HAL Id: hal-01974344
https://hal.sorbonne-universite.fr/hal-01974344
Submitted on 8 Jan 2019
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
non HCV-liver related cancers in HIV/HCV-coinfected
patients- ANRS-CO13 HEPAVIH cohort
Oumar Billa, Mathieu Chalouni, Dominique Salmon, Isabelle Poizot-Martin,
Camille Gilbert, Christine Katlama, Didier Neau, Julie Chas, Philippe
Morlat, Karine Lacombe, et al.
To cite this version:
Oumar Billa, Mathieu Chalouni, Dominique Salmon, Isabelle Poizot-Martin, Camille Gilbert, et al.. Factors associated with non-AIDS-defining cancers and non HCV-liver related cancers in HIV/HCV-coinfected patients- ANRS-CO13 HEPAVIH cohort. PLoS ONE, Public Library of Science, 2018, 13 (12), pp.e0208657. �10.1371/journal.pone.0208657�. �hal-01974344�
Factors associated with non-AIDS-defining
cancers and non HCV-liver related cancers in
HIV/HCV-coinfected patients- ANRS-CO13
HEPAVIH cohort
Oumar Billa1☯, Mathieu Chalouni1☯, Dominique Salmon2,3, Isabelle Poizot-Martin4,5, Camille Gilbert1, Christine Katlama6,7, Didier Neau8,9, Julie Chas10, Philippe Morlat1,9,11, Karine Lacombe12,13, Alissa Naqvi14, Karl Barange15, Anne Gervais16, Olivier Bouchaud17,18, Eric Rosenthal19,20, Caroline Lascoux-Combe21, Daniel Garipuy22, Laurent Alric23,24, Ste´phanie Dominguez25, Daniel Vittecoq26,27, Ce´cile Goujard27, Claudine DuvivierID28, Hugues Aumaitre29, Patrick Miailhes30, David Zucman31, Anne Simon32, Estibaliz Lazaro33, Franc¸ois Raffi34,35, Laure EsterleID1, Linda Wittkop1,36☯, Firouze´ Bani-Sadr37,38☯*, for the ANRS CO13 HEPAVIH Study Group¶
1 Univ Bordeaux, ISPED, Inserm Bordeaux Population Health, team MORPH3EUS, UMR 1219, CIC-EC 1401, Bordeaux, France, 2 Universite´ Paris Descartes, Paris, France, 3 Unite´ Maladies Infectieuses et Tropicales- Hoˆpitaux Universitaires Paris Centre, APHP, Paris, France, 4 Aix Marseille Universite´, APHM Hoˆpital Sainte-Marguerite, Service d’Immuno-he´matologie clinique, Marseille, France, 5 INSERM, U912 (SESSTIM)- Marseille, France, 6 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Pitie´-Salpe´trière, Service Maladies infectieuses et tropicales, Paris, France, 7 Institut Pierre Louis Epide´miologie et Sante´ Publique UPMC, Sorbonne Universite´ , Paris, France, 8 Centre Hospitalier Universitaire de Bordeaux, Service Maladies infectieuses et tropicales Bordeaux, Hoˆ pital Pellegrin, Bordeaux, France, 9 Universite´ de Bordeaux, Bordeaux, France, 10 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Tenon, Service Maladies infectieuses et tropicales, Paris, France, 11 Centre Hospitalier Universitaire de Bordeaux, Service de me´decine interne, Hoˆpital Saint-Andre´, Bordeaux, France, 12 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Saint-Antoine, Service Maladies infectieuses et tropicales, Paris, France, 13 UMR S1136, Institut Pierre Louis d’Epide´ miologie et de Sante´ Publique, Paris, France, 14 Centre Hospitalier Universitaire de Nice, Service d’Infectiologie, Hoˆpital Archet 1, Nice, France, 15 Centre Hospitalier Universitaire de Toulouse, Service Gastro-ente´ rologie et he´patologie, Hoˆpital Purpan, Toulouse, France, 16 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Bichat Claude Bernard, Service des maladies infectieuses et tropicales, Paris, France, 17 Assistance Publique des Hoˆpitaux de Paris, Hoˆ pital Avicenne, Service Maladies infectieuses et tropicales, Bobigny, France, 18 Universite´ Paris 13 Nord, Bobigny, France, 19 Centre Hospitalier Universitaire de Nice, Service de Me´decine Interne et Cance´ rologie, Hoˆpital l’Archet, Nice, France,
20 Universite´ de Nice-Sophia Antipolis, Nice, France, 21 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Saint-Louis, Service des Maladies infectieuses et tropicales, Paris, France, 22 Centre Hospitalier
Universitaire de Toulouse, Hoˆpital Purpan, Services des Maladies infectieuses et tropicales, Toulouse, France, 23 Centre Hospitalier Universitaire de Toulouse, Hoˆpital Purpan, Services de Me´decine interne-Pole Digestif, Toulouse, France, 24 UMR 152, IRD, Universite´ Toulouse III, Toulouse, France, 25 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Henri Mondor, Service Immunologie clinique et maladies
infectieuses, Immunologie clinique, Cre´ teil, France, 26 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Bicêtre, Hoˆpitaux universitaires Paris Sud, Service Maladies infectieuses et tropicales, Le Kremlin-Bicêtre, France, 27 Assistance Publique des Hoˆpitaux de Paris, Hoˆ pital Bicêtre, Hoˆpitaux universitaires Paris Sud, Service Me´decine interne et Immunologie clinique, Le Kremlin-Bicêtre, France, 28 Assistance Publique des Hoˆpitaux de Paris, Service de Maladies Infectieuses et Tropicales, Hoˆpital Necker-Enfants malades, Centre d’Infectiologie Necker-Pasteur, IHU Imagine, Paris, France, 29 Centre Hospitalier de Perpignan, Service Maladies infectieuses et tropicales, Perpignan, France, 30 Centre Hospitalier Universitaire de Lyon, Service des Maladies Infectieuses et Tropicales, Hoˆ pital de la Croix Rousse, Lyon, France, 31 Hoˆpital Foch, unite´ VIH, Suresnes, France, 32 Assistance Publique des Hoˆpitaux de Paris, Hoˆpital Pitie´-Salpe´trière, De´partement de Me´decine Interne et Immunologie Clinique, Paris, France, 33 Centre Hospitalier Universitaire de Bordeaux, hoˆpital Haut-Le´vèque, Service de Me´decine interne et Maladies Infectieuses, Pessac, France, 34 CHU de Nantes, Department of Infectious Diseases, Nantes, France, 35 Universite´ de Nantes, CIC 1413, INSERM, Nantes, France, 36 CHU de Bordeaux, Poˆle de Sante´ publique, Service d’information me´dicale, Bordeaux, France, 37 Centre Hospitalier Universitaire de Reims, Unite´ des Maladies
a1111111111 a1111111111 a1111111111 a1111111111 a1111111111 OPEN ACCESS
Citation: Billa O, Chalouni M, Salmon D,
Poizot-Martin I, Gilbert C, Katlama C, et al. (2018) Factors associated with non-AIDS-defining cancers and non HCV-liver related cancers in HIV/HCV-coinfected patients- ANRS-CO13 HEPAVIH cohort. PLoS ONE 13(12): e0208657.https://doi.org/ 10.1371/journal.pone.0208657
Editor: Cristian Apetrei, University of Pittsburgh
Centre for Vaccine Research, UNITED STATES
Received: August 29, 2018 Accepted: November 20, 2018 Published: December 18, 2018
Copyright:© 2018 Billa et al. This is an open access article distributed under the terms of the
Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement: Data cannot be
shared publicly because the ANRS CO13 HEPAVIH cohort is a French nationwide cohort sponsored by the ANRS (France REcherche Nord&sud Sida-hiv Hepatites)). Data is owned by ANRS and there are also legal restrictions to share data publicly. Data are available from the scientific committee and the ANRS which can allow a contractual assess for collaboration purposes. Applicants will be asked to complete a Research Application Form specifying details for their planned study which will then be
Infectieuses et Tropicales, Hoˆpital Robert Debre´, Reims, France, 38 Universite´ Reims Champagne Ardenne, EA-4684 / SFR CAP-SANTE, Reims, France
☯These authors contributed equally to this work.
¶ Membership of the ANRS-CO13 HEPAVIH cohort is provided in the Acknowledgments.
*fbanisadr@chu-reims.fr
Abstract
Compared to the general population, HIV-infected patients are at higher risk of developing non-AIDS-defining cancers. Chronic HCV infection has also been associated with a higher risk than that of the general population of developing cancers other than hepatocarcinoma. Evaluation of the impact of HCV-related factors on non-AIDS-defining and non HCV-liver (NANL) related cancers among HIV/HCV co-infected patients are scarce. The aim of this study was to identify the impact of HIV/HCV clinical characteristics on NANL related cancers in a large cohort of HIV/HCV-coinfected patients followed from 2005 to 2017. Cox propor-tional hazards models with delayed entry were used to estimate factors associated with NANL related cancer. Among 1391 patients followed for a median of 5 years, 60 patients developed NANL related cancers, yielding an incidence rate of 8.9 per 1000 person-years (95% CI, [6.6–11.1]). By final multivariable analysis, after adjustment for sex, tobacco or alcohol consumption, baseline CD4 cell count and HCV sustained viral response (SVR), age and a longer duration since HIV diagnosis were independently associated with a higher risk of NANL related cancer (aHR for each additional year 1.10, 95% CI 1.06–1.14, p<0.0001 and 1.06, 95% CI 1.01–1.11, p = 0.02, respectively). Duration of HCV infection, cirrhosis, HCV viral load, genotype and SVR were not associated with the occurrence of NANL related cancer. Among HIV/HCV-coinfected patients, age and the duration of HIV infection were the only characteristics found to be associated with the occurrence of NANL related cancer. In contrast, no association was observed with any HCV-related variables.
Introduction
HIV-infected patients have a higher risk of developing non-AIDS-defining cancers compared to the general population [1–4]. Age, duration of HIV infection and immune status are known risk factors for many of these non-AIDS cancers [2,5,6]. Additional factors may vary by cancer, such as poor immune control for oncogenic viruses such as Human papillomavirus (HPV) or Epstein Barr virus (EBV), and/or lifestyle choices, such as tobacco or alcohol use for lung or digestive cancers [2,4]. HCV is an oncogenic virus proven to cause hepatocarcinoma (HCC) and B-cell non-Hodgkin lymphoma [7,8]. Furthermore, some epidemiological studies suggest that patients with chronic HCV infection are also at higher risk than the general population of developing other cancers, such as cancers of the esophagus, pancreas, prostate, thyroid, breast or oral cavity [5,7,9–11]. In national surveys consecutively conducted in France in 2000, 2005 and in 2010, the rate of death attributed to non-AIDS-defining cancers and non HCV-liver (NANL) related cancers significantly increased between 2000 and 2010 (11% of deaths in 2000, 17% in 2005 and 22% in 2010, p<0.001) [12].
reviewed by the ANRS CO13 Hepavih Scientific commitee (contact by email at: secretariat-clinique@anrs.fr; project manager,laure. esterle@u-bordeaux.fr) for researchers who meet the criteria for access to confidential data.
Funding: The authors received no specific funding
for this work.
Competing interests: The authors have declared
Evaluations of factors associated with NANL related cancers among HIV/HCV co-infected patients are scarce. In particular, the influence of HCV-related factors, such as duration of HCV infection, cirrhosis, HCV genotype and HCV viral load, needs further study. We aimed to investigate the associations between HIV/HCV clinical characteristics, and patients’ socio-behavioural profiles, and the occurrence of NANL related cancers in a large cohort of HIV/ HCV-coinfected patients (ANRS CO13 HEPAVIH) followed in the era of increasing effective-ness of combination antiretroviral therapy (cART).
Methods
This study involved HIV/HCV coinfected patients enrolled in the ANRS CO13 HEPAVIH cohort, a prospective, hospital-based cohort of HIV/HCV coinfected patients created in 2005 and involving 28 hospitals in France [13]. For this study, patients with at least one follow-up visit before 31stSeptember 2017 were included. Patients with a history of cancer at inclusion, patients with spontaneously cured HCV, those without follow-up, and those with missing data for tobacco consumption, alcohol consumption or cirrhotic status were excluded. The study was approved by the institutional review board: Comite´ Ile de France 3, file n˚2234, ref CG/ LG/CC 2005-255. Each participant agreed to participate to the ANRS CO13 Hepavih cohort by written consent.
The primary outcome was the time to occurrence of a first NANL related cancer. Cancer cases were prospectively collected. Patients with cancers were identified using the medical dic-tionary for regulatory activities (MedDRA). Cancer cases were also researched in the record deaths mentioning the cause of death. Patients were censored at the date of the diagnosis of a first NANL related cancer, at their date of death, or at their last follow-up, date whichever came first.
Qualitative variables were described as number (percentage) and quantitative variables as median (interquartile range [IQR]). Independent risk factors associated with NANL related cancer were studied using survival analysis. To take into account competitive risk for death without NANL related cancer, patients were censored at their death date if they didn’t experi-ence a NANL cancer before.
First, univariable Cox proportional hazards models were used to identify factors associated with NANL related cancers. Variables included in these models were: age at inclusion (in years), sex, smoking status (never, former, current), alcohol consumption (never, former, cur-rent), time since HIV diagnosis (in years), duration of antiretroviral treatment (in years), AIDS status (yes, no), CD4 cell count nadir inferior to 200 cells/mm3 (yes, no), CD4 cell count (in cells/mm3), CD8 cell count (in cells/mm3), CD4/CD8 ratio, HIV viral load undetectable (yes, no), HCV transmission group (men who have sex with men, intravenous drug users, oth-ers), duration of HCV infection (counting from the date of the first transfusion, the date of ini-tial intravenous drug use, or the first positive HCV serology in subjects who were infected via the sexual route and who did not develop acute hepatitis C), HCV genotype 1 (yes, no). Sus-tained viral response (SVR) to anti-HCV therapy (yes, no) defined as an undetectable serum HCV RNA (< 15 IU per milliliter) 24 weeks after treatment completion was included in the models as a time dependent variable. Cirrhosis status assessed by transient elastometry (TE) or FIB-4 score calculated as previously described [14] for patients without liver stiffness measure-ment. Cut-offs defining fibrosis were liver stiffness measurement superior or equal to 12.5 kPa and FIB-4 superior or equal to 3.45. Type 2 diabetes (yes, no) defined by a preexisting diagno-sis of diabetes or an antidiabetic treatment at baseline, or a fasting blood glucose value of �7 mmol/L at least twice during follow-up. Wald’s tests were realized, and p-values were estimated.
Then a manual backward elimination procedure was realized in a Cox proportional hazards model. Variables with p-value inferior to 0.25 in univariate analysis were included in the model and factorsa priori deemed to be important predictive factors of NANL cancers (age,
sex, tobacco consumption, alcohol consumption, CD4 cell count and SVR) were forced in the model. Then variables nota priori important and with p-values superior to 0.05 were excluded
from the model. The proportional hazards assumption was tested with interactions between covariates and time.
Cumulative incidences of NANL cancer were estimated for each quartile of time since HIV diagnosis using Aalen Johansen methodology to take into account competitive risk of death without NANL related cancer. All statistical tests were two-sided, with a type I error of 5%. Sta-tistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Results
Among 1427 eligible patients, 36 were excluded due to missing data for tobacco-, alcohol-con-sumption or cirrhosis status. Therefore, a total of 1391 patients were considered for this analysis.
The demographic and clinical characteristics of the study population at baseline are shown inTable 1. Most patients were male (73%), and median age was 46 years (IQR, 43–51 years). There were respectively 69% current smokers and 49% current alcohol drinkers. The median time since HIV diagnosis was 18.6 years (IQR, 13.9–21.5). Most patients (90%) received cART, and the median duration of cART was 129 months (IQR, 82–179). AIDS was present in 25% of patients. The median CD4 cell count was 478 cells/mm3(IQR, 325–675 cells/mm3), median CD4 cell count nadir was 155 cells/mm3(IQR, 73–248 cells/mm3), and 75% of patients had an undetectable HIV viral load (<50 copies/ml). At baseline, 1173 patients (96%) had a positive HCV RNA and 27% had cirrhosis. The median duration of HCV infection was 14 years (IQR, 8.0–22).
Median follow-up was 5 years (IQR, 1.9–7.5 years). Overall, 94 patients developed cancers, 7 AIDS defining cancers, 27 HCV related cancers and 60 NANL related cancers. Median time between cohort inclusion and cancer diagnosis was 2.7 years (IQR, 1.3–5.3). The inci-dence rate of NANL related cancers was 8.9 per 1000 person-years (95% CI, [6.6–11.1]). Median age at cancer diagnosis was 52 (IQR, 48–56) years. The types of these cancers are described inTable 2. The results of any search for HPV through histological reports were not available in our cohort. However, 9 patients (15%) had a high probability of HPV-related cancers (anal cancer n = 6, tonsil cancer n = 1, tongue cancer n = 1, penile cancer n = 1). Five patients (8.3%) had EBV related cancers (Hodgkin lymphoma in all). Non-melanoma skin cancers and lung cancers were the most frequent NANL related cancers with respectively 13 and 14 cases.
By univariable analysis, the following variables were related to the outcome with a p-value
<0.25: age at baseline (HR = 1.09, 95% CI [1.05–1.15], p<0.0001) and duration since HIV
diagnosis (HR = 1.06, 95% CI [1.01–1.13], p = 0.0281). Duration of cART, CD4 cell count nadir below 200/mm3 and duration of HCV infection tended to be associated with an increas-ing risk of NANL cancers while CD8 cells count tended to be associated with a decreasincreas-ing risk. These factors were included in the multivariable analysis in addition to sex, tobacco, alcohol, CD4 cell count and SVR which forced in the model. Only age and a longer duration since HIV diagnosis remained independently associated with a higher risk for NANL related cancer (adjusted HR 1.10 for each additional year, 95% CI 1.06–1.14, p<0.0001 and adjusted HR 1.06 for each additional year, 95% CI 1.01–1.11, p = 0.02, respectively). SVR tended to decrease the
risk of NANL related cancer but the association was not statistically significant (adjusted HR 0.71, 95% CI 0.21–2.32, p = 0.56) (Table 3).
Discussion
In this large prospective cohort of HIV/HCV-coinfected patients followed between 2005 and 2017, 60 patients developed NANL related cancers. Over this period of increasing effectiveness of cART, the overall incidence of NANL related cancers was 8.9 per 1000 person-years. In
Table 1. Baseline characteristics of the 1391 HIV/HCV-coinfected patients from the ANRS CO13 HEPAVIH cohort; results are medians (IQR) or numbers (%).
Total (n = 1391) Age (years) 46 (43–51) Males 1009 (73) Smoking status • Never 190 (13.6) • Current 954 (68.6) • Former 247 (17.8) Alcohol consumption� • Never 306 (22) • Current 682 (49) • Former 403 (29) HIV infection
Time since HIV diagnosis (years) 18.6 (13.9–21.5)
AIDS 348 (25.3)
Ongoing antiretroviral treatment 1255 (90.4) Duration of antiretroviral treatment (months)�� 129.2 (82.4–178.7)
CD4 cell nadir (/mm3) 155 (73–248) CD4 cell nadir <200(/mm3) 799 (62.6)
Absolute CD4 cell count (/mm3) 478 (325–675)
Absolute CD8 cell count (/mm3) 787 (546–1084)
CD4/CD8 ratio 0.6 (0.4–0.9)
HIV viral load < 50 copies/ml 1038 (74.8)
HCV infection
HCV transmission group
•MSM��� 92 (7.6)
•Intravenous drug users 807 (66.8)
•Others 310 (25.6)
Duration of HCV infection (years) 14 (8–22) HCV treatment-naïve patients 933 (67.1)
HCV genotype 1 750 (56.7)
Positive serum HCV RNA 1173 (96.2)
Cirrhosis 378 (27.2)
HCV viral load, log10 IU/mL 6.2 (5.6–6.6)
Diabetes 86 (6.2)
HBs antigen positivity 33 (2.4)
�>50 g/daily for men; >30 g/daily for women;
��zero in patients not receiving antiretroviral therapy; ���: Men who have sex with men
keeping with previous reports showing that the most important risk factors for non-AIDS-defining cancers were advancing age and the duration of HIV infection, age and the time since HIV diagnosis, after adjustment for other factors, were the only factors found to be indepen-dently associated with a higher risk of developing NANL related cancers in our study [2].
Table 2. The 60 non-AIDS-defining cancers and non HCV-liver related cancers diagnosed in HIV/HCV-coin-fected patients.
Type of Cancer Number of patients
Nasopharynx/Tongue/Tonsil cancer 6 (10)
Lung cancer 14 (23)
Anal cancer 6 (10)
Esophagus/colon/rectal/pancreas cancer 6 (10)
Breast or ovarian cancer 2 (3)
Hodgkin’s lymphoma 5 (8)
Non-melanoma skin cancer 13 (22) Renal/urethra/prostate/penile cancer 5 (8)
Others 3 (5)
https://doi.org/10.1371/journal.pone.0208657.t002
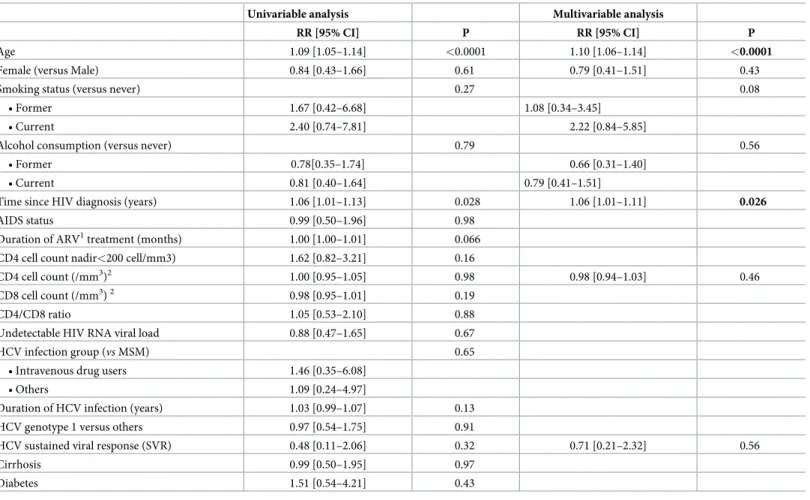
Table 3. Factors associated with non-AIDS-defining cancers and non HCV-liver related cancers among HIV/HCV-coinfected patients—Univariate and multivariate analyses from the ANRS CO13 HEPAVIH Cohort (N = 1391).
Univariable analysis Multivariable analysis
RR [95% CI] P RR [95% CI] P
Age 1.09 [1.05–1.14] <0.0001 1.10 [1.06–1.14] <0.0001
Female (versus Male) 0.84 [0.43–1.66] 0.61 0.79 [0.41–1.51] 0.43
Smoking status (versus never) 0.27 0.08
• Former 1.67 [0.42–6.68] 1.08 [0.34–3.45] • Current 2.40 [0.74–7.81] 2.22 [0.84–5.85]
Alcohol consumption (versus never) 0.79 0.56
• Former 0.78[0.35–1.74] 0.66 [0.31–1.40]
• Current 0.81 [0.40–1.64] 0.79 [0.41–1.51]
Time since HIV diagnosis (years) 1.06 [1.01–1.13] 0.028 1.06 [1.01–1.11] 0.026
AIDS status 0.99 [0.50–1.96] 0.98 Duration of ARV1treatment (months) 1.00 [1.00–1.01] 0.066 CD4 cell count nadir<200 cell/mm3) 1.62 [0.82–3.21] 0.16
CD4 cell count (/mm3)2 1.00 [0.95–1.05] 0.98 0.98 [0.94–1.03] 0.46
CD8 cell count (/mm3)2 0.98 [0.95–1.01] 0.19 CD4/CD8 ratio 1.05 [0.53–2.10] 0.88 Undetectable HIV RNA viral load 0.88 [0.47–1.65] 0.67 HCV infection group (vs MSM) 0.65
• Intravenous drug users 1.46 [0.35–6.08] • Others 1.09 [0.24–4.97]
Duration of HCV infection (years) 1.03 [0.99–1.07] 0.13 HCV genotype 1 versus others 0.97 [0.54–1.75] 0.91
HCV sustained viral response (SVR) 0.48 [0.11–2.06] 0.32 0.71 [0.21–2.32] 0.56
Cirrhosis 0.99 [0.50–1.95] 0.97
Diabetes 1.51 [0.54–4.21] 0.43
1: antiretroviral treatment 2: for a difference of 50 cells/mm3
Conversely, despite a long history of chronic HCV infection in our patients (median 14 (8–22) years), no association was observed with HCV-related variables such as HCV transmission group, duration of HCV infection, cirrhosis, HCV genotype, HCV viral load or SVR.
Many questions regarding the relationship between HCV replication and non HCV-liver related cancers remain unanswered. It is well established that HCV replication is observed in both T and B lymphocyte subsets and is associated with chronic immune activation [15]. Large cohort studies have shown that chronic HCV infection is associated with an elevated risk of developing lung, pancreatic, oropharyngeal, thyroid, skin or breast cancers in HCV mono-infected patients [7,9–11,16]. HCV eradication following anti-HCV treatment has been associ-ated with a high proportion of complete or partial response of B cell non-Hodgkin lymphoma both in HCV mono-infected patients and in HIV/HCV co-infected patients, supporting the role of chronic antigenic stimulation by HCV on lymphoma genesis [17,18]. Furthermore, HCV infection has also been associated with a higher risk of mortality from esophagus, pros-tate and thyroid cancers in HCV mono-infected patients with detectable HCV RNA, as com-pared to HCV mono-infected patients with undetectable HCV RNA [9]. In a retrospective Spanish study, HIV/HCV-coinfected patients had a higher cumulative incidence of NANL cancer than HIV-monoinfected patients (adjusted HR 1.26) [19]. However, in a study involv-ing 1625 HIV/HCV-coinfected patients who were followed up for a median of five years after the end of treatment with interferon plus ribavirin, SVR was not associated with a reduced hazard of NANL related cancers [20]. In our study, patients with SVR tended to have a lower risk of NANL related cancer (aHR 0.71, 95% CI 0.21–2.32, p = 0.56) but the association was not statistically significant.
Impaired immunity is associated with a higher risk of EBV or HPV related cancer. In HIV mono-infected patients, the most frequent non-AIDS-related cancers are oncogenic virus associated malignancies such as EBV and HPV, and low CD4 counts have been associated with a higher risk of these cancers [2,6,21–23]. HCV infection has also been associated with a higher risk of anal cancer in non HIV patients [24]. Furthermore, a higher prevalence of high risk HPV has been observed among non-HIV female liver transplant candidates with HCV chronic infection compared to women without HCV infection [25]. The higher risk of HPV related cancers in HCV mono- infected patients could be related in part to impaired immunity due to cirrhosis. Indeed, cirrhosis has been associated with decreased monocyte function, altered natural killer activity and lectin-induced proliferation of T lymphocytes [25,26]. In our study, the role of HPV was probable in 15% of the cancers (anal cancer, tonsil cancer, tongue cancer and penile cancer). We found no association between a nadir CD4 cell count below 200/mm3, low current CD4 count or cirrhosis, and the occurrence of NANL related cancers. However, we could not evaluate the duration of CD4 count below 200/mm3for most of our patients.
Surprisingly, tobacco or alcohol consumption, which are both well known risk factors for some cancers, such as oropharyngeal and lung cancers, were not found to be associated with the occurrence of NANL related cancers in our study, probably because these cancers were not specifically evaluated [27]. Low CD4/CD8 ratio has also been associated with non-AIDS defin-ing cancer in patients on antiretroviral therapy but no such association was observed in our study [28].
The major strength of this large study (1391 patients followed in 28 hospitals throughout France) is that we adjusted for most factors (including socio-behavioral characteristics) poten-tially associated with NANL related cancers in patients with long duration of both HIV and HCV infection. The main limitation of our study is that despite a median follow-up of 5 years (IQR, 1.9–7.5 years), few cases of NANL related cancers occurred. Therefore, the non-signifi-cant results observed in our study, for example regarding the impact of CD4 cell count nadir,
duration of HCV infection and SVR, could be related to a lack of power. Consequently, our results need to be confirmed in larger cohorts with longer follow-up.
In conclusion, age and the time since HIV diagnosis were the only factor found to be associ-ated with an increased risk of NANL relassoci-ated cancers in this study. In contrast, no association was observed with any HCV-related variables. Since a marked increase in the rate of viral responses is observed in HIV/HCV coinfected patients receiving direct-acting anti-HCV drugs, further research is needed to determine whether suppression of HCV replication would have an impact on occurrence of some of these NANL related cancers.
Acknowledgments
Patients of the ANRS CO13 HEPAVIH Cohort.
Scientific Committee of the ANRS CO13 HEPAVIH Study Group: D. Salmon (co-Principal investigator), L. Wittkop (co-Principal Investigator), P. Sogni (co-Principal Investigator), L. Esterle (project manager), P. Trimoulet, J. Izopet, L. Serfaty, V. Paradis, B. Spire, P. Carrieri, M.A. Valantin, G. Pialoux, J. Chas, I. Poizot-Martin, K. Barange, A. Naqvi, E. Rosenthal, A. Bicart-See, O. Bouchaud, A. Gervais, C. Lascoux-Combe, C. Goujard, K. Lacombe, C. Duvi-vier, D. Vittecoq, D. Neau, P. Morlat, F. Bani-Sadr, L. Meyer, F. Boufassa, S. Dominguez, B. Autran, A.M. Roque, C. Solas, H. Fontaine, D. Costagliola, L. Piroth, A. Simon, D. Zucman, F. Boue´, P. Miailhes, E. Billaud, H. Aumaıˆtre, D. Rey, G. Peytavin, V. Petrov-Sanchez, A. Pailhe´.
Clinical Centres (ward / participating physicians): APHP Cochin, Paris (Me´decine Interne et Maladies Infectieuses: D. Salmon, R. Usubillaga; He´pato-gastro-ente´rologie: P. Sogni; Ana-tomo-pathologie: B. Terris; Virologie: P. Tremeaux); APHP Pitie´-Salpe´trière, Paris (Maladies Infectieuses et Tropicales: C. Katlama, M.A. Valantin, H. Stitou; He´pato-gastro-ente´rologie: Y. Benhamou; Anatomo-pathologie: F. Charlotte; Virologie: S. Fourati); APHP Pitie´-Salpe´trière, Paris (Me´decine Interne: A. Simon, P. Cacoub, S. Nafissa); APHM Sainte-Marguerite, Mar-seille (Service d’Immuno-He´matologie Clinique: I. Poizot-Martin, O. Zaegel, H. Laroche; Vir-ologie: C. Tamalet); APHP Tenon, Paris (Maladies Infectieuses et Tropicales: G. Pialoux, J. Chas; Anatomo-pathologie: P. Callard, F. Bendjaballah; Virologie: C. Le Pendeven); CHU Pur-pan, Toulouse (Maladies Infectieuses et Tropicales: B. Marchou; He´pato-gastro-ente´rologie: L. Alric, K. Barange, S. Metivier; Anatomo-pathologie: J. Selves; Virologie: F. Larroquette); CHU Archet, Nice (Me´decine Interne: E. Rosenthal; Infectiologie: A. Naqvi, V. Rio; Anatomo-pathologie: J. Haudebourg, M.C. Saint-Paul; Virologie: C. Partouche); APHP Avicenne, Bobigny (Me´decine Interne–Unite´ VIH: O. Bouchaud; Anatomo-pathologie: M. Ziol; Virolo-gie: Y. Baazia); Hoˆpital Joseph Ducuing, Toulouse (Me´decine Interne: M. Uzan, A. Bicart-See, D. Garipuy, M.J. Ferro-Collados; Anatomo-pathologie: J. Selves; Virologie: F. Nicot); APHP Bichat–Claude-Bernard, Paris (Maladies Infectieuses: A. Gervais, Y. Yazdanpanah; Anatomo-pathologie: H. Adle-Biassette; Virologie: G. Alexandre); APHP Saint-Louis, Paris (Maladies infectieuses: C. Lascoux-Combe, J.M. Molina; Anatomo-pathologie: P. Bertheau; Virologie: M. L. Chaix, C. Delaugerre, S. Maylin); APHP Saint-Antoine (Maladies Infectieuses et Tropicales: K. Lacombe, J. Bottero; J. Krause P.M. Girard, Anatomo-pathologie: D. Wendum, P. Cervera, J. Adam; Virologie: C. Viala); APHP Bicêtre, Paris (Me´decine Interne: C. Goujard, Y. Quer-tainmont, E. Teicher; Virologie: C. Pallier; Maladies Infectieuses: D. Vittecoq); APHP Necker, Paris (Maladies Infectieuses et Tropicales: O. Lortholary, C. Duvivier, C. Rouzaud, J. Lou-renco, F. Touam, C. Louisin: Virologie: V. Avettand-Fenoel, A. Me´lard); CHU Pellegrin, Bor-deaux (Maladies Infectieuses et Tropicales: D. Neau, A. Ochoa, E. Blanchard, S. Castet-Lafarie, C. Cazanave, D. Malvy, M. Dupon, H. Dutronc, F. Dauchy, L. Lacaze-Buzy; Anatomo-patholo-gie: P. Bioulac-Sage; ViroloAnatomo-patholo-gie: P. Trimoulet, S. Reigadas); Hoˆpital Saint-Andre´, Bordeaux (Me´decine Interne et Maladies Infectieuses: Me´decine Interne et Maladies Infectieuses:
P. Morlat, D. Lacoste, F. Bonnet, N. Bernard, M. Hessamfar, J, F. Paccalin, C. Martell, M. C. Pertusa, M. Vandenhende, P. Mercie´er, D. Malvy, T. Pistone, M.C. Receveur, M. Me´chain, P. Duffau, C Rivoisy, I. Faure, S. Caldato; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Tri-moulet, S. Reigadas); Hoˆpital du Haut-Levêque, Bordeaux (Me´decine Interne: J.L. Pellegrin, J. F. Viallard, E. Lazzaro, C. Greib; Anatomo-pathologie: P. Bioulac-Sage; Virologie: P. Trimou-let, S. Reigadas); Hoˆpital FOCH, Suresnes (Me´decine Interne: D. Zucman, C. Majerholc; Virologie: E. Farfour); APHP Antoine Be´clère, Clamart (Me´decine Interne: F. Boue´, J. Polo Devoto, I. Kansau, V. Chambrin, C. Pignon, L. Berroukeche, R. Fior, V. Martinez; Virologie: C. Deback); CHU Henri Mondor, Cre´teil (Immunologie Clinique: Y. Le´vy, S. Dominguez, J.D. Lelièvre, A.S. Lascaux, G. Melica); CHU Hoˆtel Dieu, Nantes (Maladies Infectieuses et Tropi-cales: E. Billaud, F. Raffi, C. Allavena, V. Reliquet, D. Boutoille, C. Biron; Virologie: A. Rodal-lec, L. Le Guen); Hoˆpital de la Croix Rousse, Lyon (Maladies Infectieuses et Tropicales: P. Miailhes, D. Peyramond, C. Chidiac, F. Ader, F. Biron, A. Boibieux, L. Cotte, T. Ferry, T. Per-point, J. Koffi, F. Zoulim, F. Bailly, P. Lack, M. Maynard, S. Radenne, M. Amiri; Virologie: C. Scholtes, T.T. Le-Thi); CHU Dijon, Dijon (De´partement d’infectiologie: L. Piroth, P. Chavanet M. Duong Van Huyen, M. Buisson, A. Waldner-Combernoux, S. Mahy, R. Binois, A.L. Sim-onet-Lann, D. Croisier-Bertin); CH Perpignan, Perpignan (Maladies infectieuses et tropicales: H. Aumaıˆtre); CHU Robert Debre´, Reims (Me´decine interne, maladies infectieuses et immu-nologie clinique: F. Bani-Sadr, D. Lambert, Y Nguyen, J.L. Berger); CHRU Strasbourg (Le Trait d’Union: D Rey, M Partisani, ML Batard, C Cheneau, M Priester, C Bernard-Henry, E de Mautort, Virologie: P Gantner et S Fafi-Kremer), APHP Bichat-Claude Bernard (Pharmacolo-gie: G. Peytavin).
Data collection: F. Roustant, I. Kmiec, L. Traore, S. Lepuil, S. Parlier, V. Sicart-Payssan, E. Bedel, F. Touam, C. Louisin, M. Mole, C. Bolliot, M. Mebarki, A. Adda-Lievin, F.Z. Makhou-khi, O. Braik, R. Bayoud, M.P. Pietri, V. Le Baut, D. Bornarel, C. Chesnel, D. Beniken, M. Pau-chard, S. Akel, S. Caldato, C. Lions, L. Chalal, Z. Julia, H. Hue, A. Soria, M. Cavellec, S. Breau, A. Joulie, P. Fisher, C. Ondo Eyene, S. Ogoudjobi, C. Brochier, V. Thoirain-Galvan.
Management, statistical analyses: E. Boerg, P. Carrieri, V. Conte, L. Dequae-Merchadou, M. Desvallees, N. Douiri, L. Esterle, C. Gilbert, S. Gillet, R. Knight, F. Marcellin, L. Michel, M. Mora, S. Nordmann, C. Protopopescu, P. Roux, B. Spire, PM Carrieri, S. Tezkratt, A. Vilotitch, I. Yaya, L Wittkop.
We also thank Fiona Ecarnot (EA3920, University Hospital Besancon, France) for editorial assistance.
Author Contributions
Conceptualization: Oumar Billa, Isabelle Poizot-Martin, Linda Wittkop. Data curation: Camille Gilbert.
Formal analysis: Oumar Billa, Mathieu Chalouni.
Investigation: Dominique Salmon, Isabelle Poizot-Martin, Christine Katlama, Didier Neau,
Julie Chas, Philippe Morlat, Karine Lacombe, Alissa Naqvi, Karl Barange, Anne Gervais, Olivier Bouchaud, Eric Rosenthal, Caroline Lascoux-Combe, Daniel Garipuy, Laurent Alric, Ste´phanie Dominguez, Daniel Vittecoq, Ce´cile Goujard, Claudine Duvivier, Hugues Aumaitre, Patrick Miailhes, David Zucman, Anne Simon, Estibaliz Lazaro, Franc¸ois Raffi.
Methodology: Oumar Billa, Linda Wittkop. Project administration: Laure Esterle.
Validation: Firouze´ Bani-Sadr. Visualization: Firouze´ Bani-Sadr.
Writing – original draft: Oumar Billa, Linda Wittkop, Firouze´ Bani-Sadr.
Writing – review & editing: Mathieu Chalouni, Dominique Salmon, Isabelle Poizot-Martin,
Camille Gilbert, Christine Katlama, Didier Neau, Julie Chas, Philippe Morlat, Karine Lacombe, Alissa Naqvi, Karl Barange, Anne Gervais, Olivier Bouchaud, Eric Rosenthal, Caroline Lascoux-Combe, Daniel Garipuy, Laurent Alric, Ste´phanie Dominguez, Daniel Vittecoq, Ce´cile Goujard, Claudine Duvivier, Hugues Aumaitre, Patrick Miailhes, David Zucman, Anne Simon, Estibaliz Lazaro, Franc¸ois Raffi, Laure Esterle, Linda Wittkop, Fir-ouze´ Bani-Sadr.
References
1. Herida M, Mary-Krause M, Kaphan R, Cadranel J, Poizot-Martin I, Rabaud C, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol Off J Am Soc Clin Oncol 2003; 21:3447– 3453.
2. Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. AIDS Lond Engl 2014; 28:453–465.
3. Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol Off J Am Soc Clin Oncol 2009; 27:884–890.
4. Zucchetto A, Virdone S, Taborelli M, Grande E, Camoni L, Pappagallo M, et al. Non-AIDS-Defining Cancer Mortality: Emerging Patterns in the Late HAART Era. J Acquir Immune Defic Syndr 1999 2016; 73:190–196.
5. Riedel DJ, Tang LS, Rositch AF. The role of viral co-infection in HIV-associated non-AIDS-related can-cers. Curr HIV/AIDS Rep 2015; 12:362–372.https://doi.org/10.1007/s11904-015-0276-6PMID:
26152660
6. Reekie J, Kosa C, Engsig F, Monforte A d’Arminio, Wiercinska-Drapalo A, Domingo P, et al. Relation-ship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defin-ing malignancies. Cancer 2010; 116:5306–5315.https://doi.org/10.1002/cncr.25311PMID:20661911
7. Mahale P, Torres HA, Kramer JR, Hwang L-Y, Li R, Brown EL, et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer 2017; 123:1202– 1211.https://doi.org/10.1002/cncr.30559PMID:28117886
8. Peveling-Oberhag J, Arcaini L, Hansmann M-L, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol 2013; 59:169– 177.https://doi.org/10.1016/j.jhep.2013.03.018PMID:23542089
9. Lee M-H, Yang H-I, Lu S-N, Jen C-L, You S-L, Wang L-Y, et al. Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis 2012; 206:469–477.https://doi.org/10.1093/infdis/jis385PMID:22811301
10. Allison RD, Tong X, Moorman AC, Ly KN, Rupp L, Xu F, et al. Increased incidence of cancer and can-cer-related mortality among persons with chronic hepatitis C infection, 2006–2010. J Hepatol 2015; 63:822–828.https://doi.org/10.1016/j.jhep.2015.04.021PMID:25937437
11. Fiorino S, Bacchi-Reggiani L, de Biase D, Fornelli A, Masetti M, Tura A, et al. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: A systematic review. World J Gastroenterol 2015; 21:12896–12953.https://doi.org/10.3748/wjg.v21.i45.12896
PMID:26668515
12. Vandenhende M-A, Roussillon C, Henard S, Morlat P, Oksenhendler E, Aumaitre H, et al. Cancer-Related Causes of Death among HIV-Infected Patients in France in 2010: Evolution since 2000. PloS One 2015; 10:e0129550.https://doi.org/10.1371/journal.pone.0129550PMID:26083524
13. Loko M-A, Salmon D, Carrieri P, Winnock M, Mora M, Merchadou L, et al. The French national prospec-tive cohort of patients co-infected with HIV and HCV (ANRS CO13 HEPAVIH): early findings, 2006– 2010. BMC Infect Dis 2010; 10:303.https://doi.org/10.1186/1471-2334-10-303PMID:20969743
14. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple non-invasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol Baltim Md 2006; 43:1317–1325.
15. Sandberg JK, Falconer K, Gonzalez VD. Chronic immune activation in the T cell compartment of HCV/ HIV-1 co-infected patients. Virulence 2010; 1:177–179.https://doi.org/10.4161/viru.1.3.11206PMID:
21178437
16. Su F-H, Chang S-N, Chen P-C, Sung F-C, Huang S-F, Chiou H-Y, et al. Positive association between hepatitis C infection and oral cavity cancer: a nationwide population-based cohort study in Taiwan. PloS One 2012; 7:e48109.https://doi.org/10.1371/journal.pone.0048109PMID:23133554
17. Terrier B, Costagliola D, Prevot S, Chavez H, Missy P, Rince P, et al. Characteristics of B-cell lympho-mas in HIV/HCV-coinfected patients during the combined antiretroviral therapy era: an ANRS CO16 LYMPHOVIR cohort study. J Acquir Immune Defic Syndr 1999 2013; 63:249–253.
18. Vallisa D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, et al. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin’s lymphoma: a multicenter Italian experience. J Clin Oncol Off J Am Soc Clin Oncol 2005; 23:468–473.
19. Meijide H, Pe´rtega S, Rodrı´guez-Osorio I, Castro-Iglesias A´ , Baliñas J, Rodrı´guez-Martı´nez G, et al. Increased incidence of cancer observed in HIV/HCV-coinfected patients versus HIV-monoinfected, 1993–2014. AIDS Lond Engl Published Online First: 21 March 2017.https://doi.org/10.1097/QAD. 0000000000001448PMID:28441174
20. Berenguer J, Rodrı´guez-Castellano E, Carrero A, Von Wichmann MA, Montero M, Galindo MJ, et al. Eradication of HCV and non-liver-related non-AIDS-related events in HIV/HCV coinfection. Hepatol Bal-tim Md Published Online First: 21 January 2017.https://doi.org/10.1002/hep.29071PMID:28109003
21. Guiguet M, Boue´ F, Cadranel J, Lang J-M, Rosenthal E, Costagliola D, et al. Effect of immunodefi-ciency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009; 10:1152–1159. https://doi.org/10.1016/S1470-2045(09)70282-7PMID:19818686
22. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS Lond Engl 2014; 28:881–890.
23. Yanik EL, Napravnik S, Cole SR, Achenbach CJ, Gopal S, Dittmer DP, et al. Relationship of immuno-logic response to antiretroviral therapy with non-AIDS defining cancer incidence. AIDS Lond Engl 2014; 28:979–987.
24. Mahale P, Sturgis EM, Tweardy DJ, Ariza-Heredia EJ, Torres HA. Association Between Hepatitis C Virus and Head and Neck Cancers. J Natl Cancer Inst 2016; 108.https://doi.org/10.1093/jnci/djw035
PMID:27075854
25. Tarallo PA, Smolowitz J, Carriero D, Tarallo J, Siegel A, Jia H, et al. Prevalence of high-risk human pap-illoma virus among women with hepatitis C virus before liver transplantation. Transpl Infect Dis Off J Transplant Soc 2013; 15:400–404.
26. Ma´rquez M, Ferna´ndez-Gutie´rrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodrı´guez-Ramos C, et al. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol 2009; 158:219–229.https://doi.org/10.1111/j.1365-2249.2009.04005.xPMID:
19737142
27. Helleberg M, Gerstoft J, Afzal S, Kronborg G, Larsen CS, Pedersen C, et al. Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS Lond Engl 2014; 28:1499–1508.
28. Hema MN, Ferry T, Dupon M, Cuzin L, Verdon R, Thie´baut R, et al. Low CD4/CD8 Ratio Is Associated with Non AIDS-Defining Cancers in Patients on Antiretroviral Therapy: ANRS CO8 (Aproco/Copilote) Prospective Cohort Study. PloS One 2016; 11:e0161594.https://doi.org/10.1371/journal.pone. 0161594PMID:27548257