HAL Id: hal-03218118
https://hal.sorbonne-universite.fr/hal-03218118
Submitted on 5 May 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
ASSEMBLAGES AND FRUSTULE MORPHOLOGY
IN INDOOR MICROCOSMS
F Rimet, L Ector, A Dohet, H Cauchie
To cite this version:
F Rimet, L Ector, A Dohet, H Cauchie. IMPACTS OF FLUORANTHENE ON DIATOM
ASSEM-BLAGES AND FRUSTULE MORPHOLOGY IN INDOOR MICROCOSMS. Vie et Milieu / Life &
Environment, Observatoire Océanologique - Laboratoire Arago, 2004, pp.145-156. �hal-03218118�
IMPACTS OF FLUORANTHENE ON DIATOM ASSEMBLAGES
AND FRUSTULE MORPHOLOGY IN INDOOR MICROCOSMS
F. RIMET, L. ECTOR, A. DOHET, H.M. CAUCHIE
Public Research Center – Gabriel Lippmann, avenue de la Faïencerie, 1511 Luxembourg, Grand-Duchy of Luxembourg rimet@crpgl.lu BENTHIC DIATOMS ECOTOXICOLOGY FLUORANTHENE MICROCOSM PAH TERATOLOGICAL FORMS
ABSTRACT. – Polycyclic aromatic hydrocarbons (PAH) such as fluoranthene are frequently detected in rivers. The impact of these compounds on benthic algae is hardly known, especially concerning freshwater diatoms. To study the ecotoxicity of fluoranthene, benthic diatom communities collected from an unpolluted stream in Luxembourg were exposed to two fluoranthene concentrations in microcosms under growth conditions near to the conditions encountered in the stream: a mode-rate fluoranthene concentration of 0.05µg.l–1 and a high concentration of 16.46µg.l–1. With a moderate concentration, fluoranthene induced modifications of the assemblages and no frustule deformities were observed. With a high fluoran-thene concentration, modifications of diatom assemblages occurred and teratologi-cal forms were observed for most of the taxa. In both experiments fluoranthene sensitive and tolerant taxa could be identified.
DIATOMÉES BENTHIQUES ÉCOTOXICOLOGIE FLUORANTHÈNE MICROCOSME FORMES TÉRATOLOGIQUES HAP
RÉSUMÉ. – Les hydrocarbures aromatiques polycycliques tels que le fluoranthène sont souvent détectés dans les cours d’eau. L’impact de ces produits sur les algues benthiques est peu connu, surtout concernant les Diatomées benthiques. Pour étu-dier l’écotoxicité du fluoranthène, des communautés de Diatomées benthiques ré-coltées dans un ruisseau non pollué au Luxembourg ont été exposées à deux concentrations de fluoranthène en microcosmes dans des conditions de croissance proches de celles rencontrées dans le ruisseau: une concentration modérée de 0,05µg.l–1et une concentration forte de 16,46µg.l–1. Avec une concentration mo-dérée, le fluoranthène a induit des modifications des assemblages mais aucune dé-formation des frustules n’a été observée. Avec une forte concentration de fluoranthène, des modifications des assemblages de Diatomées et des formes térato-logiques ont été observées pour la plupart des taxons. Pour les deux expériences, les taxons sensibles et tolérants au fluoranthène ont pu être identifiés.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAH) and more particularly fluoranthene are frequently de-tected pollutants in rivers. These compounds natu-rally arise from volcanoes and forest fires, but their main sources are artificial. Fluoranthene is usually produced by incomplete combustion of petroleum products in municipal incinerators. It is emitted from coal and gasoline engines (Verschueren 1983). It has a high molecular weight and a rather low solubility in water; 0.265 ppm according to Irwin et al. (1997). In the year 2000, concentra-tions of circa 0.01µg.l–1 fluoranthene were de-tected 9 times and concentrations of 0.02µg.l–1and higher (maximum 0.12µg.l–1) 2 times in a total of 42 samples collected in the rivers of Luxembourg (Ministère de l’Environnement du Grand-Duché de
Luxembourg 2001). In wastewater and urban run-off even concentrations of 12µg.l–1 and 130µg.l–1 respectively, were reported (Irwin et al. 1997). Whereas in general fluoranthene concentrations in surface waters of rivers are near the detection lim-its (Irwin et al. 1997). When released in natural water, fluoranthene is quickly adsorbed to particles in the water column and to sediments, and is also rapidly bio-concentrated into aquatic organisms (Irwin et al. 1997, Sirota et al. 1983).
Algae and in particular diatoms constitute the essential of the photosynthetic biomass in many watercourses. Pesticide effects on benthic and planktonic algal communities, in particular those of atrazine (Solomon et al. 1996, Guasch et al. 1998, Bérard et al. 1999, Seguin et al. 2001, Leboulanger et al. 2001) or those of metals (Peres
et al. 1997, Ivorra 2000, Gold 2002) are quite well
known. In ecotoxicological studies the effects of
fluoranthene on algae are mainly expressed as acute effect concentrations such as LOEC (LOwest Observed Effective Concentration for a given pa-rameter) (Bastian & Toetz 1982) and EC50 (Effec-tive Concentration of toxicant reducing 50% of a given parameter) values (Irwin et al. 1997). How-ever, this study incorporates less traditional param-eters to describe the effects of fluoranthene to natural benthic algal communities using micro-cosm tests mimicking watercourses. Firstly, modi-fication of the structure of benthic diatom assemblage was used to characterize the toxicant effect (Dorn et al. 1997, Mundie et al. 1991, Perrin
et al. 1992). Secondly, sensitive and resistant
dia-tom taxa to fluoranthene exposure were identified. And thirdly, the presence of teratological forms was used as an indication of chemical stress (McFarland et al. 1997, Stevenson & Bahls 1999).
MATERIAL AND METHODS
Study site: For this experiment, an unpolluted stream stretch was selected in order to test fluoranthene effects on benthic algae that had never been exposed to this pol-lutant. In this framework, several sites from headwater streams showing minimal human disturbances were re-tained. Physical and chemical parameters were measured and diatoms were sampled. The best reference site ap-peared to be the Rollingerbaach stream near Rollingen (6o7’32” longitude, 49o44’3” latitude): parameters cha-racterizing pollution were very low; concentrations of PAH were below the detection limits of 0.01µg.l–1 (de-tection by HPLC, APHA 1995). Samples of benthic dia-toms were taken during 2002 and diatom indices SPI (Specific Polluosensitivity Index, Coste in Cemagref 1982) confirmed the high biological water quality of this site. This stream was characterized by a width of 2 me-ters and a depth of about 20 cm. During all the year dia-tom assemblages were dominated by Achnanthidium
biasolettianum (Grunow) Round & Bukhtiyarova, A. mi-nutissimum (Kützing) Czarnecki, Amphora pediculus
(Kützing) Grunow, Encyonema minutum (Hilse) Mann and Gomphonema pumilum (Grunow) Reichardt et Lange-Bertalot.
Artificial substrate description: Nine glass staining trays containing 10 slides each (size 76× 26 mm) were placed in a plastic cage, the slides were in a vertical po-sition, perpendicularly to the current and elevated from the bottom of the cage by a wire mesh to avoid sediment deposits. The plastic cage weighted by pavements was immersed into the stream and was left in the site during 10 days for colonisation. Hoagland et al. (1982) demons-trated that a period of about 2 weeks is sufficient to pro-duce algal developments on artificial substrates. But this colonisation time can differ depending on the river considered: about one month in French and US rivers (Eulin & Le Cohu 1998, Oemke & Burton 1986) to 4 weeks in Iraqi’s canals (Hameed 2003). At the end of this period of 10 days, the slides colonised by an imma-ture biofilm were transferred to a growth chamber containing 9 microcosms.
Microcosm description: Each microcosm was composed
of a 2 L beaker (Fig. 1), filled with 1.8 L of filtrated wa-ter coming from the stream. A 50µm filter was used to eliminate zoobenthos and coarse suspended matter. A glass plate non-hermetically closed the microcosm. Clean glass staining trays heightened by 4 cm high glass slides were placed in each microcosm. A magnetic stir-rer was placed under the glass staining tray to conti-nuously mix the water. Glass staining tray of each microcosm received slides colonised by the biofilm co-ming from a same glass staining tray which was placed during 10 days in the stream.
Air renewal in the microcosm was provided by aspi-ration and avoided contamination of a microcosm by vo-latile pollutants present in another microcosm. An electric pump was used for the aeration (1 L.min–1 air circulation in each microcosm). Air coming out of the microcosms was expulsed outside the growth chamber. During 14 days, the 9 microcosms were left in the growth chamber. Temperature was set to 12oC (average water temperature of the stream during all seasons). Light supply was given by cool white fluorescent lamps providing light between 400 and 700 nm. The measured intensity in the microcosms was of 33.1 ± 3.4µmol.s–1.cm–2. Light: dark cycle was of 16h: 8h.
Physical, chemical and biological sampling:
Conducti-vity, pH and dissolved oxygen were measured in the mi-crocosms at the beginning of the experiment (D0), after 7 days (D7) and 14 days (D14, end of the experiment). NO3 – , NO2 – , NH4 +
, chemical oxygen demand, biological oxygen demand, PO4
3–
, total phosphorus, Cl–, SiO2 –
, SO4
2-, light intensity and fluoranthene were measured at the beginning and at the end of the experiment. The che-mical analyses were performed according to standard methods; HPLC was used for fluoranthene detection (APHA 1995). Biofilm samplings were carried out once a week during two weeks; for each microcosm two slides were taken, the biofilm was sampled using a clean blade and fixed in 40 ml 4% formaldehyde. The sampled slides were replaced in the microcosm by clean ones to avoid agitation modifications. A sub-sample was treated with 40% hydrogen peroxide and 35% hydrochloride acid (Prygiel & Coste 2000). The sample was mounted in Naphrax a resin with a high refractive power (1.74). Fig. 1. – Schematic representation of a microcosm.
The identification and analysis were carried out with a light microscope (Leica DMRB) at a magnification of 1000. The treatment and count methods followed the French standard NF T 90-354 (AFNOR 2000), in parti-cular this standard requires counting 400 frustules in each sample in order to have statistically consistent in-ventories.
Experimental design: Three microcosm experiments were carried out. Additionally to the assessment of the effects of fluoranthene in the microcosm studies, a preli-minary experiment was performed to determine the ef-fects of two carrier solvents (3 to 17 September 2002). In ecotoxicological experiments, DMSO (e.g. Wiegman 2002, Dijkman et al. 1997) and acetone (e.g. Boese et al. 1998, Hatch & Burton 1998) are often used at concentra-tions of 0.05 (v/v)%. The risk of using solvents in eco-toxicological experiments is to interfere with or even mask the toxicant effect (Bérard 1996). Therefore, 3 mi-crocosms received 0.05 (v/v)% acetone, 3 others 0.05 (v/v)% DMSO. The last 3 microcosms were without any solvent addition and served as controls. No effect on the structure of diatom assemblage nor on the diversity were detected (ANOVA carried out on the Bray-Curtis indices and on Shannon & Weaver indices, P > 0.05). However for DMSO exposure, total algal biovolumes were signifi-cantly lower after 2 weeks (ANOVA, P < 0.05 and pair-wise multiple comparison procedures using the Student-Newman-Keuls Method, P < 0.05, 48% of control). No differences were found for acetone exposure. Therefore, for the exposure of assemblages to fluoranthene, acetone was used as carrier solvent.
Two concentrations of fluoranthene were tested: an experiment tested a moderate concentration and another experiment tested a high concentration. For the moderate concentration experiment an initial concentration of 2µg.l–1fluoranthene (0.05% acetone) was introduced in 5 microcosms (9 to 23 April 2003). Due to non-specific adsorption on glass and biofilms this concentration after two weeks decreased to an average of 0.05µg.l–1 fluo-ranthene (Table I). This final concentration of fluoran-thene can be observed in polluted rivers (Irwin et al. 1997). Four other microcosms received only 0.05% ace-tone at the beginning of the experiment and were used as control (no introduction of fluoranthene).
For the high concentration experiment an initial concentration of 200µg.l–1 fluoranthene (0.05% ace-tone) was introduced in 5 microcosms (22 November to 6 December 2002). After 2 weeks the concentration de-creased to an average of 16.5µg.l–1 fluoranthene (Table I). This final concentration is never observed in natural rivers but can be attained in wastewater (Irwin et
al. 1997). Four other microcosms received 0.05%
ace-tone at the beginning of the experiment and were used as control (no introduction of fluoranthene).
Data analysis: The diversity index of Shannon &
Wea-ver (1948) was calculated for each treatment at each sampling date. Shannon & Weaver (1948) index calcula-tion: h n n n n i i = −
∑
ln with: n: number of frustules counted (in our case 400); ni: number of frustules of taxon i.
Data were checked for normality and variance equali-ty with Sigma-Stat software (Jandel Corporation 1992), before launching the tests. This software was also used to test for significant differences between treatments.
Bray-Curtis similarity index were calculated between the assemblages of each microcosm and each sampling date. Bray-Curtis index calculation:
Bray Curtis x x x x jk ij ik i s ij ik i s − = − + = =
∑
∑
11with: xij: number of valves of taxon i in sample j; xik: number of valves of taxon i in sample k.
Firstly, the assemblage differences between the 3 sampling dates D0, D7 and D14 were assessed: Bray-Curtis indices calculated between diatom samples taken at the same date were compared with Bray-Curtis indices calculated between diatom samples realised at different dates (ANOVA). Secondly, for each sampling date, Bray-Curtis indices were calculated between fluoran-thene-polluted microcosms, between control micro-cosms, and between fluoranthene-polluted and control microcosms; the indices values between these 3 groups were compared (ANOVA) to test the homogeneity of the treatment and then to assess assemblage changes due to fluoranthene effects.
To visualise the differences between the diatom as-semblages, a correspondence analysis (Hill 1973) was computed using PC-Ord software (McCune & Mefford 1999).
In order to define the species characterising each treatment, the Indicator Species Analysis (Dufrêne & Legendre 1997) was used. On the basis of taxon specifi-city and faithfulness to a treatment, an index was calcu-lated for each taxon. This index was maximum when all specimens of a taxon were found in a single treatment and when the taxon occurred in all samples of this treat-ment. The statistical significance of the species indicator values was evaluated using a randomization procedure (Monte-Carlo test).
RESULTS
Physical and chemical parameters
The results of the physical and chemical mea-sures are given for the beginning and the end of the experiment in Table I. For most of the parameters no significant changes during the experiments were observed (Mann-Whitney rank sum test, P > 0.05), except for the SiO2–concentration and conductivity (Mann-Whitney rank sum test, P < 0.001 for both parameters). Both parameters decreased from an average of 6.2 mg.l–1at the beginning of the exper-iments to an average of 0.4 mg.l–1after 2 weeks for SiO2–, and from an average of 483 µS.cm–1 to an average of 290 µS.cm–1 after 2 weeks for conduc-tivity.
At the end of both experiments, no significant differences were observed between control and
polluted microcosms for all measured parameters (conductivity, pH, dissolved oxygen, dissolved or-ganic carbon, biological oxygen demand, NH4+, PO43–, total phosphorus, SiO2–, Cl–, NO2–, NO3–, Na+, K+, Mg2+ and Ca2+, Mann-Whitney rank sum test, P > 0.05), except for the measures of fluoranthene concentration (Mann-Whitney rank sum test, P=0.016 for both experiments). Fluoranthene concentrations were still much higher in the polluted microcosms.
Changes in diatom assemblages
For the moderate and the high fluoranthene con-centration experiments, respectively a total of 75 and 102 diatom taxa were identified. In the moder-ate concentration experiment, the most abundant taxa (over 5%), representing 74% of the assem-blage at D0 were: Achnanthidium biasolettianum (21%), A. minutissimum (29%), Amphora pediculus (5%), Encyonema minutum (7%), Gomphonema
pumilum (6%) and Navicula reichardtiana
Lange-Bertalot (6%). In the high concentration experiment, the most abundant taxa (over 5%) rep-resenting 68% of the assemblage at D0 were:
Achnanthidium biasolettianum (25%), A.
minutissimum (11%), Amphora pediculus (12%), Navicula tripunctata (Müller) Bory (5%) and Staurosira pinnata (Ehrenberg) Lange-Bertalot
(15%). Achnanthidium biasolettianum, A.
minutissimum and Amphora pediculus, were
present among these 10 most abundant taxa in both experiments, and represented 55% of the taxa in the moderate concentration experiment, and 48% in the high concentration experiment, the
assem-blages composition of both experiment were com-parable.
For both experiments, no significant differences in diversity were observed (Shannon & Weaver index) between the control and the fluoranthene-polluted microcosms after 7 days and after 14 days (t-test: P=0.68 and P=0.88 respectively, for the moderate concentration experiment; Mann-Whit-ney Rank Sum Test P=0.90 and t-test P=0.18 re-spectively, for the high concentration experiment). For the two experiments and for both treatment (control and fluoranthene-polluted microcosms), a decrease of diversity was observed between the be-ginning and the end of the experiments: the Shan-non & Weaver indices averages, calculated with the 9 microcosms, decreased from 3.8 at D0 to 3.4 at D14 for the moderate concentration experiment, and from 3.4 at D0 to 1.7 at D14 for high concen-tration experiment.
In the experiment with the moderate fluoranthene concentrations, diatom assemblages changed during the experiment, resulting in signifi-cant differences in Bray-Curtis indices calculated for the sampling times D0, D7 and D14 (Table II). No differences were observed between diatom as-semblages of the control and the fluoranthene ex-posed treatments at the beginning and at the end of this experiment, however the Bray-Curtis indices for the control and the fluoranthene exposed as-semblages differed significantly after 7 days of ex-posure (Table II).
For the high concentration experiment, signifi-cant differences were found with the Bray-Curtis indices between assemblages at D0, D7 and D14 (Table II), and are visualized in Fig. 2 using the correspondence analysis (Table III gives the taxa
Table I. – Physical and chemical measures carried out in the microcosms. Cond.: conductivity, BOD: biological oxy-gen demand in 5 days, Pt: total phosphorus.
Table II. – One way repeated measures ANOVA carried out on Bray-Curtis indices calculated for the different sam-pling dates D0, D7 and D14, and carried out on Bray-Curtis indices calculated between fluoranthene-polluted and con-trol microcosms at the beginning of the experiment (D0), after 7 days (D7) and after 14 days (D14). The tests were done for both experiments of fluoranthene concentrations: moderate and high. Each index calculation was carried out on 400 valves.
Table III. – Taxa codes signification of Fig. 2.
Fig. 2. – Correspondence analysis calculated with the diatom assemblages of the control and the fluoranthene polluted microcosms, from D0 to D14 during the high concentration experiment. (a) Microcosms are numbered from 1 to 9. (b) Codes of the species, signification of the codes is given in Table III (only the 50 most abundant taxa and the indicator species are mentioned).
code signification). In Fig. 2 the diatom assem-blages were well separated in time; D0 is on the right, D7 in the middle and D14 on the left of the first axis. After 14 days exposure to fluoranthene, a difference between assemblages of control and fluoranthene-polluted microcosms was observed on the multivariate analysis (Fig. 2); this was con-firmed by the tests on Bray-Curtis indices that showed a significant difference after 14 days be-tween assemblages of control and fluoranthene-polluted microcosms. No significant differences were found between the Bray-Curtis indices be-tween control and fluoranthene-polluted micro-cosms at the beginning of the experiment and after 7 days (Table II).
Indicator species analysis
An Indicator species analysis (Dufrêne & Legendre 1997) was calculated for the moderate concentration experiment (Table IV). 15 taxa char-acterized the beginning of the experiment. After 14 days, Nitzschia acicularis (Kützing) Smith indi-cated significantly the fluoranthene-polluted mi-crocosms.
Similar observations were done for the high con-centration experiment since 8 taxa characterized
significantly the beginning of the experiment (Ta-ble IV). After 7 days of exposure to fluoranthene,
Nitzschia hantzschiana Grunow and Mayamaea atomus (Kützing) Lange-Bertalot var. permitis
(Hustedt) Lange-Bertalot defined significantly the polluted microcosms. Navicula antonii Lange-Bertalot, Surirella angusta Kützing, Caloneis
bacillum (Grunow) Cleve and Nitzschia linearis
(Agardh) Smith characterized significantly the control microcosms. After 14 days, Achnanthidium
minutissimum with teratological forms indicated
significantly the polluted microcosms.
For D0, some indicator species as Achnanthidium
biasolettianum, Amphora pediculus, Navicula tripunctata, N. cryptotenella Lange-Bertalot
corre-sponded to common taxa in both experiments. The other indicator species were different from an ex-periment to the other, because even if the assem-blages of both experiments were comparable, they had some differences especially among rare taxa as
Diadesmis contenta (Grunow) Mann, Fallacia monoculata (Hustedt) Mann, Geissleria acceptata
(Hustedt) Lange-Bertalot, Navicula lanceolata
(Agardh) Ehrenberg, Nitzschia inconpicua Grunow that were present in only one experiment. The taxa
Meridion circulare (Greville) Agardh, Gomphonema olivaceum (Hornemann) Brébisson, G. pumilum, Navicula gregaria Donkin, N. reichardtiana,
Table IV. – Indicator species analysis (Dufrêne & Legendre 1997) carried out on diatom assemblages of the control and fluoranthene-polluted microcosms. The significant indicator taxa (Monte-Carlo tests, P(0.05) are ranked by abundance for each experiment, treatment and date, with their indicator value in bracket. The second value in bracket is their abun-dance. For D0, the two treatments were grouped since the biofilms were not yet in contact with the pollutant.
Nitzschia dissipata (Kützing) Grunow, N. linearis, Planothidium lanceolatum (Brébisson) Round et
Bukhtiyarova, Rhoicosphaenia abbreviata (Agardh) Lange-Bertalot and Surirella brebissonii Krammer et Lange-Bertalot were present in both experiments but were more abundant in one of the experiments.
Effect on frustule shape
After 14 days exposure to fluoranthene in the high concentration experiment, microscopic inves-tigations allowed the observation of teratological valves: microcosm 1: 4.9 ‰, microcosm 3: 4.1‰, microcosm 5: 9.1‰, microcosm 7: 11.6‰ and mi-crocosm 9: 9.7‰. These deformities were observed for most of the taxa present in the contaminated microcosms: Achnanthidium biasolettianum (aver-age abundance in the fluoranthene-polluted micro-cosms: 1.8‰), Gomphonema olivaceum (0.9‰), G.
parvulum Kützing (0.5‰), Hippodonta costulata
(Grunow) Lange-Bertalot, Metzeltin et Witkowski (0.5‰), Nitzschia archibaldii Lange-Bertalot (0.5‰), Planothidium dubium (Grunow) Round et Bukhtiyarova (2.78‰), P. frequentissimum (Lange-Bertalot) Round et Bukhtiyarova (1.4‰), P. lanceolatum (0.9‰) and Staurosira pinnata
(0.5‰). Some teratological forms are illustrated in Plate I, normal shapes of the frustules are presented in Plate II. This was also confirmed by the results of the Indicator Species Analysis (Table III), teratological forms of Planothidium dubium signif-icantly characterized the fluoranthene-polluted mi-crocosms. No deformities were observed during the moderate concentration experiment.
DISCUSSION
Evolution of fluoranthene concentration in microcosms
An important decrease of fluoranthene concen-tration was observed in the microcosms of both experiments. Fluoranthene has a low solubility (0.265 ppm in freshwater at 25 oC according to Irwin et al. 1997) and its volatilisation rate from water is less than 1% (Petrasek et al. 1983). In rivers, this pollutant is quickly adsorbed on sus-pended matter and bioconcentrated in aquatic organisms (Irwin et al. 1997). In our experiments, the fluoranthene concentration decrease was proba-bly due to adsorption into the biofilm, bio-concentration in algae and adsorption on the glass surfaces; measurements would be useful to determine the importance of each process. Bioconcentration in diatoms was probably a very important process; it was demonstrated in culture, that cell surface accumulation of PAH with marine diatoms was faster than most other environmental and biological process (Fan & Reinfelder 2003).
Fluoranthene and diatom diversity
In this study no differences were observed be-tween fluoranthene-polluted and control micro-cosms for both concentrations (moderate and high) in terms of biodiversity. This criterion did not seem to be efficient in this case to assess fluoranthene toxicity. This can probably be explained by the transformation of the assemblages with an increase after 2 weeks of resistant taxa in polluted micro-cosms and sensitive taxa in control micromicro-cosms. This kind of criteria has been also used in lotic mi-crocosms to assess the current velocity and copper effect on benthic algae, but no statistical differ-ences were established (Sabater et al. 2002). In in-door lentic microcosm experiments (Peres et al. 1997), methylmercury and inorganic mercury did not show any changes on species richness of periphytic diatoms. Diatom diversity did not ap-pear to be adapted to assess the effect of fluoranthene.
Fluoranthene and diatom assemblages structure
The diatom assemblages significantly changed between the beginning and the end of the experi-ments. Other microcosm experiments (Peres et al. 1997) showed important changes in diatom assem-blage structure. Many taxa did not resist to their transfer to microcosms, because of condition changes between river and microcosms such as cur-rent velocity and light. Nutrient competition can also play an important role during the experiment, as this was shown for instance by Sommer (1996) with phytoplankton in microcosm. Silicate deple-tion was observed in our microcosms experiments. Sommer (1988) showed that diatom taxa had dif-ferent threshold concentration for silicate; there-fore the change of taxa composition in microcosm can be due to the development of taxa tolerant to silicate depletion. Even if an important transforma-tion of the diatom assemblages was observed dur-ing the course of the experiment, differences be-tween assemblages of fluoranthene contaminated and control microcosms remained significant by testing Bray-Curtis indices and observable by us-ing multivariate analysis. Similar problematics have already been shown in microcosm studies with methylmercury (Peres et al. 1997) and cad-mium (Gold 2002) since impacts of these pollut-ants were observed, despite the transformation of the assemblages along the experiment.
Resistant and sensitive diatom taxa to fluoranthene
In situ studies in rivers have already tried to
characterize tolerant and sensitive diatom taxa to heavy metals (Deniseger et al. 1986, Rushforth et
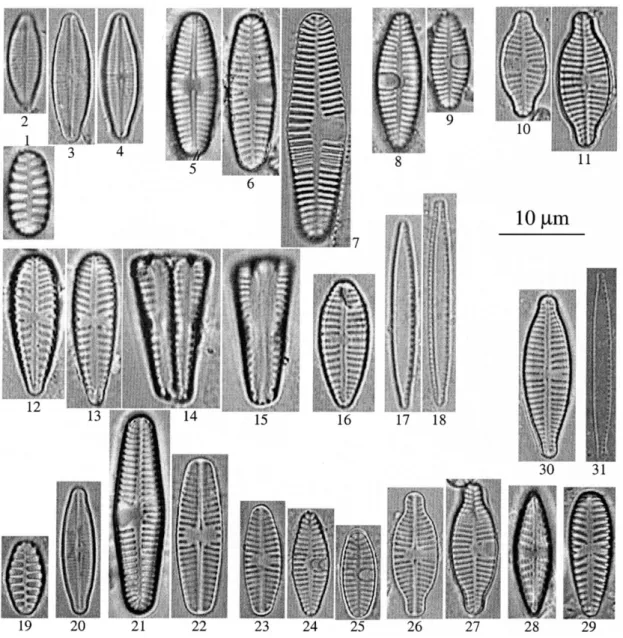
Plate I. – Teratological forms after 2 weeks exposure to fluoranthene (200µg.l–1). Microcosm 1: fig. 1-2:
Gomphone-ma olivaceum. Microcosm 3: fig. 3: Planothidium frequentissimum, fig. 4-7: P. dubium, fig. 8: Nitzschia archibaldii.
Microcosm 5: fig. 9: Staurosira pinnata, fig. 10: Achnanthidium biasolettianum, fig. 11-13: Planothidium
lanceola-tum, fig. 14-15: P. frequentissimum, fig. 16-22: P. dubium, fig. 23: Gomphonema olivaceum. Microcosm 7: fig.
24-29: Achnanthidium biasolettianum, fig. 30-32: Planothidium lanceolatum, fig. 33: P. frequentissimum, fig. 34-35: P.
dubium, fig. 36: Hippodonta costulata, fig. 37-38: Gomphonema olivaceum, fig. 39: G. parvulum. Microcosm 9:
studies to assess PAH effects on benthic algae are quite rare. Several algal species have been used to assess fluoranthene toxicity with mono-specific tests. EC50 measured on the Chlorophyceae
Selenastrum capricornutum Printz and the marine
diatom Skeletonema costatum (Greville) Cleve are high (effect on cell number 54,400µg.l–1.96 h–1 and 45,000µg.l–1.96 h–1 respectively). 14 days LOEC inhibition growth (Bastian & Toetz 1982) for the Cyanobacteria Anabaena flos-aquae
(Lyngbye) Brébisson is much lower (38µg.l–1) and nearer the concentrations of our microcosm
experi-ments. Comparisons to our microcosm study are difficult since methodologies and species used are different; but the sensitivity of our experimental design is high since concentrations of 2µg.l–1 (moderate concentration experiment) were suffi-cient to observe significant effects on diatom as-semblage structure.
Nitzschia acicularis was the only resistant taxon
for the moderate concentration experiment. This taxon is often found in lentic rivers and in plankton and is considered as eutrophic by van Dam et al. (1994).
Plate II. – Normal frustules found in the control microcosms after 2 weeks. Microcosm 2: fig. 1: Staurosira pinnata, fig. 2-4: Achnanthidium biasolettianum, fig. 5-7: Planothidium lanceolatum, fig. 8-9: P. frequentissimum, fig. 10-11:
P. dubium, fig. 12-15: Gomphonema olivaceum, fig. 16: G. parvulum, fig. 17-18: Nitzschia archibaldii. Microcosm 8:
fig. 19: Staurosira pinnata, fig. 20: Achnanthidium biasolettianum, fig. 21-22: Planothidium lanceolatum, fig. 23-24:
P. frequentissimum, fig. 26-27: P. dubium, fig. 28: Hippodonta costulata, fig. 29: Gomphonema olivaceum, fig. 30: G. parvulum, fig. 31:Nitzschia archibaldii.
In the high concentration experiment several taxa were considered as sensitive to fluoranthene after 7 days: Navicula antonii Lange-Bertalot, Surirella angusta Kützing and Caloneis bacilum
(Grunow) Cleve. Coste (Cemagref 1982) gave a good quality sensitivity value to these taxa.
Nitzschia linearis characterised control
assem-blages after 7 days but its sensitive value is moder-ate. Mayamaea atomus var. permitis was consid-ered as resistant to fluoranthene exposure after 7 days. This result was not surprising because this taxon is usually considered as polluo-resistant. The ecological classes of van Dam et al. (1994) con-sider this taxon as α–β–mesosaprobous and eutrophic, and the sensitivity values given by the different diatom indices are corresponding to bad water quality (e.g. SPI index of Coste in Cemagref 1982). This taxon is often observed in polluted rivers of Luxembourg. On another hand, Nitzschia
hantzschiana was also observed as resistant to
fluoranthene exposure after 7 days, but this taxon is usually considered as oligosaprobous and mesotrophic (van Dam et al. 1994). After 14 days, besides teratological forms, Achnanthidium
minutissimum appeared to be resistant to
fluoranthene. This taxon was also considered as re-sistant to several heavy metals (Ivorra 2000, Deniseger et al. 1986, Weber & McFarland 1981) and as cosmopolitan (Kelly & Whitton 1995). Probably its small size enables it to reproduce rap-idly and to occupy space (Sabater 2000) available after death of taxa that are sensitive to fluoranthene.
Fluoranthene and frustule deformities
When absorbed in higher plants, depending on the species studied only an average of 5% fluoranthene is metabolised to soluble metabolites (hydroxyfluoranthene). This metabolites are conju-gated with glucose, glucoronic acid and other cell components (Kolb & Harms 2000). Tests of several polynuclear aromatic compounds as benz(a)pyrene have been demonstrated to act like plant hormones, simulating growth for some algae as Chlorella,
Scenedesmus and Ankistrodesmus; others as benzfluoranthene causes depression and retardation of growth (Sims & Overcash 1983). Fluoranthene showed a negative influence on photosynthetic ac-tivity of algae in lichens (Zezulka et al. 2003). No physiological studies showed a direct effect of PAH on diatom frustules that could explain the frustule deformities observed in the high concen-tration experiment.
Frustule deformities for diatoms (teratological forms) are often caused by toxicant effect. It was demonstrated that the increase of copper concentra-tion provokes problems in the silificaconcentra-tion metabo-lism of the marine diatom Skeletonema costatum
(Greville) Cleve (Morel et al. 1978). In vitro exper-iments have shown that an increase of heavy metal concentrations caused an increase of the occur-rence of teratological frustules (McFarland et al. 1997). In the marine environment, the presence of teratological frustules can be attributed to micropollutant presence (Dickman 1998). Similar observations were done for Gomphonema
parvulum or some Eunotia in freshwater
(Murakami & Kasuya 1993, Carter 1990). This study shows that fluoranthene can also be teratogenic for freshwater diatoms at a certain dose.
CONCLUSION
As a conclusion, these microcosm experiments enabled to elucidate some effects of fluoranthene on diatom assemblages. Particularly, it was shown that these pollutants, even at a moderate concentra-tion, induced changes in diatom assemblage struc-ture and frustule shape. Some species were as-sessed as sensitive or tolerant to these pollutants and could serve as indicators of fluoranthene toxic-ity. Nevertheless, some limitations associated with microcosm experiments occurred (i.e. fluoranthene adsorption on glass and in the biofilm, no medium renewal). Consequently, different methods should be envisaged to assess more accurately and pre-cisely the sensitivity of different taxa to fluoranthene. Actually, experimentations integrat-ing different scales of complexity should be carried out in order to complete this microcosm study. The sensitive taxa and the resistant taxa identified in these experiments could be used separately in fur-ther mono-specific tests assessing the impact of fluoranthene on their productivity (EC50). Physio-logical tests as Pollution-Induced Community Tol-erance (Blanck et al. 1988) could be envisaged to measure the impact on diatom assemblage activity. Mesocosm studies, integrating several trophic lev-els of the aquatic environment, should be done to assess the effect of fluoranthene on a larger scale of complexity.
ACKNOWLEDGEMENTS. – Dr L Hoffmann provided
useful comments that improved the quality of the manus-cript. Fluoranthene detection was done thanks to Dr C Guignard & Dr JP Lickes. C Bouillon & Dr O Monnier are thanked for their help.
REFERENCES
AFNOR 2000. Norme Française NF T 90-354. Détermi-nation de l’Indice Biologique Diatomées (IBD). Association Française de Normalisation, 63 p.
APHA 1995. Standard methods for examination of water and wastewater. 19th Ed. American Public Health Assoc Washington, 1048 p.
Bastian MV, Toetz DW 1982. Effect of eight polynu-clear hydrocarbons on growth of Anabaena
flos-aquae. Environ Contam Toxicol 29: 531-538.
Bérard A 1996. Effects of four organic solvents on natu-ral phytoplankton assemblages: consequences for ecotoxicological experiments on herbicides. Bull
Environ Contam Toxicol 57: 183-189.
Bérard A, Pelte T, Druart JC 1999. Seasonal variations in the sensitivity of Lake Geneva phytoplankton com-munity structure to atrazine. Arch Hydrobiol 145: 277-295.
Blanck H, Wänkberg SA, Molander S 1988. Pollution-Induced Community Tolerance – A new ecotoxicolo-gical tool. In Cairs J, Pratt Jr, & Pratt JR eds, Functio-nal testing of aquatic biota for estimating hazards of chemicals: 219-230.
Boese B, Lamberson J, Swartz R, Ozretich R, Cole F 1998. Photoinduced toxicity of PAHs and alkylated PAHs to a marine infaunal amphipod (Rhepoxynius
abronius). Arch Environ Contam Toxicol 34:
235-240.
Carter J 1990. A new Eunotia and its great morphologi-cal variations under stress caused by a habitat loaded with copper salts. In Ricard M & Coste M eds, Ou-vrage dédié à la mémoire du Professeur Henry Ger-main (1903-1989). Koeltz Scientific Books, Koenigstein: 13-17.Cemagref 1982. Etude des mé-thodes biologiques d’appréciation quantitative de la qualité des eaux. Rapport Q.E. Lyon, Agence de l’Eau Rhône-Méditerranée-Corse – Cemagref, Lyon, 218 p.
Deniseger J, Austin A, Lucey WP 1986. Periphyton communities in a pristine mountain stream above and below heavy metal mining operations. Freshwater
Biol 16: 209-218.
Dickman M 1998. Benthic marine diatom deformities as-sociated with contaminated sediments in Hong-Kong.
Environ Int 24: 749-759.
Dijkman N, Van Vlaardingen P, Admiraal W 1997. Bio-logical variation in sensitivity to N-heterocyclic PAHs: effects of acridine on seven species of micro-algae. Environ Pollut 95: 121-126.
Dorn PB, Rodgers JH, Gillespie WB, Lizotte RE, Dunn WA 1997. The effects of a C12-13 linear alcohol ethoxylate surfactant on periphyton, macrophytes, in-vertebrates and fish in stream mesocosms. Environ
Toxicol Chem 16: 1634-1645.
Dufrêne M, Legendre P 1997. Species assemblages and indicator species: the need for a flexible asymmetri-cal approach. Ecol Monograph 67: 345-366. Eulin A, Le Cohu R 1998. Epilithic diatom communities
during the colonization of artificial substrates in the river Garonne (France). Comparison with natural communities. Arch Hydrobiol 143: 79-106.
Fan CW, Reinfelder JR 2003. Phenanthrene accumula-tion kinetics in marine diatoms. Environ Sci Technol 37: 3405-3412.
Gold C 2002. Etude des effets de la pollution métallique (Cd/Zn) sur la structure des communautés de diato-mées périphytiques des cours d’eau. Approches expé-rimentales in situ et au laboratoire. PhD thesis, Univ Bordeaux I, 175 p.
Guasch H, Ivorra N, Lehmann V, Paulsson M, Real M, Sabater S 1998. Community composition and sensiti-vity of periphyton to atrazine in flowing waters: the role of environmental factors. J Appl Phycol 10: 203-213.
Hameed HA 2003. The colonization of periphytic dia-tom species on artificial substrates in the Ashar canal, Basrah, Iraq. Limnologica 33: 54-61.
Hatch AC, Burton GA 1998. Effects of photoinduced toxicity of fluoranthene on amphibian embryos and larvae. Environ Toxicol Chem 17: 1777-1785. Hill MO 1973. Reciprocal averaging: an eigenvector
me-thod of ordination. J Ecol 61: 237-249.
Hoagland KD, Roemer SC, Rosowski JR 1982. Coloni-zation and community structure of two periphyton as-semblages, with emphasis on the diatoms (Bacillariophyceae). Am J Bot 69: 188-213.
Irwin RJ, VanMouwerik M, Stevens L, Seese MD, Bas-ham W 1997. Environmental contaminant encyclope-dia. Fluoranthene entry. Water Ressources Division National Park Service. Fort Collins, Colorado: Fede-ral Government of Colorado, 44 p.
Ivorra N 2000. Metal induced succession in benthic dia-tom consortia. PhD thesis, Univ Amsterdam, 163 p. Jandel Corporation 1992. SigmaStat for Windows
ver-sion 1.0.
Kelly MG, Whitton BA 1995. The Trophic Diatom Index: a new index for monitoring eutrophication in rivers. J Appl Phycol 7: 433-444.
Kolb M, Harms H 2000. Metabolism of fluoranthene in different plant cell cultures and intact plants. Environ
Toxicol Chem 19: 1304-1310.
Leboulanger C, Rimet F, Hême de Lacotte M, Bérard A 2001. Effects of atrazine and nicosulfuron on fresh-water microalgae. Environ Int 26: 130-135.
McCune B, Mefford MJ 1999. Multivariate analysis of ecological data, version 4.01. MjM software, Glene-den Beach, Oregon, USA.
McFarland BH, Hill BH, Willingham WT 1997. Abnor-mal Fragilaria spp. (Bacillariophyceae) in streams impacted by mine drainage. J Freshwater Ecol 12: 141-152.
Ministère de l’Environnement du Grand-Duché de Luxembourg 2001. Rapport d’activité 2000, 364 p. Morel NML, Rueter JG, Morel FMM 1978. Copper
toxi-city to Skeletonema costatum (Bacillariophyceae). J
Phycol 14: 43-48.
Mundie JH, Simpson KS, Perrin CJ 1991. Responses of stream periphyton and benthic insects to increases in dissolved inorganic phosphorus in a mesocosm. Can
J Fish Aquat Sci 48: 2061-2072.
Murakami T, Kasuya M 1993. Teratological variations of Gomphonema parvulum Kützing in a heavily pol-luted drainage channel. Diatom 8: 7-10.
Oemke MP, Burton TM 1986. Diatom colonization dy-namics in a lotic system. Hydrobiologia 139: 153-166.
Peres F, Coste M, Ribeyre F, Ricard M, Boudou A 1997. Effects of methylmercury and inorganic mercury on periphytic diatom communities in freshwater indoor microcosms. J Appl Phycol 9: 215-227.
Perrin CJ, Wilkes B, Richardson JS 1992. Stream peri-phyton and benthic insect responses to additions of treated acid mine drainage in a continuous-flow
on-site mesocosm. Environ Toxicol Chem 11: 1513-1525.
Petrasek AC, Kugelman IJ, Austern BM, Pressley TA, Winslow LA, Wise RH 1983. Fate of toxic organic compounds in wastewater treatment plants. J Water
Pol Control Fed 55: 1286-1296.
Prygiel J, Coste M 2000. Guide méthodologique pour la mise en œuvre de l’Indice Biologique Diatomées. NF T 90-354. Agences de l’eau – Cemagref, Bordeaux, 133 p.
Rushforth SR, Brotherson JD, Funglada N, Eveson WE 1981. The effects of dissolved heavy metals on atta-ched diatoms in the Uintah Basin of Utah, U.S.A.
Hy-drobiologia 83: 313-323.
Sabater S, Navarro E, Guasch H 2002. Effects of copper on algal communities at different current velocities. J
Appl Phycol 14: 391-398.
Sabater S 2000. Diatom communities as indicators of en-vironmental stress in the Guadiamar River, S-W Spain, following a major mine tailings spill. J Appl
Phycol 12: 113-124.
Seguin F, Leboulanger C, Rimet F, Druart JC, Bérard A 2001. Effects of atrazine and nicosulfuron on phyto-plankton in systems of increasing complexity. Arch
Environ Contam Toxicol 40: 198-208.
Shannon CE, Weaver W 1948. The mathematical theory of communication. Univ Illinois Press, Urbana, 117 p.
Sims RC, Overcash MR 1983. Fate of polynuclear aro-matic compounds (PNAs) in soil-plant systems.
Re-sidue Rev 88: 1-68.
Sirota GR, Uthe JF, Streedharan A, Matheson R, Musial GJ, Hamilton K 1983. Polynuclear aromatic hydro-carbons in Lobster (Homarus americanus) and in the vicinity of a coking facility. In Cooke M & Dennis AJ eds, Polynuclear Aromatic Hydrocarbons Formation, Metabolism and Measurement. Batelle Press, Colum-bus, Ohio: 1123-1136.
Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, La Point TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy J, Lenwood WH, Williams WM 1996. Ecological risk assessment of atrazine in North
American surface waters. Environ Toxicol Chem 15: 31-76.
Sommer U 1988. Growth and survival strategies of planktonic diatoms. In Sandgren CG ed, Growth and reproductive strategies of freshwater phytoplankton. Cambridge Univ Press: 227-266.
Sommer U 1996. Nutrient competition experiments with periphyton from Baltic sea. Mar Ecol Prog Ser 140: 161-167.
Stevenson RJ, Bahls LL 1999. 6. Periphyton protocols.
In Barbour MT, Gerritsen J, Snyder BD & Stribling
JB eds, Rapid bioassessment protocols for use in wa-deable streams and rivers: periphyton, benthic ma-croinvertebrates, and fish. EPA 841-B-99-002. US Environmental Protection Agency, Office of Water, Washington DC: 1-22.
van Dam H, Mertens A, Sinkeldam J 1994. A coded checklist and ecological indicator values of freshwa-ter diatoms from the Netherlands. Neth J Aquat Ecol 28: 117-133.
Verschueren K 1983. Handbook of environmental data on organic chemicals. 2nd Ed. Van Nostrand Rein-hold, New-York, 192 p.
Weber CI, McFarland BH 1981. Effects of copper on the periphyton of a small calcareous stream. In Bates JM & Weber CI eds, Ecological assessments of effluent impacts on communities of indigenous aquatic orga-nisms. Conference proceedings, American Soc Tes-ting & Materials. ASTM STP 730: 101-131. Wiegman S 2002. Photoenhanced toxicity of azaarenes
to marine phytoplankton. PhD thesis, Univ Amster-dam, 127 p.
Williams LG, Mount DI 1965. Influence of zinc on peri-phytic communities. Am J Bot 52: 26-34.
Zezulka S, Kummerová M, Kmentová E, Barták M 2003. The effect of fluoranthene on primary photochemical processes of symbiotic algae in lichen thalli. Book of Abstracts. Plant Physiol Conf PhD Students and Young Scientists, Brno: 127-128.
Reçu le 24 octobre 2003; received October 24, 2003 Accepté le 5 février 2004; accepted February 5, 2004