Improving image quality of mid-trimester fetal sonography
in obese women: role of ultrasound propagation velocity
B. CHAUVEAU
1,2, C. AUCLAIR
1,3, A. LEGRAND
1,4, R. MANGIONE
5, L. GERBAUD
1,3,
F. VENDITTELLI
1,4, L. BOYER
1,2and D. L ´EMERY
1,41Universit´e Clermont Auvergne, CNRS-UMR 6602, Institut Pascal, Axe TGI, Clermont-Ferrand, France;2P ˆole Radiologie, Centre
Hospitalier Universitaire Gabriel Montpied, Clermont-Ferrand, France;3Service de Sant´e Publique, Centre Hospitalier Universitaire de
Clermont-Ferrand, Clermont-Ferrand, France;4P ˆole Femme Et Enfant, Centre Hospitalier Universitaire de Clermont-Ferrand,
Clermont-Ferrand, France;5Coll`ege Franc¸ais d’Echographie Foetale (CFEF), France
K E Y W O R D S: fetal sonography; image quality; obesity; ultrasound propagation velocity
ABSTRACT
Objective The quality of ultrasound images is impaired
in obese patients. All ultrasound scanners are calibrated for an ultrasound propagation velocity of 1540 m/s, but the propagation in fatty tissue is slower (in the order of 1450 m/s). The main objective of this study was to evaluate the quality of images obtained with different ultrasound propagation velocity settings during the mid-trimester fetal ultrasound examination in obese patients.
Methods This was a cross-sectional study using image
sets of four recommended scanning planes collected from 32 obese pregnant women during their mid-trimester fetal scan. Each image set comprised three images obtained successively at three different propagation velocity settings (1540 m/s, 1480 m/s and 1420 m/s). A panel of 114 experts assessed the quality of 100 image sets, grading them from A (most acceptable) to C (least acceptable). Scanning-plane-specific indicators of adiposity (fatty layer thickness, probe-to-organ distance) were analyzed for each scanning plane.
Results The experts had a mean of 18.1± 10.2 years
of experience. The grade distribution (A, B, C) differed significantly (P < 0.0001) between the three propagation velocity settings tested; at the lower speed of 1480 m/s, images were most often graded A, while at the conventional speed of 1540 m/s, they were most often graded C. Regardless of the scanning plane, the thicker the fatty layer of the abdominal wall in a given plane, the lower the preferred speed (P < 0.0001).
Conclusion The construction of images taking into
account ultrasound propagation velocities lower than 1540 m/s can improve significantly the quality of images obtained during mid-trimester fetal ultrasonography in obese women. Copyright© 2018 ISUOG. Published by John Wiley & Sons Ltd.
Correspondence to: Prof. D. L´emery, P ˆole Femme Et Enfant, H ˆopital Estaing, Centre Hospitalier Universitaire de Clermont-Ferrand, 1 Place
L&R Aubrac, 63003 Clermont-Ferrand Cedex 1, France (e-mail: dlemery@chu-clermontferrand.fr)
Accepted: 5 January 2018
INTRODUCTION
The rising epidemic of obesity affects all age groups and social strata. In the USA, the prevalence of obese women aged 20–39 years rose from 28.4% in 1999 to 31.9% in 20101. A high incidence of obesity is also
observed in Europe, with a prevalence of 10%, ranging from 7.1% in Poland to 20.7% in Scotland2.
In pregnant women, obesity may lead to complica-tions for the mother and infant, into childhood and beyond. Obesity during pregnancy is associated with an increase in the use of healthcare services3 and with
a higher risk of fetal abnormalities4–8. Unfortunately, despite the improvement in ultrasound imaging (e.g. compound and tissue harmonic image processing)9 and even when examinations are repeated10,11, the
assess-ment of fetal anatomy is less thorough in this high medicolegal-risk group12,13, and the ability to detect
malformations decreases as body mass index (BMI) increases13–15.
Ultrasound image construction uses equations that contain the value of the propagation velocity of sound waves; this velocity is assumed conventionally to be constant and equal to 1540 m/s in the human body. Since 1977, all manufacturers of ultrasound scanners have used this value, which was first established in 195016–20. In
fatty tissue, however, the actual propagation velocity is only in the order of 1450 m/s21. Several studies have assessed the effect of propagation velocity on image quality according to the tissue traversed, especially in fatty tissues such as the breast; their findings suggest that taking into account slower propagation speeds would improve the quality of images obtained from fatty tissues22–24. This
topic has not been studied in other, more heterogeneous but largely fatty, propagation media such as in obese patients. Currently, obstetric ultrasound examinations are performed based on a constant propagation velocity of 1540 m/s.
The main objective of this study was to evaluate how taking different ultrasonic propagation velocities into account during image construction can influence the intrinsic quality of the image, in terms of sharpness and precision, in obese women during the second trimester of pregnancy. The secondary objective was to assess this influence according to different fetal organs and their tissue characteristics, analyzing whether adiposity and characteristics of the maternal abdominal wall could affect the choice between the conventional velocity of 1540 m/s and lower velocities, in order to obtain the most clinically acceptable image quality.
PATIENTS AND METHODS Study design
This descriptive cross-sectional study took place from 1 June to 15 July 2015, using data collected previously. The protocol was submitted to the appropriate French ethics committee, who determined that the study was not subject to French laws on biomedical research and did not require specific approval.
Scanning planes from 32 obese pregnant women under-going mid-trimester fetal ultrasound were collected for the study. These transabdominal ultrasound assessments were performed using an Aixplorer™ ultrasound machine (SuperSonic Imagine, Aix-en-Provence, France) with a curvilinear probe of 1–6-MHz and without elevation of the panniculus, if present. Per manufacturer’s spec-ifications, the machine offers four presets to take into account the following ultrasound propagation velocities: 1420 m/s, 1480 m/s, 1540 m/s and 1600 m/s. Two physi-cians (B.C. and D.L.) performed all ultrasound examina-tions and acquired the images between 1 December 2014 and 25 May 2015, at the Clermont-Ferrand University Hospital Center, France, and consensually validated them to standardize the selection. Both operators participated simultaneously in the examinations, one holding the probe and both observing the screen.
The images were obtained during the standard mid-trimester ultrasound examination between the 18th
and 26th weeks of gestation in adult women who were
obese according to the definition of the USA National Heart, Lung and Blood Institute, based on a prepregnancy BMI of ≥ 3013,14,25–27. We used no images showing
fetal malformations or abnormal volume of amniotic fluid. The women were informed that photographs of the images from the fetal scan would be recorded and potentially used in this study; we did not use images from women who objected.
Ultrasound images were obtained according to national and international guidelines28–31. We selected images from four fetal-specific scanning planes with different tissue characteristics that are considered to be difficult to obtain in obese women12: (1) the transverse plane of the biparietal diameter through the subarachnoid space, Sylvian fissure and both thalami; (2) the transverse four-chamber view of the heart; (3) the transverse abdominal plane showing the kidneys; and (4) the longitudinal plane used for femur-length measurement. Each of these four views was acquired in each patient at three different sound-velocity settings over the briefest possible period (a few seconds). The three images in each set were therefore identical except for the propagation velocity (Figure 1). Of the velocity presets available on the ultrasound machine, we chose to evaluate the conventional velocity of 1540 m/s, and the velocities 1480 m/s and 1420 m/s. All other setting parameters were identical between the three images in each set. The sequence was excluded if there was any fetal movement between two successive images in a set. Twenty sets of each of the four planes were thus obtained (i.e. 80 sets) from the 32 women. Moreover, five sets of each of the four planes (i.e. 20 sets) were duplicated randomly to assess intraobserver agreement. Therefore, 100 sets were available for assessment by the expert panel.
The following information about the mothers was collected during the examination: maternal prepregnancy weight and height, and gestational age at examination. During the examination, we measured ‘standardized’ indicators of adiposity for each patient at a point midway between the umbilicus and the pubic symphysis, i.e. the
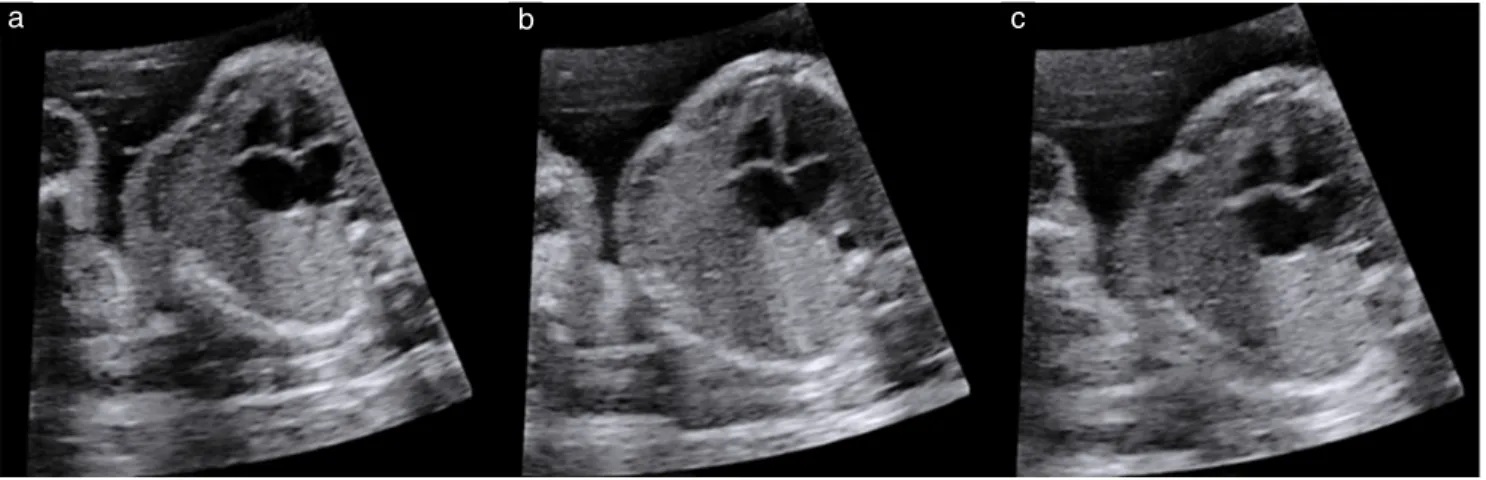
Figure 1 Set of three images of four-chamber view of fetal heart obtained at ultrasound propagation velocity settings of 1420 m/s (a),
1480 m/s (b) and 1540 m/s (c) in an obese woman during mid-trimester examination. All setting parameters apart from propagation velocity were identical between images, which were obtained successively within a few seconds.
subumbilical fatty layer thickness of the abdominal wall (SU-FLT) and the subumbilical probe-to-amniotic fluid distance (SU-PAFD).
In each organ-specific scanning plane, the fatty layer thickness (SSP-FLT) and the specific scanning plane probe-to-organ distance (SSP-POD) were measured as ‘scanning-plane-specific’ indicators of adiposity. In each of the four views, the organ landmarks for the SSP-POD measurement were defined as: the midline in the encephalic plane, the crossing of the interatrial and interventricular septa in the four-chamber view of the heart, the renal pelvis closest to the probe in the transverse abdominal plane through the kidneys and the midpoint of the femur in the longitudinal plane. These thickness measurements were performed by ultrasound using the preset propagation velocity of 1540 m/s.
All images were anonymized and exported from the hard drive of the ultrasound scanner in lossless JPEG format (720× 680 dpi). They were then treated using Photofiltre 7.0 software (Antonio Da Cruz, France) only to delete the velocity setting information usually displayed on the screenshot. The image sets were ordered randomly, as was the position of each of the three images in each set. These random distributions were established using the RAND and secondarily the IRR (in increasing order) functions of Microsoft Excel 2013 (Microsoft, Redmond, WA, USA).
The group of raters was composed of medical doctors who were members of the French College of Fetal Ultrasound, and certified to perform fetal ultrasound. They were asked by e-mail to participate in the study. Their participation, from 1 June to 15 July 2015, was voluntary, anonymous and unremunerated.
The 100 sets of images were assembled into an online questionnaire (accessible only on computer and not on mobile devices) using Limesurvey™ software, Version 1.92+ Build 120 718 (LimeSurvey Project Team, Carsten Schmitz, Hamburg, Germany). The duration of the connection necessary to complete the questionnaire (i.e. duration of participation) was recorded. Only questionnaires fully completed during a single online session were included.
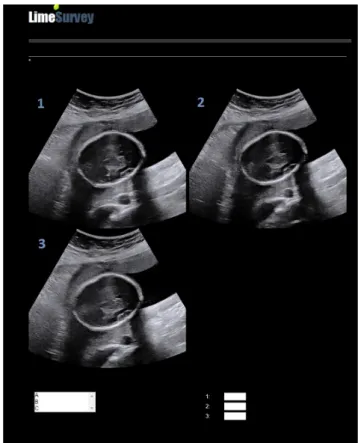
The anonymous questionnaire had two parts. The first part provided short guidance about the criteria of judgment of a picture (i.e. ‘how informative is the image regardless of any anatomical considerations’) and collected demographic data about the participating raters (age, sex, time since certification). The second part pre-sented the 100 image sets one at a time; all three images of each set appeared simultaneously on the screen (Figure 2). Raters, blinded to the propagation velocity settings, were asked to grade the quality of the three images in each set by ranking them from A (most acceptable) to C (least acceptable) and validating the grade. Each validation was final, and it was not possible to go back to any image. Statistical analysis
Quantitative data were expressed as mean± SD; all vari-ables were tested for normality (Kolmogorov–Smirnov
Figure 2 Screenshot of online questionnaire image. Raters were
asked to rank from A (most acceptable) to C (least acceptable) quality of each of three images (1,2,3) in same ultrasound plane acquired at three different ultrasound propagation velocity settings.
and Q-Q plots). Qualitative data were expressed as n (%) with contingency tables. Intrarater agreement was assessed using the kappa coefficient, good intrarater agree-ment (consistency) being defined as kappa≥ 0.532.
The distributions of the A, B and C grades according to the three velocities tested were compared using the chi-square test. Acceptable images (i.e. A and B) were merged together and compared with the less acceptable (i.e. C) in order to assess the secondary objectives. Conventional ultrasound velocity (1540 m/s) was compared with both non-conventional lower velocities (1420 m/s and 1480 m/s). Both groups of velocity were related to mater-nal BMI and each ultrasound measurement of adiposity (i.e. SU-FLT, SU-PAFD, SSP-FLT and SSP-POD) using Student’s t-test or the non-parametric Mann–Whitney
U-test.
Statistical significance was set at P≤ 0.05, and Bonferroni correction was used to adjust for post-hoc multiple testing33. SAS software version 9.4 (SAS Inc.,
Cary, NC, USA, 2002–2010) was used for analysis.
RESULTS
Obtaining 80 exploitable image sets required 32 patients, whose characteristics with respect to adiposity are shown in Table 1.
The questionnaire was completed by 114 raters with a mean time since certification of 18.1± 10.2 years. Mean
age of the raters was 49.8± 10.6 years, and 67.5% of them were women. Mean time spent rating the 100 sets of images was 55± 33.4 min. Mean intrarater agreement (kappa) was 0.35± 0.19. Thirty-five raters had a kappa ≥ 0.5 and could therefore be considered as consistent; their characteristics did not differ from those of the other 79 raters with respect to sex (P= 0.55), time since certification (P= 0.30) or time spent on completing the questionnaire (P= 0.62).
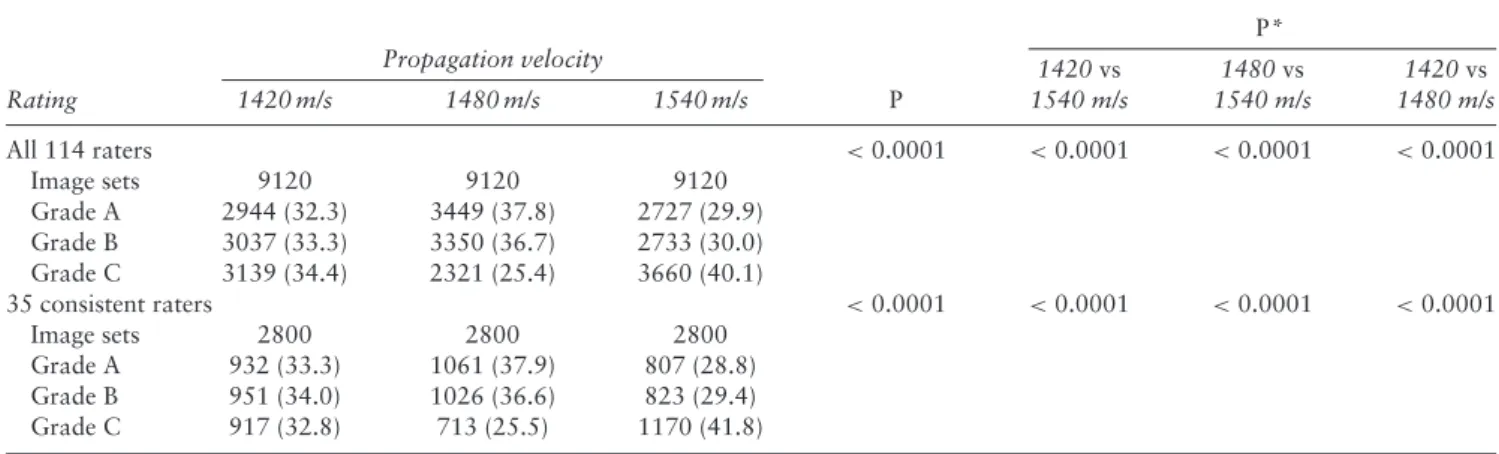
The distribution of A, B and C Grades, attributed by all 114 raters to each of the three presets of ultrasound propagation velocity evaluated, showed a significant difference in favor of 1480 m/s (P < 0.0001); the same was observed when considering the results of the 35 consistent raters (P < 0.0001) (Table 2).
Rating of image quality by the 35 consistent raters for each ultrasound velocity according to each specific scanning plane evaluated is presented in Table 3. Analysis of the distribution showed a significant difference in favor of a propagation velocity of 1480 m/s for the encephalic and cardiac images and of 1420 m/s for the femur (P < 0.0001 for all tests performed). No significant difference was observed between the three propagation velocities for the abdominal plane at the level of the kidneys.
Considering standardized indicators of adiposity, regardless of the scanning plane evaluated, images classified as acceptable (Grade A or B) by the 35 consistent Table 1 Adiposity characteristics of 32 obese patients undergoing
mid-trimester ultrasound examination
Standardized indicators of adiposity
GA (weeks) BMI (kg/m2) SU-FLT (mm) SU-PAFD (mm) 23.2± 1.8 40.5± 5.0 46.7± 11.4 76.9± 23.0 (18.9–26.4) (30.1–51.6) (14.0–67.0) (22.0–120.0)
Data are presented as mean± SD (range). BMI, body mass index (prepregnancy); GA, gestational age; SU-FLT, subumbilical fatty layer thickness; SU-PAFD, subumbilical probe-to-amniotic fluid distance.
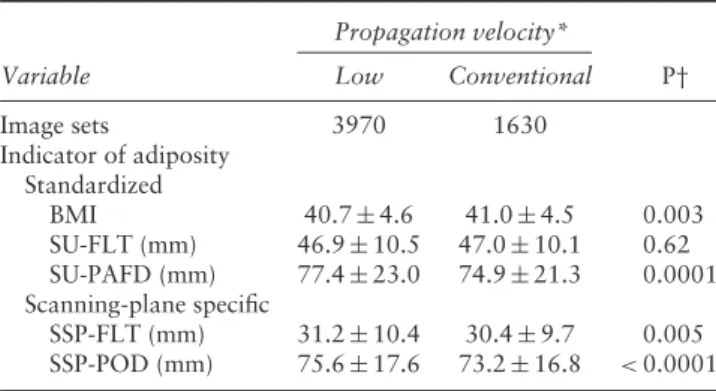
raters were obtained more often using lower velocities rather than the conventional velocity in cases with higher SU-PAFD (P= 0.0001), but no significant difference was observed with respect to SU-FLT (Table 4). When taking into account scanning-plane-specific indicators of adiposity, i.e. SSP-FLT and SSP-POD, the preference for images obtained using a lower velocity, compared with the conventional velocity, was also significant (P= 0.005 and P < 0.0001, respectively).
Regarding visualization of each organ, the same trend, favorable to images obtained using lower velocities, was observed when taking into account scanning-plane-specific indicators of adiposity (i.e. SSP-FLT and SSP-POD) for biparietal diameter (for SSP-FLT only), kidneys and femur, but not for the heart. For standardized indicators of adiposity, the trends were heterogeneous (Table 5).
DISCUSSION
The principal objective of this study was to assess the influence of considering different propagation velocities of ultrasound on the intrinsic quality of ultrasound images, in terms of sharpness and precision, obtained during the second trimester of pregnancy in obese women. Our results, based on 27 360 image ratings, show clearly that velocity settings lower than the conventional velocity of 1540 m/s significantly improve image quality in obese women.
Slower velocities improve visualization of the fetal brain and heart, which are usually difficult to observe in this population9,12, and of the femur, which requires clear
edge definition. Such improvement is not observed for the kidney, a structure that remains more difficult to image whatever the woman’s BMI34.
Standardized maternal indicators of adiposity are not relevant for guiding the choice of an optimal ultrasound velocity in obese women. On the other hand, scanning-plane-specific indicators of adiposity seem to be relevant for selecting a velocity that is able to provide optimal image quality for the plane under consideration. Table 2 Rating of image quality of 80 sets of images of any scanning plane obtained using three different ultrasound propagation velocity
settings, by all 114 raters and by subgroup of 35 raters with good intrarater agreement
P* Propagation velocity Rating 1420 m/s 1480 m/s 1540 m/s P 1420 vs 1540 m/s 1480 vs 1540 m/s 1420 vs 1480 m/s All 114 raters <0.0001 <0.0001 <0.0001 <0.0001 Image sets 9120 9120 9120 Grade A 2944 (32.3) 3449 (37.8) 2727 (29.9) Grade B 3037 (33.3) 3350 (36.7) 2733 (30.0) Grade C 3139 (34.4) 2321 (25.4) 3660 (40.1) 35 consistent raters <0.0001 <0.0001 <0.0001 <0.0001 Image sets 2800 2800 2800 Grade A 932 (33.3) 1061 (37.9) 807 (28.8) Grade B 951 (34.0) 1026 (36.6) 823 (29.4) Grade C 917 (32.8) 713 (25.5) 1170 (41.8)
Table 3 Rating, by 35 raters with good intrarater agreement, of image quality of 80 sets of images obtained using three different ultrasound
propagation velocity settings, according to scanning plane
P* Propagation velocity Scanning plane/ Grade 1420 m/s 1480 m/s 1540 m/s P 1420 vs 1540 m/s 1480 vs 1540 m/s 1420 vs 1480 m/s
Transverse encephalic plane of BPD through subarachnoid space and both thalami
Image sets 700 700 700 <0.0001 0.001 <0.0001 <0.0001
Grade A 159 (22.7) 328 (46.9) 213 (30.4)
Grade B 249 (35.6) 255 (36.4) 196 (28.0)
Grade C 292 (41.7) 117 (16.7) 291 (41.6)
Four-chamber view of heart
Image sets 700 700 700 <0.0001 <0.0001 <0.0001 <0.0001
Grade A 188 (26.9) 297 (42.4) 215 (30.7)
Grade B 269 (38.4) 258 (36.9) 173 (24.7)
Grade C 243 (34.7) 145 (20.7) 312 (44.6)
Transverse abdominal plane through kidneys
Image sets 700 700 700 0.23 — — —
Grade A 225 (32.1) 231 (33.0) 244 (34.9)
Grade B 256 (36.6) 225 (32.1) 219 (31.3)
Grade C 219 (31.3) 244 (34.9) 237 (33.9)
Longitudinal plane used to measure femur length
Image sets 700 700 700 <0.0001 <0.0001 <0.0001 <0.0001
Grade A 360 (51.4) 205 (29.3) 135 (19.3)
Grade B 177 (25.3) 288 (41.1) 235 (33.6)
Grade C 163 (23.3) 207 (29.6) 330 (47.1)
Data are presented as n or n (%). *Significant P after adjustment for multiple comparisons (Bonferroni correction) < 0.017. BPD, biparietal diameter.
Table 4 Ultrasound propagation velocity range preference
according to indicators of adiposity, based on images rated as ‘acceptable’ (Grade A or B) by 35 consistent raters, in all scanning planes combined
Propagation velocity*
Variable Low Conventional P†
Image sets 3970 1630 Indicator of adiposity Standardized BMI 40.7± 4.6 41.0± 4.5 0.003 SU-FLT (mm) 46.9± 10.5 47.0± 10.1 0.62 SU-PAFD (mm) 77.4± 23.0 74.9± 21.3 0.0001 Scanning-plane specific SSP-FLT (mm) 31.2± 10.4 30.4± 9.7 0.005 SSP-POD (mm) 75.6± 17.6 73.2± 16.8 <0.0001
Data are presented as n or mean± SD. *Low velocity = 1420 m/s or 1480 m/s; conventional velocity= 1540 m/s. †P after adjustment for multiple comparisons (Bonferroni correction) < 0.017. BMI, body mass index; SSP-FLT, specific scanning plane fatty layer thickness; SSP-POD, specific scanning plane probe-to-organ distance; SU-FLT, subumbilical fatty layer thickness; SU-PAFD, subumbilical probe-to-amniotic fluid distance.
In fact, standardized indicators are approximate markers of adiposity whereas scanning-plane-specific indicators reflect the actual anatomical distribution of fat in the locations in which the ultrasound probe operates.
To our knowledge, no study has examined the influence of the ultrasound propagation velocity setting parameter in optimizing sonographic imaging in obese women, whether pregnant or not. Several studies have attempted to estimate ultrasound propagation velocity in tissues from algorithms of reconstruction22,24,35–38, but none
as a function of image quality or with such a large representative panel of experienced raters.
In the present study, 1540 m/s was chosen as the universally accepted conventional ultrasound propagation velocity18, while 1420 m/s and 1480 m/s were selected from the available presettings of the ultrasound scanner because they are close to the velocity of ultrasound in fatty tissues (1430–1470 m/s), such as breast tissue22–24,29.
In contrast to studies intended to assess the anatomical quality of a scanning plane40, and given the lack of a
published score enabling an objective assessment of the intrinsic quality of images, our study was based on sub-jective qualitative assessment by raters of images whose quality on the computer screen remained unchanged for the entire questionnaire period, which took place in a sin-gle session. Moreover, the raters’ judgment was inevitably based on still images, which reflect imperfectly what the eyes perceive during a real-time ultrasound examination. On the other hand, in order to avoid any heterogeneity in the quality of the acquired images, both operators par-ticipated simultaneously in all ultrasound examinations, one holding the probe and both watching the screen.
The quality of ultrasound images constructed by the ultrasound software does not depend only on insonation depth, energy absorption and dispersion of the ultrasound beam9; it is also governed in part by a distance–duration
relationship according to the equation Z= cT/2 (where Z= depth, c = velocity of ultrasound propagation in a homogeneous medium and T= duration of the round trip of the wave between its source and target). Accordingly, construction of an image by the scanner based on the conventional velocity of 1540 m/s when the actual
Table 5 Ultrasound propagation velocity range preference
according to indicators of adiposity, based on images rated as ‘acceptable’ (Grade A or B) by 35 consistent raters, for each scanning plane
Propagation velocity* Scanning plane/
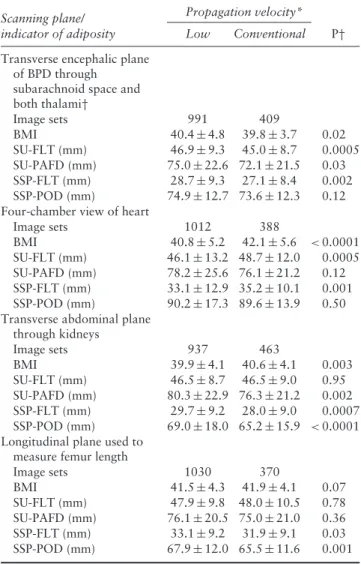
indicator of adiposity Low Conventional P† Transverse encephalic plane
of BPD through subarachnoid space and both thalami† Image sets 991 409 BMI 40.4± 4.8 39.8± 3.7 0.02 SU-FLT (mm) 46.9± 9.3 45.0± 8.7 0.0005 SU-PAFD (mm) 75.0± 22.6 72.1 ± 21.5 0.03 SSP-FLT (mm) 28.7± 9.3 27.1± 8.4 0.002 SSP-POD (mm) 74.9± 12.7 73.6 ± 12.3 0.12
Four-chamber view of heart
Image sets 1012 388 BMI 40.8± 5.2 42.1± 5.6 < 0.0001 SU-FLT (mm) 46.1± 13.2 48.7 ± 12.0 0.0005 SU-PAFD (mm) 78.2± 25.6 76.1 ± 21.2 0.12 SSP-FLT (mm) 33.1± 12.9 35.2 ± 10.1 0.001 SSP-POD (mm) 90.2± 17.3 89.6 ± 13.9 0.50
Transverse abdominal plane through kidneys Image sets 937 463 BMI 39.9± 4.1 40.6± 4.1 0.003 SU-FLT (mm) 46.5± 8.7 46.5± 9.0 0.95 SU-PAFD (mm) 80.3± 22.9 76.3 ± 21.2 0.002 SSP-FLT (mm) 29.7± 9.2 28.0± 9.0 0.0007 SSP-POD (mm) 69.0± 18.0 65.2 ± 15.9 < 0.0001
Longitudinal plane used to measure femur length
Image sets 1030 370 BMI 41.5± 4.3 41.9± 4.1 0.07 SU-FLT (mm) 47.9± 9.8 48.0 ± 10.5 0.78 SU-PAFD (mm) 76.1± 20.5 75.0 ± 21.0 0.36 SSP-FLT (mm) 33.1± 9.2 31.9± 9.1 0.03 SSP-POD (mm) 67.9± 12.0 65.5 ± 11.6 0.001
Data presented as n or mean± SD. *Low velocity = 1420 m/s or 1480 m/s; conventional velocity= 1540 m/s. †Significant P after adjustment for multiple comparisons (Bonferroni correction)
<0.017. BMI, body mass index; BPD, biparietal diameter; SSP-FLT, specific scanning plane fatty layer thickness; SSP-POD, specific scanning plane probe-to-organ distance; SU-FLT, subumbilical fatty layer thickness; SU-PAFD, subumbilical probe-to-amniotic fluid distance.
velocity of propagation in the tissue studied is slower, leads to discrepancies in the distances measured, thus the reconstructed image of the target is represented at a site and scale different from reality22. Speckling
is increased and lateral resolution and contrast are poor, so that a punctate object appears as a segment (‘moustache effect’)22. Accordingly, correction of the
ultrasound velocity in imaging of the breast (mainly composed of fat) improves image quality22–24,29. This tool
for correcting image degradation appears to be useful9;
indeed, part of the round trip journey of the ultrasound wave takes place in an environment in which the velocity of propagation is low (i.e. fat), whereas in the other part it is conventional (i.e. muscle and other soft tissues). This heterogeneous constitution of the path traveled by
the ultrasound wave explains why an image construction based solely on an ultrasound propagation velocity of 1540 m/s cannot provide an optimal image when fatty tissue represents a large part of its path.
These physical facts of tissue-related propagation velocity of ultrasound also address questions about biometry and morphology, based on the old consensus of a conventional velocity of 1540 m/s. What margin of error is acceptable in measurements if they are distorted because they are based on an inaccurate propagation velocity? This phenomenon is obviously marginal for measurements in the order of centimeters, but it is probably more significant for measurements at the precision level of a tenth of a mm, such as first-trimester nuchal translucency28. This issue is compounded by the
impairment of lateral resolution, which increases the difficulty of assessing criteria such as caliper placement, when the observed structures appear with fuzzy edges. This may, at least partly, explain the small but significant positive relationship observed between maternal weight and nuchal translucency thickness41.
Obstetric ultrasound assessment of obese patients is associated with high medicolegal risk, because of a higher rate of malformations in addition to impaired screening and diagnosis in these patients4–8,12–15. Any tool that
improves image sharpness and precision in this population is therefore a major improvement.
This study was conducted in the context of obstetric ultrasound, which is a well-standardized practice28–31.
Although no comparable studies have been published, we feel that our results could be extrapolated to other less standardized ultrasound domains, such as abdominal ultrasound.
Finally, our study shows that the ability to adapt ultrasound velocity to the thickness of the fatty layer and the probe-to-organ distance in any given scanning plane is a major advantage in an obese population. Modeling the velocity as a function of these scanning-plane-specific indicators of adiposity would be helpful in developing algorithms offering more relevant settings. Validation of the homogeneity of the biometric measurements according to the ultrasound propagation velocity remains an unanswered issue.
In conclusion, construction of a sonographic image using the conventional propagation velocity of ultrasound waves of 1540 m/s is not the most appropriate technique for the mid-trimester fetal scan in obese pregnant women, therefore, the option of using a lower velocity setting would be helpful for improving image quality and sharpness in such cases.
REFERENCES
1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012; 307: 491–497.
2. EURO-PERISTAT P. European Perinatal Health Report 2010. Health and Care of Pregnant Women and Babies in Europe in 2010. http://www.europeristat.com/ reports/european-perinatal-health-report-2010.html (accessed 5 June 2015). 3. Chu SY, Bachman DJ, Callaghan WM, Whitlock EP, Dietz PM, Berg CJ,
O’Keeffe-Rosetti M, Bruce FC, Hornbrook MC. Association between obesity during pregnancy and increased use of health care. N Engl J Med 2008; 358: 1444–1453.
4. Stothard KJ, Tennant PWG, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009; 301: 636–650.
5. Nuthalapaty FS, Rouse DJ. The impact of obesity on obstetrical practice and outcome. Clin Obstet Gynecol 2004; 47: 898–913; discussion, 980–981.
6. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015; 16: 621–638.
7. Shaw GM, Velie EM, Schaffer D. Risk of neural tube defect-affected pregnancies among obese women. JAMA 1996; 275: 1093–1096.
8. Werler MM, Louik C, Shapiro S, Mitchell AA. Prepregnant weight in relation to risk of neural tube defects. JAMA 1996; 275: 1089–1092.
9. Paladini D. Sonography in obese and overweight pregnant women: clini-cal, medicolegal and technical issues. Ultrasound Obstet Gynecol 2009; 33: 720–729.
10. Hendler I, Blackwell SC, Bujold E, Treadwell MC, Wolfe HM, Sokol R, Sorokin Y. The impact of maternal obesity on midtrimester sonographic visualization of fetal cardiac and craniospinal structures. Int J Obes Relat Metab Disord 2004; 28: 1607–1611.
11. Hendler I, Blackwell SC, Bujold E, Treadwell MC, Mittal P, Sokol RJ, Sorokin Y. Suboptimal second-trimester ultrasonographic visualization of the fetal heart in obese women: should we repeat the examination? J Ultrasound Med 2005; 24: 1205–1209; quiz, 1210–1211.
12. Fuchs F, Houllier M, Voulgaropoulos A, Levaillant J-M, Colmant C, Bouyer J, Senat MV. Factors affecting feasibility and quality of second-trimester ultrasound scans in obese pregnant women. Ultrasound Obstet Gynecol 2013; 41: 40–46.
13. Dashe JS, McIntire DD, Twickler DM. Effect of maternal obesity on the ultrasound detection of anomalous fetuses. Obstet Gynecol 2009; 113: 1001–1007. 14. Dashe JS, McIntire DD, Twickler DM. Maternal obesity limits the ultrasound
evaluation of fetal anatomy. J Ultrasound Med 2009; 28: 1025–1030.
15. Thornburg LL, Miles K, Ho M, Pressman EK. Fetal anatomic evaluation in the overweight and obese gravida. Ultrasound Obstet Gynecol 2009; 33: 670–675. 16. Ludwig GD. The Velocity of Sound through Tissues and the Acoustic Impedance of
Tissues. J Acoust Soc Am 1950; 22: 862–866.
17. Szabo TL. Introduction. In Diagnostic Ultrasound Imaging: Inside Out (2ndedn).
Szabo TL (ed). Academic Press: Boston, MA, USA, 2014; 1–37.
18. Wells PNT. Biomedical Ultrasonics. Academic Press: London, UK, 1977; 120–125. 19. Nelson TR, Pretorius DH. The Doppler signal: where does it come from and what
does it mean? AJR Am J Roentgenol 1988; 151: 439–447.
20. Jensen JA. Medical ultrasound imaging. Prog Biophys Mol Biol 2007; 93: 153–165. 21. Wells PNT. Ultrasonic imaging of the human body. Rep Prog Phys 1999; 62:
671–722.
22. Napolitano D, Chou CH, McLaughlin G, Ji TL, Mo L, DeBusschere D, Steins R. Sound speed correction in ultrasound imaging. Ultrasonics 2006; 44 (Suppl 1): e43–46.
23. Piccoli CW, Forsberg F. Advanced ultrasound techniques for breast imaging. Semin Roentgenol 2011; 46: 60–67.
24. Barr RG, Rim A, Graham R, Berg W, Grajo JR. Speed of sound imaging: improved image quality in breast sonography. Ultrasound Q 2009; 25: 141–144.
25. Davies GAL, Maxwell C, McLeod L, Gagnon R, Basso M, Bos H, Delisle MF, Farine D, Hudon L, Menticoglou S, Mundle W, Murphy-Kaulbeck L, Ouellet A, Pressey T, Roggensack A, Leduc D, Ballerman C, Biringer A, Duperron L, Jones D, Lee LS, Shepherd D, Wilson K; Society of Obstetricians and Gynaecologists of Canada. SOGC Clinical Practice Guidelines: Obesity in pregnancy. No. 239, February 2010. Int J Gynaecol Obstet 2010; 110: 167–173.
26. Wolfe HM, Sokol RJ, Martier SM, Zador IE. Maternal obesity: a potential source of error in sonographic prenatal diagnosis. Obstet Gynecol 1990; 76: 339–342. 27. Khoury FR, Ehrenberg HM, Mercer BM. The impact of maternal obesity on
satisfactory detailed anatomic ultrasound image acquisition. J Matern Fetal Neonatal Med 2009; 22: 337–341.
28. American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med 2013; 32: 1083–1101.
29. Cargill Y, Morin L; DIAGNOSTIC IMAGING COMMITTEE. Content of a complete routine second trimester obstetrical ultrasound examination and report. J Obstet Gynaecol Can 2009; 31: 272–275.
30. Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez-Andrade E, Johnsen SL, Kalache K, Leung KY, Malinger G, Munoz H, Prefumo F, Toi A, Lee W; ISUOG Clinical Standards Committee. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2011; 37: 116–126. 31. Viossat P, Ville Y, Bessis R, Jeny R, Nisand I, Teurnier F, Coquel P, Lansac J. [Report of the French Comit´e national technique de l’´echographie de d´epistage pr´enatal (CNTEDP) – Recommendations for second line prenatal ultrasound]. Gynecol Obstet Fertil 2014; 42: 51–60.
32. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960; 20: 27–46.
33. Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ 1995; 310: 170.
34. Bowie JD, Rosenberg ER, Andreotti RF, Fields SI. The changing sonographic appearance of fetal kidneys during pregnancy. J Ultrasound Med 1983; 2: 505–507. 35. Cho MH, Kang LH, Kim JS, Lee SY. An efficient sound speed estimation method to enhance image resolution in ultrasound imaging. Ultrasonics 2009; 49: 774–778. 36. Qu X, Azuma T, Liang JT, Nakajima Y. Average sound speed estimation using
speckle analysis of medical ultrasound data. Int J Comput Assist Radiol Surg 2012; 7: 891–899.
37. Yoon C, Kang J, Han S, Yoo Y, Song TK, Chang JH. Enhancement of photoacoustic image quality by sound speed correction: ex vivo evaluation. Opt Express 2012; 20: 3082–3090.
38. Atlas de la d´emographie m´edicale 2015 — Conseil National de l’Ordre des M´edecins. http://www.conseil-national.medecin.fr/node/1607 (accessed 17 August 2015). 39. Chen Q, Zagzebski JA. Simulation study of effects of speed of sound and attenuation
on ultrasound lateral resolution. Ultrasound Med Biol 2004; 30: 1297–1306. 40. McLennan A, Schluter PJ, Pincham V, Hyett J. First-trimester fetal nasal bone audit:
evaluation of a novel method of image assessment. Ultrasound Obstet Gynecol 2009; 34: 623–628.
41. Cowans NJ, Spencer K. The relationship between maternal body mass, smoking status and ethnicity and first trimester nuchal translucency thickness. Prenat Diagn 2011; 31: 446–449.