; Allen, H. C.
488
Sodium-carboxylate contact ion pair formation induces stabilization of palmitic acid monolayers at
489
high pH. Phys. Chem. Chem. Phys. 2017, 19 (16), 10481-10490.
490
(5) Tseng, R. S.; Viechnicki, J. T.; Skop, R. A.; Brown, J. W. Sea-to-air transfer of surface-active
491
organic-compounds by bursting bubbles. J. Geophys. Res.: Oceans 1992, 97 (C4), 5201-5206.
492
(6) Tervahattu, H.; Hartonen, K.; Kerminen, V. M.; Kupiainen, K.; Aamio, P.; Koskentalo, T.; Tuck,
493
A. F.; Vaida, V. New evidence of an organic layer on marine aerosols. J. Geophys. Res. 2002, 107
494
(D7-D8), AAC1-AAC9.
495
(7) Cochran, R. E.; Laskina, O.; Jayarathne, T.; Laskin, A.; Laskin, J.; Lin, P.; Sultana, C.; Lee, C.;
496
Moore, K. A.; Cappa, C. D. Analysis of organic anionic surfactants in fine and coarse fractions of
497
freshly emitted sea spray aerosol. Environ. Sci. Technol. 2016, 50 (5), 2477-2486.
498
(8) Lin, W.; Clark, A. J.; Paesani, F. Effects of surface pressure on the properties of Langmuir
499
monolayers and interfacial water at the air-water interface. Langmuir 2015, 31 (7), 2147-2156.
500
(9) Tervahattu, H.; Hartonen, K.; Kerminen, V. M.; Kupiainen, K.; Aarnio, P.; Koskentalo, T.; Tuck,
501
A. F.; Vaida, V. New evidence of an organic layer on marine aerosols. J. Geophys. Res.: Atmos. 2002,
502
107 (D7), 4053. 503
(10) Donaldson, D. J.; Vaida, V. The influence of organic films at the air-aqueous boundary on
504
atmospheric processes. Chem. Rev. 2006, 106 (4), 1445-1461.
505
(11) Ellison, G. B.; Tuck, A. F.; Vaida, V. Atmospheric processing of organic aerosols. J. Geophys.
506
Res.: Atmos. 1999, 104 (D9), 11633-11641. 507
(12) Feingold, G.; Chuang, P. Y. Analysis of the influence of film-forming compounds on droplet
508
growth: Implications for cloud microphysical processes and climate. J. Atmos. Sci. 2002, 59 (12),
509
2006-2018.
510
(13) Vaida, V. Atmospheric radical chemistry revisited Sunlight may directly drive previously
511
unknown organic reactions at environmental surfaces. Science 2016, 353 (6300), 650-650.
512
(14) Zhang, T.; Cathcart, M. G.; Vidalis, A. S.; Allen, H. C. Cation effects on phosphatidic acid
513
monolayers at various pH conditions. Chem. Phys. Lipids 2016, 200, 24-31.
514
(15) Li, S. Y.; Du, L.; Wei, Z. M.; Wang, W. X. Aqueous-phase aerosols on the air-water interface:
515
Response of fatty acid Langmuir monolayers to atmospheric inorganic ions. Sci. Total Environ. 2017,
516
580, 1155-1161. 517
(16) Larsen, M. C. Binary phase diagrams at the air-water interface: An experiment for undergraduate
518
physical chemistry students. J. Chem. Educ. 2014, 91 (4), 597-601.
519
(17) Adams, E. M.; Casper, C. B.; Allen, H. C. Effect of cation enrichment on
520
dipalmitoylphosphatidylcholine (DPPC) monolayers at the air-water interface. J. Colloid Interface
521
Sci. 2016, 478, 353-364. 522
(18) Rouviere, A.; Ammann, M. The effect of fatty acid surfactants on the uptake of ozone to aqueous
523
halogenide particles. Atmos. Chem. Phys. 2010, 10 (23), 11489-11500.
524
(19) Shrestha, M.; Luo, M.; Li, Y.; Xiang, B.; Xiong, W.; Grassian, V. H. Let there be light: stability
525
of palmitic acid monolayers at the air/salt water interface in the presence and absence of simulated
526
solar light and a photosensitizer. Chem. Sci. 2018, 9 (26), 5716-5723.
527
(20) Mochida, M.; Kitamori, Y.; Kawamura, K.; Nojiri, Y.; Suzuki, K. Fatty acids in the marine
528
atmosphere: Factors governing their concentrations and evaluation of organic films on sea-salt
529
particles. J. Geophys. Res.: Atmos. 2002, 107 (D17), 4325.
530
(21) Sicre, M. A.; Marty, J. C.; Saliot, A. n-Alkanes, fatty acid esters, and fatty acid salts in size
531
fractionated aerosols collected over the Mediterranean Sea. J. Geophys. Res.: Atmos. 1990, 95 (D4),
532
3649-3657.
533
(22) Cincinelli, A.; Stortini, A.; Perugini, M.; Checchini, L.; Lepri, L. Organic pollutants in
sea-534
surface microlayer and aerosol in the coastal environment of Leghorn—(Tyrrhenian Sea). Mar. Chem.
535
2001, 76 (1-2), 77-98.
536
(23) Ehrhardt, M.; Osterroht, C.; Petrick, G. Fatty-acid methyl esters dissolved in seawater and
537
associated with suspended particulate material. Mar. Chem. 1980, 10 (1), 67-76.
538
(24) Mukherjee, S.; Datta, A. Langmuir-Blodgett deposition selects carboxylate headgroup
539
coordination. Phys. Rev. E 2011, 84 (4), 041601.
540
(25) Tang, C. Y.; Huang, Z. S. A.; Allen, H. C. Binding of Mg2+ and Ca2+ to palmitic acid and
541
deprotonation of the COOH headgroup studied by vibrational sum frequency generation spectroscopy.
542
J. Phys. Chem. B 2010, 114 (51), 17068-17076. 543
(26) Gericke, A.; Brauner, J. W.; Erukulla, R. K.; Bittman, R.; Mendelsohn, R. In-situ investigation
544
of partially deuterated fatty acid and phospholipid monolayers at the air-water interface by IR
545
reflection-absorption spectroscopy. Thin Solid Films 1996, 284 (1-2), 428-431.
546
(27) Tang, C. Y.; Allen, H. C. Ionic binding of Na+ versus K+ to the carboxylic acid headgroup of
547
palmitic acid monolayers studied by vibrational sum frequency generation spectroscopy. J. Phys.
548
Chem. A 2009, 113 (26), 7383-7393. 549
(28) Teer, E.; Knobler, C. M.; Lautz, C.; Wurlitzer, S.; Kildae, J.; Fischer, T. M. Optical
550
measurements of the phase diagrams of Langmuir monolayers of fatty acid, ester, and alcohol
551
mixtures by brewster-angle microscopy. J. Chem. Phys. 1997, 106 (5), 1913-1920.
552
(29) Simon-Kutscher, J.; Gericke, A.; Huhnerfuss, H. Effect of bivalent Ba, Cu, Ni, and Zn cations
553
on the structure of octadecanoic acid monolayers at the air-water interface as determined by external
554
infrared reflection-absorption spectroscopy. Langmuir 1996, 12 (4), 1027-1034.
555
(30) Sinnamon, B. F.; Dluhy, R. A.; Barnes, G. T. Reflection-absorption FT-IR spectroscopy of
556
pentadecanoic acid at the air/water interface. Colloids Surf., A 1999, 146 (1-3), 49-61.
557
(31) Nikolova, G. S.; Zhang, L.; Chen, X.; Chi, L.; Haufe, G. Selective synthesis and self-organization
558
at the air/water interface of long chain fluorinated unsaturated ethyl esters and alcohols. Colloids Surf.,
559
A 2008, 317 (1-3), 414-420. 560
(32) Nieto-Suarez, M.; Vila-Romeu, N.; Prieto, I. Influence of different factors on the phase
561
transitions of non-ionic Langmuir monolayers. Appl. Surf. Sci. 2005, 246 (4), 387-391.
562
(33) Nieto-Suarez, M.; Vila-Romeu, N.; Dynarowicz-Latka, P.; Prieto, I. The influence of inorganic
563
ions on the properties of nonionic Langmuir monolayers. Colloids Surf., A 2004, 249 (1-3), 11-14.
564
(34) Pelletier, I.; Bourque, H.; Buffeteau, T.; Blaudez, D.; Desbat, B.; Pezolet, M. Study by infrared
565
spectroscopy of ultrathin films of behenic acid methyl ester on solid substrates and at the air/water
566
interface. J. Phys. Chem. B 2002, 106 (8), 1968-1976.
567
(35) Gericke, A.; Hühnerfussf, H. The conformational order and headgroup structure of long-chain
568
alkanoic acid ester monolayers at the air/water interface. Ber. Bunsenges. Phys. Chem. 1995, 99 (4),
569
641-650.
570
(36) Gericke, A.; Huhnerfuss, H. In-situ investigation of saturated long-chain fatty-acids at the
air-571
water-interface by external infared reflection-absorption spectrometry. J. Phys. Chem. 1993, 97 (49),
572
12899-12908.
573
(37) Brzozowska, A. M.; Duits, M. H. G.; Mugele, F. Stability of stearic acid monolayers on artificial
574
sea water. Colloids Surf., A 2012, 407, 38-48.
575
(38) Kester, D. R.; Duedall, I. W.; Connors, D. N.; Pytkowicz, R. M. Preparation of artificial seawater.
576
Limnol. Oceanogr. 1967, 12 (1), 176-179. 577
(39) Forestieri, S. D.; Staudt, S. M.; Kuborn, T. M.; Faber, K.; Ruehl, C. R.; Bertram, T. H.; Cappa,
578
C. D. Establishing the impact of model surfactants on cloud condensation nuclei activity of sea spray
579
aerosol mimics. Atmos. Chem. Phys. 2018, 18 (15), 10985-11005.
580
(40) Khattari, Z.; Sayyed, M. I.; Qashou, S. I.; Fasfous, I.; Al-Abdullah, T.; Maghrabi, M. Interfacial
581
behavior of myristic acid in mixtures with DMPC and cholesterol. Chem. Phys. 2017, 490, 106-114.
582
(41) Hao, C. C.; Sun, R. G.; Zhang, J. Mixed monolayers of DOPC and palmitic acid at the liquid-air
583
interface. Colloids Surf., B 2013, 112, 441-445.
584
(42) Griffith, E. C.; Guizado, T. R.; Pimentel, A. S.; Tyndall, G. S.; Vaida, V. Oxidized
aromatic-585
aliphatic mixed films at the air-aqueous solution interface. J. Phys. Chem. C 2013, 117 (43),
22341-586
22350.
587
(43) Minh Dinh, P.; Lee, J.; Shin, K. Collapsed States of Langmuir Monolayers. J. Oleo Sci. 2016,
588
65 (5), 385-397. 589
(44) Kaganer, V. M.; Mohwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers.
590
Rev. Mod. Phys. 1999, 71 (3), 779-819. 591
(45) Hasegawa, T.; Nishijo, J.; Watanabe, M.; Umemura, J.; Ma, Y. Q.; Sui, G. D.; Huo, Q.; Leblanc,
592
R. M. Characteristics of long-chain fatty acid monolayers studied by infrared external-reflection
593
spectroscopy. Langmuir 2002, 18 (12), 4758-4764.
594
(46) Adams, E. M.; Allen, H. C. Palmitic acid on salt subphases and in mixed monolayers of
595
cerebrosides: Application to atmospheric aerosol chemistry. Atmosphere 2013, 4 (4), 315-336.
(47) Nutting, G. C.; Harkins, W. D. Pressure-area relations of fatty acid and alcohol monolayers. J.
597
Am. Chem. Soc. 1939, 61, 1180-1187. 598
(48) Sierra-Hernandez, M. R.; Allen, H. C. Incorporation and exclusion of long chain alkyl halides in
599
fatty acid monolayers at the air-water interface. Langmuir 2010, 26 (24), 18806-18816.
600
(49) Brzozowska, A.; Mugele, F.; Duits, M. Stability and interactions in mixed monolayers of fatty
601
acid derivatives on artificial sea water. Colloids Surf., A 2013, 433, 200-211.
602
(50) Yue, X. L.; Steffen, P.; Dobner, B.; Brezesinski, G.; Mohwald, H. Monolayers of mono- and
603
bipolar palmitic acid derivatives. Colloids Surf., A 2004, 250 (1-3), 57-65.
604
(51) Gershevitz, O.; Sukenik, C. N. In situ FTIR-ATR analysis and titration of carboxylic
acid-605
terminated SAMs. J. Am. Chem. Soc. 2004, 126 (2), 482-483.
606
(52) Johann, R.; Vollhardt, D. Texture features of long-chain fatty acid monolayers at high pH of the
607
aqueous subphase. Mater. Sci. Eng., C 1999, 8, 35-42.
608
(53) Wang, L.; Jacobi, S.; Sun, J.; Overs, M.; Fuchs, H.; Schaefer, H. J.; Zhang, X.; Shen, J.; Chi, L.
609
Anisotropic aggregation and phase transition in Langmuir monolayers of methyl/ethyl esters of 2,
3-610
dihydroxy fatty acids. J. Colloid Interface Sci. 2005, 285 (2), 814-820.
611
(54) Wang, Y.; Du, X.; Guo, L.; Liu, H. Chain orientation and headgroup structure in Langmuir
612
monolayers of stearic acid and metal stearate (Ag, Co, Zn, and Pb) studied by infrared
reflection-613
absorption spectroscopy. J. Chem. Phys. 2006, 124 (13), 134706.
614
(55) Chen, Q. B.; Kang, X. L.; Li, R.; Du, X. Z.; Shang, Y. Z.; Liu, H. L.; Hu, Y. Structure of the
615
Complex Monolayer of Gemini Surfactant and DNA at the Air/Water Interface. Langmuir 2012, 28
616
(7), 3429-3438.
617
(56) Pelletier, I.; Laurin, I.; Buffeteau, T.; Desbat, B.; Pézolet, M. Infrared study of the molecular
618
orientation in ultrathin films of behenic acid methyl ester: comparison between single
Langmuir-619
Blodgett monolayers and spin-coated multilayers. Langmuir 2003, 19 (4), 1189-1195.
620
(57) Muro, M.; Itoh, Y.; Hasegawa, T. A conformation and orientation model of the carboxylic group
621
of fatty acids dependent on chain length in a Langmuir monolayer film studied by
polarization-622
modulation infrared reflection absorption spectroscopy. J. Phys. Chem. B 2010, 114 (35),
11496-623
11501.
624
(58) Levin, I. W.; Thompson, T. E.; Barenholz, Y.; Huang, C. Two types of hydrocarbon chain
625
interdigitation in sphingomyelin bilayers. Biochemistry 1985, 24 (22), 6282-6286.
626
(59) Aoki, P. H. B.; Morato, L. F. C.; Pavinatto, F. J.; Nobre, T. M.; Constantino, C. J. L.; Oliveira,
627
O. N., Jr. Molecular-Level Modifications Induced by Photo-Oxidation of Lipid Monolayers
628
Interacting with Erythrosin. Langmuir 2016, 32 (15), 3766-3773.
629
(60) Huang, C. H.; Lapides, J. R.; Levin, I. W. Phase-transition behavior of saturated, symmetric
630
chain phospholipid-bilayer dispersions determined by Raman-spectroscopy-correlation between
631
spectral and thermodynamic parameters. J. Am. Chem. Soc. 1982, 104 (22), 5926-5930.
632
(61) Sakai, H.; Umemura, J. Molecular orientation in Langmuir films of 12-hydroxystearic acid
633
studied by infrared external-reflection spectroscopy. Langmuir 1998, 14 (21), 6249-6255.
634
(62) Le Calvez, E.; Blaudez, D.; Buffeteau, T.; Desbat, B. Effect of cations on the dissociation of
635
arachidic acid monolayers on water studied by polarization-modulated infrared reflection-absorption
636
spectroscopy. Langmuir 2001, 17 (3), 670-674.
637
(63) Avila, L.; Saraiva, S.; Oliveira, J. Stability and collapse of monolayers of stearic acid and the
638
effect of electrolytes in the subphase. Colloids Surf., A 1999, 154 (1-2), 209-217.
639
(64) Griffith, E. C.; Adams, E. M.; Allen, H. C.; Vaida, V. Hydrophobic collapse of a stearic acid
640
film by adsorbed l-phenylalanine at the air-water interface. J. Phys. Chem. B 2012, 116 (27),
7849-641
7857.
642
(65) Gilman, J. B.; Tervahattu, H.; Vaida, V. Interfacial properties of mixed films of long-chain
643
organics at the air-water interface. Atmos. Environ. 2006, 40 (34), 6606-6614.
(66) Gilman, J. B.; Eliason, T. L.; Fast, A.; Vaida, V. Selectivity and stability of organic films at the
645
air-aqueous interface. J. Colloid Interface Sci. 2004, 280 (1), 234-243.
646
(67) Tervahattu, H.; Juhanoja, J.; Kupiainen, K. Identification of an organic coating on marine aerosol
647
particles by TOF-SIMS. J. Geophys. Res.: Atmos. 2002, 107 (D16), 4319.
648
(68) Noziere, B.; Baduel, C.; Jaffrezo, J.-L. The dynamic surface tension of atmospheric aerosol
649
surfactants reveals new aspects of cloud activation. Nat. Commun. 2014, 5, 3335.
650
(69) Farmer, D. K.; Cappa, C. D.; Kreidenweis, S. M. Atmospheric Processes and Their Controlling
651
Influence on Cloud Condensation Nuclei Activity. Chem. Rev. 2015, 115 (10), 4199-4217.
652
(70) Gorbunov, B.; Hamilton, R.; Clegg, N.; Toumi, R. Water nucleation on aerosol particles
653
containing both organic and soluble inorganic substances. Atmos. Res. 1998, 48, 271-283.
654 655