ContentslistsavailableatScienceDirect
Scientia
Horticulturae
j o u r n a l ho me p a g e :w w w . e l s e v i e r . c o m / l o c a t e / s c i h o r t i
The
interaction
between
arbuscular
mycorrhizal
fungi
and
Piriformospora
indica
improves
the
growth
and
nutrient
uptake
in
micropropagation-derived
pineapple
plantlets
B.C.
Moreira
a,
F.C.
Mendes
a,
I.R.
Mendes
a,
T.A.
Paula
a,
P.
Prates
Junior
a,
L.C.C.
Salomão
b,
S.L.
Stürmer
c,
W.C.
Otoni
d,
A.
Guarc¸
oni
M.
e,
M.C.M.
Kasuya
a,∗aLaboratóriodeAssociac¸õesMicorrízicas/BIOAGRO,DepartamentodeMicrobiologia,UniversidadeFederaldeVic¸osa,36570-900,Vic¸osa,MG,Brazil bDepartamentodeFitotecnia,UniversidadeFederaldeVic¸osa,36570-900,Vic¸osa,MG,Brazil
cDepartamentodeCiênciasNaturais,Fundac¸ãoUniversidadeRegionaldeBlumenau,89012-900Blumenau,SC,Brazil
dLaboratóriodeCulturadeTecidos(LCTII)/BIOAGRO,DepartamentodeBiologiaVegetal,UniversidadeFederaldeVic¸osa,36570-900,Vic¸osa,MG,Brazil eInstitutoCapixabadePesquisa,AssistênciaTécnicaeExtensãoRural,29375-000,VendaNovadoImigrante,ES,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received14April2015 Receivedinrevisedform 22September2015 Accepted23September2015 Availableonline9October2015 Keywords: Ananascomosus AMF P.indica Phosphoruslevels
a
b
s
t
r
a
c
t
Arbuscularmycorrhizalfungi(AMF)andPiriformosporaindicaarewellknownforpromotinggrowth, development,andnutrientuptakeandforimprovingplantphotosynthesis.Thesefungirepresent promis-ingtoolssupportingmicropropagatedplantsduringtheacclimatizationstage,andtheirusecanreduce theapplicationofphosphatefertilizers,providingeconomicandenvironmentalbenefits.Therefore,this studyaimedtoevaluatethebenefitsofinoculationwithAMFandP.indicaforthegrowthofplantletsof theImperialcultivarofpineappleinoculatedduringtheacclimatizationstageandgrownwithdifferent levelsofphosphorus(P).TheexperimentconsistedofsixPlevels(0,20,40,80,160and320mgkg−1soil) withinoculationofClaroideoglomusetunicatum,Dentiscutataheterogama,Rhizophagusclarus,P.indica,a mixtureofallfungi(Mix),orcontrol(noinoculation).Theparametersvegetativegrowth,thenutrient contentsintheplants,photosyntheticefficiency,andthecomponentsofdependenceandcolonization byfungiwereassessed.Thefungalinoculationwaseffectiveforplantletgrowth,especiallyuptoaPdose of40mgkg−1,increasingbothplantbiomassandtheabsorptionofallevaluatednutrients.WithPat 80mgkg−1,onlythetreatmentswithC.etunicatumandMixproducedplantletsofbetterqualitythanthe non-inoculatedcontrol.ThecolonizationbyAMFandP.indicawasnotaffectedbytheadditionofPtothe soil,althoughfungaldependencedecreasedundertheseconditionsandcouldbeconsideredmoderate evenat40mgkg−1forplantsinoculatedwithC.etunicatum,R.clarus,P.indicaorMix.Theinoculation ofpineappleplantletsisapromisingmethodthatcanbeemployedtoproducehigh-qualitypropagative materialforthemarket.
©2015ElsevierB.V.Allrightsreserved.
1. Introduction
Pineapple(Ananascomosus(L.)Merrill;Bromeliaceae)isnative
to the southern and southeastern regions of Brazil, Argentina
and Uruguay(Melo etal., 2006).It is a cropof great economic
importancein manytropical countries(Beand Debergh, 2006),
andapproximately23.34milliontonsofthisfruitwereproduced
worldwidein2012(FAOSTAT,2014).Brazilianpineapple
produc-∗ Correspondingauthorat:LaboratóriodeAssociac¸õesMicorrízicas/BIOAGRO, DepartamentodeMicrobiologia,UniversidadeFederaldeVic¸osa,Campus Univer-sitário,AvenidaPeterHenryRolfss/n,UniversidadeFederaldeVic¸osa,36570-900, Vic¸osa,MG,Brazil.Fax:+553138992573.
E-mailaddresses:mkasuya@ufv.br,catarinakasuya@gmail.com
(M.C.M.Kasuya).
tioncorrespondsto10.6%oftotalworldwideproduction,occupying
the equivalentof 6% of thetotal areaof pineapple plantations
worldwide. Brazil ranks third worldwide in the production of
pineapplefruit,behindThailandandCostaRica(FAOSTAT,2014).
Pineapple is vegetatively propagated,and thequality of the
propagationmaterialsignificantinfluencesplanthealth,
develop-mentandyield(BeandDebergh,2006;Kapooretal.,2008;Souza
et al., 2013).A wide variety of plant material can beused for
propagationofpineapple,includingfruitcrown,lateralbranches
(suckersandslips),andseedlingsgrownfromstemsectionsorvia
micropropagation(Hepton,2003).Researchhasbeenconducted
to enhance themultiplication of pineappleby means of tissue
culturetechniques(Smithetal.,2003;Souzaetal.,2013).
Pineap-ple explantscan be multiplied in vitro onsolid and liquid MS
medium(MurashigeandSkoog,1962)(BeandDebergh,2006;Silva
http://dx.doi.org/10.1016/j.scienta.2015.09.032
etal.,2007).Thismediumcanbesupplementedwithsucroseand
cytokininsorauxins(dependingonthepurpose)asa meansto
initiateculture,proliferationorgrowthoftheshoots,orevenas
a meansof rooting(Smithet al., 2003;Be and Debergh, 2006;
Silvaetal.,2007).Toachievelarge-scaleproductionofpineapple
plantletsatareducedcost,Escalonaetal.(1999)proposed
micro-propagationusingtemporaryimmersionsystemsinabioreactor,
andthisapproachhasbeenwidelyusedsince.
Micropropagatedplantsare moreuniformand showgreater
synchronyoffloweringandfruitinginthefield(Singhetal.,2012).
Micropropagationalsofacilitatestheintroductionofplantletsin
newareas(Escalonaetal.,1999),theproductionofhigh-quality
materialinanyseason(Chandraetal.,2010),large-scaleproduction
andpathogen-freecrops(Routetal.,2006;Souzaetal.,2013).This
techniqueisusedtoobtainalargenumberofplantsthatare
genet-icallyidenticaltotheparentplant.Forpineapple,thistechnique
hasseveraladvantagesoverconventionalmethodsofpropagation,
includingarapidandefficientincreaseintheproductionofplantsof
selectedvarieties(González-Olmedoetal.,2005;Farahani,2013).
Althoughmicropropagationisefficient,ahighmortalityratehas
beenobservedduringtheacclimatizationprocessdueto
physiolog-icalchangescausedbytheinvitroenvironment,suchaschanges
inthefunctionofthestomataandroots,undevelopedcuticles,and
photosyntheticinefficiency(Pospíˇsilováetal.,1999;Hazarikaetal.,
2002;Hazarika,2006;Xiaoetal.,2011;KumarandRao,2012;Singh etal.,2012).
Ifestablishedduringtheearlystageofacclimatization,an
asso-ciationwithbeneficialfungicanreducethestressofacclimatization
andpromotethegrowthofmicropropagatedplants(Kapooretal.,
2008;Singhetal.,2012;Yadavetal.,2013a,b).Inoculationwith
arbuscularmycorrhizalfungi(AMF)(Glomeromycota)and
Pirifor-mosporaindica(rootendophyticfungus,Basidiomycota)hasproven
tobeapromisingalternativefortheproductionofplantletsof
supe-riorquality(SahayandVarma,1999;Kapooretal.,2008;Yadav
etal.,2013a,b).Thesefungihavealsobeenreportedtoincrease
plants’nutrientuptake(Smithetal.,2010;Varmaetal.,2012),
tol-erancetodroughtandsaltstresses(Augé,2001;Varmaetal.,2012),
resistancetotheeffectsofheavymetals(Azcón-Aguilaretal.,1997;
Varmaetal.,2012)andphotosyntheticefficiency(Estrada-Lunaand Davies,2003;Achatzetal.,2010;Boldtetal.,2011;Yadavetal., 2013a,b).
Phosphorus (P) fertilization and phytosanitary control play
important roles in the production of high-quality pineapple
propagules.ThemanagementofPinthesoilisamajorfactorin
achievingsustainableagriculturalsystems(Kahiluotoetal.,2000).
PlantsexposedtohighlevelsofPinthesoilshowreduced
mycor-rhizalcolonization(Mengeetal.,1978;Kahiluotoetal.,2000;Grant
etal.,2005).Thus,itishighlyimportanttoidentifytheamountof
PthatwillmaximizetheeffectofbothAMFandP.indicaandallow
properuptakeofthisnutrienttoprovideanadequatenutritional
statusfortheplant.Likewise,itishighlyimportanttoestablishan
associationwiththesefungithatwillenabletheplanttoextract
themaximumbenefitfromthesymbiosis.
Theaimofthisstudywastoobtainhigh-qualitypropagation
materialbyevaluatingthebenefitsofinoculationwithAMFand/or
P.indicaattheacclimatizationstageandbyexaminingtheroles
ofthesefactorsinthegrowth,nutrientuptakeandphotosynthetic
efficiencyofpineappleplantletsunderdifferentPlevels.
2. Materialsandmethods
2.1. Invitroculture
Micropropagatedplantletsofpineapple,cultivarImperial,were
subcultivated in liquid MS (Murashige and Skoog, 1962)
cul-turemediumformultiplication.Themediumwassupplemented
with30gL−1sucrose,1.8mgL−1␣-naphthaleneaceticacid(NAA),
2mgL−1indole-3-butyricacid(IBA),and2.1mgL−1kinetin(KIN)
atpH5.5.Theculturesweregrownin250-mLglassjars
contain-ing15mLofculturemediumandsealedwithrigidpolypropylene
covers.Cultureswerekeptina growthroomat 26±2◦Cunder
a photoperiodof16hlight/8hdarkand underan irradianceof
36molm−2s−1,providedbywhitefluorescentlamps.Subcultures
wereperformedevery40d(seeSupplementarymaterial).
2.2. Fungalinoculants
Isolates of AMF Dentiscutata heterogama PNB102A (=
Scutel-lospora heterogama), Claroideoglomus etunicatum RJN101A (=
Glomus etunicatum) and Rhizophagus clarus RJN102A (= Glomus
clarum)wereobtainedfromtheInternationalCultureCollection
ofGlomeromycota(CICG,www.furb.br/cicg)attheUniversidade
RegionaldeBlumenau,SantaCatarina,Brazil.Singlecultureswere
establishedfollowingtheproceduresadoptedattheCICG.Briefly,
sporeswereextractedfromtrapcultures,separatedby
morpho-typesandinoculatedontherootsof15-day-oldSorghumbicolor
seedlingsthat hadbeengrown onsterilizedsubstrate.Sorghum
seedlingswerethentransplantedtocones(270cm3)ina
steril-izedsand:expandedclay:soil (2:2:1v:v:v)mixandgrown for4
monthsundergreenhouseconditions.Afterthatperiod,coneswere
checkedforsporulation.Plantswereallowedtodry insitu,and
thecontentsofconeswerestoredinziplockplasticbagsat4◦C
for6months.TheinvitrocultureofP.indicawasobtainedfrom
themicrobialcollectionoftheLaboratoryofMycorrhizal
Associ-ationsofUniversidadeFederaldeVic¸osa—MinasGeraisand was
maintainedandmultipliedinKaefermedium(KM)(HillandKaefer,
2001)andstoredinthedarkat30◦C(Kumaretal.,2011)for30d.
2.3. Characteristicsandsoilpreparation
Thesubstratewassterilizedinanautoclavefor1hat121◦C
and was composed of a mixture of soil and sand (1:1 v:v)
withthefollowingcharacteristics:pH(water)=4.9,P=1.1mgdm−3
(Mehlich 1),K=34mgdm−3, Ca=0.2cmolcdm−3,Mg and Al=0,
sumofexchangeablebases (SB)=0.29cmolcdm−3,organic
mat-ter (OM)=1.1dagkg−1 and Premaining=6.6mgL−1. Liming was
performed according toSouza et al. (1999), following the
rec-ommendationsforpineapplecultivationtomeettheCaandMg
requirements for 2cmolcdm−3. The substrate was moistened,
placedinplasticbagsandallowedtorestfor30datroom
tempera-ture.Afterthisperiod,phosphorusfertilizationwasperformedfor
eachdoseofP(0,20,40,80,160and320mgkg−1 soil)usingan
aqueoussolutionofKH2PO4justbeforetransplantationatthetime
ofacclimatization.
2.4. Experimentaldesignandinoculation
Theexperimentwithpineappleplantlets(cultivarImperial)was
conductedinagreenhouseandconsistedofsixdosesofP(0,20,
40,80,160and320mgkg−1ofsoil)andinoculationwithC.
etuni-catum,D.heterogama,R.clarus,P.indica,amixtureofallfungi(Mix)
oranon-inoculatedcontrol(Cont).Therewerefourreplicates,and
theexperimentswereperformedfollowingacompletely
random-izeddesignwitha6×6factorialarrangement(seeSupplementary
material).
The50-day-oldplantlets,withanaverageheightof4.4cmand
12–15leaves,weretransplantedintoplasticpotscontaining1kg
ofsterilizedsubstrate.Whentheplantletsweretransplanted,the
substratewasinoculatedneartheroot,usinganaverageof120
sporesoftheAMFperplantlet.InoculationwithP.indicawas
(hyphaeandchlamydospores).IntheMixtreatment,40sporesof
eachAMFandtwo1-cmdiscswithKMcontainingP.indicawere
usedforinoculation.Controlplantsreceivednofungalinoculum.
Themoistureofthesubstrateinthepotswascorrected
periodi-callywithdistilledwater.Clark’snutrientsolution(50mL)without
Pwasappliedevery20days(Clark,1975).
2.5. Plantgrowthmeasurementsandshootnutrientanalysis
At210daftertransplanting,theplantheight(H),thenumber
ofleaves(NL),andtheshootdrymatter(SDM)weredetermined.
The SDMwas determinedafter drying toa constant weightat
70◦Cinanovenunderforcedventilation.Thismaterialwasthen
groundinaWileymillwitha0.420-mmsieveandsubjectedto
nitroperchloricdigestion(JohnsonandUlrich,1959)todetermine
theconcentrationsofthenutrientsP,K,Ca,andMg.These
nutri-ents,exceptP,weremeasuredbyopticalemissionspectrometry
withinductivelycoupledplasma(ICP-OES)(Optima8300ICP-OES
Spectrometer,PerkinElmer).ForNanalysisoftheshoots,the
min-eralizationwasperformeddry,bydirectincinerationofthesample
inmuffles,anditscontentwasdeterminedbytheKjeldahlmethod
(EMBRAPA,1999).ThePcontentoftheshootswasdetermined
col-orimetricallybythevitaminCmethodasmodifiedbyBragaand
Defelipo(1974).
2.6. Photosyntheticparameters
Thevaluesofthephotosyntheticparameterswereassessedwith
aPAMfluorometer[JUNIOR-PAMTeachingChlorophyll
Fluorome-ter(Walz,Mess-undRegeltechnik)].Thefollowingphotosynthetic
parameterswereusedforanalysis:maximumphotochemical
effi-ciency or maximum quantum yield (Fv/Fm) and quenching or
non-photochemicaldissipation(qNandNPQ).Thesevalueswere
obtainedfromintermediateandfullyexpandedleaves.The
min-imal fluorescence (Fo) was measured after the application of
modulatedlight (<0.1molphoton m−2s−1)toleavesthat had
beendark-adaptedforatleast30min.Themaximalfluorescence
(Fm)wasobtainedbyimposingapulsesaturationof10,000molm
−2s−1for0.6s.
2.7. Fungalcolonization
Approximately0.5gofrootsystemperplantwasdiaphanized
in10%KOH(w:v)for12h,followedbythreesuccessivewashes
intapwater.Afterwashing,therootsystemwasplacedinHCl2%
(w:w)for5minandthenwasstainedwith0.05%trypanbluein
lactoglycerol(w:v)at70◦Cfor30–40min.Thesamplewasthen
storedinlactoglycerol(PhillipsandHayman,1970;Brundrettetal.,
1996).Rootcolonizationwasquantifiedbyusingthegridline
inter-sectmethod(Giovannettiand Mosse,1980)withastereoscopic
microscope.
The mycorrhizal fungidependence was determined
accord-ing to Plenchette et al. (1983) with modifications, using the
equation:(FD)={(SDMofinoculatedplants−SDMnon-inoculated
plants)/SDMof inoculatedplants}×100. Thesameformulawas
usedtocalculatetheP.indicadependence.So,wearedenominating
fungaldependence(FD)toreferatbothfungi.
2.8. Statisticalanalysis
Thedata weresubjected toanalysisof variance (ANOVA)at
an˛ levelof 5%.Themeanswerecompared usingTukey’s test
(p≤0.05).Quantitativedataweresubjectedtoaregression
anal-ysis,andtheregressioncoefficientswereanalyzedusingStudent’s
t-test.Thedatarelatingtofungalcolonizationwerefirst
normal-izedviaanarcsin√(x/100)transformationforasubsequentANOVA.
TheFD datawere classifiedaccordingtoHabte andManjunath
(1991):>75%=excessivedependence;50–75%=highdependence;
25–50%=moderatedependence;<25%=marginaldependence;and
noresponsetoinoculation=independence.
3. Results
3.1. Plantgrowthmeasurements
ThepineappleplantletsrespondedtoinoculationwithbothAMF
and P. indica. A quadratic regression model successfully fit the
responseofthegrowthparameterstoincreasesintheapplication
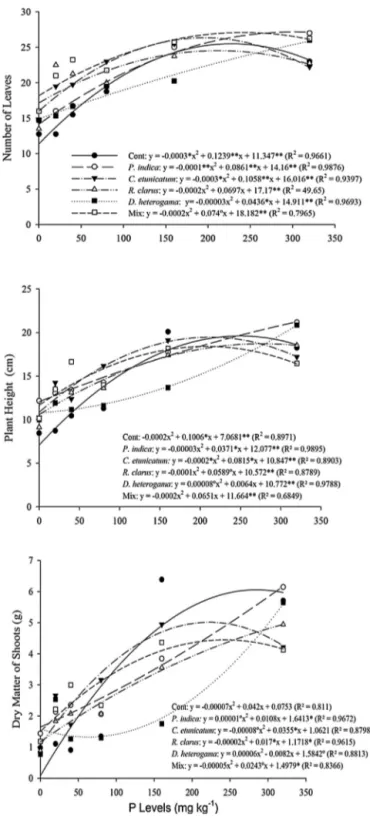
ofPtothesubstrate(Fig.1).
Ingeneral,theinoculatedplantletsgrewbetterthantheir
non-inoculated counterparts at doses of P up to40mgkg−1 for all
treatmentswithfungi(Fig.1).However,withincreasingamountsof
Pinthesubstrate,thisgrowthwaslesspronounced.At80mgkg−1
P, only the C. etunicatum and Mix treatments showed positive
effects onSDMafterinoculationcompared withthecontrol. At
160mgkg−1,theSDMinthecontroltreatmentwassuperiortothe
correspondingvaluesintheothertreatments(Fig.1).
AtlowerdosesofP(0–40mgkg−1P),dependingonthe
inoc-ulatedfungi,pineappleplantletsshowedasignificantincreasein
thegrowthparametersdependingonfungalidentity.Atthedoseof
0mgkg−1,therewereincreasesinHof70.8,53.4,49.5,52.4and65%
inplantletsinoculatedwithP.indica,C.etunicatum,R.clarus,D.
het-erogamaandMix,respectively,whereasNLincreasedby24.7,41.1,
51.3,31.4and60.2%,respectively,forthesamefungi.Forthissame
doseofP,SDMincreasedby2079.6,1310.4,1456.1,2003.8and
1889.2inresponsetoP.indica,C.etunicatum,R.clarus,D.heterogama
andMix,respectively.
At40mgkg−1,thisbenefitwaslower,butincreasesof25.4,27.9,
18.5,1.1and29.4%inHandof10.2,24.9,24.1,4.9and31.5%inNL
wereobservedforP.indica,C.etunicatum,R.clarus,D.heterogama
andMix,respectively.Further,theSDMincreasedby27.1,43.2,10.7
and45.4%forP.indica,C.etunicatum,R.clarusandMix,respectively.
Atthe40mgkg−1dose,theplantletsinoculatedwithD.heterogama
showedadecreaseof17.71%inSDM(Fig.1).Ingeneral,thebest
resultsforgrowthparameterswereobservedwhenplants were
treatedwiththemixedfungalinoculum,C.etunicatumorR.clarus.
Theplantletsthatreceived80mgkg−1Pandwereinoculated
withR.clarusorP.indicashowed19.53%and13.99%decreasesin
SDM,respectively.Atthesamedose, theC.etunicatumandMix
treatmentsforthesameparametersproducedincreasesinSDMof
4.50%and13.48%,respectively(Fig.1).
3.2. Nutritionalcontentofplants
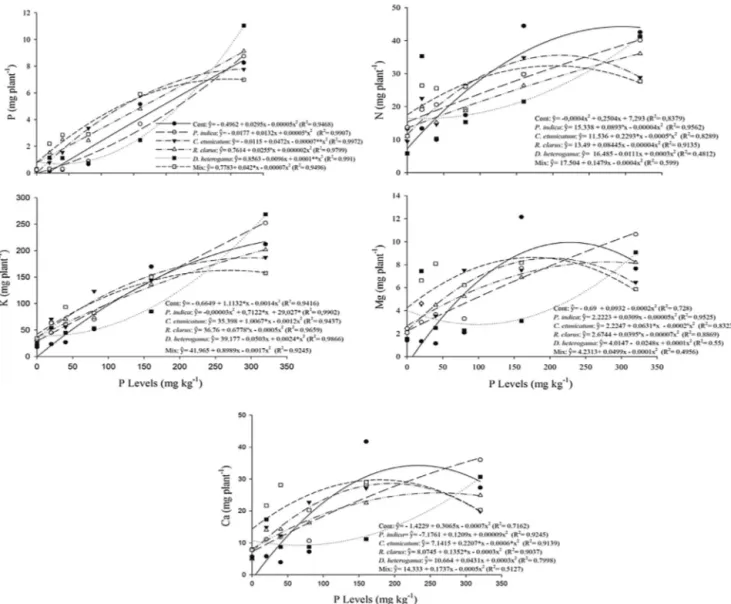
Thefungal inoculation(AMFand/or P.indica)wasbeneficial
(p≤0.05)forthenutritionalstatusofthepineappleplantlets.The
increasedPinthesubstrateinfluencedthecontent(mgplant−1)
ofPandoftheothermacronutrients,N,K,CaandMg(Fig.2).The
levelsofthesenutrientsincreasedwiththeapplicationofhigher
dosesofPforalltreatments.Atthelowestdoses(0–40mgkg−1),
thetreatmentswithfungishowedhighervaluesthanthosefound
inthecontrolplants.AtthehigherdosesofP,increasescompared
tothecontrolswereobservedinthecurvesforonlyafewfungal
treatments(Fig.2).
GreatereffectsoffungalinoculationonPuptakebyplantswere
observedatlowerdosesofP,andthemagnitudeoftheseeffects
variedinrelationtothecontroldependingontheinoculated
fun-gus.Atthedoseof 20mgkg−1,the plantletsinoculated withP.
indicashowedanincreaseofapproximately260%overthecontrol,
whereasC.etunicatum,R.clarus,D.heterogamaandMixtreatments
Fig.1.Numberofleaves(NL),plantheight(H)andshootdrymatter(SDM)ofpineappleplantletsmicropropagatedandinoculatedwithC.etunicatum,D.heterogama,R. clarus,P.indica,Mixorcontrol.Measurementswereobtained210daysafterinoculationinthegreenhousewithdifferentPlevelsinthesoil.◦,*and**,significantat10,5
and1%probability,respectively.
thePcontentperplant.TheincreasedPuptakewasconsiderably
lesspronouncedatthehigherPdoseof40mgkg−1.Thesevalues
were−2,192,194,4and228%forP.indica,C.etunicatum,R.clarus,
D.heterogamaandMix,respectively.Atdosesabove80mgkg−1,
onlythefungiC.etunicatum,R.clarus,andMixwereeffectivefor
increasingthecontentofP.Atthisdose(80mgkg−1),theC.
etuni-catum,R.clarus,andMixtreatmentsproducedgainsof114,80and
139%,respectively,relativetothecontrol(Fig.2).
TheeffectsofthetreatmentsonNcontentweresimilartotheir
effectsonPuptake.Atthedoseof0mgkg−1,increasesinNcontent
of110,58,84,126and140%wereobservedforthetreatmentswith
P.indica,C.etunicatum,R.clarus,D.heterogamaandMix,
respec-tively.ThesevalueswerealsolowerwithincreasingdosesofP,
showinggainsinNcontentof13,19,0.8,−0.88and36%,
respec-tively,forthesametreatmentsatthedoseof40mgkg−1(Fig.2).
At80mgkg−1,onlytheC.etunicatumandMixtreatmentsshowed
significantgainsintheNcontentperplant,namely7.7%forC.
etuni-catumand36.6%forMix.Fortheothertreatmentsatthe80mgkg−1
doseand foralltreatments atgreater doses,thecontrol plants
showedahighermeanNcontent.
ThelevelsofKandMgwerealsoinfluencedbyfungal
Fig.2.Content(mgplant−1)ofP,N,K,MgandCainmicropropagatedpineappleplantletsinoculatedwithC.etunicatum,D.heterogama,R.clarus,P.indica,Mixorcontrol.
Measurementswereobtained210daysafterinoculationinthegreenhousewithdifferentPlevelsinthesoil.◦,*and**,significantat10,5and1%probability,respectively.
20mgkg−1,theplantletsinoculatedwithP.indica,C.etunicatum,R.
clarus,D.heterogamaandMixshowedincreasesof105,161,138,85
and181%inKcontentandof157,211,214,315and374%inMg
con-tent,respectively.At80mgkg−1,mostoftheinoculatedplantlets
stillshowedrelativeincreasesintheabsorptionofKof8,36,10,
and29%forP.indica,C.etunicatum,R.clarus,andMix,respectively.
OnlytheplantletsinoculatedwithC.etunicatum,D.heterogamaand
MixshowedahigherMgcontentthanthecontrols,withgainsof
9,21and38%,respectively,whenPwasappliedat80mgkg−1soil
(Fig.2).
TheCacontentwasalsoaffectedbytheinoculationofpineapple
plantletswiththesefungi.TheabsorptionofCaincreasedmainly
underexposuretolowerdosesofP,reaching91%(P.indica),126%(C.
etunicatum),113%(R.clarus),133%(D.heterogama)and253%(Mix)
ofthecontrollevelatadoseof20mgkg−1.ThishighCacontent
comparedwiththecontrolalsodecreasedwithincreasingPdoses.
ForCa,thestrongestpositiveresponsesat80mgkg−1werethose
forC.etunicatumandMix(Fig.2).
3.3. Measurementsofphotosyntheticparameters
P.indicaandAMFpositivelyinfluencedthephotosynthetic
effi-ciencyoftheplants,especiallyatlowdosesofP(P≤40mgkg−1)
(Fig. 3).The Fv/Fm parameter, which indicatestheefficiency at
whichlightenergycapturedinPSIIistransferredtoother
devel-opingphotochemicalreactions,waslowerinthecontrolsthanin
theothertreatmentsuptoadoseof40mgkg−1(oruptoadose
of320mgkg−1inthecaseoftheP.indicaandMixtreatments).At
80and160mgkg−1,nodifferencesinFv/Fmwerefoundamongthe
treatments.Fortheparametersmeasuringtheamountofenergy
capturedbyPSIIthatwasdissipatedasheat(qNandNPQ),the
controlsweresuperiortomosttreatmentsatvariousdosesofP.
TheMixtreatmentshowedthelowestqNandNPQatvirtuallyallP
levels,followedbytheremainingAMF,C.etunicatum,R.clarusand
D.heterogama,whichbehavedsimilarly.ThefungusP.indicawas
lessefficientthantheAMFinreducingqNandNPQbutwasmore
efficientthanthecontrolatdosesof40and80mgkg−1(Fig.3).
3.4. Fungalcolonization
Thefungal colonizationwassuccessful bothfor plants
inoc-ulated with AMF and for those inoculated with P. indica, with
structurescharacteristicofintraradicularcolonizationobservedin
treatedplantsandnocolonizationinthecontrolplants(Fig.4).
Withinthegroupsoftreatedplantsthatreceivedeachindividual
AMF,therewasnodifference(p≥0.05)inthepercentageof
fun-galcolonization,evenwhenhigherdosesofPwereappliedtothe
Fig.3.Maximumphotochemicalefficiency(Fv/Fm)andquenching(qN)ornon-photochemicaldissipation(NPQ)inmicropropagatedpineappleplantletsinoculatedwithC.
etunicatum,D.heterogama,R.clarus,P.indica,Mixorcontrol(notinoculated).Measurementswereobtainedafter210daysofgrowthinthegreenhousewithdifferentPlevels inthesoil.MeansfollowedbythesameletterwithinthesamedoseofPdonotdiffer(Tukey’stest,5%probability).
Table1
PercentageofcolonizationbyP.indicaandmycorrhizalfungiinmicropropagation-derivedpineappleplantletsunderdifferentphosphorus(P)levelsafter210daysof acclimatizationinthegreenhouse.
P(mgkg−1) P.indica R.clarus C.etunicatum D.heterogama Mix
0 20.94±9.54 bA 67.40±10.41 aA 37.64±27.10 abA 57.75±30.66 abA 59.50±11.39 AB 20 22.78±10.12 cA 72.88±7.03 aA 26.11±22.31 cA 45.49±19.06 bcA 67.82±7.58 abAB 40 28.11±6.32 bA 69.41±7.89 aA 46.95±20.54 abA 42.72±27.27 abA 76.50±15.59 aAB 80 27.31±5.81 bA 74.35±3.58 aA 69.08±15.79 aA 37.45±25.82 abA 74.19±9.01 aAB 160 21.13±5.36 dA 71.07±5.76 abA 46.53±13.24 bcA 39.67±23.02 cdA 75.50±3.70 aAB 320 18.71±6.79 bA 69.95±7.43 abA 41.49±31.66 abA 40.56±22.38 bA 81.50±6.56 aA Meansfollowedbythesamelowercaseletterinthesamerowandmeansfollowedbythesamecapitalletter,inthesamecolumn,donotdifferbyTukeytestat5%probability.
colonizationbutdidnotdifferfromtheotherfungaltreatments underthesameconditionsofPfertilization(Table1).FortheAMF
treatmentswiththesamedoseofP,thetreatmentsinoculatedwith
MixorR.clarushadthehighestaveragepercentageofmycorrhizal
colonizationatthehigherdosesofP(Table1).Furthermore,the
percentageofcolonizationwithP.indicadidnotvarywith
increas-ingdosesofP,andremainedlowerthanthatforAMF-inoculated
Fig.4. TypicalstructuresobservedinassociationwitharbuscularmycorrhizalfungiandPiriformosporaindica.(A)Controlwithoutfungalcolonization;(B,DandF)colonization witharbuscularmycorrhizalfungi,withthepresenceofarbuscules,vesiclesandhyphae;(C)colonizationwithP.indicaintherootcortex;and(E)colonizationbyP.indica intheroothairs,withhyphaeandchlamydospores.Theassessmentwasperformed210daysafterinoculationinagreenhouseinthepresenceofvariousPlevelsinthesoil.
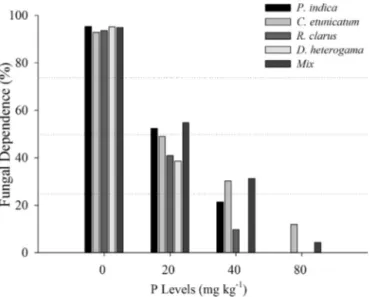
3.5. Fungaldependence
Thefungaldependenceofthepineappleplantletswasexcessive
at0mgkg−1andwasmoderatetohighatdosesof20,40mgkg−1
P in the soil for all fungal treatments (Fig. 5). At 80, 160 and
320mgkg−1Pinthesoil,theplantsdidnotdependonfungal
col-onizationforgrowthanddevelopmentintermsoftheresponseof
shootbiomass.
4. Discussion
StudiesontheeffectofAMFontissueculture-derivedpineapple
plantletsarescarce.However,paststudiesonthistopichaveused
variousAMFspecies,includingClaroideoglomusclaroideum(=
Glo-musclaroideum),Rhizophagusfasciculatus(=Glomusfasciculatum)
(Gutiérrez-Olivaetal.,2009)andFunneliformismosseae(=Glomus
mosseae)(Rodríguez-Romeroetal.,2011),whichwerenotusedin
thecurrentstudy.
Ingeneral,plantscolonizedbyAMFandP.indicashowedbetter
growthandincreasedabsorptionofnutrients,especiallyifallfungi
wereinoculatedtogether(Mix)andatlowerdosesofP.Positive
responsestocolonizationbyAMFhavealreadybeenreportedin
pineapple(Gutiérrez-Olivaetal.,2009;Rodríguez-Romeroetal.,
2011).Note,however,thatthisstudyisthefirstreportevaluating
thecolonizationofP.indicaanditseffectsonpineappleplantlets
andshowingroothaircolonization(Fig.4)asnotedbyWalleretal.
(2005).
Strongresponsesinthegrowthanddevelopmentofplants
inoc-ulatedwithAMFhavebeenwelldocumentedforseveralspeciesof
plants,e.g.,maize(ZeamaysL.)(Cozzolinoetal.,2013),oregano
(Ori-ganumonites),mint(Mentharequienii)(Karagiannidisetal.,2011),
papaya(Caricapapaya)andpineapple(Rodríguez-Romeroetal.,
Fig.5. FungaldependenceinpineappleplantletsinoculatedwithC.etunicatum,D. heterogama,R.clarus,P.indicaandMix.Measurementswereobtained210daysafter inoculationinthegreenhousewithdifferentPlevels.>75%=excessivedependence; 50–75%=high dependence; 25–50%=moderate dependence; <25%=marginal dependence;noresponsetoinoculation=independence.
L.(Yadavetal.,2011),GlycyrrhizaglabraL.(Yadavetal.,2013a)and
GloriosasuperbaL.(Yadavetal.,2013b).P.indicainoculationhas
beenreportedtobenefitbarley(Hordeumvulgare)(Walleretal.,
2005), cabbage(Brassica rapa)(Sunet al.,2010)and beet(Beta
vulgaris)(Varmaetal.,2012),particularlyifthesoilPlevelsare
low.
Althoughthepositiveresponsesofthegrowthparameterswere
stronglyrelatedtothecontrolsatsmallerdosesofP,thegrowth
ofinoculatedplantletsatthedoseof80mgkg−1wassuperiorto
theirgrowthatthedoseof40mgkg−1.Thisresultindicatesthat
bothofthesedoses,whichareabovethatrecommended
(approx-imately36mgkg−1)forcultureunderconditionsoflowsoilPin
thefield(Souzaetal.,1999),aregoodoptionsfortheproduction
ofplantletsinoculatedwiththesefungi.TheincreasesinNL,Hand
SDMresultingfromassociationswithAMFand/orP.indica,
espe-ciallyatrelativelylowdosesofP,arecloselylinkedwiththefungal
colonizationandmorphologyoftheroots.Thepineapplehasashort
andcompactrootsystemnearthestem,withmanythickrootsand
limitedbranching(d’EeckenbruggeandLeal,2003).This
character-isticindicatesthatfungalcolonizationplaysanimportantrolein
theuptakeofwaterandnutrients.Colonizedrootscanexplorea
greatervolumeofsoil(SmithandRead,1997)andshowmorethan
100timesthecoverageareaoftherootsofanon-colonizedplant
(Sieverding,1991).Moreover,thesmaller-diameterhyphalfungi
allowaccesstothesoilmicropores(Grantetal.,2005),andthe
broadgrowthofthecolonizedrootsresultsinawell-developed
networkofseveralcentimetersinextent,whichcanreachareas
wherePisnotdepleted(Smithetal.,2011).Incontrast,plantsnot
colonizedwiththesesymbioticfungicannotexploretheseareas.
NutrientabsorptionwasconsiderablyinfluencedbyAMFand
P.indica,especiallyuptoaPdoseof40mgkg−1(Fig.2).Thefungi
increasedthecontentsofP,N,K,CaandMgcomparedwiththe
non-inoculatedcontrols.However,theseedlingsinoculatedwithMix
andC.etunicatum,followedbyR.clarus,presentedbetter
absorp-tionformanyofthesenutrients,evenataPdoseof80mgkg−1.
Thesebenefits observedin colonized plantlets have oftenbeen
describedfortheuptakeofP(Kahiluotoetal.,2000;Yadavetal.,
2010;Rodríguez-Romeroetal.,2011;Tongetal.,2013;Xieetal., 2014),ofN(Azcónetal.,2008;Oelmülleretal.,2009; Rodríguez-Romeroetal.,2011;Tongetal.,2013),ofK(Rodríguez-Romero etal.,2011),ofS(Oelmülleretal.,2009),ofCuandZn(Kahiluoto
etal.,2000;Karagiannidisetal.,2011;Tongetal.,2013),andofCa, FeandMn(Karagiannidisetal.,2011).Thesefindingsincludethe
resultsofstudiesofpineappleplantlets(Rodríguez-Romeroetal.,
2011)andmayvarybasedonthespeciesofplant-colonizingAMF
incombinationwiththeconcentrationofPinthesoil(
Gutiérrez-Olivaetal.,2009).Inaddition,differencesinthesebenefitsmay
dependontheplantvarietystudied(Karagiannidisetal.,2011)or
thehosttissueanalyzed(Tongetal.,2013).
For the pineapple plantlets that received more than
160mgPkg−1, the controls presented better growth
parame-ters and nutrient contents per plant, indicating that the fungi
tend tohave deleteriouseffects onthese characteristicsat this
highlevelofPinthesoil.TheincreasingavailabilityofPinsoil,
incombinationwiththehighrateofcolonization,canreducethe
growthoftheplantduetothehighercostofcarbonsynthesizedby
theplanttosupplythefungianddecreasedbenefitsfornutrient
absorptionbecausetheiravailability isgreater(Kahiluotoetal.,
2000).
AtlowerdosesofP,pineappleplantletsinoculatedwithAMF
showedhigher efficiencyof PSIIin capturingenergy, which is
senttootherphotosyntheticreactions.However,thecontrolslost
moreenergyasheat,showinghigherqNandNPQvaluesthanthe
inoculatedplants,particularlyatdosesof20,40and80mgkg−1
(Fig.3).Asaresult,thephotosyntheticrateofinoculatedplantlets
washigherthanthatofthecontrols.Thisbehaviorwasalsofound
intomatoplantsinoculatedwithF.mosseae(Boldtetal.,2011).In
barleyseedlings,ahigherphotosyntheticratewasobservedunder
lowlightconditionswhenplantswereinoculatedwithP.indica
(Achatzetal.,2010).Investmentsofplantresourcesin the
syn-thesisofphotoassimilatestobetransferredtofungisuchasAMF
andP.indicaareoffset,inpart,bythehigherphotosyntheticrates
(Balotaetal.,2011;Smithetal.,2011)and/orbysavingsresulting
fromthereductioninrootproduction (Smithetal.,2011).
Fac-torsthat enhancephotosynthesisbymycorrhizalplants include
increasesinthetransportofinorganicelementsfromthesoilto
theplant(Soleimanzadeh,2010),inchlorophyllcontent(
Estrada-LunaandDavies,2003;Yadavetal.,2013a,b),andintheratesof
photosyntheticstorageandexport(Augé,2001).
Thesecharacteristicsofgrowthpromotion,improvednutrient
contentandenhancedphotosyntheticefficiencyhavemotivated
increasingresearchactivityontheinoculationofplantlets with
AMF(Estrada-LunaandDavies,2003;Kapooretal.,2008;Singh etal.,2012;Yadavetal.,2013a,b)andwithP.indica(Sahayand Varma,1999)toaidin theacclimatizationstage.Forpineapple,
theinoculated plantletsweremorevigorous andshowedbetter
developmentduringthisstage.
Ingeneral,increasesintheavailabilityofsoilPtendtodecrease
mycorrhizalcolonization(Kahiluotoetal.,2000;Soleimanzadeh,
2010;Balotaetal.,2011;Smithetal.,2011;Shuklaetal.,2012).This
decreaseinrootcolonizationmayberelatedtotheincreased
con-centrationofPpergramofdrymatterintheplant(Liuetal.,2000).
However,here,inpineappleplantletsexposedtothesame
treat-ments,thepercentagesoffungalcolonizationremainedhigh,even
athighdosesofP(Table1).Similarresultswerenotedinthe
stud-iesofXavierandGermida(1997),Cozzolinoetal.(2013)andTong etal.(2013),andthecolonizationpercentagesweremuchhigher
thanthatfoundbyRodríguez-Romeroetal.(2011)inpineapple.
Thismaybeduetothedilutioneffectofthenutrientsinplant
tis-sue,inwhichincreasedabsorptionproducesahigheramountof
biomassratherthanagreaterconcentrationofnutrients,increasing
theexudationofcarbohydratesandthusstimulatingmycorrhizal
colonization(Liuetal.,2000;Azcónetal.,2003).
Foralmostallevaluatedparameters,thetreatmentcontaining
amixtureofallfungiwasmoreeffectiveinpromotingthegrowth
oftheplantlets,whichhasbeenobservedinthestudiesofYadav
ofdifferentspeciesallocatedindifferentorders(Glomeralesand
Diversisporales—Redeckeretal.,2013)hasbeenshowntohavea
positiverelationshipwithbiomassproductioninPlantago
lanceo-lata(MaheraliandKlironomos,2007).BecausedifferentAMFmay
transmitdifferentamountsofPtotheplant,theireffectsonplant
growthmaybedifferent(Shuklaetal.,2012).However,because
theplantscanbecolonizedsimultaneouslybyfungifrom
differ-enttaxa(Smithetal.,2011),thebeneficialcharacteristicsofeach
funguscouldpotentiallybeexploitedbytheplant.Thispatternis
observedunderfieldconditions,inwhichthesumofallthe
bene-fitsofcolonizationbydifferentfungicontributestothesuccessof
mycorrhizaeinfacilitatinggrowthandreproduction(Smithetal.,
2011).
ThedecreaseobservedinfungaldependencewithincreasingP
levelssuggeststhattheeffectoffungalinoculationismore
pro-nouncedatlowerdosesofP(Kahiluotoetal.,2000;Balotaetal.,
2011).However,evenwithnodependenceoftheplantletsatthe
80mgkg−1doseandmoderatedependenceatthe40mgkg−1dose
forC.etunicatumandMix,thehighrateofcolonizationunderthese
conditionsishighlysignificantforpineapplecultivationbecause
therecommendedsoilPlevelforthiscultureinsoilswithalowP
levelisapproximately35mgkg−1 (Souzaetal.,1999).Thiseffect
maybemoresignificantunderfieldconditions,wherethesoilisless
homogeneousandmycorrhizalassociationcanimprovenutrient
absorption.
Inthefield,pineappleis considerednon-responsiveto
phos-phatefertilizationintermsoffruitproduction(Spironelloetal.,
2004; Guarc¸oni M. and Ventura, 2011; Caetano et al., 2013),
withmycorrhizalcolonizationconsideredoneofthemainfactors
responsibleforthesupplyofPtoplants(Guarc¸oniM.andVentura,
2011).Therefore,inviewoftheimportanceofPnutritioninthe
earlystagesofcropdevelopment,theroleofmycorrhizal
associa-tionattheseedlingstageremainstobeestablishedinthecontextof
theexplorationoftheoverallproductivepotentialofplants(Grant
etal.,2005).
For pineapple plantlets, inoculation withAMF and P. indica
confersmanybenefitsanddoesnotinterferewiththeprocessof
producingpropagativematerialovertime.Inaddition,itdoesnot
addalabor-intensivecomponenttotheproductionchainbecause
theinoculationmethodissimpleandplantletsmustremaininthe
greenhouseforapproximatelysixmonths(Farahani,2013).
5. Conclusions
MicropropagatedpineappleplantletscolonizedbyAMFandP.
indicashowenhancedgrowth,nutrientuptakeandphotosynthetic
efficiency,particularlyatlow soilPlevels(≤40mgkg−1).AtaP
levelequivalenttotherecommendedfertilizationrate(35mgkg−1
inthefield),inoculatedplantletsarebetternourishedand
vigor-ous,especiallywheninoculatedwithC.etunicatumorMix.Further
fieldevaluationsareneededtodeterminewhetherinoculation
dur-ingtheseedlingstageisreflectedinproductivityorcanserveasa
meanstoreducechemicalfertilization.Suchoutcomeswouldresult
notonlyintheoptimaluseoffertilizerbutalsoineconomicbenefits
fortheproducers.
Acknowledgments
TheauthorsthanktheConselhoNacionaldeDesenvolvimento
CientíficoeTecnológico(CNPq),Coordenac¸ãodeAperfeic¸oamento
de Pessoal de Nível Superior (CAPES), Fundac¸ão de Amparo à
PesquisadoEstadodeMinasGerais(FAPEMIG)andFundac¸ãode
AmparoàPesquisaeInovac¸ãodoEstadodeSantaCatarina(FAPESC)
forfinancialsupport.TheauthorsalsothankUniversidadeRegional
de Blumenau-Santa Catarinafor providing theinoculum
main-tainedattheInternationalCultureCollectionofGlomeromycota
(CICG).
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,
intheonlineversion,athttp://dx.doi.org/10.1016/j.scienta.2015.
09.032.
References
Achatz,B.,Rüden,S.,Andrade,D.,Neumann,E.,Pons-Kühnemann,J.,Kogel,K.H., Franken,P.,Waller,F.,2010.RootcolonizationbyPiriformosporaindica enhancesgrainyieldinbarleyunderdiversenutrientregimesbyaccelerating plantdevelopment.PlantSoil333,59–70.
Augé,R.M.,2001.Waterrelations,droughtandvesicular-arbuscularmycorrhizal symbiosis.Mycorrhiza11,3–42.
Azcón,R.,Ambrosano,E.,Charest,C.,2003.Nutrientacquisitioninmycorrhizal lettuceplantsunderdifferentphosphorusandnitrogenconcentration.Plant Sci.165,1137–1145.
Azcón,R.,Rodríguez,R.,Amora-Lazcano,E.,Ambrosano,E.,2008.Uptakeand metabolismofnitrateinmycorrhizalplantsasaffectedbywateravailability andNconcentrationinsoil.Eur.J.SoilSci.59,131–138.
Azcón-Aguilar,C.,Cantos,M.,Troncoso,A.,Barea,J.M.,1997.Beneficialeffect arbuscularmycorrhizasonacclimatizationofmicropropagatedcassava plantlets.Sci.Hortic.72,63–71.
Balota,E.L.,Machineski,O.,Truber,P.V.,Scherer,A.,Souza,F.S.,2011.Physicnut plantspresenthighmycorrhizaldependencyunderconditionsoflow phosphateavailability.Braz.J.PlantPhysiol.23,33–44.
Be,L.V.,Debergh,P.C.,2006.Potentiallow-costmicropropagationofpineapple (Ananascomosus).S.Afr.J.Bot.72,191–194.
Boldt,K.,Pörs,Y.,Haupt,B.,Bitterlich,M.,Kühn,C.,Grimm,B.,Franken,P.,2011.
Photochemicalprocesses,carbonassimilationandRNAaccumulationof sucrosetransportergenesintomatoarbuscularmycorrhiza.J.PlantPhysiol. 168,1256–1263.
Braga,J.M.,Defelipo,B.V.,1974.Determinac¸ãoespectofotométricadefósforoem extratosdesoloseplanta.Rev.Ceres21,73–85.
Brundrett,M.,Bougher,N.,Dell,B.,Grove,T.,Malajczuk,N.,1996.Workingwith MycorrhizasinForestryandAgriculture.ACIARMonograph.
Caetano,L.C.S.,Ventura,J.A.,Costa,A.F.S.,Guarc¸oni,R.C.,2013.Efeitodaadubac¸ão comnitrogêniofósforoepotássionodesenvolvimentonaproduc¸ãoena qualidadedefrutosdoabacaxi‘Vitória’.Rev.Bras.Frutic.35,883–890.
Chandra,S.,Bandopadhyay,R.,Kumar,V.,Chandra,R.,2010.Acclimatizationof tissueculturedplantlets:fromlaboratorytoland.Biotechnol.Lett.32, 1199–1205.
Clark,R.B.,1975.Characterizationofphosphatasesofintactmaizeroots.J.Agric. FoodChem.23,458–460.
Cozzolino,V.,DiMeo,V.,Piccolo,A.,2013.Impactofarbuscularmycorrhizalfungi applicationsonmaizeproductionandsoilphosphorusavailability.J.Geochem. Explor.129,40–44.
d’Eeckenbrugge,G.C.,Leal,F.,2003.Morphology,anatomyandtaxonomy.In: Bartholomew,D.P.,Paull,R.E.,Rohrbach,K.G.(Eds.),ThePineapple:Botany, ProductionandUses.CABIPublishing,Wallingford,U.K,pp.13–32.
EmpresaBrasileiradePesquisaAgropecuária—EMBRAPA,1999.Manualdeanálises químicasdesolos,plantasefertilizantes.CentroNacionaldePesquisadeSolos, RiodeJaneiro.
Escalona,M.,Lorenzo,J.C.,González,B.,Daquinta,M.,González,J.L.,Desjardins,Y., Borroto,C.G.,1999.Pineapple(AnanascomosusL.Merr.)micropropagationin temporaryimmersionsystems.PlantCellRep.18,743–748.
Estrada-Luna,A.A.,Davies,J.F.T.,2003.Arbuscularmycorrhizalfungiinfluence waterrelations,gasexchange,abscisicacidandgrowthofmicropropagated chileanchopepper(Capsicumannuum)plantletsduringacclimatizationand post-acclimatization.J.PlantPhysiol.160,1073–1083.
FAOSTATAcessoemjulhode,2014.Disponívelem:<http://faostat.fao.org/site/ 339/default.aspx/>.
Farahani,F.,2013.Growth,floweringandfruitinginvitropineapple(Ananas comosusL.)ingreenhouseconditions.Afr.J.Biotechnol.12(15),1774–1781.
Giovannetti,M.,Mosse,B.,1980.Anevaluationoftechniquesformeasuring vesiculararbuscularmycorrhizalinfectioninroots.NewPhytol.84,489–500.
González-Olmedo,J.L.,Fundora,Z.,Molina,L.A.,Abdulnour,J.,Desjardins,Y., Escalona,A.,2005.Newcontributionstopropagationofpineapple(Ananas comosusL.Merr.)intemporaryimmersionbioreactors.InVitroCell.Dev.Biol. Plant41(1),87–90.
Grant,C.,Bittman,S.,Montreal,M.,Plenchette,C.,Morel,C.,2005.Soilandfertilizer phosphorus:effectsonplantPsupplyandmycorrhizaldevelopment.Can.J. PlantSci.85,3–14.
Guarc¸oniM,A.,Ventura,J.A.,2011.Adubac¸ãoN-P-Keodesenvolvimento, ProdutividadeequalidadedosfrutosdoAbacaxi‘GOLD’(MD-2).Rev.Bras. Cienc.Solo35,1367–1376.
Gutiérrez-Oliva,V.F.,Abud-Archila,M.,Flores-Pérez,A.,Alvarez-Solis,J.D., Gutiérrez-Miceli,F.A.,2009.Influenciadeloshongosmicorrizicosarbusculares sobreelcrecimientodevitroplantulasdepi ˜na(Ananascomosus(L.)Merr.)Con diferentesnivelesdefosforo.GayanaBot.66(1),1–9.
Habte,M.,Manjunath,A.,1991.Categoriesofvesicular-arbuscularmycorrhizal dependencyofhostspecies.Mycorrhiza1,3–12.
Hazarika,B.N.,2006.Morpho-physiologicaldisordersininvitrocultureofplants. Sci.Hortic.108,105–120.
Hazarika,B.N.,Parthasarathy,V.A.,Bhowmik,G.,2002.Thephysiologicalstatusof micropropagatedplants—areview.Agric.Rev.23(1),53–58.
Hepton,A.,2003.Culturalsystem.In:Bartholomew,D.P.,Paull,R.E.,Rohrbach,K.G. (Eds.),ThePineapple:Botany,ProductionandUses.CABIPublishing, Wallingford,U.K,pp.109–142.
Hill,T.W.,Kaefer,E.,2001.Improvedprotocolsforaspergillusmedium:trace elementandminimalmediumsaltstocksolutions.FungalGenet.News48, 20–21.
Johnson,C.M.,Ulrich,A.,1959.AnalyticalMethodsforUseinPlantsAnalyses. UniversityofCalifornia,Bulletin766,LosAngeles,pp.32–33.
Kahiluoto,H.,Ketoja,E.,Vestberg,M.,2000.Promotionofutilizationofarbuscular mycorrhizathroughreducedPfertilization1:bioassaysinagrowthchamber. PlantSoil227,191–206.
Kapoor,R.,Sharma,D.,Bhatnagar,A.K.,2008.Arbuscularmycorrhizain micropropagationsystemsandtheirpotentialapplications.Sci.Hortic.116, 227–239.
Karagiannidis,N.,Thomidis,T.,Lazari,D.,Panou-Filotheou,E.,Karagiannidou,C., 2011.EffectofthreeGreekarbuscularmycorrhizalfungiinimprovingthe growth,nutrientconcentration,andproductionofessentialoilsoforeganoand mintplants.Sci.Hortic.129,329–334.
Kumar,K.,Rao,I.U.,2012.Morphophysiologicalsproblemsinacclimatizationof micropropagatedplantsin—exvitroconditions—areview.J.Ornam.Hortic. Plants2(4),271–283.
Kumar,V.,Sahai,V.,Bisaria,V.S.,2011.High-densitysporeproductionof Piriformosporaindica,aplantgrowth-promotingendophyte,byoptimizationof nutritionalandculturalparameters.Bioresour.Technol.102,3169–3175.
Liu,A.,Hamel,C.,Hamilton,R.I.,Smith,D.L.,2000.Mycorrhizaeformationand nutrientuptakeofnewcorn(ZeamaysL.)hybridswithextremecanopyand leafarchitectureasinfluencedbysoilNandPlevels.PlantSoil221,157–166.
Maherali,H.,Klironomos,J.N.,2007.Influenceofphylogenyonfungalcommunity assemblyandecosystemfunctioning.Science316,1746–1748.
Melo,A.S.,Netto,A.O.A.,Neto,J.D.,Brito,M.E.B.,Viégas,P.R.A.,Magalhães,L.T.S., Fernandes,P.D.,2006.Desenvolvimentovegetativo,rendimentodafrutae otimizac¸ãodoabacaxizeirocv.Pérolaemdiferentesníveisdeirrigac¸ão.Cienc. Rural36,93–98.
Menge,J.A.,Steirle,D.,Bagyaraj,D.J.,Johnson,E.L.V.,Leonard,R.T.,1978.
Phosphorusconcentrationsinplantsresponsibleforinhibitionofmycorrhizal infection.NewPhytol.80,575–578.
Murashige,T.,Skoog,F.,1962.Arevisedmediumforrapidgrowthandbioassays withtobaccotissuecultures.Physiol.Plant15,473–497.
Oelmüller,R.,Sherameti,I.,Tripathi,S.,Varma,A.,2009.Piriformosporaindica,a cultivablerootendophytewithmultiplebiotechnologicalapplications. Symbiosis49,1–17.
Phillips,J.M.,Hayman,D.S.,1970.Improvedproceduresforclearingrootsand stainingparasiticandvesicular-arbuscularmycorrhizalfungiforrapid assessmentofinfection.Br.Mycol.Soc.55,158–161.
Plenchette,C.,Fortin,J.A.,Furlan,V.,1983.Growthresponsesofseveralplant speciestomycorrhizaeinasoilofmoderatePfertility.I.Dependencyunder fieldconditions.PlantSoil70,199–209.
Pospíˇsilová,J.,Tichá,I.,Kadleˇcek,P.,Haisel,D.,Plzáková, ˇS.,1999.Acclimatization ofmicropropagatedplantstoexvitroconditions.Biol.Plant42(4),481–497.
Redecker,D.,Schüßler,A.,Stockinger,H.,Stürmer,S.L.,Morton,J.B.,Walker,C., 2013.Anevidence-basedconsensusfortheclassificationofarbuscular mycorrhizalfungi(Glomeromycota).Mycorrhiza23,515–531.
Rodríguez-Romero,A.S.,Azcón,R.,Jaizme-Vega,M.C.,2011.Earlymycorrhization oftwotropicalcrops,papaya(CaricapapayaL.)andpineapple[Ananascomosus (L.)Merr.],reducesthenecessityofPfertilizationduringthenurserystage. Fruits66(1),3–10.
Rout,G.R.,Mohapatra,A.,MohanJain,S.,2006.Tissuecultureofornamentalpot plant:acriticalreviewonpresentscenarioandfutureprospects.Biotechnol. Adv.24,531–560.
Sahay,N.S.,Varma,A.,1999.Piriformosporaindica:anewbiologicalhardeningtool formicropropagatedplants.FEMSMicrobiol.Lett.181,297–302.
Shukla,A.,Kumar,A.,Ajit,J.A.,N.Rao,D.V.K.,2012.Phosphorusthresholdfor arbuscularmycorrhizalcolonizationofcropsandtreeseedlings.Biol.Fertil. Soils48,109–116.
Sieverding,E.,1991.Vesicular-ArbuscularMycorrhizaManagementinTropical Agrosystems.TechnicalCooperation,Eschborn,FederalRepublicofGermany.
Silva,A.B.,Pasqual,M.,Teixeira,J.B.,Araújo,A.G.,2007.Métodosde
micropropagac¸ãodeabacaxizeiro.Pesqui.Agropec.Bras.42(9),1257–1260.
Singh,N.V.,Singh,S.K.,Singh,A.K.,Meshrama,D.T.,Suroshe,S.S.,Mishra,D.C.,2012.
Arbuscularmycorrhizalfungi(AMF)inducedhardeningofmicropropagated pomegranate(PunicagranatumL.)plantlets.Sci.Hortic.—Amst.136,122–127.
Smith,M.K.,Ko,H.L.,Hamill,S.D.,Sanewski,G.M.,Graham,M.W.,2003.
Biotechnology.In:Bartholomew,D.P.,Paull,R.E.,Rohrbach,K.G.(Eds.),The Pineapple:Botany,ProductionandUses.CABIPublishing,Wallingford,U.K,pp. 57–68.
Smith,S.E.,Facelli,E.,Pope,S.,Smith,F.A.,2010.Plantperformanceinstressful environments:interpretingnewandestablishedknowledgeoftherolesof arbuscularmycorrhizas.PlantSoil326,3–20.
Smith,S.E.,Jakobsen,I.,Gronlund,M.,Smith,F.A.,2011.Rolesofarbuscular mycorrhizasinplantphosphorusnutrition:interactionsbetweenpathwaysof phosphorusuptakeinarbuscularmycorrhizalrootshaveimportant implicationsforunderstandingandmanipulatingplantphosphorus acquisition.PlantPhysiol.156,1050–1057.
Smith,S.E.,Read,D.J.,1997.MycorrhizalSymbiosis.AcademicPress,Cambridge, MA.
Soleimanzadeh,H.,2010.EffectofVA-mycorrhizaongrowthandyieldof sunflower(HelianthusannuusL.)atdifferentphosphoruslevels.WorldAcad. Sci.Eng.Technol.4,375–378.
Souza,F.V.D.,Souza,A.S.,Santos-Serejo,J.A.,Souza,E.H.,Junghans,T.G.,Silva,M.J., 2013.Micropropagac¸ãodoAbacaxizeiroeOutrasBromeliáceas.In:Junghans, T.G.,Souza,A.S.(Eds.),AspectosPráticosdaMicropropagac¸ãodePlantas.,2ed. EMBRAPA,DF,pp.188–218.
Souza,M.,Guimarães,P.T.G.,Carvalho,J.G.,Fragoas,J.C.,1999.Abacazixeiro.In: Ribeiro,A.C.,Guimarães,P.T.G.,Alvarez,V.V.H.(Eds.),Recomendac¸õesParaO UsoDeCorretivosEFertilizantesEmMinasGerais.,1◦ed.SBCS,Vic¸osa.
Spironello,A.,Quaggio,J.A.,Teixeira,L.A.J.,Furlani,P.R.,Sigrist,J.M.M.,2004.
PineappleyeldandfruitqualityeffectedbyNPKfertilizationinatropicalsoil. Rev.Bras.Frutic.26,155–159.
Sun,C.,Johnson,J.M.,Cai,D.,Sherameti,I.,Oelmüller,R.,Lou,B.,2010.
PiriformosporaindicaconfersdroughttoleranceinChinesecabbageleavesby stimulatingantioxidantenzymes,theexpressionofdrought-relatedgenesand theplastid-localizedCASprotein.J.PlantPhysiol.167,1009–1017.
Tong,Yu.,Gabriel-Neumann,E.,Ngwene,B.,Krumbein,A.,Baldermann,S., Schreiner,M.,George,E.,2013.Effectsofsingleandmixedinoculationwith twoarbuscularmycorrhizalfungiintwodifferentlevelsofphosphorussupply on-caroteneconcentrationsinsweetpotato(IpomoeabatatasL.)tubers. PlantSoil372,361–374.
Varma,A.,Bakshi,M.,Lou,B.,Hartmann,A.,Oelmueller,R.,2012.Piriformospora indica:anovelplantgrowth-promotingmycorrhizalfungus.Agric.Res.1(2), 117–131.
Waller,F.,Achatz,B.,Baltruschat,H.,Fodor,J.,Becker,K.,Fischer,M.,Heier,T., Huckelhoven,R.,Neumann,C.,Wettstein,D.V.,Franken,P.,Kogel,K.,2005.The endophyticfungusPiriformosporaindicareprogramsbarleytosalt-stress tolerance,diseaseresistance,andhigheryield.PNAS102(38),13386–13391.
Xavier,L.J.C.,Germida,J.J.,1997.GrowthresponseoflentilandwheattoGlomus clarumNT4overarangeofPlevelsinaSaskatchewansoilcontaining indigenousAMfungi.Mycorrhiza7,3–8.
Xiao,Y.,Niu,G.,Kozai,T.,2011.Developmentandapplicationofphotoautotrophic micropropagationplantsystem.PlantCellTissueOrganCult.105,149–158.
Xie,X.,Weng,B.,Cai,B.,Dong,Y.,Yan,C.,2014.Effectsofarbuscularmycorrhizal inoculationandphosphorussupplyonthegrowthandnutrientuptakeof Kandeliaobovata(Sheue,Liu&Yong)seedlingsinautoclavedsoil.Appl.Soil Ecol.75,162–171.
Yadav,K.,Singh,N.,Aggarwal,A.,2011.Influenceofarbuscularmycorrhizal(AM) fungionsurvivalanddevelopmentofmicropropagatedAcoruscalamusL. duringacclimatization.J.Agric.Technol.7,775–781.
Yadav,K.,Aggarwal,A.,Singh,N.,2013a.Arbuscularmycorrhizalfungiinduced acclimatizationandgrowthenhancementofGlycyrrhizaglabraL.:apotential medicinalplant.Agric.Res.2,43–47.
Yadav,K.,Aggarwal,A.,Singh,N.,2013b.Arbuscularmycorrhizalfungi(AMF) inducedacclimatization,growthenhancementandcolchicinecontentof micropropagatedGloriosasuperbaL.plantlets.Ind.CropsProd.45,88–93.
Yadav,V.,Kumar,M.,Deep,D.K.,Kumar,H.,Sharma,R.,Tripathi,T.,Tuteja,N., Saxena,A.K.,Johri,A.K.,2010.Aphosphatetransporterfromtheroot endophyticfungusPiriformosporaindicaplaysaroleinthephosphate transporttothehostplant.J.Biol.Chem.285,26532–26544.