HAL Id: hal-02156397
https://hal.archives-ouvertes.fr/hal-02156397
Submitted on 18 Jun 2019
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
CHARACTERIZATION OF MUSCARINIC BINDING
SITES IN THE ADULT AND DEVELOPING RAT
COCHLEA
Sylvain Bartolami, Myriam Planche, Remy Pujol
To cite this version:
Sylvain Bartolami, Myriam Planche, Remy Pujol. CHARACTERIZATION OF MUSCARINIC
BIND-ING SITES IN THE ADULT AND DEVELOPBIND-ING RAT COCHLEA. Neurochemistry International,
Elsevier, 1993, 23 (5), pp.419-425. �hal-02156397�
,'¢et, o(Iwm Int Vol 23, No 5. pp 419 425, 1993 0197-0186,935600+000
P]lnled in Great Britain Pergamon Pres,~ Lid
C H A R A C T E R I Z A T I O N OF M U S C A R I N I C B I N D I N G SITES
IN THE A D U L T A N D DEVELOPING RAT COCHLEA
SYLVAIN BARTOLAMI,* MYRIAM PLANCHE a n d RI~My PUJOL Laboratolre de Neuroblologm de 1"Audition, 1NSERM U-254 & Umverslt6 Montpellier II,
CHU St. Charles, 34059 Montpelher, Cedcx l, France
( Receu'ed 5 March 1993. accepted 24 Ma3 1993)
A b s t r a e ~ T h e maturation of the chohnerglc innervatmn of the rat cochlea is associated with a transient increase in the muscarmlc-receptor activated mosltol phosphate synthesis In order to investigate the mechamsms revolved in this transient enhancement of the lnOSltol phosphate response, the binding prop- emes of the cochlear muscarmlc receptors were studied during rat cochlear development. Incubating the membranes from 4-day-old, 12-day-old and adult cochleas with [~H]quinuchdmyl benzylate indicates that thmr respective, mean concentrahons of chohnoceptors are 454 _+ 51 (+_ SEM), 39 _+ 2 and 42 _+ 3 fmol,,'mg of protein. The dissociation constants at equihbrmm are 207 + 80, 42_+ 7 and 28_+_ 3 pM for the binding sites of the 4-day-old, 12-day-old and adult cochleas, respectively. Pharmacological charactenzatlon of the binding, using selective antagonists, shows that M3 cholinoceptors are expressed in developing and adult cochleas The data demonstrate that changes m muscanmc receptor affinity and number do not correlate with the previously observed peak of the lnositol phosphate metabolism. The transient enhanced mosltol phosphate response is therefore not due to changes in chollnoceptors, but probably due to alterations involving the intrinsic achvlty of the phosphohpase C and/or the efficacy of coupling of the transductlon system.
A c e t y l c h o l i n e is the m a i n n e u r o t r a n s m ~ t t e r o f the efferent a u d i t o r y system (for review see E y b a h n , 1993) t h a t leeds back from the superior olivary complex to the cochlea. This n e u r o t r a n s m i t t e r mediates slow m e t a b o t r o p i c effects within t a r g e t cells, via the acti- v a t i o n o f the m u s c a r i n i c receptors (for reviews see N a t h a n s o n , 1987 : H u l m e el al., 1990 ; Hosey, 1992). T h e i r presence m the cochlea has been clearly dem- o n s t r a t e d by b o t h b i n d i n g ( J a m e s et al., 1983; W h i p p l e a n d Drescher, 1984 : van M e g e n et al., 1988) a n d f u n c t i o n a l studms ( G u i r a m a n d el al., 1990; Bar-
tolami el al., 1990, 1992a: Niedzielsky et al., 1992).
These latter investigations d e m o n s t r a t e d t h a t the
*To v, hom correspondence should be addressed Dr S. Bar- tolanal, Laboratolre de Neurophyslologm Sensorlelle, Unl~crsltd Montpelher 1I, Place Eugene Batalllon, 34095 Montpelher, Cedex 5. France
4hbrel mlron.s AF-DX 116, I 1-((2-((dmlhylamlnoI-methyl)-
I -plperldlnyl)acetyl)-5, 11 -dihydro-6H-pyrido(2,3-b)-
(l,4)-benzodiazepme-6 one: B ... maximum con-
centratmn of blndmg site; 4-DAMP, 4-dlphenylacetoxy- N-methyl plperldlne methiodide: lPs, inosltol phos- phates, Kj, dissociation constant at equilibrium : K,, lnhl- bmon constant at equlhbrmm ; QNB, qumuchdinyl ben- zylate.
m u s c a r i n i c receptors are linked to the inosltol p h o s - p h a t e (IP) signalling p a t h w a y . M 3 m u s c a n n i c recep- tors have been c h a r a c t e r i z e d in the 12-day-old rat cochlea ( G u i r a m a n d et al., 1990: B a r t o l a m i et al.,
1992a) a n d e x p e r i m e n t s using the p o l y m e r a s e c h a i n reaction have identified m R N A s e n c o d i n g for m l, m3 a n d m5 subtypes o f m u s c a r i n i c receptor in the m o u s e cochlea ( D r e s c h e r et al., 1992). T h e f o r m e r two molec- ular f o r m s o f the c D N A - c l o n e d receptors c o r r e s p o n d to the M1 a n d M 3 receptors, defined in functional studies by m e a n s o f selective a n t a g o n i s t s ( B o n n e t ,
1989; H u l m e et al., 1990)
D u r i n g p o s t n a t a l cochlear d e v e l o p m e n t , the acti- v a t i o n o f m u s c a r l n i c receptors is c h a r a c t e r l s e d by a t r a n s i e n t e n h a n c e m e n t o f the s t i m u l a t e d IP synthesis which peaks at day 12 ( B a r t o l a m i el al., 1990, 1992a). The t r a n s i e n t increase o f the IP response occurs con- c o m i t a n t l y with b o t h the f o r m a t i o n of the efferent synapses on the o u t e r h a i r cells (Lenoir et al., 1980; S i m m o n s et al., 1990 ; Cole a n d R o b e r t s o n , 1992) a n d the onset o f physiological m a t u r a t i o n o f the cochlea (Uzlel et al., 1981 ; Puel a n d Umel, 1987; Lenoir a n d Puel, 1987; R y b a k et al., 1992). Since a similar cor- relation between a m u s c a r i n i c receptor activated IP synthesis a n d the efferent synaptogenesis has been
421) SYLVAIN BARTOLAMI et al
observed in the bird cochlea (Bartolami et al., 1992b). These findings suggest t h a t the IP t u r n o v e r coupled to muscarinic receptors m a y be implicated in the func- tional m a t u r a t i o n o f the cochlea. In addition, the tran- sient peak o f the IP synthesis m response to activation o f c h o l i n o c e p t o r s is c o n c o m i t a n t with a period at which o u t e r hair cells are hypersensitive to ototoxicity induced by a m i n o g l y c o s i d e antibiotics (Pujol, 1986). This suggests a relationship between the anaino- glycoside toxicity and the activation o f the IP m e t a b - o h s m (Schacht, 1976, 1986; Bartolami et al., 1993).
A l t o g e t h e r these o b s e r v a t i o n s indicate that the d e v e l o p m e n t a l p a t t e r n o f the stimulation o f musca- rinic receptors is o f i m p o r t a n c e for the physiology a n d p a t h o p h y s i o l o g y o f the cochlea. In the present study, we investigate w h e t h e r the transient e n h a n c e m e n t o f the muscarinic r e c e p t o r activated IP synthesis was due to d e v e l o p m e n t a l changes in binding properties o f muscarinic receptors in the cochlea. The high affinity, but nonetheless non-specific a n t a g o n i s t quinuclidinyl benzylate ( Y a m a m u r a and Snyder, 1974) was used to d e t e r m i n e d the c o n c e n t r a t i o n a n d affinity o f musca- rinic receptors in developing a n d adult rat cochleas. In addition, the binding sites were pharmacologically characterized using the selective a n t a g o n i s t s piren- zepine. A F - D X 116 a n d 4 - D A M P , respectively spe- cific for M1, M2 and M3 muscarinlc receptors ( H a m - mer c t a l . , 1980 ; Ladinsky et al., 1988 ; Barlow et al.,
1990). This study particularly focuses on three stages: 4-day-old, 12-day-old and adult which are rep- resentative o f the three phases o f the d e v e l o p m e n t a l profile o f the muscarinlc receptor activated IP synthesis, The first stage c o r r e s p o n d s to the onset o f the inositol p h o s p h a t e response, the second one to the peak response, and third one to m a t u r i t y o f the response (Bartolami et al., 1990~ 1992a).
E X P E R I M E N T A L P R O C E D U R E S
AlaterutLs
Trltated qulnuchdlnyl benzylate (QNB, speclliC activity' 45.4 Ci mmol) was purchased from New England Nuclear Boston, MA, atropine and plrenzepine from Sigma Chemical C o , St Louis MO and 4-dlphenylacetoxy-N-methyl plp- erldine methlodlde (4-DAMPI from Research Biochemical Inc. Natlck, MA. Bovine serum albumin was obtained from Boerhlnger Mannhelm, Indianapolis, IN and l l-((2-((dl- ethylatnlnol - methyl)- 1 - plperldlnyl)acetyl) - 5,11 - dlhydro - 6H-pyrldo(2,3-b)-(1,4)-benzodiazeplne-6 one (AF-DX 116) was a gift from Boehringer lngelheim (Reims, France). All other compounds were of analytical grade.
Dis.section ol the cochleas
Adult, [2 find 4-day-old Wlstar rats were stunned and killed by decapitation. The cochleas were quickly dissected
out from the temporal bones, the stria ~,ascularls were removed and the cochleas were collected in ice-cold phos- phate buffered saline pH 7.4 (PBS 50 mM Na,HPO~ 50 mM KH.,PO4 125 mM NaCI). The dissected cochleas were then composed of the organ of Corti, the spiral ganglion and the modiolus that contains the distal (peripheral) segment of the cochlear nerve.
Membrane pr¢Taralton
The dissected cochleas were washed 4 times with PBS at 0 4 C and then homogenized in PBS using an all glass homogenizer at the concentration of 12 cochleas/ml. The resulting homogenates were centrifuged at 300 j., for 10 rain, the supernatants containing the cochlear membranes were taken, and then stored at - 7 0 C before use
As.sa) s o / [ ~H]QNB binding
The method was derived from the technique optimized by Yamamura and Snyder (1974). Membrane ahquots (320 id) were mixed with 40/A PBS and [~H]QNB at concentrations varying from 0.01 to 5 nM, and then incubated at 25 C for 90 mln. The binding reaction was stopped by filtration of the samples, under vacuum, onto Whatman GF/B filters and by washing the filters with 3 x 4 ml PBS at 0 4 C The amount of [']QN B retalned/'filter was measured by liquid scintillation counting ~lth an efficacy of 50%. This procedure allows the determination of the quantity of total bound QNB. In ordel to measure the non-specific binding, 1 ILM atropine was added to the incubation medium. In this condition, atropine is used at a 1000-fold higher concentration than the QNB concentration find therefore it competitively prevents the binding of QNB to muscarlnlC sites In the presence of atro- pine, QNB binds soleI~ to non-specific sites. The amount of specific bound QNB was obtained by subtracting the values of the non-specific binding from the total binding. Trials of displacement of the binding of [~H]QNB were conducted by adding the specific, muscarinlc antagonists 4-DAMP (M3), plrenzeplne (MI) and AF-DX 116 (M2) to the reaction medium The results of the binding assays were normalized to the amount of protein present in the membrane preparations, which was measured according to Lowr~ et al (1951) using bowne serum albumin as standard
SlallSllCa/ atla]l'MS arid data expres.~ton
The results were analysed using the LIGAND computer program (Munson and Rodbard, 1980) and were expressed either as ratios of the amount of bound QNB to the quantity of protein contained in the samples (fmol of QNB/mg of protein), or as percentages of the amount of specificall~ bound QNB The data are the lneans±SEM of three indi- vidual e~perlments, at least Each experiment ~as performed In duphcate The statistical significance of the data was deter- named using one way ANOVA
RESULTS
Linearity o[ the [ ~H]QNB binding as a / u n c t i o n o! the concentration o['cochlear membrane
C o c h l e a r m e m b r a n e s o b t a i n e d f r o m adult, 12 and 4-day-old rat cochleas, were diluted in PBS at con- c e n t r a t l o n s ranging from 2 to 12 cochleas/ml a n d used for binding assays with Q N B at the c o n c e n t r a t i o n
Muscarmlc binding sites m the developing cochlea 421 of 0.08 nM. The specific Q N B binding is linear for
concentrations o f cochlear m e m b r a n e ranging from 111 to 186 #g/ml (6 10 cochleas/ml) in the case o f adult cochleas, from 57 to 143 /~g/ml ( 4 - I 0 coch- leas/ml) for the 12-day-old cochleas and from 52 to 317 ttg/nll (2-12 cochleas/ml) for the 4-day-old coch- leas (data not shown). The mean amounts (-+SEM)
of protein/cochlea are 26.4 4-5.9
#g,
14.3 4- 1.8Itg
and18.6_+2.2 Itg lbr the 4-day-old, 12-day-old and adult cochleas, respectwely.
Saturation of the [ W]QNB binding
Membranes obtained from adult, 12-day-old and 4-day-old rat cochleas were diluted to concentrations of 149, 114 and 211 #g protein/ml respectively (8 coch- leas/ml) and incubated with increasing concentrations of ['H]QNB. The saturation curves were established, for each stage o f maturation and their respective Scat- chard analyses were carried out. F r o m these analyses, the binding constants were obtained (Table I). The dissociation constants (Kj) are 207-+80 (-+SEM), 42-+ 7 and 28 :t- 3 pM for the muscarinic binding sites of the 4-day-old, 12-day-old and adult cochlear mem- branes, respectively. Each Kd value is significantly different from the other two (P < 0.001) The con- centratlons of binding sites (B.,~,) within the 4-day- old, 12-day-old and adult m e m b r a n e preparations are, respectively, 454 + 51 ( -+ SEM), 39-+ 2 and 42-+ 3 fmol of Q N B/mg of protein. The B ... values of the 12-day- old and adult cochlear m e m b r a n e s are not statistically different. The saturation of the specific binding is reached with concentrations o f Q N B starting at 0.1 nM in the cases of the adult and 12-day-old cochleas and, 0.5 nM for the 4-day-old cochlea. The Hill coefficients are in all cases less than but close to unity (Table 1 ).
hlhtbmon o/ the speci#c QNB binding by selective
I?llISC(II'InlC anld, qoniAts
In this series of experiments, every m e m b r a n e prep- aratlon was diluted to the concentration of 6 coch- leas/ml. This concentration corresponds to about 111,
Table I C o n s t a n t s o f the binding of Q N B at e q m h b r m m , m the cochlear m e m b r a n e s
A g e n A,,(pM) Bin,, ( f m o l / m g ) Hdl n u m b e r 4-day-old 8 207+_80*** 4 5 4 + 5 1 " * * 0 . 9 4 _ + 0 0 2
12-day-old 6 4 2 + 7 " * * 3 9 + 2 0 . 9 2 ± 0 0 5 Adult 6 2 8 + 3 4 2 ± 3 0 9 7 ± 0 . 0 2 All the d a t a are m e a n s ± S E M o f experiments conducted In duphcate
The n u m b e r o f expernnent is n and the n u m b e r o f tested Q N B concentraUon', experiment ranges from 5 to I I *** different from its c o r r e s p o n d i n g adult p a r a m e t e r with P < 0 001
86 and 158 /tg protein/ml for the adult, 12-day-old and 4-day-old m e m b r a n e preparations, respectively. The subtype-specific muscarlnlC antagonists piren- zepine (M1), A F - D X 116 (M2) and 4 - D A M P (M3) were used to displace the specific Q N B binding obtained by incubating the samples of adult and 12- day-old cochlear membranes with 0.08 nM of Q N B and the 4-day-old m e m b r a n e samples with 0.4 nM of QNB. These concentrations of Q N B were selected from the saturation study.
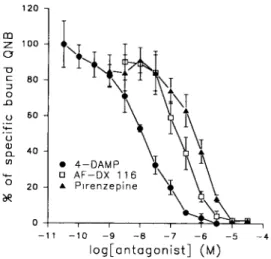
As for the adult samples, the subtype-specific antag- onists inhibit the specific binding of QNB, that is 38_+9 (_+SEM) fmol o f Q N B / m g o f protein, in a dose-dependent m a n n e r (Fig. 1) with a rank order of potency : 4 - D A M P > A F - D X 116 > pirenzeplne (Table 2) The inhibition constants (K,) are sig- nificantly different, with P < 0.01 for 4 - D A M P : p l - renzepine and with P < 0.05 for A F - D X l l6/piren- zepine and 4 - D A M P / A F - D X 116.
In the 12-day-old preparation, the specific binding of Q N B is 4 5 + 10 fmol of Q N B / m g o f protein. It is Inhibited In a dose-dependent manner (Fig. 2) with the following rank order of potency : 4 - D A M P > A F - D X 116 = pirenzepine. The h; values (Table 2) are
1 2 0 -ID C 80 t) 60 tn • 4 - D A M P 2 0 e e 0 i l - 1 1 - - 1 0 - 9 - 8 - 7 --6 - 5 - 4 I o g [ o n t a g o n i s t ] ( a )
Fig. l. lnhibmon of the speofic binding of QNB by musca- rlnlC antagonists in adult cochlear membranes. The membranes, diluted to a concentraUon of 11[_+ 13 /~g/ml, were incubated with 0 08 nM of [~H]QNB and exposed to
the selective muscanmc antagonists 4-DAMP, AF-DX 116 and pirenzeplne. The results are given as percentages of the specific binding of QNB, obtained in the absence of these muscarinlc blockers, which is equal to 384-9 ( _+ SEM) fmol of QNB/mg of protein The data points are means of 3 replications, at least. Each replication was performed in duplicate. The error bars are SEM and are not shown when
422 SYLVAIN BARTOLAMI el a/.
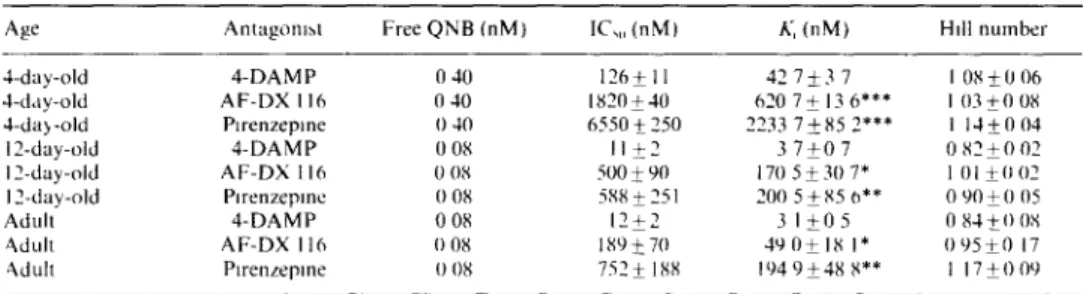
Table 2 lnhxbltory p a r a m e t e r s of the displacement o f the specific binding o f Q N B in the cochlear m e m b r a n e s Age Antagom~t Free Q N B ( n M ) IC,. ( n M ) A, ( n M ) Hdl n u m b e r 4-day-old 4 - D A M P 0 40 1 2 6 + 11 42 7_+3 7 I 0 8 + 0 06 4-day-old A F - D X 116 0 40 1820+40 620 7 + 13 6*** I 03_+0 08 4-day-old Plrenzepme 0 40 6550+_250 2233 7_+85 2*** I 14_+0 04 12-day-old 4 - D A M P 0 08 II + 2 3 7 + 0 7 0 8 2 + 0 02 12-day-old A F - D X I 16 0 08 500 + 90 170 5 + 30 7* 1 01 + 0 02 12-day-old PJ renzepme 0 08 588 + 251 2(10 5 + 85 0"* 0 90 + (I 05 Adult 4 - D A M P 0 08 1 2 + 2 3 I + 0 5 0 84-+(10S ~dult A F - D X 116 0 08 189-+70 49 0 + 18 I* 0 9 5 + 0 17 ~dull Plrenzepme 0 08 752 + 188 194 9 + 48 8** I 17 + 0 09 The d a t a are m e a n s + S E M o f experiments conducted In duphcate A t least 3 identical experiments were done m each
case K, values were calculated by the e q u a t m n o f C h e n g and Prusoff11973) A, - ( I + C / h a ) x IC,,, ~,,lth ( ' being the c o n c e n t r a t m n o f free Q N B , the K,~ valuc~ are given m Table 2 F o r a gwen developmental stage, K, values are dlfl'elent from the K, value obtained wllh 4 - D A M P , wHh P < 0 001 1"**), P < 0 01 (**) and P < 0 05 (*)
stgnificantly different for 4 - D A M P / A F - D X 116 ( P < 0.01) a n d for 4 - D A M P / p l r e n z e p i n e ( P < 0.05).
E x p o s u r e o f the 4-day-old m e m b r a n e s to antag- omsts also results m d o s e - d e p e n d e n t inhibitions o f the specific b m d i n g o f Q N B , that is 479__+28 fmol o f Q N B / m g protein (Fig. 3). The relative o r d e r o f p o t e n c y is 4 - D A M P > A F - D X 116 < plrenzeplne. The K, values o f the three a n t a g o n i s t s statistically differ ( P < 0.001, m every case, Table 2). A t all stages, the Htll n u m b e r s are close to umty, w h a t e v e r the a n t a g o n i s t (Table 2).
D I S C L S S I O N
Chan.qes in muscarinic hinding properties are not invoh,ed in the enhanced IP metaholR'm
The affinity o f the cochlear m u s c a n m c receptor increases during m a t u r a t i o n , while the c o n c e n t r a t i o n o f receptor decreases with age. W h e n c o m p a r e d with the d e v e l o p m e n t a l p a t t e r n o f the muscarinic receptor activated IP synthesis (Bartolami et al., 1990, 1992a), the d a t a d e m o n s t r a t e that binding properties o f muscarinic sites and changes in the muscarlnic sttmu-
120 Z 100 (Dr -0 E 80 0
J
.2 60o
l \
© ca_ 40 o3 ~- • 4 - D A M P o 2O o A F - D X 1 1 6t
• P I r e n z e p i n e - 1 1 - 1 0 - 9 - B - 7 - 6 - 5 - 4tog[antGgonist] (M)
Fig. 2 Inhibition of the specttic bmding of QNB by musca- nmc antagonists in membranes from 12-day-old cochleas. The membranes, dtluted to a concentration of 86_+ I 1 pg/ml,
were incubated with 0.08 nM of ['H]QNB and exposed to the selective muscarimc antagomsts 4-DAMP, AF-DX 116 and plrenzeplne. The fesults are given as percentages of the specific binding of QNB. obtained in the absence of these muscarlntc blockers, which is equal to 45 4- 10 ( 4- SEM) fmol of QNB/mg of protein The data points are means of 3 replications, at least. Each replication was performed m duplicate. The error bars are SEM and are not shown when
they are smaller than the symbols.
120 c- 8 0 o x3
._
6 0 o 20 • , P l r e n z e p l 0 i "~A i - 1 1 - 1 0 - 9 - 8 - 7 - 6 - 5 -,4 - 3log [AntGgonist] (M)
Fig 3 Inhibition of the specific binding
of
QNB by mUSCd- rlnlC antagonists in membranes from 4-day-old cochleas The membranes, diluted to a concentration of 158_+35 /~g, ml, were incubated with 0 4 nM of 3H-QNB and exposed to the selective muscarmlc antagonists 4-DAMP, AF-DX 116 and p l r e n z e p l n e , used at various concentrations T h e results are given as percentages of the specific blndlng of QNB, obtained in the absence of these muscarlnlC blocker~, which is equal to 479 + 28 ( _+ SEM) fmol of QN B/rag of protein The data points are means of 3 replications, at least. Each replication was performed in duplicate. The error bars are SEM and arcMuscarlnic binding sites m the developing cochlea 423 lation of the signalling pathway diverge during matu-
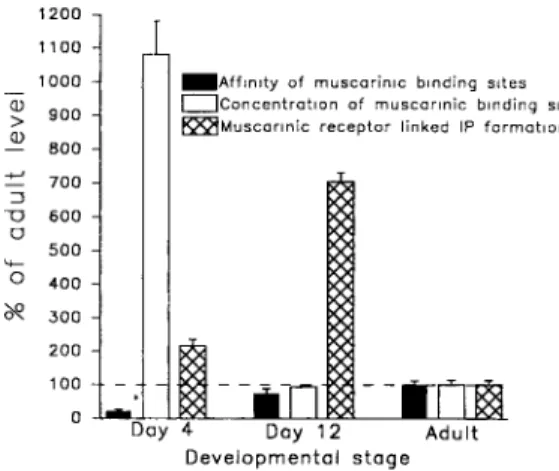
ration of the auditory organ. F o r instance, the IP synthesis decreases from day 12 to a d u l t h o o d whereas, during the same period, the receptor affinity increases and the concentration o f muscarinic site does not change significantly (Fig. 4). Likewise, from day 4 to day 12 the IP formation increases while the con- centration of muscarimc sites falls drastically (Fig 4). Such a difference between muscarimc binding prop- ertles and muscarmlc agonist-elicited IP turnover have also been shown during cortical ontogeny (Balduinl et
al., 1987 : Heacock etal., 1987 : R o o n e y and Nahorski, 1987). This conclusion rules out the possibility that the transient enhancement of the IP response peaking at day 12 (Bartolami et al., 1990, 1992a), is due to an increase m e~ther the concentration o f muscarinic receptors or the affinity of the binding sites.
Pharmacoh¥l.)' o / t h e hindinq sites
Alternatively, a variation in the receptor phenotype could explain the transient, high IP response. The expression of M3 receptors, characterized at day 12 ( G u i r a m a n d ¢t al., 1990: Bartolami et al., 1992a), may decline and switch to the synthesis o f muscarlnic
> (1) -+~ ~D E/ q-- 0 1200 1100 1000 9 0 0 8 0 0 7 0 0 6 0 0 5 0 0 4 0 0 3 0 0 2 0 0 100 0 I A f f l n l t y of rnuscarimc b(ndlng sites [ ~ ] C o n c e n t r a t l o n of muscarmie binding sites ~ M u s c a r m ; c receptor linked IP formation
D a y 4 D a y 1 2
ir11
A d u l t D e v e l o p m e n t o l s t e g eFig. 4. Relat~onshlp between muscanmc binding properties and the stimulated IP formation during cochlear maturation. Concentrations and affimtle~ of muscarlnic receptors are obtained from the saturation studies Receptor affinities were calculated from A'~ values (affinity = I/Kj) Data obtained m each saturation experiment were expressed as percentage of the mean affimt2¢ and B,n.,, values determined at adulthood These percentage levels were combined for each devel- opmental stage and their means ( _+ SEM, n = 6 at least) are shown The adult mean B ... value is given in Table 1 and the adult mean aftimty value is 38.81 +4.20 nM ~. The IP responses are from Bartolaml et al. (1992a, Fig. 1) The IP synthesis was stimulated by carbachol (1 mM), the basal level taken to be 100% of IP formation in adult cochleas is 0 043±0.006 dpm~H-IPs/dpm [~H]myo-lnosltol taken up/
cochlea
receptors which are not coupled to phospholipase C, but rather linked to inhibition of adenylyl cyclase such as m2 or m4 receptors (Nathanson, 1987; Bonner, 1989: H u l m e et al., 1990; Hosey, 1992). However, the possibility o f such a change in the muscarinic phenotype can be dismissed because the molecular analysis o f m R N A s encoding cochlear muscarinlc receptors did not detect m2 and m4 m R N A s in coch- lear cells (Drescher et al., 1992).
Drescher and co-workers (1992) also identified m l, m3 and m5 proteins m R N A s in adult mice cochleas, suggesting the presence o f several muscarinic receptor subpopulations. In the present study, the very low affinity of pirenzepme for the binding s~tes argues against the functional expression of M I receptors in the auditory organ. The absence o f M I receptors is further supported by an extended characterization which showed that both antagonist and agonist M1 ligands have a low affimty for IP synthesis-coupled muscarlnic receptors in the cochlea (Bartolami et al., 1992a). As for M5 receptors, the lack of a specific hgand prevents their pharmacological identification. On the other hand, in the present work Hill numbers are close to unity (Tables 1 and 2). In agreement with other surveys, this indicates the existence o f solely one population of muscarlnlC sites (James et al., 1983: Whipple and Drescher, 1984 : van Megen et al., 1988). This population is highly sensitive to 4 - D A M P which is at least 15-fold more efficient than A F - D X 116 and plrenzeplne in inhibiting the Q N B binding (Table 2). Therefore, the cochlear muscarinic population is likely of the M3 subtype, as previously reported (Gui- ramand et al., 1990 : Bartolami et al., 1992a). Together with m R N A analysis (Drescher et al., 1992), our data suggest that the synthesis of MI and M5 receptors may be regulated posttranscriptlonally. In addition, M3 receptors have a pharmacology proper to the cochlea in that A F - D X 116 is a better antagonist than pirenzepine at the binding sites. Such a profile was also found m ~'ico (Kujawa et al., 1993) and in patch- clamp studies using isolated outer hair cells (Kakehata
et al., 1993).
Concerning the m u s c a n m c sites of the 4-day-old cochlea, their pharmacologic profile fits the M3 type but their K,, and K, parameters are much higher than the ones generally obtained with M3 sites. This dis- crepancy may reflect a possible molecular maturation of muscarinic proteins during cochlear development, as has been shown in the retina (Cho and Klein, 1988).
E n h a n c e m e n t o / p h o s p h o l i p a s e C actit'io'
F r o m the previous conclusions, it appears that the transitional peak activation of muscarinic receptors is
424 SYLVAIN BARTOLAMI et al
accounted for by changes in activities o f other proteins in the signalling pathway, lying downstream from muscarmic sites. These changes may be clther an increase m the efficacy o f coupling between receptors
and phospholipases C, as reported in the developing
cortex (Balduini et al., 1987~ Heacock et al., 1987:
R o o n e y and Nahorskl, 1987) or an alteration in the intrinsic actwity o f phospholipase C.
Concerning couphng efficiency, besides regulation by anteracting molecules, the improvement o f the coupling efficiency could be due to the transitional expression of G proteins that are more potent in sti- mulating phosphohpases C. Indeed, it has been reported that several G proteins able to activate phos- phohpases C with various efficiencies exist (Ashkenazi
et al.. 1989: Lechleiter et al., 1991). Alternatively, a variation in the turnover of G protein a n d / o r pho- spholipase C could be involved. This would modify the stoichlometry o f interactions between receptors, G proteins and phospholipases C, thus resulting in a greater number of phospholipases C achvated by a single muscarmic receptor.
As for alterations o f phosphohpase C intrinsic acuvity, these modulations may be achieved by several mechanisms : ( 1 ) phosphorylation o f phospholipase C b~ various kmases (Crooke and Bennet, 1989: Maj-
erus et al., 1990, Rhee, 1991 ; Guillon et al.~ 1992),
some of these kinases being present in the cochlea (Coling and Schacht, 1991): {2) regulation of the mtracellular calcmm level (Crooke and Bennet, 1989 : G m l l o n et a/., 1992); (3) inhibition by phosphollpids
such as phosphatidylcholine (Majerus et al., 1986):
(4) regulation o f phospholipase C translocation (Lee
et al., 19871 and (5) changes in the substrate supply due either to complex formations between substrate molecules and proteins such as alpha actmine, pro- fillne and profilactine (Lassing and Lindberg, 1985: G o l d s h m i d t - C l e r m o n t et al., 1990; F u k a m i et al.,
1992) or to alteration of substrate synthesis (Gmllon
et al., 1992). Actually. since an enhancement o f the basal IP turnover parallels the transitional rise m the
stunulated IP response (Bartolami et al., 1990, 1992a),
an alteration of the intrinsic achvity o f phosphohpase C Is favoured with regard to changes in the couphng efficiency.
In summary, the developmental divergcnce between the muscarinic binding properties and the pattern o f stimulated IP metabolism suggests that changes in the properttes o f muscarinic receptors do not underlie the transaent rise o f the inositol phosphate response. Therefore, the transient increase, observed during the maturation of the rat cochlca, in both the basal
and muscarinic receptor-mediated synthesis o f
mos~tol phosphates appears to be likely due to an enhancement o f the intrinsic activity of phospho- hpase C.
.4cknowh,~tqements--We gratefully thank Prof Jochen Schacht and Drs Ohvler Manzom, Guy Richardson and Ebraham Mayat for their useful comments
REFERENCES
Ashkenazi A., Peralta E. G , Wmslow J. W., Ramachandran
J. and Capon D. J (1989) Funchonally &stinct G proteins
selechvely couple different receptors to P1 hydrolysas in the same cell. Cell 56, 487 493.
Balduinl W., Sheldon M. D and Costa L G. (1987) Devel- opmental changes in muscarmac receptor stamulatcd
phosphomositade metabohsm an rat brain. J. Pharmac
exp. Ther 241,421 427.
Barlow R B., Shepherd M. K and Veale M A (1990) Sume
dlfferentml effects of 4-&phenylacetoxy-N-(2-chlo-
roethyl)-plperldme hydrochlorlde on guinea-pig atria and
ileum. J. Pharm. Pharmac. 42, 412 418
Bartolaml S., Gmramand J., LenoJr M., Pujol R. and Reca- sens M (1990) CarbachoMnduced mosllol phosphate for-
mahon during rat cochlea development. Hear Re,s. 47,
229 234
Bartolann S., Lebrun F. Mayat E, Gmramand J , Lenoar M., Rebfllard G , Lappe W. R , Pujol R. and R&asens M (1992a) Pharmacological characterizauon of muscaNmc receptors and thear putatwe involvement m the developing peripheral auditory organ. In : Recent Ath'ance.~ m Celhdar and Moh'cular Bwk~gy (Wcgmann R. J. and Wegmann M A , eds), Vol 3, pp 251 260. Peeters Press. Leuvcn. Bartolaml S., Mayat E.. Lippe W. R., Rcbfllard G and Pujol
R (1992b) Actavation of muscanmc chohnerglc receptors stamulates inositol phosphates s)nthesls in the developing avmn cochlear duct, Int. J. Det" Nt'uro~'ct. 10, 31 36 Bartolaml S, Planche M and Pulol R. (1993) lnhibahon of
the carbachol-evoked synthesJ~ of mos~tol phosphates b~ ototoxac drugs an the rat cochlea Ht'~ll Rex 67, 203 210 Bonner T. 1 (1989) The molecular baslb ofmuscarlmc recep-
tor di~ersaty. Trends' Neuroscl 12, 148 151.
Cheng Y. C. and PrusoffW. H. (1973) Relauonshlp between
the mhab~tion constant (K,) and the concentrahon of
inhibitor whach causes 50 perccnt inhibition (IC~,) of an
enzymatac reacnon. Btochem. Pkarmac. 22, 3099 3108.
Cho N. J. and Klein W L. (1988) Muscanmc acetylcholine receptors from avian retana and heart undergo different
patterns of molecular maturation J Neurochcm 50, 1403
1411
Cole K. S and Robcrtson D (1992) Early efferent lnnei- ration of the developing rat cochlea studaed wuh a car-
bocyanme dye. Brain Res. 575, 223 230.
Colmg D E and Schacht J. (1991) Protein phosphorylat~on m the organ of Corti. differential regulataon by second
messenger~ between basa and apex Hear Rev 57, 113
120.
Crooke S T. and Bennett C. F (1989) Mammahan phos-
phoinoslhde-speclfic phosphohpase C isoenzymes Cell
Cah'. 10, 309 323.
Drescher D. G , Upadhyay S., Wilcox E and Fex J. (1992) Analysis of nauscarmlc receptors ,,,ubtypes in the mouse
Muscarlmc binding sites in the developing cochlea 425 cochlea by m e a n s of the polymerase chain reaction J.
Neuro~hem. 59, 765 767.
Eyhahn M (1993) Ncurotransmltters and n e u r o m o d u l a t o r s of the m a m m a h a n cochlea Phy,wol Ret. 73, 309 373. Fukaml K , Furuhashi K., Inagaki M , Endo T., H a t a n o S.
and T a k e n a w a T (1992) Requirement o f p h o s p h a - lldylmosllol 4,5-blsphosphate for alpha actmln function
Nature 359, 150 152.
Goldschmldt-Clermont P J., Machesky k. M., Baldassare J J and Pollald T D (1990) The actm-bindlng protein profihn brads to PIP, and inhibits Its hydrolysis by phos- phohpase C Sewm'e 247, 1575 1578.
Gulllon G . Moulllac B. and Savage A. L (1992) Modulation of hormone-senslll~e p h o s p h o h p a s e C. Cell. SIq 4, I 1 23
G t n r a m a n d J , M a ) a t E , Bartolaml S , Lenolr M , R u m i g n y J F., Pujol R and Recabens M. (1990) A M3 muscarlnic receptor coupled to lnosltol phosphate formation in the rat cochlea" Bto~hem Pharmae. 39, 1913 1919
H a m m e r R , B e r N e C P , B u ' d s a l l N J . M . , B u r g e n A . S . V and H u l m e E C (1980) Plrenzepme dlstlngmshes between different subclasses o f muscarinlc receptors Nature 283, 91) 92
Heacock A M , Fisher S K. and A g r a n o f f B. W. (1987) Enhanced c o u p h n g o f neonatal muscarlmc receptor in rat brain to phosphomosltlde turnover. J Neltroe]lem 48,
191)4 1911
Hosey M. M (1992) Diversity of structure, slgnalhng and regulation within the famllty of muscarlnlC chohnergic receptors. FASEB JI 6, 845 852
Hulme E C , Blrdsall N J M and Buckley N. J. (1990) MuscarlnlC receptors subtypes. A. Ret pllarntac. Torte 30, 633 673
James W M , C h e a t h a m M A. and Klein W. L (1983) Muscarunc acetylchohne receptor binding in the guinea pig cochlea. Hear Res. 9, I 13 121
Kakehata S , N a k a g a w a T , T a k a s a k a T and A k m k e N. (1993) Cellular lnechanlsm of acetylchohne-mduced response In dissociated outer hair cells of guinea-pig coch- lea Y Phy.wol, LomL 463, 227 224
KuJawa S G , Glattke T. J., Fallon M. and Bobbin R. P. 11993) Effect of chohnerglc a n t a g o m s t s on contralateral suppression o f DPOAEs. Ahstr. Assoc. Res Otolarvnqol (St Petersburg beach, FL) 16, 394
Ladlnsky H , Gn'aldo E., Monferml E., Schlavl G. B., Vlgano M A , De (.'ontl L , Mlchelettl R. and H a m m e r R (1988) Muscarunc leceptor heterogeneity in s m o o t h muscle. binding and functional studies with A I ' - D X 116 Trends
Ptlartnae Set. February Suppl., 44 48
Lassmg l and Lmdberg U (1985) Specllic interaction between phosphatldyhnosltol 4.5-blsphosphate and pro- lilactm Nature 314, 472 474.
kechlelter ,1., Girard S , C l a p h a m D. and Peralta E (1991) Subcelluhlr patterns of calcium release determined b? G proteln-speclliC residues o f muscarlnlc receptors Nalure 350, 505 508
Lee K Y . Ryu S H., Suh P. G., Chol W. C. and Rhee S G (I987t P h o s p h o h p a s e C associated with particulate fraction of bovine brain Pro~ hath. ,4~ ad. SOl. U.S.A. 84, 5540 5544
Lcnolr M and Puel J L. t 1987) Development of2f~-L otoac- oustJc emissions in the rat Hear. Res. 29, 265 271 Lenolr M , Shncrson A. and Pujol R. (1980) Cochlear recep-
tor development m the rat with emphasis on synap- togenesls Anat Embo'ol 160, 253 262.
Lowry O H., Rosebrough N. J., Farr A L. and Randall R. J. (1951) Protein m e a s u r e m e n t with the fohn phenol reagent J. Inol Chem 193, 265 275.
Majerus P. W., Connolly T. M., Deckmyn H . Ross T. S., Bross T. E.. Ishu H., Bansal V. S. and Wdson D B. (1986) The metabolism of phosphomositlde-denved messenger molecules. Scu'nee 234, 1519 1526.
Majerus P W , Ross T. S., C u n n l n g h a m T W., Caldwell K K., Bennet Jefferson A. and Bansal V S. (1990) Recent insights In phosphatldyhnositol signaling Cell 63, 459 465
van Megen Y. J B., Klaassen A B. M., R o d n g u e s de Miranda J. F. and Kuopers W. (1988) Chohnerglc musca- rlmc receptors m rat cochlea. Brain Res. 474, 185 188. M u n s o n P. J. and Rodbard D (1980) L I G A N D : a versatile
computerized approach for the characterization of hgand binding systems Anah't &oehem. 107, 220 239 N a t h a n s o n N M. ( 1987i Molecular properties of the musca-
rnnc acetylchohne receptor. A Ret Neuro.s¢'; 10, 195 236.
Nledzlelskl A , O n o T and Schacht J (1992) Chohnerglc regulation of the phospholnositlde second inessenger sys- tem in the guinea pig organ o f C o r t l Hea;. Re~. 59, 250 254
Palazzl E , Fehnska S , Zambelh M , Flsone G., Bartfal T. and Consolo S. (1991~ G a l a m n reduces carbachol stimu- lation o f phospholnosihde turnover m rat ventral hlp- pocampus by lov, ering Ca z~ influx through voltage-sen- sitive Ca 2 + channels. J. Neuroehem. 56, 739 747 Puel J. L and Uzlel A (1987) Correlative development of
cochlear action potential sensitivity, latency, and fie- quency selectivity Dell Brain Res. 37, 179 188. PuJol R (1986) Periods of sensfllvlty to antibiotic treatment
Acta OIolarvng Suppl. 429, 29 33
Rhee S G (1991) lnosltol phosphohpld-~pccllic phos- pholipase C ' interaction of the g a m m a I lSoform with tyroslnc klnase. Trends' Btoehem Set. 16, 297 3111 Rooney T. A and Nahorski S R. ( 19871 Postnatal ontogeny
of agonist and depolarization-reduced phospholnoslhde hydrolysis m rat cerebral cortex. J PharnlaC. evp. Ther. 243, 333 341
Rybak L. P., Whltworth C. and Scott V. (1992) Development of endocochlear potentml and c o m p o u n d achon potential m the rat. Heat Res 59, 189 194.
Schacht J. (1976) Inhibition by neomycin of poly- phospholnositlde turnover m subcellular fi'actlon of guinea-pig cerebral cortex m I';tro. J. ,¥euroe/le.1 27, I 119
1124.
Schacht J (1986) Molecular m e c h a m s m s of drug-reduced hearing loss Hear Res. 22, 297 304.
Simmons D. D., Manson-Gleseke L., ttendNx T W and McCarter S (1990) Reconstructions uf efferent tibers In the postnatal hamster cochlea. Hear. Res. 49, 127 1411. Uzlel A., R o m a n d R and Marot M (1981) Development of
cochlear potentials in rats Audiology 20, 89 100 Whipple M. R and Drescher D. G (1984) Muscarmlc recep-
tol's m the cochlear nucleus and audltorv nerve of the guinea pig J Neurmhem 43, [92 198.
Y a m a m u r a H I and Snyder S. H. (1974) Muscarlmc chol- lnerglc binding m the rat brain. Proc naltt ,4cad Set