Antibody-Modified Conduits for Extracorporeal
Selective Cytokine Filtration in Sepsis
by
Morgan K. Moroi
Submitted to the Department of Mechanical Engineering
in partial fulfillment of the requirements for the degree of
Bachelor of Science in Engineering as Recommended by the
Department of Mechanical Engineering
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
June 2016
@
Massachusetts Institute of Technology 2016. All rights reserved.
Author
....
Signature redacted
6'
Department of Mechanical Engineering
May 6, 2016
Cetfidby.Signature
redacted
Certified
by...
... ...
Robert S. Langer
David H. Koch Institute Professor
Thesis Supervisor
Antibody-Modified Conduits for Extracorporeal Selective
Cytokine Filtration in Sepsis
by
Morgan K. Moroi
Submitted to the Department of Mechanical Engineering on May 6, 2016, in partial fulfillment of the
requirements for the degree of
Bachelor of Science in Engineering as Recommended by the Department of Mechanical Engineering
Abstract
Sepsis kills millions of people worldwide each year and occurs when microorganisms enter the bloodstream of an infected host. The presence of microorganisms in the bloodstream triggers the body to produce many inflammatory proteins, known as cytokines, that cause damage to blood vessels and vital organs. This leads to cap-illary leak, failing organs, and often death. We have developed a novel approach to modulate the inflammatory response, using antibody-modified conduits (AMCs) to filter harmful cytokines selectively from the circulation and in a time-specific manner. Here, we characterize variables that affect AMC performance to determine optimal
AMC conditions for later use downstream.
Thesis Supervisor: Robert S. Langer Title: David H. Koch Institute Professor
Acknowledgments
The author gratefully acknowledges Dr. J. Brian McAlvin, Dr. Daniel S. Kohane, and Dr. Robert S. Langer for their guidance throughout the completion of this work. The author would also like to thank all family and friends for their continual support.
Contents
1 Introduction
13
2 Materials and Methods
17
2.1 M aterials . . . . . 17
2.2 Preparation of Antibody-Modified Conduits . . . . 18
2.2.1 Synthesis and Dialysis of Furan-Functionalized Antibody . . . 18
2.2.2 Formation of Antibody-Modified Conduits . . . . 18
2.3 In Vitro Demonstration of Selective Cytokine Filtration . . . . 21
2.4 Influence of Flow Rate on Elimination Kinetics . . . . 22
2.5 Influence of Antibody Surface Packing Density on Elimination Kinetics 23 2.6 Influence of Surface Area to Volume Relationships on Elimination Ki-netics . . . . 23
2.7 Influence of PEG Spacer Length on Elimination Kinetics . . . . 23
2.8 Determination of Cytokine Binding Capacity . . . . 24
2.9 Statistics . . . . 24
3 Results
25
3.1 In Vitro Demonstration of Selective Cytokine Filtration . . . . 253.6
Determination of Cytokine Binding Capacity . . . .
30
4 Discussion
31
5 Conclusion
35
List of Figures
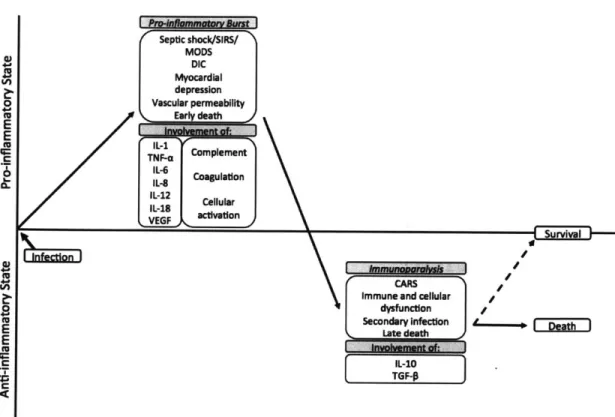
1-1 Pathophysiology of the systemic inflammatory response in sepsis . . . 14
2-1 Schema of formation of an antibody-modified conduit . . . . 20 2-2 Experimental setup for testing selective TNF-a filtration with
infliximab-m odified conduits . . . . 21
3-1 Type of cytokine filtered was determined by the selection of AMC surface antibody . . . . 26 3-2 a filtration by infliximab-modified conduits, where the BSA
TNF-a solution wTNF-as circulTNF-ated through AMCs TNF-at vTNF-arying flow rTNF-ates . . . . 27 3-3 TNF-a filtration by infliximab-modified conduits prepared with
vary-ing infliximab concentrations . . . . 28 3-4 TNF-a filtration by infliximab-modified conduits with varying surface
area to volume ratios . . . . 29 3-5 TNF-a filtration by infliximab-modified conduits with varying PEG
spacer lengths... ... 30 3-6 TNF-a filtration by ideal infliximab-modified conduits (2.50 surface
area to volume ratio, NHS-PEGIO-Maleimide, 1 mg/mL infliximab so-lution) . . . . 30
List of Tables
Chapter 1
Introduction
Septic shock affects millions of people worldwide every year and remains the lead-ing cause of death in critically ill patients in the United States [1-4]. Sepsis occurs when microorganisms (most commonly bacteria) enter the bloodstream and cause a dynamic, unregulated systemic inflammatory response [2] (Figure 1-1). The early stage of sepsis is dominated by a surge of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-a) [5], interleukin 1 beta (IL-1#) [5,6], interleukin 6 (IL-6)
[5], and vascular endothelial growth factor (VEGF) [7]. As sepsis persists, a decrease
in pro-inflammatory cytokines and an increase in anti-inflammatory cytokines occurs, resulting in immunoparalysis [2,5]. Recovery from this immunosuppressive state may be associated with positive clinical outcomes [8].
Pro-in ammato Burst Septic shock/SIRS/ MODS DIC Myocardial depression Vascular permeability Early death Involvement of: Complement IL-6 8 CoagulatIon IL-12 IL-12 ellular VEGF activation 0 to E 0 0 to E
E
to F DeathFigure 1-1: Pathophysiology of the systemic inflammatory response in sepsis.
Investigators have tried for decades to modulate the immune response during sepsis in an effort to restore homeostasis [5]. Unfortunately, most attempts, including corticosteroids [5,9], antiendotoxin antibodies [5,10], tumor necrosis factor antagonists [5,11], interleukin-1-receptor antagonists [5,6], and ibuprofen [12], have largely proven unsuccessful. Additionally, efforts to non-specifically remove both pro-inflammatory and anti-inflammatory cytokines, by dialysis [13] or therapeutic plasma exchange (TPE) [14], have also failed to improved survival. One reason for these failures may be because the complex pathophysiology of sepsis changes over time. For example, many targeted immune therapies that are beneficial during the pro-inflammatory surge exacerbate the immunosuppression that is present in the later phase of sepsis. We presume that previous attempts to modulate the immune system have been unsuccessful due to prolonged inimunosuppression (via anti-inflammatory therapies) or non-s)ecific removal of cytokines that would have been better left in the circu-lation (via dialysis or TPE). Furthermore, we hypothesize that successful immune
Immuno oral sis CARS
Immune and cellular
dysfunction Secondary infection Late death Involvement of: IL-10 TGF-P
modulating therapies for sepsis must remove deleterious cytokines selectively and in a time-specific manner. Pro-inflammatory cytokines should only be removed during the pro-inflammatory burst to prevent long-lasting immune suppression. Similarly, anti-inflammatory cytokines should only be removed during immunoparalysis to aid in the restoration of homeostasis.
To accomplish this, silicone tubes were individually surface modified with antibod-ies against a particular cytokine. Known as an antibody-modified conduit (AMC), each tube is capable of removing one type of cytokine from blood that extracorpo-really circulates through it. If installed in parallel and for patient-specific durations of time, AMCs may prevent multi-organ injury and mortality by allowing for spe-cific temporally controlled manipulations of the cytokine cascade in sepsis. Here, we specifically focus on the characterization of variables that affect AMC filtration performance.
Chapter 2
Materials and Methods
2.1
Materials
Polydimethylsiloxane (PDMS) tubing was purchased from Cole-Parmer Instrument Company (Vernon Hills, IL). (3-Aminopropyl)trimethoxy-silane (APTMS) was pur-chased from Sigma-Aldrich (St. Louis, MO). Heterobifunctional poly(ethylene glycol) with N-hydroxysuccinimide ester and maleimide moieties (NHS-PEGa-Maleimide, where n is the molecular weight of the PEG spacer) was purchased from Rapp Polymere (Tuebingen, Germany). 2-Furoic acid, N-(3-Dimethylaminopropyl)-N
'-ethylcarbodiimide hydrochloride (EDC), and N-Hydroxysuccinimide (NHS) were pur-chased from Sigma-Aldrich (St. Louis, MO). Infliximab (Janssen Biotech, Inc.,
Hor-2.2
Preparation of Antibody-Modified Conduits
2.2.1
Synthesis and Dialysis of Furan-Functionalized
Anti-body
Furan with N-hydroxysuccinimide ester (NHS-furan) was synthesized by dissolving 1 molar equivalent of furoic acid, 3 molar equivalents of EDC, and 3 molar equivalents of NHS in dichloromethane (DCM). The aqueous solution was continuously stirred for
13 hours at room temperature and purified using a CombiFlash@ Rf 150 purification
system (Teledyne Isco, Inc., Lincoln, NE) with a 40-gram high performance silica column (Teledyne Isco, Inc., Lincoln, NE). Elution fractions containing the product were combined and the liquid was evaporated using a Rotavapor@ (Buchi Corpora-tion, New Castle, DE) set to rotate at 45 'C. The final product was lyophilized (SP
Scientific VirTis Lyophilizer, Warminster, PA) for 16 hours.
NHS-furan was dissolved in dimethyl sulfoxide (DMSO) (45 mg/mL) and added to an aqueous antibody solution (10 - 25 mg/mL) to form a 10:1 molar mixture of
NHS-furan to antibody. The mixture was rotated (VWR@ Tube Rotator, Radnor, PA) for 2 hours at room temperature to encourage the formation of furan-functionalized
antibody.
The resulting solution was placed into the lumen of a Slide-A-LyzerTM dialysis
cassette with a 10 kDa molecular weight cutoff (Thermo Scientific, Rockford, IL). The dialysis bag was placed into 4L 1X phosphate buffered saline (PBS) (pH 7.4) and incubated at 2 - 8 'C with continuous stirring. The PBS buffer was exchanged
every 8 hours for 24 hours, and the final dialyzed solution was stored at 2 - 8 *C until
use.
2.2.2
Formation of Antibody-Modified Conduits
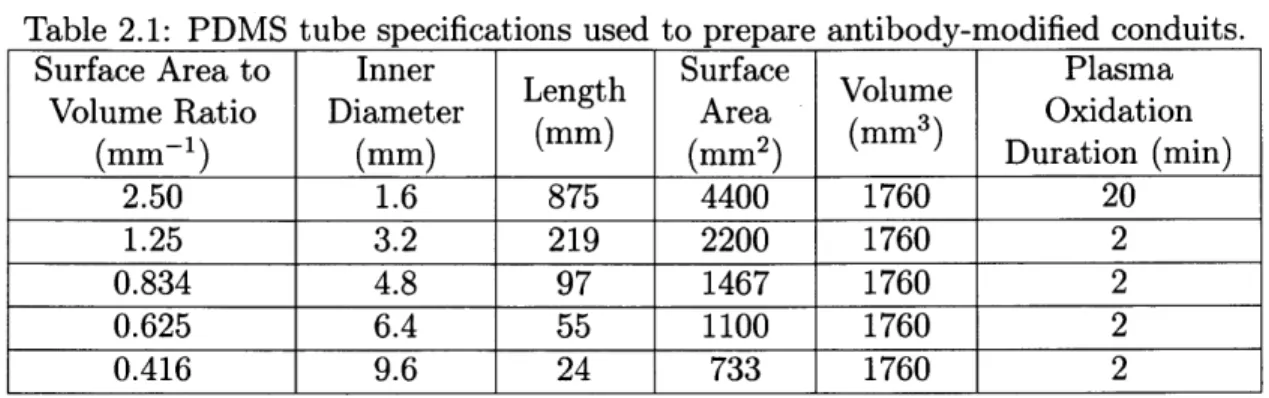
PDMS tubes were cut to length based on the desired surface area to volume ratio and plasma oxidized (Harrick Plasma Cleaner, PDC-001, Ithaca, NY) at 1.5 mbar for the corresponding duration (Table 2.1) (Figure 2-1). Tubes with the largest surface area
to volume ratio (i.e. long length, small diameter) were plasma oxidized for a longer duration of time to ensure complete diffusion of plasma throughout the tube.
Table 2.1: PDMS tube specifications used to prepare antibody-modified conduits.
Surface Area to Inner Surface Plasma
. .Length Srae Volume
Volume Ratio Diameter Area Oxidation
(mm- 1) (mm) (mm) (mm2) (mm Duration (min) 2.50 1.6 875 4400 1760 20 1.25 3.2 219 2200 1760 2 0.834 4.8 97 1467 1760 2 0.625 6.4 55 1100 1760 2 0.416 9.6 24 733 1760 2
Oxidized PDMS tubes were connected in series and 5% (v/v) APTMS in acetone was circulated through the tubes at room temperature for 1 hour using a peristaltic
pump (Watson-Marlow, Inc., 120U/DV, Wilmington, MA) set at 40.0 rpm (Figure 2-1). The resulting aminopropyl silane coated tubes were flushed with ethanol followed by forced air to remove any residual solution. NHS-PEG,-Maleimide in 1X PBS (pH 8.5) (2 mg/mL) was then circulated at room temperature for 1 hour at 160.0 rpm
to produce Maleimide-PEG, modified tubes (Figure 2-1). Tubes were then flushed with 10 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 5.5) followed by forced air. To quench residual amines, 200 mM acetic anhydride in methanol (MeOH) was circulated at room temperature for 2 hours at 160.0 rpm (Figure 2-1). Tubes were then flushed with 10 mM MES (pH 5.5) followed by forced air. The furan-functionalized antibody solution was diluted with 10 mM MES (pH 5.5) to an antibody concentration of 0.5 mg/mL and circulated at 37 'C for 13 hours at 160.0 rpm (Figure 2-1). Before use, antibody-modified conduits were flushed with sterile IX PBS (pH 7.4).
Antibody-modified conduits were stored in sterile IX PBS (pH 7.4) at 2 - 8 'C
CHI 3 CH3 CH3 I I H3Cnmo' P'CH Unmodified PDMS 0 NH 0 NH 0 0 O 0 0 0 HN HN 0 0 NH NH NH F S ' Si ' I I |
Antibody Modified Conduit (AMC) Air Plasma LI NH
F-uran- Functic Antibody in pH 5.5 OH OH OH I I I HO' I 1 0 'OH Oxidized PDMS 0 N 0 N7 0 0 0 0 0 HN HN 0 0 NH NH NH n lzed2) MES. 2 i' ' Si Si 0100
Maleinide-PEG , Modified Surf, (Residual amines blocked by acyl
with acetic anhydride)
H3CO H3CO-Si NH2 H3CO 5%' (v/v) APTMS in acetone NH2 NH2 NH2 HO' I 10 OH Aminopropyl Silane Coated Surface 0 0 HN 0 O NHS-PEG,-Maleimide in PBS (pH 8.5)
Acetic Anhydride in MeOH 0 0
ace ation
Figure 2-1: Schema of formation of an antibody-modified conduit. Conjugation of antibodies onto the inner surface of silicone tubes was accomplished through four sequential reactions. The first step was to activate the silicone surface by oxygen plasma oxidation to create reactive silanol species (Si-OH). The anchoring molecule (APTMS) was then covalently grafted to the Si-OH moieties. Next, a spacer molecule (NHS-PEG,-Maleimiide) was attached to the primary amine of the APTMS anchor. In the final step, the terminal naleimide on the PEG spacer was reacted with the furan moieties that were added to the antibodies.
2.3
In Vitro Demonstration of Selective Cytokine
Filtration
Antibody-modified conduits with a surface area to volume ratio of 0.834 (Table 2.1)
were prepared with NHS-PEG10-Maleimide and a 0.5 mg/mL infiiximab solution and
tested to determine if selective TNF-a filtration could be achieved (PEG10 , 10 kDa
molecular weight PEG) (infiiximab is a monoclonal antibody that binds TNF-a).
Solutions of 53 (w/v) BSA in sterile lX PBS (pH 7.4) were enriched with 2000
pg/mL TNF-a and 2000 pg/mL VEGF-A and circulated through infiiximab-modified
conduits using the experimental setup in Figure 2-2. The cytokine concentrations were chosen to be an order of magnitude greater than what would be encountered
clinically during an episode of sepsis to sufficiently challenge AMCs.
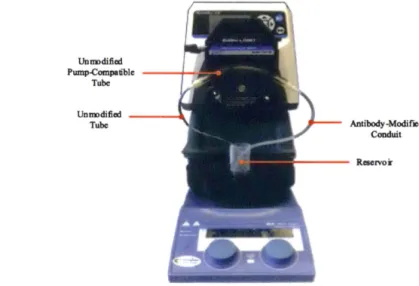
Unm>dified Pump-Compa111>le - - - 4 " ' -·.ll Tube Unm>dified -Tube Antibody-Modified Conduit
Figure 2-2: Experimental setup for testing selective TNF-a filtration with infiiximab-modified conduits. An infiiximab-modified conduit was connected in series with two
other tubes: (1) an unmodified tube (Cole-Parmer Instrument Company, PVC, ID
(Cole-Reservoirs were filled with 20 mL of BSA cytokine solution and a 0.5 mL sample
was taken at time zero. The cytokine solution was circulated at 20 mL/min and
addi-tional 0.5 mL samples were obtained at 0.5, 1, 2, 3, and 4 hours. The reservoir volume
and circulation rate were chosen in anticipation of in vivo experiments (rat model of
sepsis), where the rat circulating blood volume is 20-25 mL and the extracorporeal
circulation rate will be 20 mL/min. Samples were stored at -20 'C until analysis.
VEGF-A and TNF-a concentrations in the samples were determined using VEGF-A
and TNF-a enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc.,
Minneapolis, MN).
This experiment was repeated with bevacizumab-functionalized AMCs with a
sur-face area to volume ratio of 0.834, using a 0.5 mg/mL bevacizumab solution
(beva-cizumab is a monoclonal antibody that binds VEGF-A).
2.4
Influence of Flow Rate on Elimination Kinetics
Infliximab-modified conduits with a surface area to volume ratio of 0.834 (Table 2.1)
were prepared with NHS-PEGiO-Maleimide and tested to characterize how flow rate
affects cytokine filtration. Solutions of 5% (w/v) BSA in sterile 1X PBS (pH 7.4)
were enriched with 2000 pg/mL TNF-a and circulated through infliximab-modified
conduits using the experimental setup in Figure 2-2. Experiments were performed
with flow rates of 10, 20, 30 and 40 mL/min to determine the influence of flow rate
on cytokine elimination kinetics. TNF-a concentration was measured by ELISA at
0, 0.5, 1, 2, 3, and 4 hours.
The rate constant, k (min-
1), was determined for each flow rate as a measure
of how fast cytokines were cleared from the solution. Assuming a one phase decay
model, each rate constant was determined using a nonlinear curve fit.
2.5
Influence of Antibody Surface Packing Density
on Elimination Kinetics
Antibody-modified conduits with a surface area to volume ratio of 0.834 (Table 2.1) were prepared with NHS-PEGIO-Maleimide, followed by the addition of infliximab us-ing circulatus-ing antibody concentrations of 0.05, 0.5, 1.0, 1.5 and 2.0 mg/mL. Solutions of 5% (w/v) BSA in sterile 1X PBS (pH 7.4) were enriched with 2000 pg/mL TNF-a and circulated through infliximab-modified conduits at a flow rate of 20 mL/min, us-ing the experimental setup in Figure 2-2. Samples were obtained at 0, 0.5, 1, 2, 3 and 4 hours, and TNF-a concentrations were determined by ELISA. The rate constant,
k, was determined for each antibody concentration used.
2.6
Influence of Surface Area to Volume
Relation-ships on Elimination Kinetics
Infliximab-modified conduits with various surface area to volume relationships were prepared with NHS-PEGIO-Maleimide (Table 2.1). Solutions of 5% (w/v) BSA in sterile IX PBS (pH 7.4) were enriched with 3000 pg/mL TNF-a and circulated at 20 mL/min through infliximab-modified conduits, using the experimental setup in Figure 2-2. Samples were obtained at 0, 0.5, 1, 2, 3 and 4 hours, and TNF-a concentrations were measured by ELISA. The rate constant, k, was determined for each surface area to volume ratio.
Elimina-TNF-a and circulated through infliximab-modified conduits at a flow rate of 20
mL/min, using the experimental setup in Figure 2-2. Samples were obtained at
0, 0.5, 1, 2, 3, and 4 hours, and TNF-a concentrations were measured by ELISA. The
rate constant, k, was determined for each PEG spacer length.
2.8
Determination of Cytokine Binding Capacity
Infliximab-modified conduits were prepared with the highest performing
characteris-tics (2.50 surface area to volume ratio, NHS-PEGiO-Maleimide, 1 mg/mL infliximab
solution) to determine the maximum cytokine binding capacity for AMCs. Solutions
of 5% (w/v) BSA in sterile 1X PBS (pH 7.4) were enriched with 80,000 pg/mL
TNF-a to sTNF-aturTNF-ate AMCs TNF-and circulTNF-ated through infliximTNF-ab-modified conduits, using the
experimental setup in Figure 2-2. Samples were obtained at 0, 0.5, 1, 2, 3, 4, 5, 6, 7,
and 8 hours, and TNF-a concentrations were measured by ELISA.
Cytokine binding capacity was calculated using the following equation:
Capacity = ([Cytokine]initiai - [Cytokine]saturation) x Volumecirculating fluid (2.1)
2.9
Statistics
Statistical analyses were performed with Prism (Graph-Pad Software Inc., San Diego,
CA). All data were presented as mean
SD. In Sections 3.2
-
3.4, one-way analysis
of variance (ANOVA) was performed to evaluate the significance of the data and
calculate the P value. In Section 3.5, unpaired Student t-tests were performed to
evaluate the significance of the data and calculate the P value.
Chapter 3
Results
3.1
In Vitro Demonstration of Selective Cytokine
Filtration
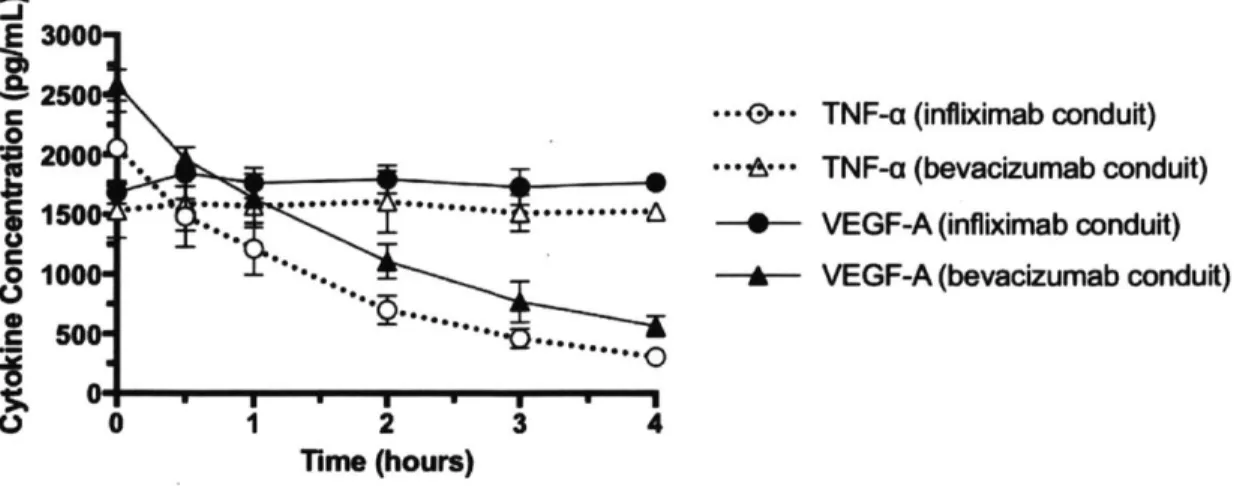
AMCs performed selective cytokine filtration by only clearing the cytokine they were designed to filter. AMCs modified with infliximab removed 82% of the TNF-a present at the starting concentration after 4 hours, while VEGF-A levels remained unaffected (Figure 3-1). AMCs modified with bevacizumab removed 78% of the VEGF-A present at the starting concentration after 4 hours, while TNF-a levels remained unaffected
E 3000 CL 2500 C 2000 '. ... m 1000e'-h..s S 0 1 2 34 Time (hours)
TNF-a (infliximab conduit) TNF-a (bevacizumab conduit) VEGF-A (infliximab conduit) VEGF-A (bevacizumab conduit)
Figure 3-1: Type of cytokine filtered was determined by the selection of AMC surface antibody. Conduits modified with infliximab (monoclonal antibody against TNF-o) removed TNF-o from the circulation. Conduits modified with bevacizumab (mono-clonal antibody against VEGF-A) removed VEGF-A. Data were presented as mean
SD.
9
3.2
Influence of Flow Rate on Elimination Kinetics
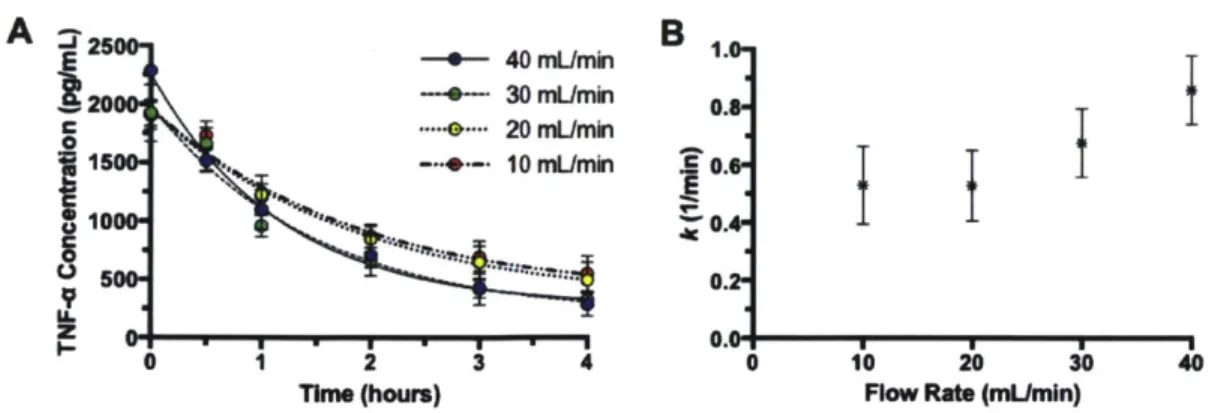
Rate of TNF-a elimination was directly proportional to flow rate (Figure 3-2). Curve fitting analysis revealed that, TNF-a elimination followed a single phase, exponential decay with strong correlation (R2 > 0.90 for all groups) (Figure 3-2A). The
differ-ences in k values were determined to be statistically significant (P = 0.0004, one-way ANOVA) (Figure 3-2B). Higher flow rates were correlated with higher k values, sug-gesting that faster and more favorable kinetics were achieved at higher flow rates
A -25 _ B E - 40 mL/min 1 i20 30 mUmin 0.8 -- ** -.. 20 mUmin 0 150--- -- 10 mUmin .50.6-1000- -0.4-L 5 0.2 z
~..
II0.0-0 1 2 3 4 0 10 20 30 40Time (hours) Flow Rate (mUmin)
Figure 3-2: n filtration by infliximab-modified conduits, where the BSA
TNF-a solution wTNF-as circulTNF-ated through AMCs TNF-at vTNF-arying flow rTNF-ates. All conduits hTNF-ad TNF-a surface area to volume ratio of 0.834 (Table 2.1) and were prepared with NHS-PEGio-Maleimide and a 0.5 ing/mL infliximab solution. (A) TNF-a elimination profiles for each group. (B) Exponential decay rate constants (k) for each group. Data were presented as mean SD.
3.3
Influence of Antibody Surface Packing Density
on Elimination Kinetics
Rate of TNF-a elimination was affected by antibody surface packing density (Figure 3-3). Curve fitting analysis revealed that TNF-a elimination followed a single phase,
exponential decay (R2 = 0.64, 0.77, 0.44, 0.93, 0.89, 0.94 for 2.0 ng/mL, 1.5 ng/mL, 1.0 mg/mL, 0.5 mg/mL, 0.1 mg/mL, 0.05 mg/mL, respectively) (Figure 3-3A). The differences in k values were determined to be statistically significant (P = 0.0104, one-way ANOVA) (Figure 3-3B). AMC performance (i.e. rate of TNF-a elimina-tion) was directly proportional to infliximab concentration used to prepare AMCs for concentrations up to 1.0 mg/mL (Figure 3-3B). AMCs prepared with infliximab concentrations of 1.0 ing/mL or higher had similar k values (Figure 3-3B).
A
B
-J -- 2.0 mg/mL 30- 1.5 mg/mL '-'250 0 1.0 mg/mL = 200 0.5 mg/mL r C 150 -- * 0.1mg/mL 1 0.05 mg/mL .V 0 100 500 * 0 1 2 3 4 0.0 0.5 1.0 1.5 2.0Time (hours) Infliximab Concentration (mg/mL)
Figure 3-3: TNF-o filtration by infliximab-modified conduits with a surface area to volume ratio of 0.834 (Table 2.1). AMCs were prepared with NHS-PEGio-Maleimnide.
Surface antibody addition was performed with solutions of varying infliximab con-centration. (A) TNF-o elimination profiles for each group. (B) Exponential decay rate constants (k) for each group. Data were presented as mean SD.
3.4
Influence of Surface Area to Volume
Relation-ships on Elimination Kinetics
Rate of TNF-o elimination was directly proportional to the ratio of AMC surface
area to volume (Figure 3-4). Curve fitting analysis revealed that TNF-a elimination followed a single phase, exponential decay with strong correlation (R' > 0.93 for all groups) (Figure 3-4A). The differences in k values were determined to be statistically significant (P < 0.0001, one-way ANOVA) (Figure 3-4B). Higher surface area to volume ratios were correlated with higher k values., suggesting that faster and more
favorable kinetics were achieved by AMCs with higher surface area to volume ratios (Figure 3-4B).
-- +*-- 2.50 mm-1 . -~1 - 0.
4-..
---- 0. --- + --I
-7.'
--- I.
A
-4000 . S3000 0 r 2000-0 1000-ILL 0 0B
25 mm 834 mm-1 625 mm-1 416 mm-1 4. 3-1. * 0.0 0.5 1.0 1.5 2.0 2.5 3.0 Surface Area to Volume Ratio (1/mm)Figure 3-4: TNF-i filtration by infliximab-modified conduits with varying surface area to volume ratios. All conduits were prepared with NHS-PEG1 0-Maleimide and a 0.5 mig/niL infliximab solution. (A) TNF-o elimination profiles for each group. (B) Exponential decay rate constants (k) for each group. Data were presented as mean
SD.
3.5
Influence of PEG Spacer Length on
Elimina-tion Kinetics
AMC perfornanice was enhanced by increasing PEG spacer length (Figure 3-5). Curve fitting analysis revealed that TNF-a elimination followed a single phase. exponential decay with strong correlation (B2 = 0.73, 0.99 for 3 kDa PEG. 10 kDa PEG. respec-tively) (Figure 3-5A). The difference in k values was determined to be statistically significant (P = 0.0422, unpaired Student t-test) (Figure 3-5B). AMCs prepared with
NHS-PEG1O-Maleimide (PEGio, 10 kDa PEG) had higher k values, suggesting that
faster and more favorable kinetics were achieved by AMCs with a longer PEG spacer
length (Figure 3-5B).
1 2 3 4
A-
B
E10000 - 3 kDa PEG 2.
.- e- 10 kDa PEG C 8000 . 1. 0 6000 --. .... . ... ... 4000- \ % 20000 0 1 2 3 4 0 5 10
Time (hours) PEG Spacer Length (kDa)
Figure 3-5: TNF-o filtration by inflixinab-iodified conduits. Conduits with a surface area to volune ratio of 2.50 were prepared with varying PEG spacer lengths and a 0.5 nig/mL inflixiinab solution. (A) TNF-i elimination profiles for each group. (B) Exponential decay rate constants (k) for each group. Data were presented as mean
SD.
3.6
Determination of Cytokine Binding Capacity
Ideal AICs (NHS-PEG o-Maleimide. 1.0 mg/nL antibody loading concentration, 2.50 surface area to voluie ratio) performed selective cytokine filtration until satu-ration (Figure 3-6). Using Equation 2.1, the binding capacity for an ideal AMC was
found to be 394 47 pg/nun2. 2 '1000000. C 80000 0 .1 0* 60000 C 40000, 0 20000-0'* 0 1 2 3 4 5 6 7 8 Time (hours)
Figure 3-6: TNF-o, filtration by ideal infliximab-modified conduits (2.50 surface area to volume ratio, NHS-PEGIO-Maleinide, 1 ng/niL infliximab solution). Cytokine binding capacity for this ideal type of AMC was determined to be 394 47 pg/1i11112.
Chapter 4
Discussion
The purpose of this thesis was to demonstrate the concept of selective blood filtra-tion, using cytokines as the model target. Ultimately, these data will be used to develop a novel therapeutic approach to the treatment of sepsis, where circulating blood is purified by selective removal of the signaling molecules that drive systemic inflammation. Here, we successfully characterized the variables that influence AMC performance.
Ideally, AMCs must fulfill the following criteria: they must (1) only bind the targeted cytokine, and (2) be able to reduce clinically relevant cytokine levels in a timely manner and maintain them at zero. Objective (1) was fulfilled by circulating two types of cytokines through an AMC and observing only the removal of the type of cytokine targeted by the antibody (Section 3.1). Objective (2) was fulfilled by characterizing AMC variables and designing an ideal AMC to have quick elimination kinetics and a large cytokine binding capacity (Sections 3.2 - 3.6).
AMCs were determined to be capable of selective cytokine filtration. Conduits cleared targeted cytokines, whereas non-targeted cytokines remained unaffected and
Before moving forward with expansion and further testing, it was important to characterize variables that affected AMC performance so that the highest performing AMCs could be used for future experimentation. These variables included: fluid flow rate, antibody loading concentration, tubing dimension, and PEG spacer length.
In varying flow rates, we observed that higher flow rates were correlated with faster elimination kinetics. By analogy, solute clearance with traditional hemodialysis is enhanced by increasing the flow rate of blood [16]. Given that the highest flow rate tested (40 mL/min) achieved the best kinetics, a flow rate of 40 mL/min will be used in future tests. 40 mL/min is equivalent to 100 mL/kg/min for a 400 gram rat, and this normalized flow rate is in range with those achieved during extracorporeal membrane oxygenation (ECMO) in children and infants (75 - 150 mL/kg/min) [17].
In varying antibody loading concentration, we discovered that AMC performance was enhanced up to an antibody loading concentration of 1.0 mg/mL. Above 1.0 mg/mL, the kinetics remained the same. We hypothesize that this occurred because either: (1) the AMCs became saturated with antibodies when circulated with con-centrations of 1.0 mg/mL or higher, or (2) the AMCs became oversaturated with an-tibodies when circulated with concentrations greater than 1.0 mg/mL, increasing the extent of steric hindrance and preventing antibodies from favorably binding cytokines
[18]. In the future, AMCs will be prepared with an antibody loading concentration
of 1.0 mg/mL.
In varying AMC dimensions, we observed that AMCs with higher surface area to volume ratios had faster elimination kinetics. This is most likely because AMCs with a large surface area to volume ratio possess a large blood-circuit interface, allowing their surface antibodies to be exposed to a greater proportion of the circulating fluid. Given this information, future AMCs will be prepared with silicone tubing with a surface area to volume ratio of 2.50. It is important to note, however, that changing the surface area to volume ratio will also change the fluid dynamics of the system. The resistance to blood flow is directly proportional to the length and inversely pro-portional to the radius to the fourth power. This could possibly cause hemolysis due to increased shear forces, as increased resistance has been shown to cause hemolysis in
extracorporeal circuits [19]. Future in vitro studies will need to be conducted, using blood as the circulating fluid, to determine whether AMCs with an aspect ratio of
2.50 cause hemolysis.
In varying PEG spacer length, we found that AMCs prepared with a 10 kDa PEG spacer outperformed those prepared with a 3 kDa PEG spacer. The use of spacers to distance immobilized antibodies from a surface has been shown to introduce greater freedom of antibody motion [20], reduce steric interference [20,21], and improve sur-face antibody orientation [21]. Additionally, it is known that the use of PEG spacers increases antibody-antigen interactions by up to 90% and is dependent upon spacer length [21]. Thus, it is not surprising that the longer spacer displayed faster elimina-tion kinetics. Moving forward, AMCs will be prepared with NHS-PEGiO-Maleimide
(PEG10, 10 kDa PEG).
Given these findings, ideal tubes (NHS-PEGio-Maleimide, 1.0 mg/mL antibody loading concentration, 2.50 surface area to volume ratio, 40 mL/min flow rate) were tested, and the total cytokine binding capacity was calculated. The total binding capacity was determined to be 394 47 pg/mm2. This capacity will be helpful in
estimating the number of AMCs needed for use in various in vivo models.
We now know that selective cytokine filtration with AMCs yields rapid elimination of a specific cytokine with the capacity to sustain elimination for prolonged periods. Moving forward, we plan to modulate cytokine concentrations in vivo. While we have demonstrated proof of concept, a crucial test of this technology will be to use AMCs, singly or in combination, to regulate cytokine levels in rats rendered septic. In testing our device in vivo, we hope to validate that customized AMC combinations can provide precise temporal control over circulating cytokine concentrations, thereby preventing organ failures and death.
manner, has the potential to aid in the critical dissection of the impact of events in the systemic inflammatory response and become a useful tool for scientific investigation as well as therapeutic intervention.
Chapter 5
Conclusion
In summary, antibody-modified conduits (AMCs) were capable of selective and timely cytokine filtration. By characterizing variables that affect AMC performance, ideal
AMC conditions (NHS-PEGio-Maleimide, 1.0 mg/mL antibody loading
concentra-tion, 2.50 surface area to volume ratio, 40 mL/min flow rate) were determined for later use downstream. Next steps include in vivo testing to demonstrate that AMCs can modulate cytokine levels, singly or in combination, in a temporally controlled manner in animals rendered septic.
Chapter 6
References
[1] Angus, D.C., Linde-Zwirble, W.T., Lidicker, J., Clermont, G., Carcillo, J. and Pinsky, M.R., 2001, "Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care," Critical Care Medicine, 29(7), 1303-1310.
[2] Hotchkiss, R.S. and Karl, I.E., 2003, "The pathophysiology and treatment of
sepsis," New England Journal of Medicine, 348(2), 138-150.
[3] Martin, G.S., Mannino, D.M., Eaton, S. and Moss, M., 2003, "The epidemiology of sepsis in the United States from 1979 through 2000," New England Journal of
Medicine, 348(16), 1546-1554.
[4] Proulx, F., Fayon, M., Farrell, C.A., Lacroix, J. and Gauthier, M., 1996,
"Epi-demiology of sepsis and multiple organ dysfunction syndrome in children," Chest,
109(4), 1033-1037.
[5] Hall, M.W. and Muszynski, J.A., 2009, "Immune modulation in sepsis," Journal
of Pediatric Infectious Disease, 4(2), 127-136.
2008, "Vascular endothelial growth factor in severe sepsis and septic shock,"
Anes-thesia and Analgesia,
106(6),
1820-1826.
[8] Frazier, W.J. and Hall, M.W., 2008, "Immunoparalysis and Adverse Outcomes
from Critical Illness," Pediatric clinics of North America, 55(3), 647-668.
[9] Bone, R.C., Fisher, C.J., Clemmer, T.P., Slotman, G.J., Metz C.A. and Balk, R.A.,
1987, "A controlled clinical trial of high-dose methylprednisolone in the treatment of
severe sepsis and septic shock," New England Journal of Medicine, 317(11), 653-658.
[10] Ziegler, E.J., Fisher, C.J., Sprung, C.L., Straube, R.C., Sadoff, J.C., Foulke,
G.E., Wortel, C.H., Fink, M.P., Dellinger, R.P. and Teng, N.N., 1991, "Treatment
of gram-negative bacteremia and septic shock with HA-1A human monoclonal
anti-body against endotoxin: a randomized, double-blind, placebo-controlled trial," New
England Journal of Medicine, 324(7), 429-436.
[11] Abraham, E., Wunderink, R., Silverman, H., Perl, T.M., Nasraway, S., Levy,
H., Bone, R., Wenzel, R.P. Balk, R. and Allred, R., 1995, "Efficacy and safety of
monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis
syndrome: a randomized, controlled, double-blind, multicenter clinical trial," JAMA,
273(12), 934-941.
[12] Bernard, G.R., Wheeler, A.P., Russell, J.A., Schien, R., Summer, W.R.,
Stein-berg, K.P. Fulkerson, W.J., Wright, P.E., Christman, B.W., Dupont, W.D., Higgins,
S.B. and Swindell, B.B., 1997, "The effects of ibuprofen on the physiology and survival
of patients with sepsis," New England Journal of Medicine, 336(13), 912-918.
[13] Payen, D., Mateo, J., Cavaillon, J.M., Fraisse, F., Floriot, C. and Vicaut, E.,
2009, "Impact of continuous venovenous hemofiltration on organ failure during the
early phase of severe sepsis: a randomized controlled trial," Critical Care Medicine,
37(3), 803-810.
[14] Qu, L., Kiss, J.E., Dargo, G. and Carcillo, J.A., 2011, "Outcomes of previously
healthy pediatric patients with fulminant sepsis-induced multisystem organ failure
receiving therapeutic plasma exchange," Journal of Clinical Apheresis, 26(4),
208-213.
and Lamy, M., 1992, "Cytokine serum level during severe sepsis in human IL-6 as a marker of severity," Annals of Surgery, 215(4), 356-362.
[16] Hauk, M., Kuhlmann, M.K., Riegel, W. and Kohler, H., 2000, "In vivo effects of
dialysate flow rate on Kt/V in maintenance hemodialysis patients," American Journal of Kidney Diseases, 35(1), 105-111.
[17] Laquier, L., Horton, S.B., McMullan, D.M. and Bartlett, R.H., 2013,
"Extra-corporeal Membrane Oxygenation Circuitry," Pediatric Critical Care Medicine, 14,
S7-12.
[18] Xu, H., Lu, J.R. and Williams, D.E., 2006, "Effect of Surface Packing Density
of Interfacially Adsorbed Monoclonal Antibody on the Binding of Hormonal Antigen Human Chorionic Gonadotrophin," Journal of Physical Chemistry, 110(4), 1907-1014.
[19] Polaschegg, H.D., 2009, "Red blood cell damage from extracorporeal circulation
in hemodialysis," Seminars in Dialysis, 22(5), 524-531.
[20] Weimer, B.C., Walsh, M.K. and Wang, X., 2000, "Influence of a poly-ethylene glycol spacer on antigen capture by immobilized antibodies," Journal of Biochemical and Biophysical Methods, 45(2), 211-219.
[21] Cao, T., Wang, A., Liang, X., Tang, H., Auner, G.W., Salley, S.O. and Ng, K.Y., 2007, "Investigation of spacer length effect on immobilized Escherichia coli pili-antibody molecular recognition by AFM," Biotechnology and Bioengineering, 98(6),
1109-1122.
[22] Trager, K., Schutz, C., Fischer, G., Schroder, J., Skrabal, C., Liebold, A. and Reinelt, H., 2016, "Cytokine Reduction in the Setting of an ARDS-Associated In-flammatory Response with Multiple Organ Failure," Case Reports in Critical Care.