Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez
pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the
first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Paper (National Research Council of Canada. Institute for Research in
Construction); no. IRC-P-1376, 1986
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=4a648c77-ae66-4ecf-97fb-367adf6ddac2
https://publications-cnrc.canada.ca/fra/voir/objet/?id=4a648c77-ae66-4ecf-97fb-367adf6ddac2
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. /
La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version
acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien
DOI ci-dessous.
https://doi.org/10.4224/40001803
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
TGA/APCI/MS/MS: a new technique for the study of pyrolysis and
combustion products
Str
T r n
I
N21d
National Research
Consell national
no.
1376
Council Canada
de
recherches Canada
c .
2
BXSG
Institute for
Research in
lnstitut de
recherche en
-
Construction
construction
TGA/APCI/MS/MS, A New
Technique for the Study of
Pyrolysis and Combustion
Products
by Y. Tsuchiya
ANALYZED
Reprinted from
Fire Safety Science
-
Proceedings of the First
International Symposium
Gaithersburg, MD, 9
-
11 October 1985
p. 411 -420
(IRC Paper No. 1376)
Price $2.00
NRCC 25923
La Section d16tude
du feu, Division des rechercnes en oacimenc,
Conseil national de recherches du Canada,
a
fait l'acquisition
d'un
SM/SM ICPA (ionisation chimique sous pression
atmosph6rique) SCIEX TAGA 6000, appareil d'analyse d'une
sensibilit6 et d'une rapidit6 de traitement exceptionnelles.
Celui-ci est capable de dktecter simultankment un grand nombre
de gaz produits lors de la pyrolyse/combustion.
Coup16
3 un
analyseur thermogravim6trique (ATG) Dupont
951, il a servi
a
6tudier les produits de pyrolyse du polyacrylonitrile (PAN)
a
diff6rents stades de ce processus.
Un
6chantillon de
1 mg a 6t6 pyrolysk dans 1'ATG sous courant
d'azote ou d'air et les produits ont 8t6 introduits dans le
SM/SM ICPA
a
l'aide d'un court capillaire en verre.
Les
mol6cules du produit ont kt6 ionis6es sous pression
atmosph6rique, et elles ont 6tE analvs6es
en
tamps reel au
moyen de
'ce.
Les
principa.
tque et
une skr
nTGAIAPCIIMSIMS, A New Technique
6for the Study of Pyrolysis
and Combustion Products
YOSHIO TSUCHIYADivision of Building Research National Research Council of Canada + Ottawa, Ontario, K I A OR6
ABSTRACT
The Fire Research Section, Division of Building Research, National
Research Council of Canada has acquired a SCIEX TAGA 6000 APCI (atmospheric
pressure chemical ionization)/MS/MS, an analytical instrument unique for its
high sensitivity and high speed in analysis. The instrument is capable of
monitoring simultaneously many types of gases generated in
pyrolysis/combustion. Coupled with a Dupont 951 thermogravimetric analyser
(TGA), it has been used for studying the pyrolysis products of
polyacrylonitrile
(PAN) at different stages of the pyrolysis process.
A 1
mgspecimen was pyrolyzed in the TGA in a stream of nitrogen or air
and the products were introduced to the APCIIMSIMS through a short glass
capillary. The molecules of the product were ionized under atmospheric
pressure, and analyzed in real time with three serial quadrupole mass filters.
The main products were HCN, acetic acid, and a series of nitriles. The
generation of each product is discussed in the light of the thermogravimetric
analysis.
KEYWORDS
APCIIMSIMS, TGAIMS, polyacrylonitrile, combustion products,
nitriles, HCN.
INTRODUCTION
A combination of thermogravimetric analysis (TGA) and mass spectrometry
(MS) is a powerful tool for studying thermal degradation of materials. With a
TGA, a specimen of the material can be thermally degraded at accurately
controlled temperatures in a desired atmosphere. The effluent degradation
products from the TGA can be analyzed by a highly sensitive MS.
It is not
surprising that many applications of TGAIMS, using various types of
instrumentation, have been reported in the past 15 years, as shown in Table 1
(1-151, although the table is not intended to present a complete list.
The conventional MS, used in most of the earlier studies, had some
-
limitations. The electron impact (EI) ionization extensively fragmented the
molecules of the degradation products at the ion source, making the mass
spectra complicated and restricting the use of the technique to the analysis
of relatively simple degradation products. Chemical ionization (CI) has
simplified the spectra, but the information on molecular weights supplied with
TABLE 1 TGA/MS S t u d i e s i n L i t e r a t u r e
I
Author Year TGA MS E I / C I mL/min OC/min M a t e r i a l
Zitomer 1968 Dupont 950 Bendix T-0-F E I 100 15 P o l y e t h y l s u l f i d e
Chang 1971 Dupont 900/PE 881 GC Dupont CEC 21-llOB 60-80 10-15 Ethyl v i n y l a c e t a t e
Gibson 1972 M e t t l e r F i n n i g a n 1015 E I 2 , 4 , 6 Geochemical samples
Mol 1974 M e t t l e r I UTI l O O C EI Vacuum 2,4,6
PVC,
ABS, PU, P o l y e s t e rTsur 1974 PE TGSl F i n n i g a n 1015 E I A , A i r 18 16 Polybenzimidazole
K l e i n b e r g 1974 Cahn RH Dupont 21-491 DF EI Oxid. 5-25 PVC, M , PU
2
N Baumgartner 1977 M e t t l e r TAC F i n n i g a n 3200 EI-CI 60-80 4 Ca o x a l a t e
Muller 1977 M e t t l e r B a l z e r s Quad MS EI 10 Ca oxalate
M o r i s a k i 1978 IR r a y thermobalance Quad MS Analyzer EI He, Air 150 PTFE
Shimizu 1979 D i r . i n s e r t . probe Dupont 21-llOB DF C I PVC, PMM
Chiu 1980 Dupont 990 Dupont 21-104 E I He 60 5-10 Ca a c e t . P o l y a c e t a l
Yuen 1982 M e t t l e r TA1 HP 5992 EI H e , 0 2 / H e 1 5 Ca oxalate, SB e t c .
I
Chan 1982 Temp. prog. f r a c t i o n . H i t a c h i RMS-4 EI 50 PS, peanut o i l
Dyszel 1983 PE TGS 2 SCIEX TAGA 3000 APCI N p 80 Guar gums
Whiting 1984 Dupont 951/HP 5710 GC LKB 9000 EI He 100 10 Coal
I
t h e u s e of CI i s o f t e n n o t s u f f i c i e n t t o i d e n t i f y t h e d e g r a d a t i o n p r o d u c t s . I n s t u d y i n g o x i d a t i v e d e g r a d a t i o n , which i s of p a r t i c u l a r i n t e r e s t t o f i r eI r e s e a r c h e r s , t h e r e i s a l s o a t e c h n i c a l d i f f i c u l t y : an o x i d a t i v e atmosphere
c a u s e s d r a s t i c r e d u c t i o n i n t h e l i f e of t h e i o n s o u r c e .
I n t h e p r e s e n t s t u d y an APCI/MS/MS (tandem) was u s e d f o r t h e a n a l y s i s of e f f l u e n t d e g r a d a t i o n p r o d u c t s of p o l y a c r y l o n i t r i l e (PAN) from t h e TGA. T h i s MS i s c o m p a t i b l e w i t h TGA, s i n c e t h e p r o d u c t s c a n b e a n a l y z e d i n a i r u n d e r
a t m o s p h e r i c p r e s s u r e . EXPERIMENTAL
-
M a t e r i a l sAn Orlon c l o t h t h a t c o n t a i n e d more t h a n 85% p o l y a c r y l o n i t r i l e was u s e d w i t h o u t f u r t h e r t r e a t m e n t . For comparison a 100% p u r e s o l i d amorphous p o l y a c r y l o n i t r i l e (Cellomer Assoc. I n c . C a t # 134c) was a l s o used. I n s t r u m e n t a t i o n
A Dupont 951 TGA and t h e SCIEX TAGA 6000 APCI/MS/MS were t h e two main i n s t r u m e n t s u s e d i n t h i s s t u d y . The SCIEX TAGA 6000 APCI/MS/MS h a s been d e s c r i b e d e l s e w h e r e ( 1 6 ) . U n l i k e o t h e r MS i n s t r u m e n t s , i t employs a l a r g e volume of sample g a s c o n t i n u o u s l y i n t r o d u c e d i n t o t h e i n s t r u m e n t ( t y p i c a l l y 2 L/min). Components of t h e g a s a r e i o n i z e d by a c o r o n a d i s c h a r g e under a t m o s p h e r i c p r e s s u r e u s i n g oxygen o r w a t e r i n t h e sample g a s a s t h e chemical r e a g e n t . A n i t r o g e n f l o w forms a g a s e o u s membrane between APCI s o u r c e and t h e h i g h vacuum a n a l y z e r s e c t i o n t o p r e v e n t un-ionized m o l e c u l e s from g o i n g i n t o t h e a n a l y z e r . The i o n i z e d m o l e c u l e s ( p a r e n t i o n s ) p e n e t r a t e t h e membrane and a r e s e p a r a t e d a c c o r d i n g t o t h e i r m a s s l c h a r g e r a t i o (M/Z) a t t h e f i r s t q u a d r u p o l e mass f i l t e r . Argon g a s f l o w s p e r p e n d i c u l a r t o t h e p a t h of p a r e n t i o n s a t t h e second mass f i l t e r . P a r e n t i o n s c o l l i d i n g a g a i n s t argon atoms f r a g m e n t w i t h a p a t t e r n c h a r a c t e r i s t i c of t h e i o n . The p a t t e r n i s a n a l y z e d by t h e t h i r d mass f i l t e r .
The MS/MS and t h e TGA were coupled w i t h a s h o r t g l a s s c a p i l l a r y (0.5 mm
d i a . , 20 mm l o n g ) . The f l o w of e f f l u e n t from t h e TGA t o t h e MS/MS t h r o u g h t h e c a p i l l a r y was measured from t h e p r e s s u r e d i f f e r e n c e a c r o s s t h e c a p i l l a r y and c o n t r o l l e d by a 'dump' v a l v e f i t t e d w i t h a micrometer. The whole i n t e r f a c e assembly was h e a t e d t o p r e v e n t t h e c o n d e n s a t i o n of d e g r a d a t i o n p r o d u c t s . To t h e e f f l u e n t f l o w , a make-up f l o w of 2 L/min of z e r o a i r ( p u r i f i e d a i r w i t h p r a c t i c a l l y n o o r g a n i c g a s e s ) was added. The s c h e m a t i c d i a g r a m of t h e
i n t e r f a c e i s shown i n Fig. 1. A H e w l e t t Packard 5996 GC/MS/Data s y s t e m w i t h a CDS 100 p y r o l y s i s u n i t was u s e d s e p a r a t e l y t o a n a l y z e t h e p y r o l y s i s p r o d u c t s and t h e r e s u l t s were compared w i t h t h o s e from t h e APCI/MS/MS.
RESULTS AND DISCUSSION
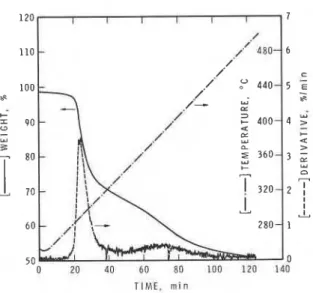
The p y r o l y s i s p r o d u c t s of PAN a n a l y z e d by t h e p y r o l y s i s GC/MS a r e shown i n Fig. 2. The p y r o l y s i s o c c u r r e d i n 3.6 atms of helium. The main p r o d u c t s were HCN, a s e r i e s of n i t r i l e s and a c e t i c a c i d . The l a s t was n o t g e n e r a t e d when t h e e x p e r i m e n t was r e p e a t e d w i t h 100% p u r e s o l i d p o l y a c r y l o n i t r i l e . I n Fig. 3, t h e r e s u l t s of TGA of PAN i n a t m o s p h e r i c n i t r o g e n a r e shown; t h e y may b e compared w i t h t h e t o t a l i o n v s t i m e from MS/MS a n a l y s i s of t h e e f f l u e n t ( F i g . 4 ) . The t o t a l c u r r e n t v a r i e d i n a manner s i m i l a r t o t h e f i r s t
d e r i v a t i v e of TGA curve. The t o t a l i o n i s t h e sum of e a c h of t h e p a r e n t i o n s ;
TAGA 6000 APCl l M S l M S
EFFLUENT FROM TGA 300 mLlmin
--+
FIGURE 1. TGA/MS/MS i n t e r f a c e .
loco 0
m
0.4 0.8 1.2 1.6 2.0 2.4 2.8 3,2 3.6 4.0 4.4 4.8 5.2 5 . 6 6.0 6.4 6.8 7.2 TIME. m i n
TIME, rnin
FIGURE
3.
Thermogravimetric analysis of PAN in nitrogen.T I M E . rnin
FIGURE 4. Pyrolysis of PAN in nitrogen, total ion in M S b S analysis, positive mode.
38 50 60 70 80 90 100 110 120 130 140 150 NORMALIZED VS MAX INT MASS. 60 (100 = 160663. COUNTS). MI2
FIGURE 5. P y r o l y s i s of PAN i n n i t r o g e n , MShS a n a l y s i s , p a r e n t s c a n , p o s i t i v e mode.
T I M E , min
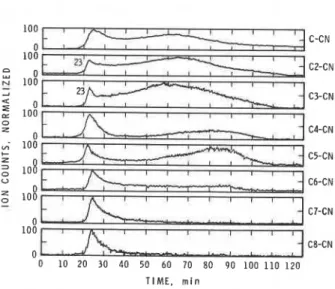
FIGURE 6. P y r o l y s i s of PAN i n n i t r o g e n , MShS a n a l y s i s , s i n g l e i o n monitoring.
From both t h e GChS and t h e MS/MS experiments, i t was c l e a r t h a t a s e r i e s of n i t r i l e s were formed. M/Z = 60 was acetamide, which was very e a s i l y i o n i z e d and was dominating i n t h e p a r e n t s c a n , but i t s a c t u a l c o n c e n t r a t i o n
i s shown a g a i n s t time. Some n i t r i l e s were g e n e r a t e d i n two s t e p s ; t h e f i r s t
!
s t e p o c c u r s a t t h e same t e m p e r a t u r e (290°C) f o r a l l t h e n i t r i l e s ; t h e second*
s t e p a t i n c r e a s i n g l y h i g h e r t e m p e r a t u r e s f o r h i g h e r n i t r i l e s . T h i s phenomenon i s n o t s i m p l y e x p l a i n e d by t h e d i f f e r e n c e i n t h e i r b o i l i n g p o i n t s . For example, b u t a n e n i t r i l e (shown a s C3-CN i n Fig. 6 ) and pentane n i t r i l e (C4-CN) have b o i l i n g p o i n t s of 117.6 and 140.7"C, r e s p e c t i v e l y , w h i l e t h e r e c o r d e d t e m p e r a t u r e s i n t h e TGA a t t h e peak of g e n e r a t i o n were 372 and 410°C, r e s p e c t i v e l y . The e l u c i d a t i o n of t h e mechanism i s l e f t f o r f u t u r e s t u d i e s .The MS/MS can d e t e c t e i t h e r p o s i t i v e i o n s o r n e g a t i v e i o n s . I n t h e p r e s e n t s t u d y , t h e p o s i t i v e i o n mode was used f o r n i t r i l e s and t h e n e g a t i v e i o n mode f o r a c i d s . Fig. 7 shows
MSIMS
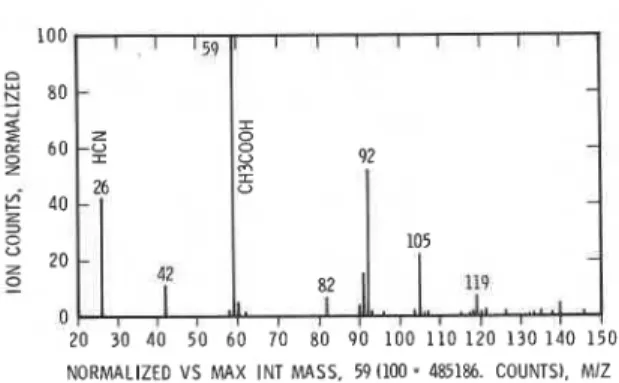
a n a l y s i s i n t h e n e g a t i v e mode. A p a r e n t s c a n a t t h e peak i s shown i n Fig. 8; major components were HCN and a c e t i c a c i d .z
=
0 0 10 20 30 40 50 60 70 80 90 100 110 120 130 T I M E , m l n FIGURE 7. P y r o l y s i s of PAN i n n i t r o g e n , t o t a l i o n i n MS/MS a n a l y s i s , n e g a t i v e mode.NORMALIZED V S MAX INT MASS. 59 (100 = 485186. COUNTS), MIZ
FIGURE 8. P y r o l y s i s of PAN i n n i t r o g e n , MS/MS a n a l y s i s , p a r e n t s c a n . n e g a t i v e mode.
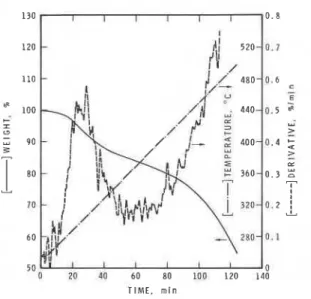
The TGA of PAN i n a i r i s shown i n Fig. 9. PAN degraded i n two s t e p s ,
b o t h i n a i r and i n n i t r o g e n ; t h e f i r s t s t e p i s a t 290°C and t h e second a t
400-500°C. I n a i r , t h e w e i g h t l o s s i n t h e f i r s t s t e p was s m a l l e r and t h e
second s t e p was l a r g e r t h a n t h e c o r r e s p o n d i n g s t e p s i n n i t r o g e n . G e n e r a t i o n
of HCN, a s determined by t h e MS/MS, i s shown i n Fig. 10. The c u r v e was
s i m i l a r t o t h e f i r s t d e r i v a t i v e of t h e TGA curve. The HCN was a l s o g e n e r a t e d
i n two s t e p s .
FIGURE 9. Thermogravimetric a n a l y s i s of PAN i n a i r .
T I M E , rnln
1 The f i r s t d e r i v a t i v e of t h e TGA c u r v e determined f o r t h e s o l i d specimen,
;
and t h e t o t a l i o n c u r r e n t determined from t h e p y r o l y s i s p r o d u c t s i n t h e g a s.
phase, were n e a r l y i d e n t i c a l i n s h a p e i n n i t r o g e n and i n a i r . Although t h i si s e x p e c t e d , s i n c e t h e weight l o s s i s a r e s u l t of g a s i f i c a t i o n ( g e n e r a t i o n of gaseous p r o d u c t s ) of t h e s o l i d specimen, t h e f i n d i n g u n d e r l i n e s t h e v a l i d i t y of t h e t e c h n i q u e . The two-step g e n e r a t i o n of HCN may be e x p l a i n e d by t h e u n z i p p i n g of molecules i n t h e f i r s t s t e p and t h e decomposition of N-containing c h a r i n t h e second s t e p . HCN i s t h e major t o x i c component i n t h e t h e r m a l decomposition p r o d u c t s of PAN (17) and o t h e r N-containing o r g a n i c
m a t e r i a l s ( 1 8 ) . CONCLUSION
The p y r o l y s i s of PAN i n n i t r o g e n and i n a i r was s t u d i e d by
TGA/APCI/MS/MS. HCN, a s e r i e s of n i t r i l e s , and a c e t i c a c i d were i d e n t i f i e d . The PAN degraded i n two s t e p s . N i t r i l e s and HCN were g e n e r a t e d i n b o t h s t e p s i n n i t r o g e n and i n a i r .
The TGA/APCI/MS/MS was found t o be an e f f e c t i v e t o o l f o r s t u d y i n g t h e p y r o l y s i s of polymers. P y r o l y s i s p r o d u c t s can be i d e n t i f i e d and t h e i r g e n e r a t i o n a t d i f f e r e n t s t a g e s of p y r o l y s i s can be c l o s e l y observed. F u r t h e r s t u d i e s a r e planned.
ACKNOWLEDGEMENT
The a u t h o r t h a n k s J.B. S t e w a r t f o r h i s a s s i s t a n c e i n r u n n i n g e x p e r i m e n t s and p r o c e s s i n g d a t a . T h i s paper i s a c o n t r i b u t i o n from t h e D i v i s i o n of B u i l d i n g Research, N a t i o n a l Research Council of Canada.
REFERENCES
1. Zitomer, F.: T h e r m o g r a v i m e t r i c l n a s s s p e c t r o m e t r i c a n a l y s i s , Anal. Chem., 40: 7, 1091, 1968.
2. Chang, T.L., and Mead, T.E.: Tandem t h e r m o g r a v i m e t r i c analyzer-GC-high r e s o l u t i o n MS system, Anal. Chem.,
43:
534, 1971.3. Gibson, E.K. J r . , and Johnson, S.M.: Thermogravimetric-quadrupole mass s p e c t r o m e t r i c a n a l y s i s of geochemical samples, Thermochim. Acta,
4:
49, 1972.4. Mol, G.J.: Simultaneous thermogravimetry and mass s p e c t r o m e t r y i n polymer c h a r a c t e r i z a t i o n , Thermochim. A c t a , 259, 1974.
5. T s u r , Y., F r e i l i c h , Y.L., and Levy, M.: TGA-MS d e g r a d a t i o n s t u d i e s of some new a l i p h a t i c - a r o m a t i c p o l y b e n z i m i d a z o l e s , J . Polym. S c i . , Chem. ed.,
12:
1531, 1974.6. K l e i n b e r g , G.A., Geiger, D.L., and Gormley, W.T.: Rapid d e t e r m i n a t i o n of k i n e t i c p a r a m e t e r s f o r t h e t h e r m a l d e g r a d a t i o n of h i g h polymers u t i l i z i n g a computerized t h e r m o g r a v i m e t r i c a n a l y z e r - m a s s s p e c t r o m e t e r system, Makromol. Chem.,
175:
483, 1974.7. Baumgartner, E., and Nachbaur, E.: Thermogravimetry combined w i t h chemical i o n i z a t i o n mass s p e c t r o m e t r y : a new t e c h n i q u e i n t h e r m a l a n a l y s i s ,
Thermochim.
19: 3, 1977..
-8.
Muller-Vonmoos, M., Kahr, G., and Rub, A.: Quantitative determination ofH20, CO and C02 by evolved gas anslysis with a MS, Thermochim. Acta,
p
f20: 387, 1977. 4
-
9. Morisaki,
S.:
Simultaneous thermogravimetrylnass spectrometry and pyrolysis-gas chromatography of fluorocarbon polymers,25: 171, 1978.
-
10. Shimizu,
Y.,
and Munson, B.: Pyrolysis/chemical ionization mass spectrometry of polymers, J. Polymer Sci., Chem., 1991, 1979. 11. Chiu, J., and Beattie, A.J.: Techniques for coupling mass spectrometryto thermogravimetry, Thermochim. Acta,
40:
251, 1980.12. Yuen, H.K., Mappes, G.W., and Grote, W.A.:
An
automated system for simultaneous thermal analysis and mass spectrometry, partI,
Thermochim. Acta 52- 143, 1982.-,
--
13. Chan, K.C., Tse, R.S., and Wong, S.C.: Temperature programmed fractionation inlet system for mass spectrometers, Anal. Chem., 54: 1238, 1982.
-
14. Dyszel, S.M.: Thermogravimetry coupled with atmospheric pressure
ionization mass spectrometry, a new combined technique, Thermochim? Acta, 61: 169, 1983.
-
15. Whiting, L.F., and Langvardt, P.W.: On-column sampling device for
thermogravimetry/capillary gas chromatography/mass spectrometry, Anal.
e,
56:
1755, 1984.16. French, J.B., Davidson, W.R., Reid, N.M., and Buckley, J.A.: "Trace monitoring by tandem mass spectrometry," in
P.W. McLafferty, ed., John Wiley & Sons, New York, 1983. 17. Tsuchiya, Y., and S u d , K.: Thermal decomposition products of
polyacrylonitrile, J. Appl. Polym. Sci.,
21:
975, 1977.18. Tsuchiya, Y.: Significance of HCN generation in fire gas toxicity,