Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Division of Building Research); no.
DBR-P-1199, 1983
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=6056a64a-3ab5-4b9f-9f27-cf94262b2694 https://publications-cnrc.canada.ca/fra/voir/objet/?id=6056a64a-3ab5-4b9f-9f27-cf94262b2694
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/40001788
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Ignition of low-density polyethylene slabs by a small flame
Ser
TE3.lN218
'
National Research
Conseil national
I
no.
1199
I
c
ouncil Canada
de recherches Canada
I c . 2
I BLDG I
IGNITION OF LOW-DENSITY POLYETHYLENE SLABS BY A SMALL FLAME
Reprinted from
Journal of Polymer Science
Polymer Chemistry Edition, Vol. 21, 1983 p. 3225
-
3232N ~
-
CC I S T I1
BLDG.RES.
by F.R.S. Clark L ~ B R A R Y
DBR Paper No. 1199
Division of Building Research
~ ' ; ~ 3 ' " 1 2 ~ 1 6 ' - * Price $1.00 OTTAWA
04-
06-
3
0
I - . ! :;-y 4 t - J ~ -
..
2
'
:
:
,
! NRCC 23394Den p l a q u e s de p a l y € t h y l h e de F a i b l e densit* o n t B t K plscks dane l e a i l l a g e d e l a Clamme p l a n e d ' u n d l a n g e p s u v r e e n hydroghe e t e n oxyghe. Le d E l s i d'ailumage e t l a v i t e s a e I n i t i s l e de p r o p a g a t i o n d a l a flamme a p r h L'aLlumage o u t hcB mesurSe en f o n c t i o n d e p l r r s i e u r s r a p p a r t n d'€qulvnlcnce g a r a i r
e t B d i v e r s e s distances de l a Elamme d'allumage. A p r b
l ' e l l n m a g e , l a ternpikacure euperf l c i e k l e B t ait de beaucmp I n f B r i e u r e c e l l e c e q u i s e pour l a p y r o l y s e en l ' a b s e n c e U'oxygine. De p e t i t e s quantit'es de p r o d u i t s de c a r b o n i s a t i o n s e s o n t f o r k s 3 l a s i r r f a c e du polymsre pendant l e d f A a i , ce
q u i concorde a v e c l e a r h u l t a t s relatifs a u rale d e l'oxygsne
d a n s l e p r o c e s s u s de pr&llumsge an phase ~ o l i d e . La v a r i a t i o n d e , I n ( d b l a i ) en f o n c t l m de I/ (temp&ature a b a o l u e ) k t a i t ,-=-a r e e t l ' d n e r g i e d ' a c t i v a t l o n , 64 k 10 kJ molee1, a 6t'e t i c de 1 ' 6 q u a r l o n o p a g a t t o n de l a f l l e polym8re e t 1 n t a u x v i S e s s i po~yrmecny~mecnacrylate)
.
e l l e a St a + e n t l n o e p e n a a n t e s ae l a d u r k du ddLai d'ellumage. s m f d a n s l e c a r 03 l e polymPre f t a t t t r b p r b de Is E l a m . Lee r f i s u l t a t s sont e x p l i q u k B l ' a i d e d ' u n madale dans l e p u e l l e d i a l d ' a l l m a e e e t - l a v l t e s a e ' c o n c e n t r propagar d'Arrh5niu amme d i d 1 n Flamm e a o b c e IS. Lem tuafent l o a u g m n t a l n u e s S T-
. . v i t e u s e s c s q u e l a . t n a i s , r e c . l e-
6e B par lee de p r ICC e n t r e r a i r e m e 8 . .-
- . A 8.n-l c a l c u l i n f t i a d l s t a n c o n t.
,Ignition of Low-Density Polyethylene Slabs by a Small
Flame
FERRERS R. S. CLARK, Division of Building Research, National Research Council Canada, Ottawa, Canada K I A OR6
Synopsis
Slabs of low-density polyethylene (LDPE) were exposed to the wake of a lean hydrogen-oxygen flat flame. The ignition delay and initial flame velocity after the ignition were measured at several gas-air equivalence ratios and distances from the igniting flame. When ignition occurred, the surface temperature was far lower than that required for pyrolysis in the absence of oxygen. Small amounts of char formed on the polymer surface during the delay, consistent with the involvement of oxygen in solid-phase preignition processes. Plots of ln(de1ay) versus l/(absolute temperature) were linear and the activation energy was derived from the Arrhenius equation, 64 f 10 kJImol. Initial rates of flame development decreased with increased separation between the polymer and the igniting flame, but unlike those reported for poly(methy1 methacrylate), they were independent of the du- ration of the preceding delay except when the polymer was very close to the flame. The results are explained by a model in which both the ignition delay and the subsequent rate of flame development depend on the concentration of species associated with the chain-propagation steps of the combustion process.
INTRODUCTION
In a previous p u b l i ~ a t i o n , ~ ignition data for poly(methy1 methacrylate (PMMA) slabs exposed to a hydrogen-oxygen flat flame were reported. The thermal decomposition of polyethylene occurs in a manner different from that of PMMA.2 To probe the generality of the conclusions reached in the earlier work, the ignition behavior of low-density polyethylene (LDPE) was investigated using the same apparatus and conditions as in the study on PMMA.
EXPERIMENT
For general experimental details, ref. 1 should be consulted. LDPE samples 50 X 50 X 6 mm of unknown origin were used throughout. The following thermal property values (all determined at room temperature and treated as if they were independent of temperature) are used in the discussion: thermal conductivity,
K, 0.33 W m-l K-l; specific heat, C,, 2.3 X
lo3
J kg-I K-l; specific gravity, p,900 kg m-3; thermal diffusivity, k , 1.6 X m2 s-I (ref. 3).
Slabs of LDPE were placed on the sample plate of the apparatus previously described1 (Fig. 1). The assembly was raised to a predetermined distance, or "separation," from the sinter on which was stabilized a lean hydrogen-oxygen flat flame. The temperature was controlled by varying the equivalence ratio (defined as the volume of oxygen repaired for complete conversion of the hy- drogen to water divided by the actual volume of oxygen supplied) from 0.1 to 0.21. The variation in gas temperature with equivalence ratio and separation is dis-
Journal of Polymer Science: Polymer Chemistry Edition, Vol. 21,3225-3232 (1983)
CLARK
I
p
T H E R M O C O U P L Ec
---- ~ t;---a- i *2++!
---:!
+-- " e H 2 W A T E R O U T C H A M B E R \ r 1 1 , / J A C K E T ( B R A S S ) B R O N Z E S I N T E Rb-
2 6 . 3 4 m m S A M P L E P L A T E W A ~ E R w r i ~ ~ u O U TFig. 1. Burner assembly.
played in Figure 2 of ref. 1. The exit velocity of burner gases from the sinter, assuming complete combustion and a gas temperature of 1000°C, is 2.4 f 0.3 mls, and the Reynolds number is about 560. When measured as previously de- scribed,l the radiant heat contribution from the flame was at most 3 kW m-2. This is small compared with the total flux delivered to a heat flux gauge mounted at the sample position. At an equivalence ratio of 0.1, the total flux varied from 11.2
kW
m-2 a t 30 mm separation to 19.7kW
m-2 a t 5 mm.T I M E , s
IGNITION OF LDPE 3227
TABLE I
Ignition Delays for LDPE (in s)
Equivalence Separation (mm)
ratio 5 10 15 20 25 30
IGNITION DELAY
The nearly invisible blue reaction zone of the hydrogen-oxygen flame did not alter when samples were raised into position beneath it. The zone between the polymer and the igniting flame did not exhibit the blue preignition glow observed in the study on PMMA. The end of the delay was indicated by the sudden ap- pearance of a bright yellow or white flame adjacent to the polymer surface. When the samples were removed from the reaction zone, they generally continued to burn, indicating that the time to so-called transient ignition was essentially indistinguishable from the time to self-sustained combustion in these condi- tions.
Ignition delays were measured in quadruplicate for LDPE slabs at six sepa- rations from the burner and at eight equivalence ratios. The coefficient of variation among the delays in each set of four delay times did not exceed 5%. A summary of the data thus obtained appears in Table I.
APPLICABILITY OF THE THERMAL MODEL
Hunter and Hosha114 predicted that a simple thermal model would explain the delays recorded for polymers exposed to a flat flame similar to that used in the present experiment. In this model, ignition will occur as soon as the polymer surface reaches a critical temperature characteristic of the material. This temperature will be achieved in a time determined by the rate of heat transfer to the polymer surface and by the rate at which heat is conducted away from the surface into the polymer. Assuming that conduction of heat within the sample is the same as in an inert, semi-infinite solid, it may be shown that:5
(T,
-
Tr)/(Tg - T,) = 1 - exp X2.erfc X (1) X = ( h l K ) (ktig)1/2 (2) In these equations, T,, T,, and Tr are the temperatures of the flame wake, the sample surface at ignition and the room, respectively; ti, is the ignition delay and h is the heat-transfer coefficient. These expressions are eq~ivalent to those proposed by Hunter and H o ~ h a l l . ~To test the applicability of this thermal model to the ignition of LDPE, surface temperatures of LDPE slabs were measured during the delay, using chromel- alumel butt-welded thermocouples of nominal bead diameter 0.12 mm, sus-
3228 CLARK
pended so that the bead was in contact with the polymer surface. The surfaces of the LDPE slabs, like those of the PMMA slabs, reached temperatures before ignition that depended on the heating conditions and were reproducible in any set of conditions. However, the observed temperature of the bead actually dropped toward the end of the delay in some conditions, probably because it had become immersed in the melted surface. For those cases~where the temperature rose smoothly to a maximum temperature, and then remained quite constant, the maximum temperature recorded was 177 f 3g°C, the value within this range depending on the equivalence ratio and separation. As with PMMA, the tem- perature achieved increased with increasing gas temperature and decreasing separation.
Following the method of Kanury,5 plots of X from eqs. (1) and (2) against (Ts
- T,)/(T, - T,) were prepared using the delay data in Table I for LDPE, both for the value of h expected for this stagnation-point flow system: i.e., 22 W m-2 K-l, and for a wide range of other values of h. As for PMMA,l no value of h was found for which the experimental values of X from eq. (2) could be reconciled with those predicted by the thermal model [eq. (I)]. Iteration of Ts alone and together with h, over wide ranges of values, gave no better result. The same was true a t all separations. Clearly, as with PMMA, the simple thermal model does not adequately explain the ignition delays of LDPE in these conditions.
IMPORTANCE OF PYROLYSIS
A previous study of PMMA ignited in the same apparatus led to the suggestion that pyrolysis rate and energetics strongly influenced the duration and nature of the ignition delay for that po1ymer.l Since the simple thermal model failed to explain the delays for either polymer, it was thus interesting to explore the role of pyrolysis in LDPE ignition.
For the cases in which the thermocouple bead measuring surface temperature did not become submersed in the melt, it was possible to calculate a heat- transfer coefficient at any time, using eqs. ( I ) and (2). It was found that the heat-transfer coefficient quickly reached a relatively constant value, about 100 f 50 W m-2 K - l In contrast, when experiments were conducted with PMMA, delays were generally longer, and a characteristic drop in heat-transfer coefficient with time was 0bserved.l I t was concluded that endothermic pyrolysis of PMMA during the delay influenced the surface temperature. Pyrolysis of LDPE is almost certainly also endothermic although its value will vary with the conditions. The lack of depression of the heat-transfer coefficient with time for LDPE indicates a lower energy demand for pyrolysis during the delay period than is the case for PMMA.
One possible reason for this reduced energy demand may be that in air, and especially in the very oxygen-rich environment of this experiment, exothermic oxidative pyrolysis reactions may occur before ignition on the solid surface for LDPE that did not occur for PMMA. One such indicator is the temperature of the polymer a t the time of ignition. In inert atmospheres,'polyethylene is thermally stable until a temperature of about 400°C is reached, yet decomposi- tion clearly began in the author's experiments a t temperatures a t least 200°C 1ower.I On the other hand, PMMA begins to decompose at around 170°C,s in the absence of oxygen, lower than the surface temperatures a t igniti0n.l In
IGNITION OF LDPE 3229
addition, a small quantity of black char always formed on the surface of the polymer melt for LDPE by the end of the delay, which could only have been owing to oxidative reaction. In contrast, char did not form on PMMA samples in the same conditions. These observations are consistent with those of Burge and T i ~ p e r , ~ who found that oxidative pyrolysis was more important in the candle-like combustion of polyethylene than for PMMA.
As with the ignition delays for PMMA,l those for LDPE could be expressed by an Arrhenius-type equation:
tig = A exp (EIRT) (3)
where A is a constant, R is the universal gas constant, T is the absolute temper- ature and E is the so-called "activation energy." Plots of log (ti,) vs. 1IT for all data acquired for LDPE fit linear regression lines with correlation coefficients exceeding 0.99 for separations 5 to 25 mm; the correlation coefficient a t 30 mm separation is 0.979. The slopes of these lines are very similar and allow an esti- mate of the activation energy to be made. This estimation is made assuming that the reaction controlling the rate occurs in the gas phase, where the tem- peratures were measured. If the reaction occurs a t a lower temperature in the condensed phase, the activation energy estimated from the gas temperature will be an overestimate of the actual value. The value obtained, 64 f 10 kJImol, is far less than the activation energy of the pyrolysis of the polymer in the absence of oxygen (273 f 2 kJImol, ref. 10). I t is also less than the estimated activation energy of oxidative pyrolysis of polyethylene, 80 kJImol (ref. 10). As with PMMA, the duration of the ignition delay of LDPE in these conditions is not, therefore, governed by the rates of either pyrolysis or oxidative pyrolysis. Nevertheless, it is clear that oxidative pyrolysis is relatively important in the preignition states of LDPE.
GAS PHASE
Since it appeared that ignition begins in the gas phase in a plane close to the polymer surface, and that the solid-state pyrolysis reactions do not determine the ignition delay, events occurring in the gas phase were studied.
As noted previously, no preignition glow was observed above LDPE but was always seen above PMMA. However, as with PMMA, C 0 2 was found above LDPE during the delay. When a sample of LDPE was exposed to the hydro- gen-oxygen flame (equivalence ratio 0.1) a t a separatian of 5 mm for 3.0, small gas samples taken from the boundary layer contained on average 144 ppm Con, as determined by gas chromatography. This oxidation product could have been formed either in the gas phase or as a result of the condensed-phase reactions discussed previously.
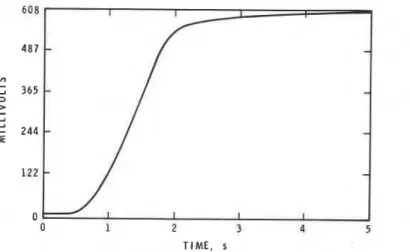
The rate of development of the flame generated immediately at the end of the delay was measured with the technique described for PMMA.l As with P M W , the first light emitted by the flame was confined to a narrow strip just above the polymer surface. Viewed with a silicon photodiode detector, the emission ap- peared as a bright line which increased in length as the flame spread. The rate of increase of the detector signal was considered to be a measure of the rate of development of the flame. As with PMMA, there was an interval during. the flame spread for LDPE when the velocity was constant (Fig. 2).
CLARK
T E M P E R A T U R E . "C
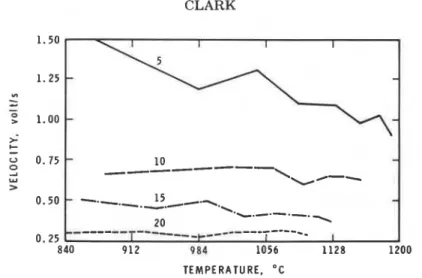
Fig. 3. Rates of flame development for LDPE; figures on the graph are separations in mm.
The rates of flame development of LDPE samples were recorded a t several separations and equivalence ratios. For each of the conditions, five experiments were conducted; the output of the silicon detector was recorded with an eight-bit transient digitizer and transferred to a desk-top computer for manipulation and display. The linear portion of each light emission time curve was identified and its slope calculated using linear regression methods. Agreement between the rates obtained in identical conditions was such that the percent standard de- viation among the five replicates was generally less than 10, but a few were as high as 27.
Figure 3 shows the variation of the mean initial flame development rates with separation and gas temperature. At 5 mm separation the rate dropped with increasing gas temperature, in a manner analogous to development rates found for flames above recently ignited PMMA samples a t the same or greater sepa- ration between the samples and the burner was greater than 5 mm, the post- ignition flame spread a t a rate independent of the gas temperature. Further, the development rate decreased as the separation increased; when the data for 5 mm separation are included, the flame development rate at any separation may be estimated from the equation: rate, V/s = -0.0526 (separation, mm)
+
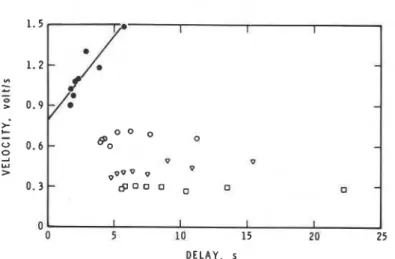
1.28.Finally, a remarkable dependence of flame development rate on the preceding delay for PMMA was noted in the earlier study.1 A similar correlation was sought between the initial rates of development of flames above LDPE and the preceding ignition delays. From Figure 4 i t is clear that for LDPE in these conditions, the flame development rate is not coupled with the delay, except perhaps at a separation of 5 mm, where an ill-defined dependence exists.
MECHANISM OF IGNITION
I t has been demonstrated in this paper that, as with PMMA,l the most com- monly used theory of ignition does not apply to ignition of LDPE in the condi- tions used. However, there are two differences between the ignition perfor- mances of the two polymers that force a fresh look a t the probable mechanism
IGNITION OF LDPE
I I I I I
0 5 10 15 20 25
D E L A Y , s
Fig. 4. Correlation between rate of flame development and ignition delay for LDPE. (a) 5 mm,
(0) 10 mm, (v) 15 mm, (0) 20 mm.
of LDPE ignition. First, the influence of oxidative pyrolysis on the preignition behavior for LDPE appears greater than on that of PMMA. Second, while a linear relationship exists between the ignition delay and the initial flame velocity for PMMA, such a relationship does not exist for LDPE.
The data for PMMA were shown in the early study1 to be consistent with a model of ignition in which exposing the polymer to a flame caused pyrolysis and subsequent release of combustible gases into the zone above the surface. The duration of the delay, it was postulated, depended on the concentration of species associated with the chain-propagation steps of the combustion process. When the concentration of these species reached a critical level, determined by the separation and the gas temperature, the reaction rate rapidly accelerated and the visible flame appeared, signalling the end of the delay. During this process, the concentration of pyrolysis gases in the zone above the polymer built up a t a rate relatively independent of the elapsed time. Thus at the end of any delay, the concentration of the pyrolysis gases present will be proportional to the du- ration of the delay. The flame generated at the end of the delay, it was suggested, has a rate of development directly proportional to the concentration of the py- rolysis gases. This mechanism explained the dependence of the rate on the delay and the reason for its decrease as the gas temperature increased.
To explain the data for LDPE, this model of ignition must be modified. Ex- posing the polymer to the igniting flame causes pyrolysis, in this case largely oxidative pyrolysis. From this process, the gas phase will contain mostly non- combustible gases; in addition, nonoxidative pyrolysis will generate combustible gases, probably largely methane, acetylene, propane, and n - b ~ t a n e . ~ As with PMMA, ignition occurs when the concentration of chain-propagating radicals reaches a critical level; the time required to reach this level depends on the gas temperature. The activation energy needed for ignition, 64 kJ/mol, is lower than the corresponding process for PMMA (96 kJ/mol) (see ref. 1). The subsequent rate of flame development for LDPE is governed by the concentration of chain-propagating radicals and not, as with PMMA, by the concentration of combustile pyrolysis gases present a t the end of the delay.
3232 CLARK
separation. The concentration of radicals in the gas phase, particularly OH,
would be expected to increase as the separation decreases. The mediation of this radical in chain-propagation reactions could cause the observed variation in rate of flame development. The dependence of rate on delay a t 5 mm sepa-
ration (Fig. 4) probably results from mechanistic transitions; in this case, as with
PMMA at all separations, the delay is so short that the concentration of pyrolysis gases governs the rate of flame development.
CONCLUSIONS
The results of this study and the previous study on PMMA1 indicate that the mechanism by which ignition occurs, a t least in conditions of predominantly convective heat transfer in the presence of excess oxygen, varies with the polymer ignited. Caution is thus indicated when seeking generalized mechanisms of ignition. Studies are being continued to further delineate the mechanistic variation possible for ignition processes of other polymers in these conditions.
T h e author gratefully acknowledges discussions with his colleagues and t h e assistance o f Mr. Raymond Flaviani in conducting the experiments reported here. T h i s article is a contribution from the Division o f Building Research, National Research Council o f Canada, and is published with the approval o f t h e Director o f the Division.
References
1. F. R. S. Clark, J. Polym. Sci. Polym. Chem. Ed., 21,2323 (1983).
2. C. F. Cullis and M. M. Hirschler, T h e Combustion of Organic Polymers, Clarendon, Oxford,
1981.
3. F. Rodriguez, Principles of Polymer Systems, McGraw-Hill, New Y o r k , 1970, p. 524.
4. L. W. ~ u n t e r and C.H. Hoshall, Fire Mater., 4(4), 201 (1980).
5. A. M. Kanury, Fire Res. Abst. Rev., 14,14 (1972).
6. W. M. Kays, Convective Heat and Mass Transfer, McGraw-Hill, New Y o r k , 1966.
7. C. F. Cullis and M. M . Hirschler, T h e Combustion of Organic Polymers, Clarendon, Oxford,
1981, p. 129.
8. N. Grassie and H. Melville, Proc. R. Soc. London Ser. A , 199,14 (1949).
9. S. J. Burge and C. F . H. Tipper, Combustion and Flame, 13(5), 495 (1969).
10. B. Dickens, J. Polym. Sci. Polym. Chem. Ed., 20(4), 1065 (1982).
Received February 24,1983 Accepted April 25,1983
T h i s paper, w h i l e being d i s t r i b u t e d i n r e p r i n t form by t h e D i v i s i o n of B u i l d i n g Research, remains t h e c o p y r i g h t of t h e o r i g i n a l p u b l i s h e r . It should n o t be reproduced i n whole o r i n p a r t w i t h o u t t h e permission of t h e p u b l i s h e r . A l i s t of a l l p u b l i c a t i o n s a v a i l a b l e from t h e D i v i s i o n may be o b t a i n e d by w r i t i n g t o t h e P u b l i c a t i o n s S e c t i o n , D i v i s i o n of B u i l d i n g R e s e a r c h , N a t i o n a l R e s e a r c h C o u n c i l of Canada, O t t a w a , O n t a r i o , KIA 0R6.