Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Division of Building Research); no.
DBR-P-710, 1976-11
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=d66152e0-61f8-4a3a-b62c-cd418a69a173
https://publications-cnrc.canada.ca/fra/voir/objet/?id=d66152e0-61f8-4a3a-b62c-cd418a69a173
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001763
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Determination of hydrogen cyanide in fire gases
S e r
5;
2
4
7
TH1
I
71°
uatlonal Research
Cofwdl
Wnai
Souncil Canada
de
recherches
Canada
BLDG
DETERMINATION OF HYDROGEN CYANIDE IN
FIRE GASES
by
Yoshio Tsuchiya and K i u o SumiReprinted
fromJournal of Combustion Toxicology,
VoL
3, November 1976p 363
-
370DBR
Paper
No.
710
Division of Building
Research
SOMMAIRE
De n o m b r e w polymers contenant de llazote produisent de l'acide cyanydrique (CNH) l o r s q u l i l s sont impliques dans des f e w . Diverses methodes de ddtermination de
CNH
dans l e s gaz de combustion sont rCvis6es. L e s methodes discutkes sont l e s tubes calorim6triques. llBlectrodes pour un ion spdcifique,
llanalyse chimique d e s solutions e t la chromatographie gaseuse. Deux p r o - bl'emes communs dans la determination de CNH sont l a p e r t e de concentration par absorption e t l e s rCactions interferentes. L e s auteurs recornmandent qu'au moins d e w diffhrentes mdthodes soient employees de mani'ereh
6viter l e s e r r e u r s causees p a r 11interf6rence e t llabsorption.1111
31809002
I I I
1111ii[~1~1
710
III! 1111
:
I -Fire Research Section Division of Building Research National Research Council of Canada Ottawa, Canada K 1A OR6
DETERMINATION OF HYDROGEN CYANIDE I N FIRE GASES
Manuscript received July14,
1976
Revised manuscript received September
25,
1976
ABSTRACT: Many of the nitrogen containing polymers produce HCN when involved in fires. Various methods of HCN determination in fire gases are reviewed. The methods discussed are colorimetric tubes, specific ion electrode, wet chemical analysis and gas chromatography. Two common problems in HCN determination are concentration decrease by absorption and interferring reactions. Authors recommend that at least two different methods be employed in order to avoid errors caused by interference and absorption.
INTRODUCTION
M
ANY OF THE nitrogen containing polymers produce hydrogen cyanide wheninvolved in fires. The quantity produced depends on both h e type of poly- mer and the conditions of combustion. Because of the high toxicity of HCN, sensitive methods for its determination are often required. There are numerous methods of HCN determination and examples of applications described i n the literature. The purpose of this paper is to present practical information on the determination of hydrogen cyanide in fire gases, based on the authors' experience and knoweldge; it is not intended to be a comprehensive review. The methods discussed are colorimetric tubes, specific ion electrode, wet chemical analysis and gas chromatography.
METHODS Colorimetric Tubes
Colorimetric detector tubes are simple and sensitive devices for quick determina- tion of gases. They are not very accurate, however. A glass tube i s filled with a mixture of chemicals that react with a specific gas and exhibit a change in color. The concentration of the gas is determined by the length of the color developed. One such commercially available tube determines HCN i n a range of
2
to150
ppm[I].
The chemicals used are mercuric chloride and methyl orange. One problemYoshio Tsuchiya and Kikuo Sumi
with colorimetric tubes is possible interference caused by other gases. Although there are some provisions for eliminating interference, there i s always uncertainty about this problem because combustion products contain a large number of known and unknown components.
Specific Ion Electrode
A specific ion electrode develops an electric potential when ions to which the electrode is selective are present in a solution. The magnitude of the potential is a function of the ion activity in the solution and can be determined by forming a cell with a reference electrode. A cyanide ion electrode i s selective to CN. For the determination of HCN in a combustion gas, a known volume of the gas i s bubbled through a known volume of alkali solution in which HCN i s absorbed and the concentration of the resulting C N i s determined. To calibrate the electric potential, several standard C N solutions, usually KCN solutions, of different concentrations ranging from
lu3
to 1 r 6 are prepared and the potential is measured by a millivolt meter. The C N solution for calibration should contain the same strength of alkali as that used for absorbing the sample gases. Constant stirring of the solution must be maintained during the determination as the stirring speed significantly affects the millivolt readings. There is a linear relationship between the reading and the logarithm of ion concentration. The millivolt meter reading i s arbitrarily set at zero for a standard KCN solution having a concentration that is about the middle of the working range. Zeroing of the meter i s done every working day using the standard solution. The calibration curve remains valid for almost the life of the electrode, which i s usually from a few months to a couple of years depending on the concen- tration of the solution and the length of time the electrode i s immersed in it.There are some possible interferences in this determination [21
.
Sulfide ions, for example, must not be present in the solution when a C N determination is made; even a very small concentration of S - , insufficient to be detected by lead acetate test paper, could interfere with the measurement. Metal salts which form complex ions with cyanide should not be present. Iodide must be less than 10 times the amount of cyanide, bromide less than 5000 times and chloride less thanlo6
times. In summary, determination of HCN using a specific ion electrode is a simple, sensitive and reliable method. The chemist should be aware of possible interference, particularly from sulfide ions that might be present in the fire gases.Titrimetric Methods
Bark and Higson have reviewed a number of titrimetric methods for cyanide determination [3], some of which might be applicable to fire gas analysis. The present authors have used a modified Liebig's silver nitrate titration [4] as one of the primary methods for the analysis of HCN. The method is fairly simple and reliable though not very sensitive. In the determination, HCN is trapped in 100 ml of 0.1-N KOH solution. The solution i s titrated with 0.02-N AgN03 solution with a
Determination o f Hydrogen Cyanide in Fire Gases
p-dimethylaminobenzylidene rhodanine indicator. When the alkali solution contain- ing HCN i s colored and the end point of the titration i s not clear, the solution has to be decolored. For this purpose about
112
gram of carbon black is added to the solution and the mixture i s stirred and filtered. The decolorizing treatment causes a slight drop in HCN concentration as determined by the cyanide ion electrode. The drop i s proportional to the depth of color; sometimes it can amount to as much as20%.
The modified Liebig method is one of the methods used in a Japanese Industrial Standard for HCN determination of exhaust gases [ 5 ] . The method is stated to be suitable for 5 to
100
ppm HCN.Colorimetric Methods
There are many color reactions used in cyanide determination. The classical Prussian blue method i s one of them. These methods are also reviewed by Bark and Higson 131. Generally colorimetric methods have high sensitivity but precautions are required because of interfering reactions and instability of color. Among the many colorimetric methods, a highly sensitive method that involves conversion of HCN to cyanogen chloride followed by a color reaction with a pyridine-pyrazolone mixture 161 has been adopted both in ASTM and Japanese Industrial Standards [4, 51
.
Gas Chromatography
Gas chromatography (GC) is more versatile than the preceding methods. During HCN analysis other combustion products can be determined at the same time. HCN and cyanogen can be separated by GC whereas most of the other methods deter- mine a combined quantity of these two compounds if they occur together. TWO main considerations are a stationary phase of the gas chromatograph column by which HCN is separated from other components, and a detector for HCN. Earlier workers used polyethylene glycol [71 and triacetin
[BI
as stationary phases, and a thermal conductivity detector (TCD). More recently, stationary phases made of substituted polystyrene have been used with a flame ionization detector (FID) [91 or an alkali metal flame ionization detector for nitrogen compounds (N-FID)[lo].
The FID has certain advantages over the TCD; it has wider dynamic range, higher sensitivity to HCN, and i s insensitive to H20, CO, C02 and other inorganic gases that are found in large quantities in fire gases and could interfere with HCN deter- mination. The N-FID has much higher sensitivity to HCN than FID. In a gas chromatograph-mass spectrometer system (GC-MS), MS serves as a very sensitive detector for GC. The sensitivity and quantitative reproducibility, however, depend largely on instrumentation, which will not be discussed in this paper.The present authors have used a Porapak Q packed glass column and a N-FID detector for HCN analysis. The sensitivity of FID and N-FID to two compounds, HCN and benzene, an example of a hydrocarbon, i s presented in Table
1.
TheYoshio Tsuchiya
andKikuo Sumi
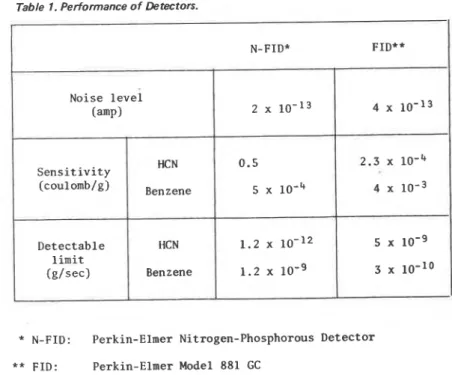
Table I . Performance of Detectors.
* N-FID:
**
FID:-
N-FID* FID**
Perkin-Elmer Nitrogen-Phosphorous Detector Perkin-Elmer Model 881 GC 2 x lo-l3 4 x 10-l3 0.5 2.3 x 5 1 0 4 4 10-3 1.2 x 10-l2 5 lo-g 1.2 lo-g 3 x lo-1o Noise level (amp)
sensitivity of N-FID depends on several variables, and the conditions used in obtain- ing these data may not have been optimum. Because N-FID i s much less sensitive to carbon compounds, simultaneous use of N-FID and FID i s an excellent technique for the analysis of combustion products of nitrogen containing polymers.
The calculated sensitivity of N-FID for HCN is not directly applicable to actual analysis of the gas because the capability of the entire GC system i s usually much less than the capability of the detector. HCN is a reactive gas and is absorbed on the surfaces of containers, injection devices and GC column during sample manipula- tion and analysis. The absorption results in an erroneously tow value for HCN especially when the concentrations are low, and produces a "ghost" peak following analysis of a high concentration of HCN. The use of a greaseless polypropylene syringe and all glass injector-column reduces this problem to a certain extent, but absorption in the GC system is s t i l l the chief limitation in direct GC analysis of HCN.
Conversion of HCN to a less reactive compound followed by GC analysis of the compound is o m solution to the absorption problem, although sample treatment requires more steps. One such method involves conversion of HCN to cyanogen chloride by the action of chloramine-T, followed by GC analysis of cyanogen chloride using the highly sensitive electron capture detector [ 11
I
.
Sensitivity (coulomb/g) HCN B~~~~~~ Detectable limit (g/sec) HCN Benzene
Determination of Hydrogen Cyanide in Fire Gases
Continuous Monitoring
Easty and others have used amperometric determination for continuous moni- toring of HCN [I21
. In order to obtain selective response to HCN, this compound
is liberated from a liquid sample and absorbed in a NaOH solution. Some monitor- ing devices are commercially available. One utilizes infrared absorption, and another, the electric conductivity change of an absorptive solution [I31.
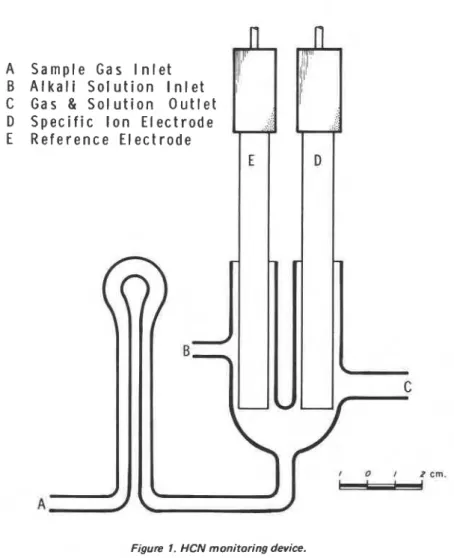
According to the literature both methods have high sensitivity but information on the selectiv- ity is not available. The present authors have used a cyanide ion electrode with simple glassware as shown in Figure 1. HCN in a gas stream i s absorbed by a flowing solution and the change of electric potential i s recorded. The rate of gas and liquidYoshio Tsucltiya and Kikuo Sumi
flow was
500
t d 1000 ml/min and 100 rnllmin respectively. The volume of the cell was 15 ml. The method has high sensitivity and good selectivity; however, it may not be accurate for low concentrations of HCN, because of the slow response of the electrode. Further refinement in the design of the electrode and glassware i s needed.GENERAL DISCUSSION
One of the common problems in HCN analysis in fire gases is the decrease in concentration during sampling. HCN i s produced in a high temperature fire gas, which contains moisture and smoke particles. These products absorb HCN when the temperature i s lowered. HCN i s also absorbed by any surface with which it comes in contact such as those of pumps, tubings, sample containers and injection devices. Heating these surfaces helps to prevent this problem. In manipulating samples, most steps such as cooling, transferring, filtering, storing etc. decrease the concentration of HCN. The decrease is sometimes quite significant; a decrease to 112 or 113 of the initial level is common when the HCN concentration i s low. Sampling should be simple, direct and short both in distance and in time. A colorimetric tube is most advantageous in this respect and gas chromatography is the least. Trapping of HCN in an alkali solution is usually effective because HCN is stabilized in the solution. Sometimes, however, increase in the concentration of C N can be observed when the solution containing HCN i s allowed to s i t for a long period.
Interference is another problem. In general, the highly sensitive techniques are more susceptible t o interference, except for gas chromatography, which is probably least affected by it because of the principle of the method. Distillation, e.g., by a standard method [4], i s a common technique used to eliminate interfering sub- stances in a solution. When S - coexists with HCN, distillation is not effective; precipitation of S-- as a heavy metal sulfide can be used instead. Polarography can also be used as an alternative technique if occlusion of HCN by the precipitate i s significant [14]. Infrared absorption spectroscopy has also been used for semi- quantitative analysis of HCN and other components in fire gases 1151. It is an advantage that several components are analysed at once without much sample manipulation, but at the same time many absorption bands due to different com- ponents complicate the spectra and could interfere with HCN analysis. In the analysis of HCN in a fire gas, the authors recommend that at least two different methods be employed in order to avoid serious errors caused by interference and absorption problems. Methods of HCN analysis discussed in this paper are sum- marized in Table 2.
CONCLUSION
Various methods for the determination of HCN in fire gases have been discussed with their advantages and disadvantages. Common problems are interference caused by other compounds and absorption of HCN. It is recommended that at least two different methods be used to ensure reliability of results.
Determination o f Hydrogen Cyanide in Fire Gases
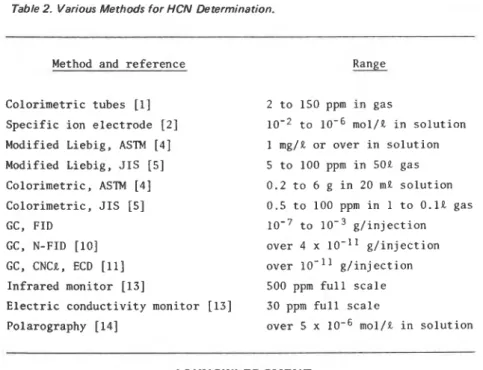
Table 2. Various Methods for HCN Determination.
Method and r e f e r e n c e
-
Range C o l o r i m e t r i c tubes [ l ] S p e c i f i c i o n e l e c t r o d e [2] M o d i f i e d L i e b i g , AS'IM [4] M o d i f i e d L i e b i g , J I S [5] C o l o r i m e t r i c , AS'IM [4] C o l o r i m e t r i c , J I S [ S ] GC, FID GC, N-FID [ l o ] GC, CNCL, ECD [ l l ] I n f r a r e d m o n i t o r [13] E l e c t r i c c o n d u c t i v i t y m o n i t o r [13] Polarography 1141 2 t o 150 ppm i n gas t o mol/L i n s o l u t i o n 1 mg/L o r over i n s o l u t i o n 5 t o 100 ppm i n 50E gas 0.2 t o 6 g i n 20 mE s o l u t i o n 0.5 t o 100 ppm i n 1 t o 0.1L gas t o g / i n j e c t i o n over 4 x 1 0 - l 1 g / i n j e c t i o n over 1 0 - l 1 g / i n j e c t i o n 500 ppm f u l l s c a l e 30 ppm f u l l s c a l e over 5 x mol/L i n s o l u t i o n ACKNOWLEDGMENTThe authors wish to thank J. Boulanger and D. W. Morwick for assistance in conducting experiments. This paper is a contribution from the Division of Building Research, National Research Council of Canada, and i s published with the approval of the Director of the Division.
REFERENCES
1. Concentration Determination with Draeger Tubes, Table 2340, 4th ed., Draegerwerk Luebeck, Luebeck, Germany (1970).
2. Guide t o Specific Ion Electrodes and Instrumentation, Orion Research Inc., Cambridge, Mass. (1969).
3. L. S. Bark and H. G. Higson. Analyst 88,751 (1963).
4. Standard Methods of Test for Cyanides in Water, ASTM D2036-74.
5. Methods for Determination of Hydrogen Cyanide in Exhaust Gases, Japanese Industrial Standard K 0109-1969.
6. J. Epstein. Anal. Chem. 19, 272 (19471.
7. K. G. Woolmington, J. Appl. Chem. 11.114 (1961 1.
8. R. E. Isbell.Ana1. Chem. 35,255 (1963).
9. R. R. Claeys and H. Freund. Environ. Sci. & Tech. 2,458 (1968). 10. M. Donike, Z. Naturforsch. Teil B. 28,533 (1973).
11. J. C. Valentour et al. Anal. Chem. 46,924 (1974). 12. D. B. Easty etal.Anal. Chem. 43,509 (1971).
13. Analyzers and Detectors for Gases and Liquids, Hartmann & Braun AG, Frankfurt, Germany (1971 ).
Yoshio Tsuchiya and Kikuo Sumi
14. D . R . Canterford.Ana1. Chem. 47.88 (1975).
15. T . A. Perenich and E . C . Tuazon, Applied Spectroscopy 30,196 (1976).
Yoshio Tsuchiya
Yoshio Tsuchiya i s a, Research Officer of the Division of Building Research, National Research Council of Canada. He received his Bachelor of Engineering and Doctor of Engineering degrees in Applied Chemistry from the University of Tokyo in 1953 and 1962 respectively. He joined the National Research Council in 1965. He has experience in fields of industrial explosives, organic peroxides and fire research.
Ki kuo Sumi
Kikuo Sumi i s a Research Officer of the Division of Building Research, Na- tional Research Council of Canada. He received his B.A.Sc. degree in Applied Science from the University of Toronto and his Ph.D. degree in Chemical Engineer- ing from the University of London. He joined the National Research Council in 1951 and has experience in various aspects of fire research such as thermal decom- position of polymers, fire extinguishment and fire prevention regulations.
This publication i s being distributed by the Division of Building R e s e a r c h of the National R e s e a r c h Council of Canada. I t should not be reproduced in whole o r in p a r t without p e r m i e s i o n of the original publisher. The Di- vision would be glad to be of a s s i s t a n c e in obtaining such p e r m i s s i o n .
Publications of the Division may be obtained by m a i l - ing the a p p r o p r i a t e r e m i t t a n c e ( a Bank, E x p r e s s , o r P o e t Office Money Order, o r a cheque, m a d e payable to the Receiver General of Canada, c r e d i t NRC) t o the National R e s e a r c h Council of Canada, Ottawa. K1A OR6.
Stamps a r e not acceptable.
A l i s t of a l l publications of the Division i s available and m a y be obtained f r o m the Publications Section, Division of Building Research, National R e s e a r c h Council of Canada, Ottawa. KIA OR 6.