Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Polymer Processing Society Asia/Australia Meeting (PPS 2004) [Proceedings], 2004-08-29
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=1a3bd94c-821e-4db4-946e-b812d7c2e4cc https://publications-cnrc.canada.ca/fra/voir/objet/?id=1a3bd94c-821e-4db4-946e-b812d7c2e4cc
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Pressure-volume-temperature measurements of polymeric nanocomposites
PVT of PNC:
Pressure-Volume-Temperature measurements
of polymeric nanocomposites
L. A. Utracki
NRCC/IMI, 75 de Mortagne, Boucherville, QC, Canada
Invited plenary lecture at the PPS-2004 Asia-Australia meeting, Gyeongju, Korea, 2004.08.29-09.01;
2
Outline
Introduction to PVT testing
Simha-Somcynsky (S-S) lattice-hole theory
Equation of state (eos)
Free volume, CED, and solubility parameters
PVT of PNC
PNC based on PA-6 and PS
PNC molecular structure from PVT-eos analysis
PVT of the PP-based PNC
Thermodynamic interactions – molecular model Free volume – a measure of clay dispersion
Free volume – correlation with PNC properties
3
Reasons for measuring PVT
To determine the P & T dependent V and derivatives, e.g.:
Isobaric thermal expansion coefficient: Isothermal compressibility:
P-dependent transition T (Ehrenfest):
To determine the free volume f = f(T, P) and derivatives:
Viscosity, diffusivity (barrier properties), surface tension, …
Physical aging: dimensional stability, mold shrinkage, creep, … Mechanical properties
Chemical reactivity.
Cohesive energy density (CED), solubility parameters, miscibility Internal pressure and internal interactions
Others, e.g.:
To estimate the bulk degree of exfoliation in PNC.
P lnV / T
α
= ∂ ∂ T lnV / Pκ
= − ∂ ∂ g dT / dP =∆κ ∆α
/4
Free volume - definitions
Free volume is that part of space that is left after
subtracting the occupied volume, defined by the atomic
dimensions.
Considering that a molecule or its segment moves in a
cage created by surrounding molecules, its free volume
is defined by the space within which its center can
freely move:
1. Total specific volume, V
2. Occupied specific volume (defined as V at T = 0 K), Vo 3. Free volume: Vf = V - Vo
4. Free volume fraction:
f = V
f/ V = 1 - (V
o/V)
5. Doolittle definition: fD = Vf/Vo = f/(1 - f)5
S-S Quasi-lattice
Hole fraction: h = 1 – y(V, T) is a measure of structural disorder.
Occupied sites Hole
(
)
(
)
V,T T F F V, T; y(V, T); c / s Minimization : F / y 0 Equation of state : P F / V = ∂ ∂ = = − ∂ ∂ Free energy
For s-mer:
Scaling parameters: P* = qz ε*/sv*; V* = v*/Mo; T* = qzε*/cR → (P*V*/T*)Mo = Rc/sv*: segmental repulsion volume
ε*: segmental attraction energy
3c/s: number of external modes (volume-dependent degrees
of freedom) per segment:
Ideal flexible linear chain: 3c/s → 1
Rigid chain: 3c/s → 0
6
S-S lattice-hole theory
The S-S theory describes the thermodynamic properties of
liquids, explicitly providing information how the hole fraction,
h = 1 - y, changes with independent variables.
From the Helmholtz free energy, F, using:
a coupled equation of states was derived.
Q
i= 1 / (y
iV
˜
i) ;
η
i= 2
−1 /6y
iQ
i1/3˜
P
iV
˜
i/ ˜
T
i= (1 − η
i)
−1+ 2y
iQ
i2(AQ
i2− B) / ˜
T
iwith:
3c
i[(
η
i−1 / 3) / (1 − η
i)
− y
iQ
i2(3AQ
i2− 2B) / 6 ˜
T
i]
+ (1 − s
i)
− s
iln[(1
− y
i) / y
i]
= 0
where: A = 1.011 and B = 1.2045
(
)
(
)
0
T V ,TP
F /
V
F / y
∂
∂
≡ −
∂
∂
=
7
Simplified dependencies
The S-S EoS predicts that
at ambient pressure
:
The temperature dependence
of PVAc reduced volume at
constant pressure is shown
(viz. Figure left).
Computed from EoS group
volumes, v*, are proportional to
the van der Waals volumes:
v* = 1.59 V
W+ 1
.Ref.: L. A. Utracki and R. Simha, ″Analytical
representation of solutions to lattice-hole theory”,
Macromol. Chem. Phys., Molecul. Theory Simul.,
10, 17 - 24 (2001). 3/ 2 0 : 0.95 1.40 : 1. ln 0.1034 23.835 2. 1 ( ) / ; ( ) 0.9473 0.9663 0.957 i i P
For polymeric melts within V
V T h = K T V K T to < < = − + × = − = ≅ -0.1 0 0.1 0.2 0.3 0.4 0 0.005 0.01 0.015 0.02 P = 0; y = -0.1034 + 23.87x; r = 0.99997 P = 0.05; y = -0.0996 + 19.53x; r = 0.9996 P = 0.1; y = -0.1016 + 16.91x; r = 0.9991 P = 0.2, y = -0.1102 + 13.66x; r = 0.9986 P = 0.3, y = -0.1199 + 11.64x; r = 0.998 y = l n ( V/ V *) x = (T/T*)3/2
8
Assuming random mixing in binary mixtures, Jain & Simha (1979) expressed the Helmholtz free energy in terms of averages, <>:
The two cross-interaction parameters are expressed as:
Fm / RT = x1 ln x1 + x2 ln x2 + (< s > /y)(1 − y)ln(1 − y) − (< s > −1)ln[(z − 1) / e] − < c > {ln[< v* > (1 − η)3 / Q] − (yQ2 / 2 ˜ T )(AQ2 − 2B)} − (3 / 2){x1c1 ln[ 2π < Mo1 > RT(NAh)−2]+ x2c2ln[2π < Mo2 > RT(NAh)−2]} < s >= x1s1 + x2s2 ; < c >= x1c1 + x2c2 ; < Mo >= (x1s1Mo1 + x2s2Mo2)/ < s > < ε* >< v* >k= X 1 2ε 11 * v11*k + X22ε22* v22*k + 2X1X2ε12* v12*k ; k = 2,4 where X1 = 1 − X2 = x1[s1(z − 2) + 2]/ < qz >
Binary mixtures
With definitions:(
)
* * * 12 11 22 v 3 * *1/ 3 *1/ 3 12 v 11 22Interaction energy :
;
1
Re pulsion volume: v
v
v
/ 8
ε εε = δ ε ε
δ ≅ δ ≅
= δ
+
9
PVT of PA-6/clay PNC 1
In binary mixtures the interaction parameters are molar averages.
Polymeric Nano-Composites (PNC) are mixtures of two components: flexible PA-6 and rigid “particles” of clay.
The idealized clay particle is 100 nm in diameter and 1 nm in
thickness, hence its “molecular mass” and “molecular volume” is:
M = NA ρπd2h/4 = 10,443 (kg/mol), and
Vplat = π(d/2)2h = 4.73×106 (mL/mol).
The lattice-hole theory assumes that the hard core volumes of the constituents do not differ too much, usually equal.
For the inherent to the theory 6-12 potential the factor 21/2 relates
the positions of potential minimum and onset of repulsion, thus for PA-6: v*hard = MsV*/21/2 = 17.60 (mL/mol)
also adopted for the clay “segment”.
Ref.: L. A. Utracki, R. Simha, and A. Garcia-Rejon, “Pressure-Volume-Temperature Relations
10
PVT of PA-6/clay PNC 2
S-S theory provides good description of the observed
PVT dependencies for PA as well as for the PNC.
0.94 0.98 1.02 1.06 480 520 560 600 experimental computed from S-S S pe c if ic v ol u m e, V (m L/ g ) T (K) P = 0.1 - 200 MPa Ube PNC 0.95 1 1.05 1.1 480 520 560 600 Ube PA-6 experimental computed from S-S S pe c if ic v ol u m e, V (m L/ g ) T (K) P = 0.1 - 200 MPa V (mL/g)
11
PVT of PA-6/clay PNC 4
To directly compare the specific volume and the hole fraction
variations with T and P for PA and PNC, the ratios V(PA)/V(PNC) and h(PA)/h(PNC) at identical T and P for each point are shown.
1 1.05 1.1 1.15 1.2 500 520 540 560 580 600 T (K) 0.1 50 110 150 190 P (MPa) 190 150 110 50 0.1 V(PA)/V(PNC) h(PA)/h(PNC)
Theoretical V-ratio for PNC with 0.64 vol% clay is: V(PA)/V(PNC) = 1.0088. The experimental ratio: V(PA)/V(PNC) = 1.008 to 1.018.
The holes ratio:
h(PA)/h(PNC) =
12
Adsorption on crystalline
solid
Israelachvili et al. 1984-8 measured 2-9 nm thick
layer of “solidified” PS from toluene solutions.
Nano-scale rheology showed that there is a
3-layer structure of PBD on mica [Luengo et al., 1997]: 1. z < 6 nm – solid-like 2. 6 < z < 100 – elastomeric 3. z > 100 nm – bulk behavior
(
)
{
0[
]
}
0 0 0 oy y
y( z )
;
z
z
z
y
y
y
exp n ( z
z ) /( z
z )
∞ ∞ ∞ ∞=
≤ <
−
−
−
−
0 40 80 0 40 80 5 4 3 2 1.5 M a tr ix i nte ra c ti on pa ram et e rs , ε 11 * or v 11 * z (nm) n13
Binary interactions in
PA-6/PNC system
The binary interaction parameters, were calculated assuming: 1. Enrobed clay 2. Average values of 1-2 interactions 3. Slightly expanded (by 10%) cell volume of dispersed phase 30 40 50 24.5 25.5 26.5 0 0.4 0.8 1.2 1.6
Interaction parameters in PA-6/PNC system
E11 E22 V11 V22 E n e rg et ic i n ter ac tio ns , ε ii V o lum et ric int er a c tio ns , v ii Clay concentration (wt%) * * ij
and v
ij
* * 221.1
11v
v
14
Viscosity vs. 1/h
Zero-shear viscosity, η0, and η
at G“ = 50 Pa are plotted vs. 1/h. The viscosity data are
interpolated, after extraction of the yield stress behavior from the experimental data
The hole fraction h was computed from PVT measurements.
The data follow a linear dependence:
Ref.: L. A. Utracki: "Temperature and Pressure Dependence of Liquid Viscosity”, Canadian
J. Chem. Eng., 61, 753 – 758 (1983). 2.4 2.5 2.6 2.7 6.6 7 7.4
Viscosity of PA-6 and its mixtures with PNC
logη 0 = 0.477 + 0.2996/h; R= 0.9775 logη(G"=50) = 1.836 + 0.0888/h; R= 0.9565 lo g η 1/h
log
η
∝
1/ h
15
PVT of PS-based PNC 1
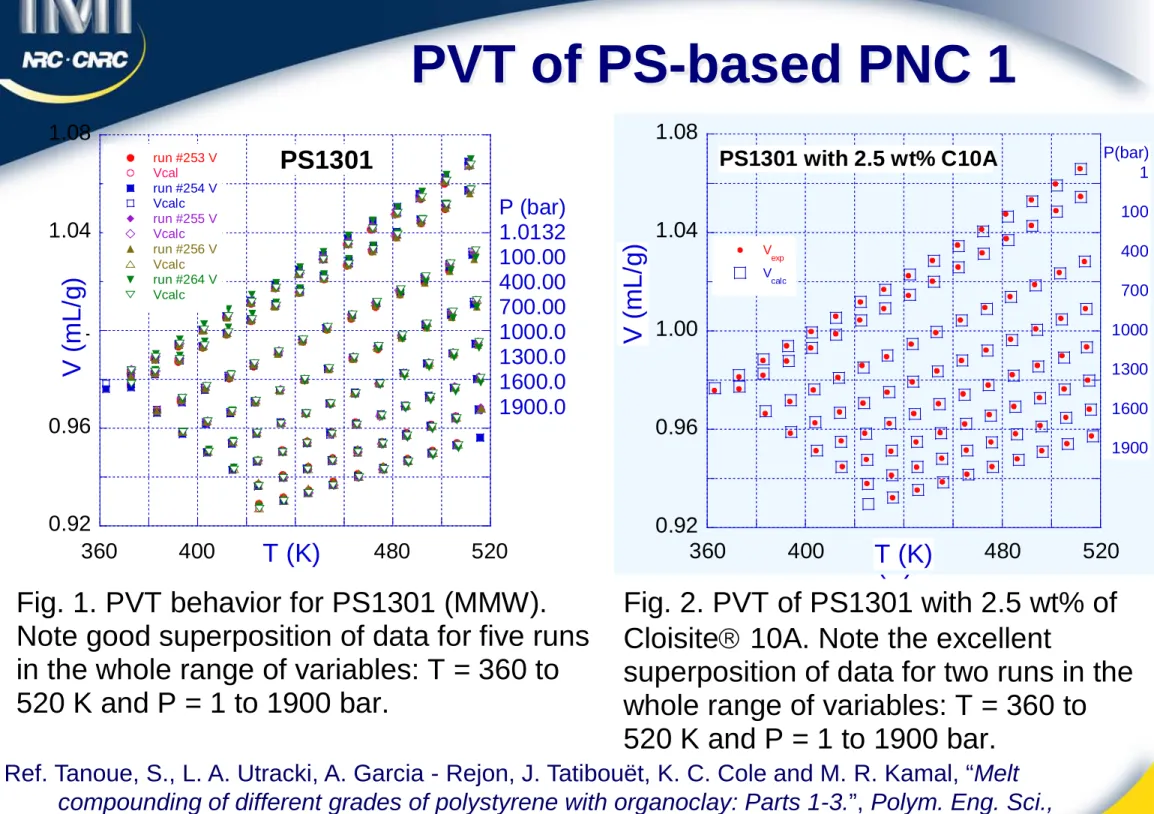
0.92 0.96 1 1.04 1.08 360 400 440 480 520 PS1301 run #253 V Vcal run #254 V Vcalc run #255 V Vcalc run #256 V Vcalc run #264 V Vcalc V ( m L /g) T (K) P (bar) 1.0132 100.00 400.00 700.00 1000.0 1300.0 1600.0 1900.0 0.92 0.96 1 1.04 1.08 360 400 440 480 520 PS1301 with 2.5 wt% C10A Run #270 Run #271 V ( m L /g) T (K)Fig. 1. PVT behavior for PS1301 (MMW). Note good superposition of data for five runs in the whole range of variables: T = 360 to 520 K and P = 1 to 1900 bar.
Fig. 2. PVT of PS1301 with 2.5 wt% of Cloisite 10A. Note the excellent
superposition of data for two runs in the whole range of variables: T = 360 to 520 K and P = 1 to 1900 bar. 0.92 0.96 1.00 1.04 1.08 360 400 440 480 520 PS1301 with 2.5 wt% C10A V exp V calc V ( m L /g) T (K) P(bar) 1 100 400 700 1000 1300 1600 1900
Ref. Tanoue, S., L. A. Utracki, A. Garcia - Rejon, J. Tatibouët, K. C. Cole and M. R. Kamal, “Melt
compounding of different grades of polystyrene with organoclay: Parts 1-3.”, Polym. Eng. Sci.,
16
From the PVT fit the reducing parameters and then the average values of the interaction parameters were calculated:
P* = zq<ε*>/(s<v*>); T* = zq<ε*>/Rc; V* = <v*>/Ms
The minimum in the hole fraction (at ca. 2 wt% C10A) corresponds to maximum clay platelets dispersion.
There is a simple relation between the free volume and the average
interaction parameter, <ε*>.
The two average interactions parameters are proportional to each other.
0.93 0.95 0.97 0.99 1.01 0 10 20
PS1301 with Cloisite 10A; P = 10 bar
T = 360 K T = 460 K T = 560 K R e la ti v e h o le fr a c ti o n , h (PN C )/ h (PS) Organoclay content, w (wt%) 1.00 32.4 32.8 33.2 43.0 43.4 43.8 44.2 44.6 0 10 20
PS1301 with Cloisite 10A
<ε ij> <v* ij> < ε ij > (k J /m o l) <v * ij > ( m L /m o l) Cloisite 10A (wt%) 0.93 0.95 0.97 0.99 1.01 32.4 32.8 33.2 h r(360) = 3.4589 - 0.075686ε* ; r 2 = 0.99992 h r(460) = 2.9245 - 0.059237ε*; r 2 = 0.99992 h r(560) = 2.6834 - 0.051815ε*; r 2 = 0.99994 h r = h (PN C )/ h (P S) <ε*> w = 0 w = 1 .4 w = 2 .8 w = 5 .7 w = 1 0 .6 w = 17. 1
PVT of PS-based PNC 2
17
PP ProFax SR256 from Himont was melt compounded with 0, 1, 2, 3 and 4 wt% of Cloisite 15A or C6A (MMT intercalated with
145% 2M2HT).
Three compatibilizers were used (see Table).
The ratio of organoclay to compatibilizer was 1:2.
The effects of Epolen® addition
was also examined.
Isothermal PVT data were
determined at P = 0 to 1900 bar,
increasing T by 10oC steps from
ambient to 550 K.
The runs were repeated until reproducibility in V was ≤ 0.02%.
PVT of PP-based PNC 1
Data from at least two runs were fitted to S-S eos, using Micromath Scientist non-linear least squares program.
E43 PP-g-MA Epolene-43 Mw = 9.1 3.81 %MA Eastman
3150 PP-g-MA Polybond-3150 Mw = 330 0.71 %MA; styrene Uniroyal
GMA6 PP-g-GMA -- Mw≈ 305 0.42 %MA; styrene IMI
PVT tests for PP with Cloisite 15A (C15A) and a compatibilizer
# Code Composition (wt%) PVT Run # Vo
PP C15A Compatibilizer (mL/g)
A PP256 100 0 0 199 1.1424 B PP256 97 3 (C6A instead C15A) 200 1.1038 0 E0-0100 100 0 (extruded) 2 (#284, 285) 1.1107 1 E0-2100 98 2 0 2 (#286, 287) 1.1030 2 E1-2-E43R 94 2 4 (E43) 2 (#357, 358) 1.1005 3 E1-2-3150 94 2 4 (3150) 2 (#304, 305) 1.0980 4 E2-2-GMA6 94 2 4 (GMA) 2 (#292, 293) 1.1042 5 E0-3200 96 4 0 2 (#298, 299) 1.0921 6 E1-4-E43 96 4 8 (E43) 2 (#306, 309) 1.090 7 E1-4-3150 96 4 8 (3150) 5 (345, 360) 1.0906 8 E2-4-GMA6 96 4 8 (GMA) 2 (#315, 341) 1.0894 9 M1 97.5 2 ½ epoxy 2 (#316, 317) 1.1004 10 M2 93.5 2 ½ epoxy + 4 wt% 3150 4 (#333, 334) 1.0977
18
Fitting the data to the theoretical equations provides two
main sets of information:
The hole fraction, h = h(P, T), and
The reducing parameters: P*, V* and T*, from which the bulk-average interaction parameters, <ε*> and <v*>, could be
computed.
The temperature and pressure dependent h-function was
shown to correlate with the degree of exfoliation.
The concentration dependent interaction parameters
have been used to examine PA-based PNC structure on
the molecular level.
All compositions were analyzed
To determine the molecular model, the data without a
compatibilizer (i.e., PP + C15A) were used.
19
PVT of PP-based PNC 3
Computed values of the characteristic reducing parameters, as well as assessment of the goodness of fit are presented above.
For the sake of comparison the data published by Zoller are also shown.
To develop a model, first only the samples: A, B, 1, and 5 were used.
Computed S-S eos parameters for PP and PP-PNC
# Code Sci# C15A Mo P* (bar) 10000V* T* (K) r2 σ
IPP Zoller ppz 0 40.853 6043 ± 79 1,1827 ± 24 10535 ± 56 0.999997 0.002045 0 PP 2845 0 50.459 4973 ± 113 1,1932± 42 10804 ± 117 0.999998 0.001706 A PP256 256 0 43.115 5727 ± 68 1,1728 ± 22 10449 ± 57 0.999998 0.001647 1 E0-2100 2867 2 48.364 5169 ± 103 1,1874 ± 37 10712 ± 98 0.999998 0.001916 B PP256 pnc 3 46.720 5457 ± 55 1,1712 ± 18 10775 ± 49 0.999998 0.001845 2 E1-2-E43 3578 2 49.432 5230 ± 117 1,2060 ± 42 11251 ± 120 0.999996 0.002505 3 E1-2-3150 3045 2 51.585 4923 ± 99 1,1971 ± 37 10970 ± 101 0.999996 0.002482 4 E2-2GMA 2923 2 47.774 5247 ± 95 1,1993 ± 34 10848 ± 90 0.999997 0.002261 5 E0-3200 2989 4 50,748 5028 ± 93 1,1857 ± 34 10917 ± 92 0.999997 0.002274 6a E1-E43 3069 0 49.194 5111 ± 95 1,1926 ± 34 10820 ± 91 0.999997 0.002350 6b E1-4-E43 367 4 45.611 5625 ± 146 1,1731 ± 48 10860 ± 132 0.999996 0.002421 7 E1-4-3150 3450 4 48.490 5266 ± 93 1,1931 ± 33 10993 ± 91 0.999996 0.002554 8 E2-4GMA 3151 4 49.412 5203 ± 96 1,1807 ± 34 10952 ± 94 0.999997 0.002272 9 M1 3167 2 52.238 4924 ± 90 1,2000 ± 34 11138 ± 95 0.999997 0.002256 10 M2 3224 2 46.893 5483 ± 105 1,1784 ± 36 10933 ± 100 0.999997 0.002251
20
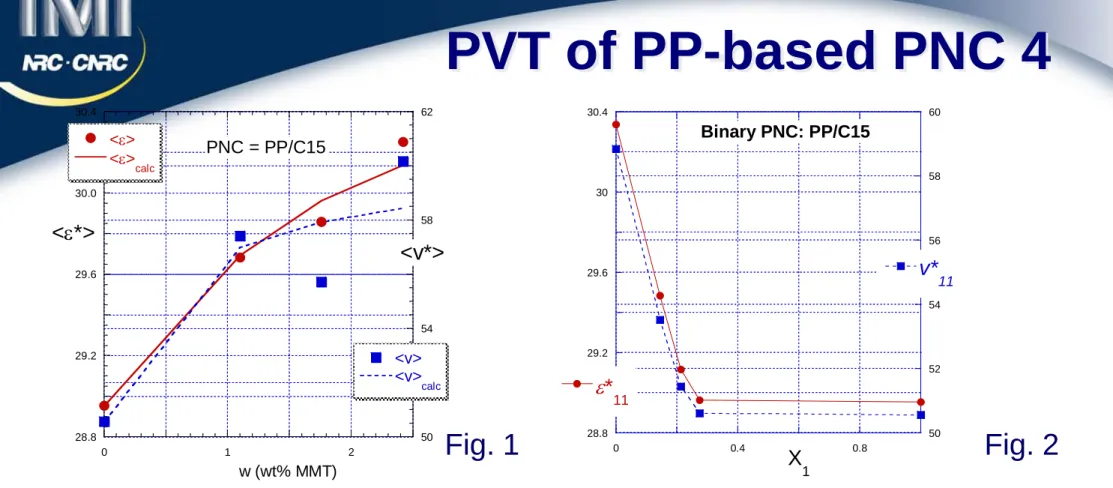
The more rigorous approach involved three steps:
Calculate ∆h as a function of clay content
Calculate and in terms of ∆h
Optimize the fit to Simha-Jain binary interactions Eqs.
The approach provides the best fit to data (see Fig. 1), and acceptable parameter values:
Figure 2 illustrates the predicted variation of matrix properties.
PVT of PP-based PNC 4
Fig. 2 Fig. 1 11 * v 11 * ε * * * * * * 22 30.3 1.0; 22 / 10 1.047; v22 58.6± 1.0; v / v22 10 1.158 ε = ± ε ε = = = 28.8 29.2 29.6 30 30.4 50 52 54 56 58 60 0 0.4 0.8 Binary PNC: PP/C15 ε* 11 v* 11 X 1 28.8 29.2 29.6 30.0 30.4 50 54 58 62 0 1 2 PNC = PP/C15 <ε> <ε> calc <v> <v> calc <ε*> <v*> w (wt% MMT)21
Next, the variation of hole
fraction with composition was examined by computing the relative rations of the specific volume and the hole fraction:
Vr ≡ V(PNC)/V(PP)
hr ≡ h(PNC)/h(PP).
The Figure for PP/C15A series shows that addition of
organoclay (no compatibilizer) has very little effect on sample density, but it reduces the free volume (at P = 10 MPa):
At 2 wt% C15A by ca. 4.7% At 4 wt% C15A by ca. 8.0%
PVT of PP-based PNC 5
0.9 0.94 0.98 1.02 440 480 520 560PP with 2 or 4 wt% C15A at P = 10 MPa
V r (2%) V r (4%) h r (2%) h r (4%) h r = h (P NC )/ h (PP) ; V r = V( PN C) /V( PP) T (K)
Owing to lower compressibility of PNC than that of a polymer the magnitude of hr increases with P.
22
The hole fraction, h = h(T), at
P = 10 MPa is shown in the
Figure for all tested samples. The data fall into 3 groups:
PP – the highest h PP + 4%C15A
PP + 2%C15A (+epoxy)
Reduction of free volume in PNC is mainly by 7 to 9%.
For 2-wt%C15 + E43 the reduction is by 12%
Addition of 2-wt% C15A + ½wt% epoxy reduces free volume by 11% (with 3150 by 8%).
Incorporation of compatibilizer reduces the clay effect.
PVT of PP-based PNC 6
0.11 0.13 0.15 0.17 0.19 440 480 520 560h = h(T) at P = 10 MPa for all samples
PP 2%C+E 4%C+E 2%C+315 4%C+315 2%C+GMA 4%C+GMA 2%C+epoxy 2%C+epoxy+4%315 H o le fr act ion, h (-) T (K)
23
Considering that h1/5 = h(T)
has a similar slope for all
tested samples, the value of h at P = 100 MPa and T = 500 K were computed.
The Figure shows that there is an excellent correlation (within the limit of experimental error indicated by the bars) between
h1/5 and <ε*>.
Thus, one can use either of these two parameters in a search for correlations with other PNC measures, viz. interlayer spacing, tensile properties, impact strength, etc.
PVT of PP-based PNC 7
6 6.4 6.8 7.2 29 30 31Inverse hole fraction vs. bulk interaction parameter for PP PNC 1/h 1/5 = -5.473 + 0.401<ε*>; r = 0.998
1
/h
1 /5 <ε*> h1/5 was computed for:
24
The interlayer spacing, d001, for
selected samples (containing 2-wt% C15A) were measured using
WAXS-XRD at Steacie Institute in Ottawa and at IMI.
Several compositions were
measured using different specimens – the standard deviation of the data was established as ± 0.15 nm.
The Figure illustrates the correlation
between d001, and computed from
the PVT data reduction of h (at 100 MPa; 500 K).
PVT of PP-based PNC 8
2 6 10 14 0 4 8 12 16Correlation for 2 wt% organoclay
PNC without compatibilizer: ∆h = 2.50 + 0.833d 011; r = 1 PP-PNC with compatibilizer: ∆h = -21.73 + 9.38d 001; r = 0.927 ∆ h ( % )= 10 0[ 1 h( P N C )/ h( pol y )] d 001 (nm) PA-6 PP PS
Two dependencies are seen:
For a two component PNC (polymer/organoclay)
For three component: PP/organoclay/compatibilizer systems.
The compatibilizers (PP grafted with MAH or GMA) dramatically reduced h with only a small effect on d001 (immiscibility?).
25
The PVT parameters indicate immiscibility in systems compatibilized with Epolene-43 and with Polybond-3150.
Similar conclusions can be made from the plot of h vs. clay content.
Immiscibility is responsible for the dramatic decrease of the impact strength.
PVT of PP-based PNC 9
28.5 29.5 30.5 31.5 0 1 2 PP-based PNC's <e> <e>cal <e>E43+0.5 <e>315+1 <e>GMA+1.5 <e>cal<
ε*>
w (wt% MMT) 50 54 58 62 0 1 2 PP-based PNC's <v> <v>cal <v>E43+0.5 <v>315+1 <v>GMA+1.5 <v>cal<
v
*>
w (wt% MMT)26
Matrix immiscibility
Microscopic drops indicating phase separation in the
mixture of PP with E43 is evident at concentrations above
6-wt%.
The new interphase significantly increases system free
volume.
27
The experimental values for Young modulus (Y), or for the notched Izod impact strength (NIRT) show poor correlation with the PVT parameters. Other properties (e.g., flexural) have shown a similar behavior.
Mechanical properties of
PP-based PNC
1 2 3 0.14 0.15 0.16All NIRT data for PP-based PNC
all NIRT no comp E43 3150 GMA epoxy No tc h I z o d a t RT , N IRT ( k J /m 2 ) Hole fraction, h (-) 1.6 2 2.4 0.14 0.15 0.16
All data for PP-based PNC
all Y-values no comp E43 3150 GMA epoxy T en s ile m odu lus , Y ( G P a ) Hole fraction, h (-) PP; Y = 1.7
28
PP-based PNC morphology
In a recent paper iPP-based PNC were prepared with w
(wt%) of ODA-MMT & 3w of MA-g-PP (Polybond®).
Top Figures show polarized light images of spherulites statically crystallized without MMT (c), and with 10-wt% ODA-MMT (f).
Bottom Figures show polarized
light micrographs of sheared at = 0.685 (1/s) PNC with
6-wt% ODA-MMT; (b) and (d) indicate iPP of Mw = 400 and 250 kg/mol, respectively.
R. Nowacki, B. Monasse, E. Piórkowska, A. Gałęski,
& J. M. Haudin, “Spherulite nucleation in isotactic PP based nanocomposites with montmorillonite under shear”, Polymer, ASAP (2004).
29
Conclusions
Simha-Somcynsky configurational thermodynamic theory provides good description of the PVT behavior of liquids, including molten polymers, their blends, foams, composites and PNC.
The computed hole fraction, h, is a measure of the free volume
fraction: f = 1 - (Vo /V).
Analysis of PVT behavior of PNC indicates the presence of a
solidified layer on the clay surface, followed by molten polymer with mobility that increases with the distance from clay surface – the “hairy clay platelet” model, HCP.
The values of h well correlate with viscosity η = η(P, T, φ), and the
interlayer spacing, d001, of PNC’s.
In the PP-based PNC, at constant organoclay loading (2-wt%) d001
well correlates with 1/h (or with h).
Addition to PP of organoclay + compatibilizer (1:2 wt ratio) results in a complex set of properties, hardly related to exfoliation.
30