Publisher’s version / Version de l'éditeur:
15th International Conference on Miniaturized Systems for Chemistry and Life
Sciences, pp. 683-685, 2011-10-02
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
High-throughput fabrication of advanced 3d microfluidic devices in
thermoplastic elastomer for biological probe immobilization
Brassard, D.; Clime, L.; Li, K.; Geissler, M.; Miville-Godin, C.; Roy, E.; Veres,
T.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=c8f3415f-0273-4b92-82c6-635ce8fe0404 https://publications-cnrc.canada.ca/fra/voir/objet/?id=c8f3415f-0273-4b92-82c6-635ce8fe0404
HIGH-THROUGHPUT FABRICATION OF ADVANCED 3D MICROFLUIDIC
DEVICES IN THERMOPLASTIC ELASTOMER FOR BIOLOGICAL PROBE
IMMOBILIZATION
D. Brassard*, L. Clime, K. Li, M. Geissler, C. Miville-Godin, E. Roy and T. Veres
National Research Council, CANADA ABSTRACT
We have developed a process based on hot-embossing lithography (HEL) to pattern at high-throughput micron-size through-holes in thin thermoplastic elastomer (TPE) membranes. This novel HEL process, which is shown to provide key ad-vantages for microfluidics applications, was applied to the fabrication of highly-integrated 3D microfluidic devices designed to precisely immobilize biomolecules on the micron-size sensing elements of microfabricated biosensor arrays. High quality deposition of DNA and proteins on a 10×10 array of 50×50 m2
isolated spots was achieved using the developed TPE micro-fluidic devices. Finally, the integration of advanced TPE devices with microfabricated biosensor arrays is demonstrated.
KEYWORDS: Thermoplastic elastomer, 3D microfluidics, Through-hole, Hot-embossing, Immobilization, Biosensor INTRODUCTION
Integrated biosensors have recently emerged as highly promising tools for the sensitive and multiplexed detection of target biomolecules [1]. However, immobilizing biomolecular probes on micrometric biosensing elements is challenging using tradi-tional techniques such as pin spotting or inkjet printing. Microfluidics offers a promising alternative to immobilize biomole-cules at high resolution by providing not only the possibility to define precisely the immobilized patterns according to the channel geometry, but also to pattern micron-size isolated spots by using a 3D channel network [2,3]. Yet, the traditional me-thods and materials used for device fabrication are hardly compatible with low-cost mass-production and the devices pro-duced to date lack the sophistication required to address large biosensors arrays. These limitations have restrained to a large extent the adoption of microfluidics as a practical tool for the immobilization of biological probes. In this paper, we present the fabrication of highly integrated 3D microfluidic immobilization devices based on a novel high-throughput manufacturing technique to produce thin thermoplastic elastomer membranes with microscopic through-holes.
THEORY
While prototype microfluidic devices are routinely fabricated using thermosetting polymers such as polydimethylsiloxane (PDMS), the elevated cost of the prepolymer components and the relatively low fabrication throughput can be problematic for mass production processes [4,5]. Thermoplastic polymers (PMMA, COC, or PC) provide an interesting alternative for the fa-brication of single-use microfluidic devices at low-cost using rapid thermoforming techniques such as hot-embossing litho-graphy or injection molding. Unfortunately, simple bonding schemes are not always available for hard thermoplastics, espe-cially when attachment of the microfluidic device to specific surfaces is required (e.g., for the immobilization of biomolecules). The patterning of high density microscopic through-holes in hard thermoplastics to create 3D microfluidic de-vices has also proved to be a very challenging task [5]. Thus, despite the wide range of materials and techniques available for microfluidic device fabrication, it is still not obvious that commercially viable mass-production of advanced 3D microfluidic devices can be achieved.
We have recently identified commercially available TPEs that can naturally conform to planar surfaces to create a reversi-ble watertight contact in a way similar to PDMS, while still providing the possibility to be processed using conventional high-throughput thermoforming methods [2,4]. TPEs also offer the advantages of lower raw material cost compared to thermoset-ting polymers while supporthermoset-ting convenient assembly with pressure free thermal bonding [4]. Figure 1 unveils a novel process we developed based on HEL to pattern rapidly and reliably micron-size through-holes in TPE membranes. We found that, by
Figure 1: Schematics of the HEL process developed for the fabrication of TPE membranes with open through-holes.
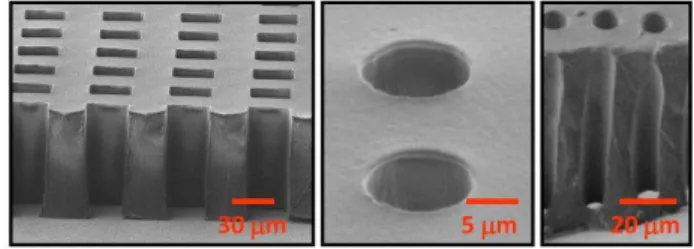
Figure 2: SEM images of TPE membranes with high aspect-ratio micrometric open-through holes.
978-0-9798064-4-5/µTAS 2011/$20©11CBMS-0001 683 Miniaturized Systems for Chemistry and Life Sciences15th International Conference on
pressing an extruded sheet of TPE until the mold nearly reaches the counter plate, trough-holes can be patterned reliably follow-ing the systematic rupture of the residual layer upon disassembly of the stack. The process not only avoids entirely the timely preparation steps (mixing, degassing, casting and curing) required for the fabrication of PDMS membranes with through-holes but also eliminates complex HEL setups, difficult demolding, and lengthy post-processing steps that complicate the patterning of through-holes in hard thermoplastics [5]. As illustrated in figure 1, the developed technique also permits the precise cutting of the TPE membranes in individual devices by placing a wall around each fluidic unit on the mold. We were thus able to routinely pre-pare multiple TPE membranes with micron-size through-holes in a single HEL run.
EXPERIMENTAL
The microfluidic devices and TPE membranes described in this paper were fabricated from Versaflex® CL30, a TPE based on styrene-ethylene/butylene-styrene block copolymer, but other commercially available TPEs would also be compati-ble with the developed process. The mold consisted of a two level SU-8 photoresist patterned on a silicon wafer using stan-dard photolithography processes. TPE was received in the form of pellets which were extruded to form 140 or 240 m thick sheets of several meters in length that were stored and used on demand. The pre-extruded sheets of TPE were placed between the mold and a counter plate and patterned in a HEL system (EVG 520) at a temperature of 170 oC and a force of 10 kN on a 4 inch diameter mold. Under such condition, through-holes could be obtained in less than 10 min embossing time. For the immobilization experiments performed with the developed TPE-based microfluidic devices, the TPE membranes were treated with O2 plasma to render their surfaces hydrophilic. Immobilization experiments were performed on activated Zeonor plastic
slides according to a process described elsewhere [2].
RESULTS AND DISCUSSION
Figure 2 presents examples of high aspect-ratio though-holes patterned in TPE membranes with the developed process. The patterning of a dense array of 30 m wide and 90 m depth square-shape through-holes in a TPE membrane is demonstrated. As shown in figure 2, high-aspect ratio through-holes can also be reliably patterned with the developed technique (10 m wide, 90 m depth). Excellent replication accuracy is achieved with only minimal deformation around the through-holes and no residual TPE layer being present.
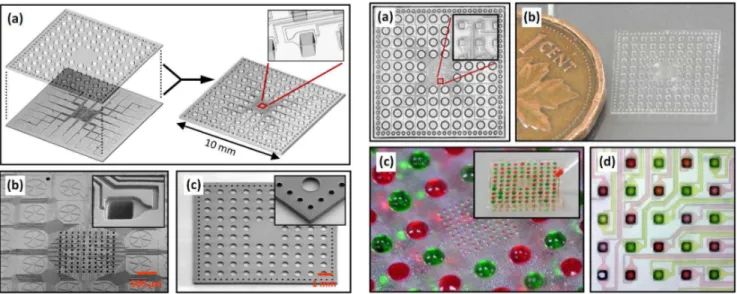
The design of a highly-integrated 3D microfluidic immobilization device based on two open-through TPE membranes of 1×1 cm2 is shown in figure 3(a). The 192 through-holes of the top membrane define the 96 inlets and 96 outlets of the device, while the bottom membrane contains 96 independent channels connected to a 10×10 array of spotting holes where the liquid en-ters in contact with the substrate on isolated spots of 50×50 m2
. The device is thus designed as a fluidic concentrator that trans-ports the liquid from macroscopic inlets to the microscopic spotting holes located in the center of the device. SEM micrographs of the replicated bottom and top TPE membranes are shown in figures 3(b) and (c), demonstrating the high replication accuracy of the developed HEL process and the possibility to precisely cut the TPE membranes to their final shape. The channel design around the spotting holes has been optimized with the help of numerical simulations based on the Lattice-Boltzmann method [6] to ensure that capillary action can be used as the sole force distributing the liquid in the channels and the spotting holes.
Figure 3: (a) Design of a highly-integrated 3D micro-fluidic immobilization device. SEM images of bottom (b) and top (c) through-hole TPE membranes of the device.
Figure 4: Photographs of the assembled 3D TPE micro-fluidic immobilization device before (a and b) and after (c and d) charging the inlets with red and green dyes.
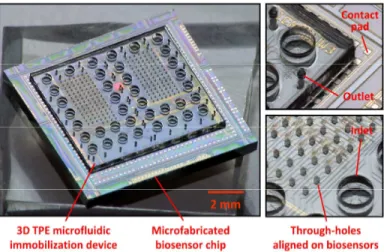
Figures 4(a) and (b) show pictures of an assembled device after alignment of the two TPE membranes. A device charged en-tirely with red and green dyes so as to create a chessboard pattern is also shown in figures 4(c) and (d), demonstrating the possi-bility to address individually the spotting holes by capillary forces. In figure 5(a), selected channels were filled with a Cy5-labeled probe DNA solution. Figure 5(b) shows the resulting fluorescence intensity of the DNA probes immobilized on the Zeonor sub-strate after removal of the TPE microfluidic device, rinsing, and drying. The fluorescence intensity of each spot reveals high spa-tial uniformity, which contrasts with the results typically obtained by techniques such as pin-spotting for which high spot unifor-mity is challenging to obtain at this scale. The immobilization of mouse and rabbit IgG proteins on 50 m size spots and subsequent high-selectivity hybridization with Cy5-labeled anti-rabbit IgG and Cy3-labeled anti-mouse IgG is also demonstrated in figure 5. Finally, an example of a TPE-based microfluidic device designed to immobilize biomolecules on the two hundred in-dependent detection elements of a 1×1 cm2 microfabricated biosensor chip is shown in figure 6. By using the developed HEL process for the fabrication of the TPE membranes with through-holes, we were able to achieve a precise alignment of the spotting holes on the detection elements of the chip and a very accurate control of the microfluidic device dimensions to avoid covering the electrical contact pads.
CONCLUSION
We have developed a process based on HEL for the high-throughput production of TPE membranes with micron-size through-holes and demonstrated the fabrication of advanced 3D microfluidic devices in TPE for the micron scale immobiliza-tion of biomolecules. We believe that the fabricaimmobiliza-tion process, material, and microfluidic devices we propose provide new key insights to solve some longstanding technical issues limiting the widespread adoption of microfluidics in many applications, including micron-scale immobilization of biological probes.
REFERENCES
1. F. Teles and L. Fonseca, “Trends in DNA biosensors,” Talanta, vol. 77, pp. 606-623 (2008).
2. M. Geissler, E. Roy, G. A Diaz-Quijada, J.-C. Galas, and T. Veres, “Microfluidic patterning of miniaturized DNA arrays on plastic substrates,” ACS Appl. Mater. Interfaces, vol. 1, pp. 1387-1395 (2009).
3. M.A. Eddings et al., “Spot and hop: internal referencing for surface plasmon resonance imaging using a three-dimensional microfluidic flow cell array,” Anal. Biochem., vol. 385, pp. 309-313, (2009).
4. E. Roy, M. Geissler, J.-C. Galas, and T. Veres, “Prototyping of microfluidic systems using a commercial thermoplastic elastomer,” Microfluid. Nanofluid., (2011). DOI:10.1007/s10404-011-0789-2
5. M. Worgull, "Hot Embossing - Theory and Technology of Microreplication", William Andrew, 2009.
6. L. Clime, D. Brassard, and T. Veres, "Numerical modeling of electrowetting processes in digital microfluidic devices,”
Comput. Fluids, vol. 39, pp. 1510-1515 (2010). CONTACT
*D. Brassard, tel: +1-450-641-5821; Daniel.Brassard@cnrc-nrc.gc.ca
Figure 5: (a) Channels and spotting holes selectively filled with Cy-5 labeled DNA solution. (b) Resulting fluorescence of immobilized DNA spots on a plastic substrate. Fluorescence of immobilized mouse and rab-bit IgG proteins after incubation with (c) Cy5-labeled anti-rabbit IgG and (d) Cy3-labeled anti-mouse IgG.
Figure 6: Images of a 3D TPE microfluidic immobilization device aligned on a microfabricated biosensor chip compris-ing two hundred of detection elements. The developed device can immobilize up to 38 different biomolecules on the 50x50 m2 sensing regions.