HAL Id: tel-02512762
https://tel.archives-ouvertes.fr/tel-02512762
Submitted on 19 Mar 2020HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Sox8 acts redundantly in place of Sox9 during
pathophysiological testicular development in R-spondin1
(RSPO1) loss-of-function mice
Nainoa Richardson
To cite this version:
Nainoa Richardson. Sox8 acts redundantly in place of Sox9 during pathophysiological testicular de-velopment in R-spondin1 (RSPO1) loss-of-function mice. Molecular biology. Université Côte d’Azur, 2019. English. �NNT : 2019AZUR6005�. �tel-02512762�
Sox8 compense la perte de Sox9
pendant le développement testiculaire
physiopathologique chez la souris
présentant une perte de fonction du
gène R-spondin1
Nainoa RICHARDSON
Laboratoire Equipe CHABOISSIER, iBV
THÈSE DE DOCTORAT
Présentée en vue de l’obtention du grade de docteur en
Interactions Moléculaires et Cellulaires d’Université Côte d’Azur
Dirigée par : Marie-Christine Chaboissier, Marie-Cécile De Cian
Soutenue le : 20 Septembre 2019
Devant le jury, composé de : Andreas Schedl, Docteur, iBV, Nice Francisco Barrionuevo, Profesor Titular de Universidad, Universidad de Granada, Granada Francis Poulat, Docteur, Institut de génétique humaine, Montpellier
Jérôme Collignon, Docteur, Institut Jacques Monod, Paris
Sox8 compense la perte de Sox9 pendant le
développement testiculaire physiopathologique
chez la souris présentant une perte de fonction
du gène R-spondin1
Jury : Rapporteurs
Francisco BARRIONUEVO, Profesor Titular de Universidad, Universidad de Granada, Granada
Francis POULAT, Docteur, Institut de Génétique Humaine UMR 9002 CNRS UM, Montpellier
Examinateur
Sox8 compense la perte de Sox9 pendant le développement testiculaire physiopathologique chez la souris présentant une perte de fonction du gène R-spondin1
Résumé
Chez les mammifères, le développement testiculaire des gonades XY est initié par les facteurs de transcription SRY/SOX9 qui promeuvent la différenciation des cellules de Sertoli. Chez l’embryon XX, la signalisation RSPO1/WNT/beta-catenin contrôle la différenciation des cellules de la granulosa et le développement ovarien. De fait, les souris XY n’exprimant pas Sox9 (KO) développent des ovaires et les souris XX n’exprimant pas Rspo1(KO) développent des ovotestis, constitués d’une partie testiculaire et une ovarienne. Leur formation est due à la différenciation précoce de cellules de granulosa et la reprogrammation d’une partie d’entre elles, en cellules de Sertoli. Chez les souris XX Rspo1 KO, SRY n’est pas nécessaire au développement testiculaire.
De plus, les gonades des souris XX et XY présentant une double inactivation des gènes
Rspo1 et Sox9 (Double Knockout/DKO) montrent une différenciation testiculaire partielle et
complète respectivement, avec un développement d’ovotestis chez les individus XX DKO et un développement de testicules hypoplasiques chez les souris XY DKO. SOX9 et/ou SRY ne sont donc pas nécessaires à la différenciation testiculaire dans ce contexte, suggérant l’implication d’autres facteurs.
L’objectif de ma thèse est de tester l’hypothèse selon laquelle SOX8, un facteur de transcription de la même famille que SOX9, pourrait induire le développement testiculaire chez les souris XX et XY DKO Rspo1 Sox9. Afin d’établir l’existence d’une compensation entre ces gènes SOX, nous avons analysé leur expression et le développement des gonades chez la souris DKO pour les gènes Rspo1 et Sox8 ou Sox9. Nous avons ensuite étudié les souris mutantes simultanément pour les gènes Rspo1, Sox8 et Sox9 (triple knockout/TKO). Notre hypothèse est qu’une perte d’expression des gènes Sox8 et Sox9 chez les souris TKO empêche la reprogrammation des cellules de la granulosa en Sertoli et par conséquent le développement testiculaire. Nous avons donc analysé la morphologie des gonades, les caractères sexuels secondaires, ainsi que l’organisation des gonades avec les différentes populations cellulaires qui les constituent par histologie et immuno-marquages à différents stades : à 17.5 jours de développement embryonnaire (E17.5) où la reprogrammation de cellules de granulosa en Sertoli commence dans la souris XX Rspo1 KO; chez les souris juvéniles au jour 10 (P10) où le développement somatique est achevé; et chez les souris adultes 40 jours après la naissance (P40).
Nos résultats montrent que SOX8 et SOX9 sont exprimés de manière indépendante dans les gonades des souris XY and XX DKO Rspo1 Sox9 et DKO Rspo1 Sox8 à E17.5 et à P10. De plus, les souris XY et XX DKO Rspo1 Sox8 développent des testicules et des ovotestis indiquant que la perte d’un seul facteur SOX n’altère pas la formation des testicules, comme dans les souris XY et XX DKO Rspo1 Sox9. Cependant, chez les souris XX et XY TKO, la reprogrammation des granulosa en Sertoli à E17.5 et le développement testiculaire postnatal ne sont plus observés, démontrant que SOX8 peut compenser la perte de SOX9. De plus, les gonades des souris XY et XX TKO sont des ovaires atrophiques, indiquant que la différenciation ovarienne peut s’opérer.
En résumé, nous avons analysé l’étiologie du développement physiopathologique des gonades chez les souris ayant une perte de fonction de RSPO1. Bien que SOX8 ne soit pas nécessaire à la différenciation testiculaire chez la souris, il peut promouvoir le développement testiculaire en l’absence de SRY et SOX9 en raison de sa redondance fonctionnelle avec SOX9. Chez l’Homme, dans les cas cliniques d’ambiguïtés sexuelles avec différenciation testiculaire, qui ne sont pas expliqués par le défaut d’expression de SRY ou SOX9, SOX8 pourrait ainsi être un facteur causatif.
Mots clés : R-spondin1, Rspo1, Sox8, Sox9, développement des gonades, inversion de sexe, modèle murin
Sox8 acts redundantly in place of Sox9 during pathophysiological testicular development in R-spondin1 (RSPO1) loss-of-function mice
Abstract
In humans and mice, testicular development in XY gonads involves SRY/SOX9 signaling to promote Sertoli cell differentiation and their formation as testis chords. For ovarian development in XX gonads, RSPO1/WNT/beta-catenin signaling is the main pathway for granulosa cell differentiation and their subsequent assembly into follicles. Indeed, XY Sox9 mutant mice develop ovaries, and XX Rspo1 mutant mice develop ovo-testes, a gonad containing a testicular and an ovarian part. In XX Rspo1 mutant mice, ovo-testicular development involves precocious differentiation of some granulosa cells and their and reprogramming as Sertoli cells. Thus, these single mutant studies demonstrated that SOX9 and RSPO1 are required for testicular and ovarian development respectively, and that SRY is dispensable for testicular development in XX Rspo1 mice.
Interestingly, gonad development in XY and XX Rspo1 Sox9 double knockout (DKO) mice has challenged the requirement of SOX9 for testicular development. In XX Rspo1 single mutants, it was assumed that Sertoli cell differentiation was SOX9-dependent, but co-inactivation of Sox9 in DKO mice does not impair the ovo-testicular phenotype. For XY Sox9 single mutant mice developing ovaries, co-inactivation of Rspo1 in XY DKO mice rescues the sex reversal, though the testes are hypo-plastic. Thus, in XY and XX Rspo1 Sox9 DKO mice, SOX9 and/or SRY are dispensable for testicular differentiation, indicating that an alternate testis factor exists.
For my research project, we hypothesized that a SOX9-related transcription factor, SOX8, acts redundantly for testicular development in XY and XX Rspo1 Sox9 DKO mice. Thus, to first establish redundancy among the SOX factors, we first analyzed their expression in Rspo1 mutant mice lacking Sox8 or Sox9, and then generated and analyzed gonad development in XY and XX
Rspo1 Sox8 DKO mice. Then to test our hypothesis, we studied Rspo1 Sox8 Sox9 triple knockout
(TKO) mice. We predicted that a loss of both Sox genes in TKO mice would prevent granulosa cell reprogramming as Sertoli cells and subsequent testicular development. To characterize gonad development and their effects in DKO and TKO mice, we performed analyses in embryonic day 17.5 (E17.5) mice, when granulosa-to-Sertoli cell reprogramming begins in XX Rspo1 single mutants; in juvenile post-natal day 10 (P10) mice, when gonad fate is set; and in young adult P40 mice. We examined a variety of parameters including gonad morphology and secondary sex characteristics, as well as gonad organization and cell population by histological and immunostaining analyses.
We report that SOX8 and SOX9 are expressed independently in XY and XX Rspo1 Sox9 DKO and Rspo1 Sox8 DKO gonads in embryonic and juvenile mice. Next, XY and XX Rspo1
Sox8 DKO mice developed testes and ovo-testes, indicating that loss of one SOX factor does not
impair testicular differentiation, as in XY and XX Rspo1 Sox9 DKO mice. In XY and XX Rspo1
Sox8 Sox9 TKO mice, granulosa-to-Sertoli cell reprogramming was impaired at E17.5 and
post-natal gonads lacked testicular development. Thus, SOX8 can compensate for the loss of SOX9 in
Rspo1 Sox9 DKO mice. In addition, gonads in XY and XX TKO mice developed as atrophied
ovaries, indicating that ovarian fate is partially maintained.
In total, we investigated the etiology of pathophysiological testicular development in RSPO1 loss-of-function mice. Remarkably, though SOX8 is dispensable for male sex determination in mice, it can promote testicular differentiation in the absence of SRY and SOX9 because of functional redundancy with SOX9. Thus, in human cases of sex reversal where testicular development cannot be explained by misexpression of SRY or SOX9, SOX8 could be a causative factor.
Table of contents
I
NTRODUCTIONChapter 1 – Opening remarks and thesis organization………..…... Chapter 2 – Reproductive anatomy and gonad biology………..
2.1 Male reproductive system………..……...………
2.2 Female reproductive system………...……….…..
Chapter 3 – SOX transcription factors, with emphasis on the E group……….…...
3.1 General SOX features………..…..
3.2 SOX organization and redundancy………..
3.3 SOX-E group (SOX8, 9, and 10).………...
3.4 SOX-E in the nervous system………..
Chapter 4 – WNT/β-catenin signaling and the R-spondin family………...
4.1 Canonical WNT/β-catenin signaling………...
4.2 R-spondin family……….
4.3 Overview of RSPO1-4………...………..
Chapter 5 – FOXL2 transcription factor………
G
ONADD
EVELOPMENTChapter 6 – Development of the bi-potential gonad………...
6.1 Overview………..
6.2 AGP formation……….
6.3 Development of the genital ridge……….
6.4 Significance of SF1……….………. 1 4 4 6 9 9 11 12 13 15 15 17 19 21 22 22 22 24 25
Chapter 7 – Testicular development, Part A………...
7.1 Overview of pathways and key events………..………....………...
7.2 SRY functions and expression……….
7.3 Up-regulation and maintenance of SOX9………
7.4 Testis chords and peritubular myoid cells………
7.5 Coelomic vessel formation………...…
7.6 Mitotic arrest of gonocytes………...…
7.7 Leydig cells………..…
Chapter 8 – Testicular development, Part B………...
8.1 AMH and the regression of Mullerian ducts………
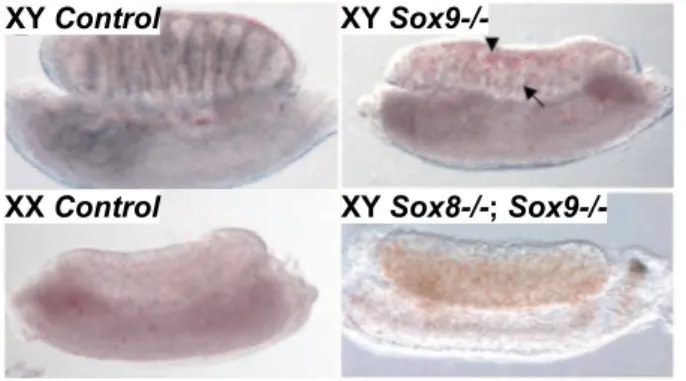
8.2 Progressive infertility in XY Sox8 mutant mice.………...…..
8.3 Cooperation of Sox8 and Sox9 in testes………...…
8.4 Control of SoxE gene expression in testes……….………..
8.5 DMRT1 in testes………..…
Chapter 9 – Ovarian development………...…
9.1 Overview of pathways and key events………...……….
9.2 Granulosa cell origins………...…………...
9.3 From germ cell meiosis to primordial follicles………
9.4 Theca cells………....………
9.5 Gonad phenotype in XX Rspo1, Wnt4, Ctnnb1, or Foxl2 mutant mice…...…………
S
PECIALT
OPICS&
P
ROJECTA
IMSChapter 10 – Gonad development in XY and XX Rspo1/Ctnnb1 Sox9
double mutant mice………...………..….……..
Chapter 11 – Thesis project overview………..
11.1 Hypothesis……….... 11.2 General approach………....………..… 26 26 27 30 32 32 33 33 35 35 35 37 39 39 40 40 41 43 44 44 47 50 50 51
R
ESULTS,
D
ISCUSSION,
&
C
ONCLUSIONChapter 12 – Manuscript entitled Sox8 and Sox9 act redundantly for ovarian- to-testicular fate reprogramming in the absence of R-spondin1 in
mouse sex reversals …….………..………...…….
Chapter 13 – Additional data from XY and XX Rspo1KO Sox8KO
and XX Rspo1KO Sox9cKO double mutants………....
13.1 Overview……….. 13.2 Quantification of Sertoli cells in XY Rspo1KO Sox8KO mice at E13.5……….
13.3 Body weight in XY and XX Rspo1KO Sox8KO mice at P40………..
13.4 Testis weight in XY Rspo1KO Sox8KO mice at P40………...
13.5 Testis cord formation and Sertoli cell maturation in XX Rspo1KO Sox8KO
mice……….. 13.6 Precocious granulosa cell differentiation in XX Rspo1KO Sox8KO and
Rspo1KO Sox9cKO double mutant gonads at E17.5………
Chapter 14 – Additional discussion and concluding remarks……….…….. References……...…..………….……… 52 87 87 87 91 93 93 94 98 100
List of Figures
Chapters 1-11
Figure 1. Signaling networks in mammalian sex determination……….. Figure 2. Male reproductive anatomy………... Figure 3. Female reproductive anatomy and folliculogenesis………...…… Figure 4. SOX family organization and E group structure.…….………..……….. Figure 5. Scheme of canonical WNT/β-catenin signaling…..………..……….. Figure 6. Scheme of human RSPO proteins and general mechanism.……….…... Figure 7. Scheme showing origin of the genital ridge and its development….……….. Figure 8. Expression of SRY and SOX9 during sex determination in mice………... Figure 9. Sex reversal in XY Sox9 versus XY Sox8 Sox9 mutant fetuses………...………... Figure 10. Scheme showing the origins of granulosa and theca cells………. Figure 11. Model for gonad sex reversal in XX Wnt4 or Rspo1 mutant mice……… Figure 12. Sertoli cell differentiation in the absence of Rspo1 or Ctnnb1 and Sox9…...…...
***** Chapter 12
Figures for the manuscript entitled Sox8 and Sox9 act redundantly for ovarian- to-testicular fate reprogramming in the absence of R-spondin1 in mouse sex reversals
Figure 1. Expression of Rspo1, Sox8, and Sox9 in E17.5 and P10 gonads………. Figure 1–figure supplement 1. Absence of β-galactosidase activity in
fetal XX Rspo1+/- and Rspo1-/- gonads.………..….…………...…..
Figure 2. Testis and ovo-testis development in adult XY and XX Rspo1KO Sox8KO
double mutant mice..……….………...……
Figure 2–figure supplement 1. Secondary sex characteristics of XY and
XX Rspo1KO Sox8KO adult P40 mice and analyses in juvenile P10
mice……….……. 2 5 7 10 16 18 23 28 38 42 46 48 58 60 62 63
Figure 3. Precocious granulosa cell differentiation in XX Rspo1KO Sox8KO
Sox9cKO triple mutant fetuses at E17.5.………...….
Figure 3–figure supplement 1. Absence of SOX9 expression in gonads
from XX Rspo1KO Sox8KO Sox9cKO fetuses and presence of
steroidogenic cells at E17.5……….
Figure 4. Lack of testis cords in XY Rspo1KO Sox8KO Sox9cKO triple mutant
fetuses at E17.5. .……….……..………..……….
Figure 4–figure supplement 1. Absence of SOX9 expression in gonads
from XY Rspo1KO Sox8KO Sox9cKO fetuses and presence of
steroidogenic cells at E17.5……….……….
Figure 5. Absence of seminiferous tubules in XY and XX Rspo1KO Sox8KO Sox9cKO
triple mutant adult mice at P40….………...………….
Figure 5–figure supplement 1. Secondary sex characteristics of XY and
XX Rspo1KO Sox8KO Sox9cKO adult P40 mice and analyses in
juvenile P10 mice…………...………...………
Figure 5–figure supplement 2. Organization of adult XY and
XX Rspo1KO Sox8KO Sox9cKO triple mutant gonads...…….…………..………
Figure 5–figure supplement 3. Rare immature Sertoli cells in XY and
XX Rspo1KO Sox8KO Sox9cKO triple mutant gonads..………
Figure 6. Gonad fate in wildtype, Rspo1, and Sox mutant mice. .………..…….……
***** Chapter 13
Figure 13. Analysis of XY Rspo1KO Sox8KO double mutant testes at E13.5
and E17.5…….………..……….…..…
Figure 14. Body weight of XY and XX, and testis weight of XY Rspo1KO Sox8KO
mice at P40. .………..……….……….……
Figure 15. Testis cord development and Sertoli cell maturation in
XX Rspo1KO Sox8KO mice.……..………..…..……….…
Figure 16. Precocious granulosa cell differentiation in gonads from
XX Rspo1KO Sox8KO and XX Rspo1KO Sox9cKO fetuses at E17.5..……….……. 66 68 71 72 74 76 78 79 82 88 92 95 96
I
NTRODUCTION
Chapter 1
Opening remarks and thesis organization
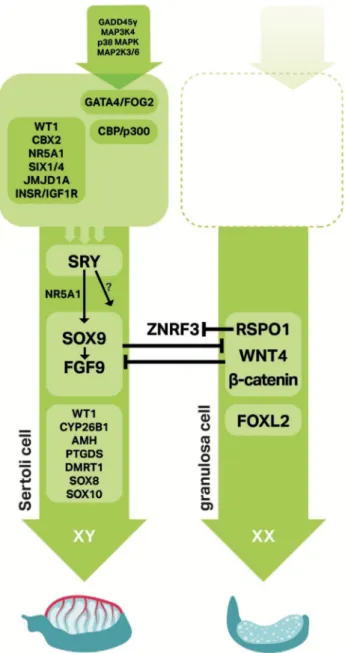
Since ancient times, how mammalian sex is determined has been an important question that was often answered by a mixture of cultural perspectives and logic (Stevant et al., 2018b). Today, sex determination is discipline in reproductive biology that explains how a bi-potential gonad can differentiate as testis or ovary containing functional germ cells for reproduction and propagation of a species. We now know from studies in humans, mice, and other models that certain genetic pathways control testis and ovarian differentiation, gonad function, and maintenance. Some key sex determination factors are highlighted in Figure 1 (Nef et al., 2019).
In humans, inappropriate regulation of sex determination genes can lead to disorders/differences in sexual development (DSD), which affects 1 in 5,500 individuals (Blackless et al., 2000; Sax 2002). Briefly, DSD defines a broad range of congenital conditions where the development of chromosomal, gonadal, or anatomical sex is atypical, and some DSD conditions lead to infertility (Lee et al., 2006). One review estimates that only about 20% of DSD cases receive a specific molecular diagnosis (Hughes et al., 2006). Another estimates only 50% of patients with XY gonadal dysgenesis receive a genetic diagnosis (Leon et al., 2019). Though modern technology like next generation sequencing will certainly aid in genetic diagnoses, functional studies for individual genes will still be necessary to confirm the causative factor.
For my thesis project, we took advantage of genetic mice models (M. musculus) to better understand the genetics of gonad development. Thus, mouse genes, proteins, and nomenclature will be used to describe sex determination, with casual references to the processes as they occur in humans or other species. If not stated explicitly, the reader should assume the example or reference is from or for mice.
This manuscript is organized into five sections: the INTRODUCTION section covers basic reproductive anatomy and function, as well as an overview of some key factors,
Figure 1. Signaling networks in mammalian sex determination. Sex determination involves many factors, some of which are shown here. In particular, SRY and SOX9 are testis factors and RSPO1/WNT4/β-catenin along with FOXL2 are ovarian factors. In general, arrows indicate positive interactions and T-bars represent antagonistic interactions (Adapted from Nef et al., 2019).
pathways, or gene families related to sex determination (Chapters 2-5); GONAD
DEVELOPMENT describes testis and ovarian development from a bi-potential gonad (Chapters 6-9); SPECIAL TOPICS & PROJECT AIMS highlights the premise of my thesis project and aims (Chapters 10-11); RESULTS, DISCUSSION, & CONCLUSION presents an article manuscript and concluding remarks (Chapters 12-14); and the BIBLIOGRAPHY section shows the references for all chapters.
Chapter 2
Reproductive anatomy and gonad biology
2.1 Male reproductive system 2.2 Female reproductive system
2.1 Male reproductive system
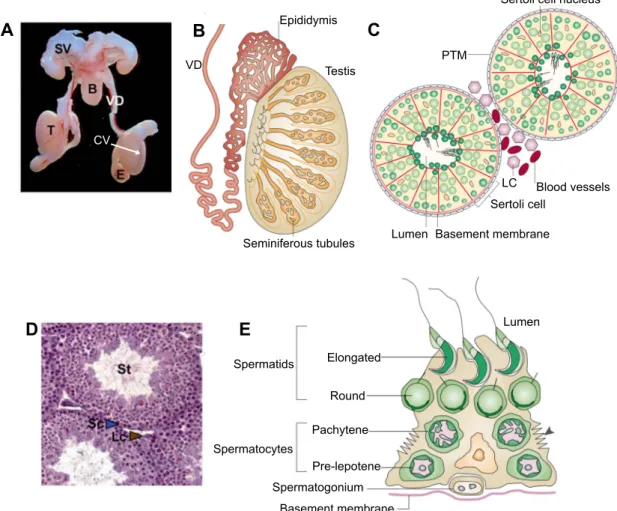
Macroscopic examination of the reproductive tract in XY mice shows epididymides, vasa deferentia, and an accessory organ called seminal vesicles (Figure 2A). These and other organs support the transport, maturation, and overall success of spermatozoa for sexual reproduction. The mammalian testis itself contains arrays of convoluted seminiferous tubules (or testis/sex cords) that converge in an area called the rete testis, which leads to the epididymis (Figure 2B) (Cooke and Saunders 2002). Testis sections show that seminiferous tubules are highly organized structures containing Sertoli and germ cells surrounded by peritubular myoid cells and by a basement membrane (Figure 2C, D). Spermatogenesis occurs in the area close to the basement membrane, which is referred to as the seminiferous epithelium (Oakberg 1956). Between testis cords exists an interstitial compartment, which contains steroidogenic Leydig cells.
During spermatogenesis, spermatogonia (diploid, 2n) undergo meiosis to form spermatozoa (haploid, 1n) in defined stages (spermatogenic cycle) (Ahmed and de Rooij 2009). This process is directional, as germ cells move from an area near the basement membrane inward, towards a lumen, until mature spermatozoa can break away to enter the reproductive tract (Figure 2E). In addition, during spermatogenesis, meiotic germ cells pass through a barrier called the blood-testis barrier or BTB, which creates a nurturing environment for meiotic and post-meiotic germ cells and protects them from immune cells, as first revealed in rat testes (Tuck et al., 1970; Evans and Setchell 1978; Setchell 1990). The BTB is formed by specialized junction proteins expressed by Sertoli cells, such as Claudin proteins (Mazaud-Guittot et al., 2010).
Beginning at puberty in humans and mice, spermatogenesis is regulated by hormones in the hypothalamic-pituitary-gonadal (HPG) axis. Briefly, the hypothalamus produces gonadotropin-releasing hormone (GnRH), which in turn stimulates secretion of luteinizing
B Seminiferous tubules Testis VD Epididymis D A C Blood vessels LC Sertoli cell PTM
Sertoli cell nucleus
Basement membrane Lumen Spermatocytes Basement membrane Spermatogonium Pre-lepotene Pachytene Round Elongated Spermatids Lumen E CV
Figure 2. Male reproductive anatomy. A – Macroscopic view of the reproductive system from adult male mice with labeled seminal vesicle (SV), bladder (B), vas deferens (VD), epididymis (E), and testis (T). The coelomic vessel (CV) is a prominent blood vessel in testes (Adapted from Chassot et al., 2008). B – Scheme of testis show convoluted seminiferous tubules. C, D – Scheme and histology of a testis section showing that seminiferous tubules (St) contain Sertoli and germ cells. Peritubular myoid cells (PTM) surround seminiferous tubules and Leydig cells (Lc or LC) are found between seminiferous tubules in the interstitial compartment. E – Scheme of the seminiferous epithelium showing meiotic cells at different maturation stages in spermatogenesis (Adapted from Cooke and Saunders 2002).
hormone (LH) and follicular stimulating hormone (FSH) by the pituitary. In testes, FSH and LH bind their receptors on Sertoli and Leydig cells respectively, to regulate testosterone. Studies in mice have demonstrated that testosterone or its main receptor, androgen receptor (AR), are essential for meiosis and male fertility (Haywood et al., 2003; Chang et al., 2004; De Gendt et al., 2004).
2.2 Female reproductive system
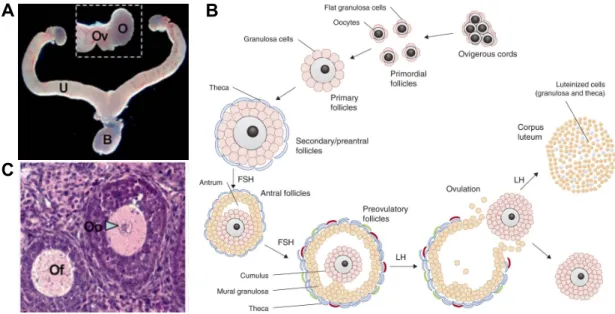
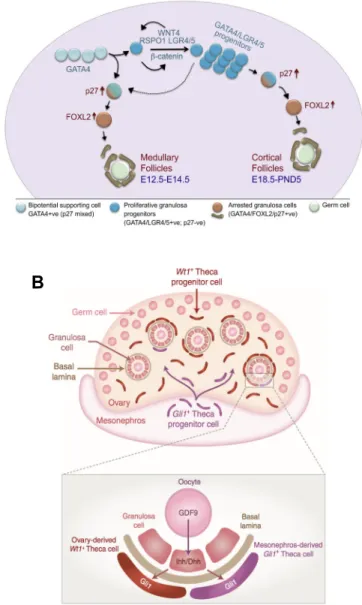
Macroscopic examination of the reproductive tract in XX mice shows oviducts and uteri (Figure 3A). In humans and mice, adult ovaries contain follicles at different stages of maturation, which is described in a process called folliculogenesis. Between follicles exists an interstitial compartment containing steroidogenic theca cells.
Follicles are composed of granulosa cells organized around a single oocyte (Figure 3B, C), which begin to assemble in developing ovaries (Tingen et al., 2009). Prior to follicle assembly, oogonia (diploid, 2n) initiate meiosis until arresting at prophase I as a primary oocyte (diploid, 2n) (Di Carlo et al., 2000; Ghafari et al., 2007; Spiller et al., 2017). Then, before birth in humans and after in mice, follicles can either enter folliculogenesis or remain dormant until activated (McGee and Hsueh 2000; Mork et al., 2012). Studies in mice have revealed a heterozygous population of follicles: medullary and cortical follicles contribute to early and late fertility, respectively (Mork et al., 2012). The cortical follicles make up a pool of primordial follicles known as the ovarian reserve of follicles.
Figure 3B shows a scheme for folliculogenesis (Georges et al., 2014). Before puberty, primordial follicles can enter folliculogenesis and mature as primary and secondary follicles with surrounding theca cells. As granulosa cells proliferate and the follicle size increases, secondary follicles can mature as antral/tertiary, then pre-ovulatory stage follicles though a GnRH-dependent selection process starting at puberty. During ovulation, the mature ovum (or ova) is released from the ovary and the remaining post-ovulatory follicle develops as
corpus lutea. In the event of fertilization, corpus lutea secrete progesterone to support
pregnancy.
The HPG axis also functions in folliculogenesis. Starting at puberty in ovaries, FSH and LH bind their receptors on granulosa cells and theca cells respectively, to promote follicle growth and the secretion of estrogens and another hormone called inhibin. At low and high estrogen levels, pituitary derived hormones (FSH and LH) are regulated in a negative and positive manner, respectively, and inhibin regulates FSH. Thus, as some follicles grow, LH and FSH levels tapers until inhibin and high estrogen levels triggers a surge of only LH,
A B
C
Figure 3. Female reproductive anatomy and folliculogenesis. A – Macroscopic view of the reproductive system from adult female mice with labeled ovary (O), oviduct (Ov), uterus (U), and bladder (B) (Adapted from Chassot et al., 2008). B – Histology showing ovarian follicle (Of) and oocyte (Oo) (Adapted from Chassot et al., 2008). C – Diagram of folliculogenesis. The ovarian reserve serves as a pool of primordial follicles that can enter folliculogenesis. Activated follicles are surrounded by theca cells and grow in size by addition of granulosa cell layers. Antral follicles enter a selection process that depends on pituitary derived follicular stimulating hormone (FSH) and luteinizing hormone (LH). One, and sometimes more follicles, can advance to ovulation, leaving behind cells that form corpus lutea (Georges et al., 2014).
and thus ovulation. In pre-ovulatory follicles, the LH surge also causes arrested primary oocytes to resume meiosis until meiotic metaphase II, as secondary oocytes (1n, haploid), and also to promote corpus lutea formation. If fertilized, the secondary oocyte will complete meiosis. Studies in XX mice have demonstrated that estrogen is essential for fertility, since estrogen receptor (ER) loss-of-function mice exhibit large follicle defects and are anovulatory (Schomberg et al., 1999).
Altogether, the testis and ovary are distinct organs and in general, a mature mammalian gonad contains supporting cells (Sertoli or granulosa), steroidogenic cells (Leydig or theca), and germ cells (spermatozoa or ova). In mice gonads, the supporting cell line is the first somatic cell type to differentiate (Karl and Capel 1998; Mork et al., 2012), and these cells can influence the differentiation and survival of other cell lines, as discussed in chapters to come.
Chapter 3
SOX transcription factors, with emphasis on the E group
3.1 General SOX features
3.2 SOX organization and redundancy 3.3 SOX-E group (SOX8, 9, and 10) 3.4 SOX-E in the nervous system
3.1 General SOX features
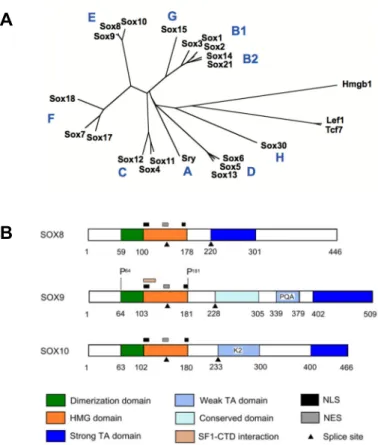
The SRY-related HMG-box (SOX) protein family is a group of transcriptional regulators capable of influencing diverse developmental and cellular processes. In 1990, SRY became the founding member of this group, as it featured a protein domain sharing homology with other DNA-binding proteins from humans, mice, and yeast (Gubbay et al., 1990; Sinclair et al., 1990). The domain is the high mobility group (HMG)-box domain, which is a evolutionarily conserved sequence of ~75-79 amino acids (Stros et al., 2007). For SOX proteins, the presence of the HMG domain with 50% or greater homology with the HMG domain of SRY initially defined this group. However, this criterion has evolved to include other SOX factors with slightly less homology. The defining feature of this group is now a signature RPMNAFMVW amino acid sequence within the HMG domain (Bowles et al., 2000).
Some additional unifying SOX features include: functional amino acid sequences within the HMG domain, the DNA consensus sequence recognized by HMG, and how SOX generally operate. The HMG domain of the SOX group is characterized by two independent nuclear localization signals (NLSs) and one leucine-rich nuclear export signal (NES) (Sudbeck and Scherer 1997), as diagramed in Figure 4B for SOX-E transcription factors (Barrionuevo and Scherer 2010). These signals can mediate shuttling of SOX proteins between the nucleus and cytoplasm in cells (Gasca et al., 2002; Malki et al., 2010). In general, SOX proteins elicit their function in part by binding a DNA consensus sequence of (A/T)(A/T)CAA(A/T)G via its HMG domain (Harley et al., 1994; Wegner 2010).
SOX factors are known to operate with partner proteins to regulate target genes and in some cases, its own expression (Kamachi et al., 2000; Kondoh and Kamachi 2010). For
A B
B
Figure 4. SOX family organization and E group structure. A – Unrooted phylogenetic tree of the high-mobility group (HMG) domains in mice. SOX transcription factors are grouped according to the degree of conservation with the HMG-box of SRY, the founding member. SOX-E transcription factors include SOX8, SOX9, and SOX10 (Adapted from Kamachi and Kondoh 2013). B – Structure of human SOX-E proteins. Among the defining features of this group is a DNA-dependent dimerization domain near the N-terminus (Adapted from Barrionuevo and Scherer 2010).
example, during lens development, SOX2 and its binding partner PAX6 cooperatively activate the d-crystallin DC5 enhancer, but engagement of the enhancer does not occur with SOX2 or PAX6 alone (Kamachi et al., 2001). In the same example, SOX2 and PAX2 are known to bind the Sox2 enhancer itself and Pax6 is also regulated by SOX2 (Inoue et al., 2007; Lin et al., 2009). Auto-regulation provides a mechanism in cells to attain or maintain adequate protein levels, which is important for SOX factors, since their function is known to be dose-dependent (Pevny and Nicolis 2010). In Chapter 7, these SOX characteristics (use of a binding partner, auto-regulation) will be recurring themes to describe testicular differentiation.
3.2 SOX organization and redundancy
In mammals, SOX factors have been organized into letter groups A-H according to conservation within the HMG domain (Figure 4A) (Bowles et al., 2000; Kamachi and Kondoh 2013). In human and mice, 20 orthologous pairs of SOX/Sox genes have been identified (Schepers et al., 2002). Beyond homology, members of the same SOX group are often expressed in the same developing tissues, may function with the same binding partners, and even co-regulate the same targets (Kamachi and Kondoh 2013). Thus, there is genetic redundancy among the members of a SOX group. Furthermore, there is evidence to show that SOX factors retain certain functions, even though they are not expressed in a particular organ during normal development.
Perhaps the best example of redundancy is in male sex determination, where all SOX-E (Sox8, Sox9, and Sox10) transcription factors are expressed in supporting cells of the XY gonad (Jameson et al., 2012; Stevant et al., 2018a), but only Sox9/SOX9 is required for testicular differentiation (Wagner et al., 1994; Chaboissier et al., 2004; Barrionuevo et al., 2006; Lavery et al., 2011). Strikingly, though Sox10 is dispensable, ectopic expression in XX gonads is sufficient for testicular differentiation (Polanco et al., 2010), indicating that SOX10 retains this capability, and perhaps even SOX8. Interestingly, the closest relative to Sry, Sox3 (SOX-B1 group) (Stevanovic et al., 1993; Wallis et al., 2008), though not or minimally expressed in XY gonads (Collignon et al., 1996; Jameson et al., 2012; Stevant et al., 2018a), is also sufficient for testicular development in XX gonads (Sutton et al., 2011). Furthermore, when the DNA binding domain (HMG box) of SRY is replaced by those belonging to SOX3 or SOX9, Sertoli cell differentiation still occurs in XY mice (Bergstrom et al., 2000). This indicates that other SOX factors may also retain this function.
There is an interesting study by Kellerer et al. worth mentioning. Though both Sox8 and Sox10 exhibit overlapping expression patterns during nervous system development, replacement of the Sox10 coding sequence by Sox8 resulted in a partial rescue of Sox10 mutant defects (Kellerer et al., 2006). This again emphasizes the idea of genetic redundancy among member of the same SOX group in developing tissue systems, while indicating that their functions are not necessarily equivalent.
3.3 SOX-E group (SOX8, 9, and 10)
SOX8, SOX9, and SOX10 exhibit a unique DNA-dependent dimerization domain proximal to N-terminus of the HMG box (Peirano and Wegner 2000; Bernard et al., 2003; Sock et al., 2003), and transactivation domains of varying strengths at the C-terminus (Figure 4B) (Sudbeck et al., 1996; Pusch et al., 1998; Pfeifer et al., 2000; Schepers et al., 2000; Schreiner et al., 2007). Within this group, the HMG domain exhibits about 95% identity, differing in only 1-4 amino acids. In addition to functions in testes (Barrionuevo and Scherer 2010; She and Yang 2017), SOX-E transcription factors have been implicated in several developmental processes including neural differentiation and nervous system development (Weider and Wegner 2017), ureter branching during kidney development (SOX8 and SOX9) (Reginensi et al., 2011), cardiac development (SOX9) (Akiyama et al., 2004), and chondrogenesis (SOX9) (Akiyama et al., 2002).
In agreement with some of the SOX-E functions, heterozygous SOX9 mutations in humans cause Campomelic dysplasia, a lethal skeletal defect, and varying degrees of male-to-female sex reversal (Foster et al., 1994; Wagner et al., 1994). Similar phenotyes have been reported in Sox9 mutant mice (Akiyama et al., 2002; Chaboissier et al., 2004). In addition,
SOX10 heterozygous mutations in humans cause neurocristropathies referred to as
Waardenburg syndrome, Hirschsprung disease and PWH, which is a combination of Waardenburg syndrome and Hirschsprung disease with peripheral neuropathy (Weider and Wegner 2017).
Though SOX8 is expressed in many organs in mice including some vital ones (brain, kidneys), loss-of-function animals are viable and exhibit mild defects (Schepers et al., 2000; Sock et al., 2001). Most prominently, Sox8 loss-of-function mice exhibit a weight reduction that is mostly attributed to defective replenishment of the adipocyte pool in adults (Guth et al., 2009), and in part due to osteopenia (Schmidt et al., 2005). In addition, XY mice exhibit progressive infertility due to a loss of integrity of the seminiferous epithelium in testes (O'Bryan et al., 2008). Recently, gonad dysgenesis and infertility has been reported in SOX8
heterozygous XY humans (Portnoi et al., 2018). It is not clear how SOX8 operates in human testicular development. Note that how SOX-E transcription factors operate in mice testes are dedicated topics in Chapter 7 and 8.
3.4 SOX-E in the nervous system
All the SOX-E group members participate in the development of the nervous system, which is divided into the peripheral nervous system (PNS) and central nervous system (CNS). During neurulation in vertebrae, an ectoderm layer called the neural plate folds upon itself to from the neural tube, which develops as the spinal cord and brain to form the CNS (Gammill and Bronner-Fraser 2003). The PNS is derived from cells from the neural crest (NC), which develops where the neural plate fuses to form the neural tube. Both the PNS and CNS contain glia cells (Schwann cells and oligodendrocytes, respectively), which associate and support various neuronal cell functions, including myelination of axons (Zuchero and Barres 2015). Myelination is important for efficient transmission of information in the form electrical impulses between cells. For differentiation and function of Schwann cells and oligodendrocytes, as well as the NC specification, SOX-E transcription factors play important roles.
During NC formation, SOX-E transcription factors operate downstream of other NC factors to promote NC cell identity, survival, and their ability to undergo epithelial-to-mesenchymal transition (Cheung et al., 2005; Taylor and Labonne 2005; Liu et al., 2013; Weider and Wegner 2017). In mammals, though Sox9 and Sox8 respectively are expressed first in NC progenitor cells, they are down-regulated before cells commit to a glial or neural fate (Cheung and Briscoe 2003; Cheung et al., 2005). After this, Sox10 plays a more prominent role in glia cells of the PNS (Britsch et al., 2001; Sonnenberg-Riethmacher et al., 2001). Specifically, Sox10 is required for early development of Schwann cells and also for their function to myelinate nerve cell axons (Schreiner et al., 2007; Finzsch et al., 2010; Bremer et al., 2011; Frob et al., 2012). Interestingly, during NC formation in Xenopus, Sox8 is expressed first among the SOX-E transcription factors and is the most important (O'Donnell et al., 2006). Thus, at least in Xenopus, Sox8 expression does not necessarily depend on Sox9.
In CNS developing tissues, all SOX-E transcription factors are expressed and in the general order of Sox9, Sox8, then Sox10 (Stolt et al., 2003; Stolt et al., 2004; Stolt et al., 2005). The expression of Sox9 promotes induction and maintenance of neural stem cells
et al., 2003; Stolt et al., 2005). After glia cells are specified, in oligodendrocyte precursors,
Sox9 expression is down-regulated, Sox8 is maintained, and Sox10 expression is up-regulated
(Stolt et al., 2003; Stolt et al., 2005). The expression of Sox10 is required for terminal differentiation of oligodendrocytes and myelination (Hornig et al., 2013), and though Sox8 expression continues, it is not sufficient to replace Sox10 functions (Kellerer et al., 2006), as mentioned earlier.
In summary, in aspects of nervous system development in mice, SOX9 and SOX10 play key roles, and SOX8 supports their functions.
Chapter 4
WNT/β-catenin signaling and the R-spondin family
4.1 Canonical WNT/β-catenin signaling 4.2 R-spondin family
4.3 Overview of RSPO1-4
4.1 Canonical WNT/β-catenin signaling
Canonical WNT signaling converges on the transcription factor β-catenin to regulate target genes (MacDonald et al., 2009). In general, the canonical pathway includes WNT ligands (wingless-type MMTV integration site family), membrane receptors, intracellular secondary messengers, and secreted agonists (such as R-spondins, discussed in Section 4.2), which together influences the levels of CTNNB1 (β-catenin) in cells. There are several WNT ligands, but for ovarian development, WNT4 is important (discussed in Chapter 9). The effector protein of canonical WNT signaling, β-catenin, is also known for cell adhesion through interaction with E-cadherin (Ozawa et al., 1989; Brembeck et al., 2006). Like SOX factors, WNT/β-catenin signaling controls many developmental processes and promotes homeostasis in various tissues.
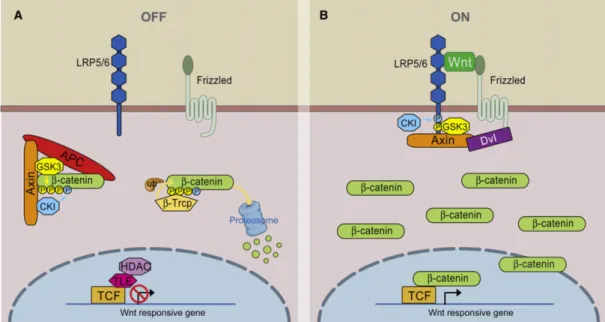
In the absence of a WNT ligand, cytoplasmic β-catenin is degraded by the action of the Axin complex, which is composed of the scaffolding protein Axin, the tumor suppressor adenomatous polyposis coli (APC), and glycogen synthase kinase 3 (GSK3) (Figure 5A) (MacDonald et al., 2009). GSK3-mediated phosphorylation of β-catenin targets it for ubiquitination and thus proteasomal degradation, and this prevents β-catenin from reaching the nucleus.
The WNT/β-catenin signaling pathway is activated when a WNT ligand binds to the transmembrane receptor Frizzled (Fz) and its co-receptor, lipoprotein receptor-related protein 5 or 6 (LRP5/6) (Figure 5B) (MacDonald et al., 2009). This leads to the intracellular recruitment of another scaffolding protein, Dishevelled (Dvl), and subsequently, Axin and GSK3. This renders GSK3 unavailable to target catenin for ubiquitination, and allows β-catenin levels to accumulate and travel into the nucleus. There, it interacts with TCF/LEF transcription factors to regulate target genes.
Figure 5. Scheme of canonical WNT/β-catenin signaling. A – In the absence WNT, a protein complex containing Axin, APC, and GSK3 targets β-catenin for ubiquitination and proteosomal degradation. B – The binding of WNT to receptors Frizzled and LRP5/6 prevents targeted ubiquitination of β-catenin by sequestering Axin and GSK3. This allows β-catenin levels to accumulate and travel to the nucleus where it can regulate target genes with TCF proteins (Adapted from MacDonald et al., 2009).
Several secreted factors have been shown to modulate WNT/β-catenin signaling. For example, secreted Frizzled-related proteins (sFRPs) and WNT inhibitory protein (WIF) can bind to WNT or Fz to prevent activation of the pathway (Bovolenta et al., 2008). In another example, Dickkopf-1 (DKK1) antagonizes WNT/β-catenin signaling through a mechanism that has been under dispute. Previously, it was suggested that DKK1 promotes internalization of the WNT co-receptor, LRP5/6 (Semenov et al., 2001). Now, it appears that DKK1 binds competitively with LRP5/6, to inhibit WNT/β-catenin signaling (Mao et al., 2002; Semenov et al., 2008).
Two examples of agonists of WNT/β-catenin signaling are Norrin and R-spondin (RSPO) proteins. Similar to WNT, Norrin activates the pathway by binding to Fz (Niehrs 2004; Xu et al., 2004). On the other hand, RSPO proteins enhance WNT/β-catenin signaling by interacting with WNT, Fz, and LRP6 (Kazanskaya et al., 2004; Kim et al., 2005; Nam et al., 2006; Wei et al., 2007). Given the importance of RSPO1 for ovarian development (Parma et al., 2006; Chassot et al., 2008), a proper introduction to the RSPO protein family is the subject of the next two sections.
4.2 R-spondin family
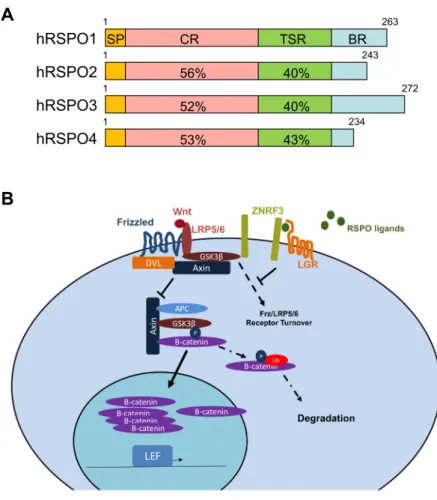
The R-spondin (RSPO) are a family of four (RSPO1-4) conserved thrombospondin type 1 repeat (TSR1) secreted proteins (Kamata et al., 2004) that control a variety of cellular and tissue functions by enhancing WNT/β-catenin signaling (Kim et al., 2008). In mammals, RSPOs show high structural similarity and ~60% sequence homology (Nam et al., 2006). The human RSPOs contains a hydrophilic, putative signal peptide sequence for secretion (SP), a cysteine-rich furin-like (CR) domain (commonly called FU domain), the TSR1 domain (TSR), and a basic amino acid-rich (BR) domain of variable length (Figure 6A) (Kazanskaya et al., 2004; Yoon and Lee 2012).
The RSPO mechanism of action has had a share of controversy. Reports indicate that RSPO binds to both Fz and LRP6 (Nam et al., 2006), or mostly LRP6 (Wei et al., 2007), but another study did not detect interaction with these receptors (Kazanskaya et al., 2004). Another model suggests that RSPO promotes WNT/β-catenin signaling by preventing DKK-mediated LRP6 receptor internalization (Binnerts et al., 2007), but as mentioned above, the DKK mechanism has also been contested. More recently, it has been shown that RSPO operates through the leucine-rich repeat-containing G-protein-coupled receptors, LGR4-6 (Carmon et al., 2011; de Lau et al., 2011; Glinka et al., 2011; Ruffner et al., 2012). This
Figure 6. Scheme of human RSPO proteins and general mechanism. A – Human RSPO proteins contain a putative secretion signal (SP), a cysteine-rich furin-like domain (CR), a TSR1 domain (TSR), and a basic amino acid-rich (BR) domain. Percentiles indicate amino acid similarities with the recognized domains among the members (Adapted from Yoon and Lee 2012). B – When RSPO binds LGR receptors, ZNFR3 cannot regulate the receptors of WNT, Frz and LRP5/6. Thus, RPSO proteins promote high levels of WNT/β-catenin signaling (Adapted from Knight and Hankenson 2014).
A
mechanism (Figure 6B) (Knight and Hankenson 2014), RSPO interaction with LGR preoccupies the transmembrane RING-type E3 ubiquitin ligases ZNRF3 or RNF43 (Hao et al., 2012; Koo et al., 2012), which regulates the Fz-LRP5/6 receptor turnover (Hao et al., 2012). Consequently, RSPO proteins promote WNT/β-catenin signaling by maintaining the WNT ligand receptors. As discussed in Chapter 7, ZNRF3 acts as an anti-WNT signaling factor that is required in XY mice gonads (Harris et al., 2018).
Though all four RSPOs can activate the WNT/β-catenin canonical pathway, RSPO2-3 are more potent than RSPO1, and RSPO4 is the weakest (Kim et al., 2008). In addition, with respect to LGR receptors, though LGR4 is essential for full potency of RSPO1-4 (Park et al., 2018), RSPO2-3 can operate independently of LGR4 (Lebensohn and Rohatgi 2018; Park et al., 2018).
4.3 Overview of RSPO1-4
Though the RSPO3 was discovered nearly 50 years ago (as PWTSR) (Baenziger et al., 1971), the RSPO family only gained notoriety three decades later with the identification of RSPO1 (Chen et al., 2002; Kamata et al., 2004), and its association with palmoplantar hyperkeratosis and predisposition to squamous cell carcinoma (Parma et al., 2006). In addition to the skin disorder, XX humans with a RPSO1 loss-of-function mutation exhibit complete female-to-male sex reversal (Parma et al., 2006), indicating a requirement for female sex determination. Mice lacking functional RSPO1 are also sex reversed (Chassot et al., 2008), and are unable to lactate because of defective duct branching and alveolar formation in mammary glands (Chadi et al., 2009). Furthermore, a RSPO1 gain-of-function mutation in mice causes ovarian defects including cysts and granulosa cell tumors, indicating that Rspo1 is oncogene in ovaries (De Cian et al., 2017).
RSPO1 has been implicated in several other processes including osteoblast differentiation and skeletal muscle repair (Lu et al., 2008; Sharma et al., 2013; Lacour et al., 2017), angiogenesis (Gore et al., 2011), as well as β-cell proliferation and neogenesis in the pancreas (Wong et al., 2011). When injected in to mice, human RSPO1 protein induced rapid onset of crypt cell proliferation in the intestinal epithelium, again indicating mitogenic influence (Kim et al., 2005). Moreover, in bone marrow mesenchymal stem cells, RPSO1 acts an anti-apoptotic factor (Cheng et al., 2019).
and hypomorphic lungs (Bell et al., 2008; Yamada et al., 2009). Transgenic expression of
Rspo2 causes asymmetric limb defects and for this reason, Rspo2Tg mice have been given the
descriptor, Footless mice (Bell et al., 2003). In ovaries, oocyte-derived RSPO2 has been shown to promote follicle development (Cheng et al., 2013).
Studies in cells show that RSPO2 expression can be a promoter of colorectal and pancreatic cancer by acting in cancer stem cells (Ilmer et al., 2015; Zhang et al., 2016), but also a repressor of colorectal cancer via non-canonical WNT signaling (Wu et al., 2014; Dong et al., 2017). Interestingly, insertion of a 3’-UTR in dog Rspo2 stabilizes mRNA and causes a more pronounced hair growth on the face and legs (Cadieu et al., 2009; Parker et al., 2010).
RSPO3 is a vascular stability and cardiac development factor. During development, RSPO3 deficient mice exhibit embryonic death around E10 due to placenta defects, which stems from a lack of vascularization and VEGF expression (Aoki et al., 2007; Kazanskaya et al., 2008). During heart development, β-catenin signaling mediated by RSPO3 promotes proliferation that is essential for coronary artery branching (Da Silva et al., 2017). In addition, RSPO3 is important for proper liver zonation (Rocha et al., 2015). In cancer, RSPO3 has been implicated in cancer stem cells in the intestines and colon (Storm et al., 2016; Dong et al., 2017). Also, RSPO3 expression also connotes invasiveness or aggressiveness of certain cancers (Gong et al., 2015; Chen et al., 2019; Mesci et al., 2019).
RSPO4 deficiency is known for its association with anonychia (Bergmann et al., 2006; Blaydon et al., 2006; Ishii et al., 2008), a rare congenital condition characterized by the absence of nails. Mutations in RSPO4 that led to anonychia appear to be clustered in the FU domain (Bruchle et al., 2008), which as mentioned earlier, is important for interaction with LGR receptors.
Chapter 5
FOXL2 transcription factor
The transcription factor FOXL2 (forkhead box transcription factor L2) requires mention, since it activates a parallel pathway to RSPO1/WNT4/β-catenin signaling for ovarian differentiation in mice (Auguste et al., 2011). As its name implies, FOXL2 belongs to the forkhead box (FOX) family of transcription factors, which share a conserved DNA binding FOX domain. Outside of the FOX domain, there is very little similarity among the family members (Carlsson and Mahlapuu 2002).
Though FOXL2 was discovered in 1998 as gene expressed in the mouse developing pituitary (Treier et al., 1998), it only gained notoriety a few years later from studies in humans and goats. In humans, mutations in FOXL2 were reported to be the underlying cause of BPES (blepharophimosis, ptosis, and epicanthus inversus syndrome) (Crisponi et al., 2001), an autosomal dominant disorder that causes craniofacial abnormalities in BPES type I, which involves premature ovarian failure (POF).
In goats, the mutation underlying the polled phenotype (no horns) and XX sex reversal, also known as polled/intersex syndrome (PIS), affects FOXL2 expression (Pailhoux et al., 2001). The phenotype in goats suggested a direct sex determination role, which was subsequently confirmed (Boulanger et al., 2014). However, Foxl2 in mice does not have an equivalent role in female sex determination, since loss-of-function mutations cause follicular defects after birth, but not sex reversal (Schmidt et al., 2004; Uda et al., 2004). Interestingly, inactivation of Foxl2 in ovaries of adult XX mice caused granulosa cells to reprogram as Sertoli cells, indicating that FOXL2 is required for the maintenance of ovarian fate in post-natal mice (Uhlenhaut et al., 2009).
In addition to craniofacial development and ovarian development and function, FOXL2 has been implicated in cartilage and skeleton development (Marongiu et al., 2015) and angiogenesis in the uterus (Bellessort et al., 2015).
G
ONAD
D
EVELOPMENT
Chapter 6
Development of the bi-potential gonad
6.1 Overview 6.2 AGP formation
6.3 Development of the genital ridge 6.4 Significance of SF1
6.1 Overview
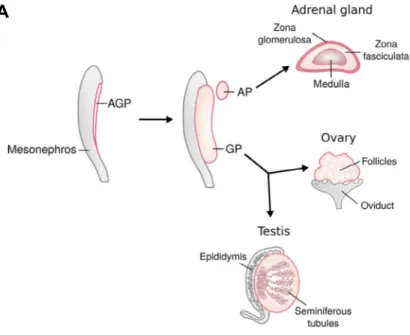
The origin of gonads can be traced back to an intermediate mesoderm derived structure, the adreno-genital primordium (AGP), which begins to form at embryonic day 9.5 (E9.5) in mice and during week 4 of gestation in human embryos (Ikeda et al., 1994; Hatano et al., 1996; Rey et al., 2000). Eventually, the AGP splits into an adrenal primordium and a gonadal primordium (Figure 7A). The gonadal primordium can then differentiate as a testis or ovary when provided with the appropriate genetic cues. Given these two possibilities, the gonadal primordium is often referred to as a bi-potential gonad.
6.2 AGP formation
At E9.5 in mice, the APG begins to develop when coelomic epithelial cells divide to cause a thickening of the ventral surface of the mesonephros (Ikeda et al., 1994; Hatano et al., 1996). At this time, the AGP contains gonad progenitor cells expressing the steroidgogenic factor 1 (SF1, also known as NR5A1) and primordial germ cells (Figure 7B) (Hatano et al., 1996; Morohashi 1997; Molyneaux et al., 2001). The germ cells arise earlier (E7) to colonize to gonad at around E10 (Ginsburg et al., 1990; Molyneaux et al., 2001; Seki et al., 2005). By E10.5, the AGP separates into an adrenal primordium and a gonadal primordium containing primordial germ cells (Ikeda et al., 1994; Hatano et al., 1996; Bandiera et al., 2013).
Though several genes have been implicated in AGP formation, the transcription factors SF1 (Luo et al., 1994), GATA binding protein 4 (GATA4) (Hu et al., 2013), and Wilms’ tumor 1 protein (WT1) (Kreidberg et al., 1993) have central roles. In mice
B
Figure 7. Scheme showing origin of the genital ridge and its development. A – Testes and ovaries in mice can be traced back to the adreno-genital primordium (AGP), which splits into an adrenal primordium (AP) and gonadal primordium (GP) by embyonic day 10.5 (E10.5). The GP or bi-potential gonad can then differentiate as a testis or ovary (Adapted from Nef et al., 2019). B – Transverse section scheme of the developing genital ridge shows germ cells (purple cells), which migrated earlier into the AGP earlier. The genital ridge develops when proliferating cells from the coelomic epithelium (green cells) ingress inward (Adapted from Stevant and Nef 2019).
developing without SF1, adrenal glands and gonads do not form (Luo et al., 1994), and SF1 expression in the AGP depends on GATA4 and WT1 (Wilhelm and Englert 2002; Hu et al., 2013). Consistent with these studies, co-immunolabeling experiments show that GATA4 and WT1 expression within the presumptive AGP at E9.5 precedes the appearance of SF1, which appear some six hours later at E9.75 (Bandiera et al., 2013).
6.3 Development of the genital ridge
The continued development of the gonadal primordium (also called genital ridge) is characterized by epithelial-to-mesenchymal transition (EMT) of cells that ingress from the proliferating coelomic epithelium (Karl and Capel 1998; Kusaka et al., 2010). By E11.5, the basement membrane beneath the coelomic epithelium is discontinuous, which allows more ingression of SF1-positive cells and growth of the genital ridge. This growth continues in both XY and XX genital ridges until E12.5 when the developing tunica albuginea in male gonads blocks the ingression of cells (Karl and Capel 1998).
For the proliferation of coelomic epithelial cells, GATA4 is required (Hu et al., 2013), and other factors including LIM homeobox 9 (LHX9) (Birk et al., 2000), insulin receptors INSR and IGF1R (Pitetti et al., 2013), WNT signaling ligands WNT4 and RSPO1 (Chassot et al., 2012), and the growth factor Neuregulin1 (NRG1) (Gregoire et al., 2018) have been implicated. Among these, INSR and IGF1R are together required for proliferation and priming of gonadal precursor cells for testicular or ovarian differentiation (Pitetti et al., 2013). Consequently, without insulin/IGF signaling in the AGP, the transcriptomes of XY and XX gonads exhibit extensive alterations at E11.5 (Pitetti et al., 2013), which is when sex determination begins in mice (Koopman et al., 1990). The outcome is failure of the testis pathway and sex reversal in XY mutant mice and delayed ovarian differentiation in both XY and XX mice.
During formation of the genital ridge, EMT requires Empty spiracle homeobox 2 (EMX2) (Kusaka et al., 2010) and Sine oculis homeobox homologs 1 and 4 (SIX1/4) (Fujimoto et al., 2013), and mice lacking either exhibit a decrease of cell ingression stemming from loss of coelomic cell polarity or delayed/reduced EMT. Furthermore, in addition to the aforementioned AGP and genital ridge factors, genital ridge development also depends on: the polycomb factor M33 (CBX2) (Katoh-Fukui et al., 2005), the transcription co-factor CITED2 (Val et al., 2007), and the homeodomain protein PBX1 (Schnabel et al., 2003). In general, inactivation of these any one of the factors leads to down-regulation of Sf1 within the developing genital ridge.
6.4 Significance of SF1
While GATA4 and WT1 expression is found within the AGP and surrounding tissues, SF1 expression is specific to the AGP (Hatano et al., 1996; Morohashi 1997). Indeed, triple immunolabeling for GATA4, WT1, and SF1 confirmed specific SF1 expression within the developing AGP (Bandiera et al., 2013). This implies that SF1 expressing cells could serve as a progenitor cell pool of all somatic cells that will develop in testes or ovaries. Indeed, lineage-tracing experiments have shown that Sertoli and granulosa cells originate from the coelomic epithelium (Karl and Capel 1998; Mork et al., 2012), and that both share a common progenitor (Albrecht and Eicher 2001). Also, pioneering work from the Nef lab recently demonstrated that an SF1-positive population of gonadal cells at E10.5 gives rise to supporting and steroidogenic cell lines in testes and ovaries, as shown by single cells transcriptomic analyses (Stevant et al., 2018a; Stevant et al., 2019). However, this “common precursor” hypothesis does not account for all somatic cells in gonads, since some theca cells originate from a GLI1-positive precursor population from the adjacent mesonephros (Liu et al., 2015). Beyond AGP and genital ridge formation, as well as marking a somatic progenitor cell pool in gonads, SF1 is co-transcription factor for various processes in ovaries and testes.
Chapter 7
Testicular development, Part A
7.1 Overview of pathways and key events 7.2 SRY functions and expression
7.3 Up-regulation and maintenance of SOX9 7.4 Testis chords and peritubular myoid cells 7.5 Coelomic vessel formation
7.6 Mitotic arrest of gonocytes 7.7 Leydig cells
7.1 Overview of pathways and key events
For testicular differentiation in XY mice, SRY up-regulation of Sox9 expression (Sekido and Lovell-Badge 2008) initiates a SOX9/FGF9 feed-forward loop, which antagonizes ovarian development via FGF9-mediated repression of WNT4 (Kim et al., 2006). Also, testicular differentiation involves the anti-WNT signaling factor ZNRF3, which promotes Sox9 expression (Harris et al., 2018). Studies in XY mice show that Sry and Sox9 are required for testicular differentiation, since loss-of-function mice develop ovaries containing granulosa cells forming follicles instead of testes containing Sertoli cells forming testis cords (Lovell-Badge and Robertson 1990; Chaboissier et al., 2004; Barrionuevo et al., 2006; Lavery et al., 2011; Kato et al., 2013). Also, loss of Znrf3 or stabilization β-catenin in XY gonads causes partial to complete sex reversal, indicating that repression of WNT/β-catenin signaling is also necessary for male sex determination (Maatouk et al., 2008; Bernard et al., 2012; Harris et al., 2018).
In XY mice, and thus in the presence of SRY (Gubbay et al., 1990; Sinclair et al., 1990), the bipotential gonad containing primordial germ cells at E10.5 can develop as a testis. The key early events include proliferation of the coelomic epithelium and ingression of cells (Karl and Capel 1998; Chassot et al., 2012) and up-regulation of testis determination genes including Sry starting at E10.5, Sox9 at ~E11, and Fgf9 at E11.5 (Koopman et al., 1990; Morais da Silva et al., 1996; Kim et al., 2006; Hiramatsu et al., 2009). These genes promote
Sertoli cell differentiation and their formation into testis cords, coelomic vessel formation, Leydig and peritubular myoid cell differentiation, which all are apparent by E12.5 (Karl and Capel 1998; Brennan et al., 2002; Yao et al., 2002; Cool et al., 2008). In developing testes, germ cells differentiate as gonocytes and enter mitotic arrest at E14.5 (Western et al., 2008). Beyond E14.5, the testis continues to increase in size and after birth at ~E19.5/P0, testis cords develop as seminiferous tubules (Barrionuevo et al., 2009).
For ease, testicular development is presented over two chapters. This chapter mostly covers events within the developing XY gonad in fetal mice. Chapter 8 covers additional select topics such as the function of AMH, SOX8, and DMRT1.
7.2 SRY function and expression
In humans and mice, SRY/Sry is both required (Berta et al., 1990; Lovell-Badge and Robertson 1990; Kato et al., 2013) and sufficient (Sinclair et al., 1990; Koopman et al., 1991) for testicular and male development. Though SRY is most known for activating Sox9 expression (Sekido and Lovell-Badge 2008), it also promotes mesonephric cell migration into the gonad (Tilmann and Capel 1999), cell proliferation (Schmahl et al., 2000; Schmahl and Capel 2003), and glycogenesis in pre-Sertoli cells (Matoba et al., 2005). These functions of SRY contribute to the overall growth of the testis, which is significantly greater than ovarian growth in fetuses (Schmahl et al., 2000). SRY expression was also reported in neurons (Dewing et al., 2006), suggesting a role in brain sex determination. However, the function of SRY outside of testis development is poorly understood.
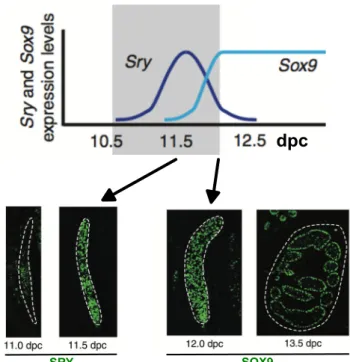
Over the years, several methodologies have been used to determine that Sry is expressed in the genital ridges of XY mice from E10.5 to E12.5 (Koopman et al., 1990; Hacker et al., 1995; Jeske et al., 1995; Albrecht and Eicher 2001; Bullejos and Koopman 2001; Sekido et al., 2004; Wilhelm et al., 2005). The pattern of Sry expression has been described as wave-like, beginning near the center of the bi-potential gonad in pre-Sertoli cell precursors and extending outward toward poles (Figure 8) (Kashimada and Koopman 2010), followed by down-regulation in the center, then anterior and posterior poles (Bullejos and Koopman 2001; Wilhelm et al., 2005). In mice, SRY must robustly activate Sox9 expression during a short and critical window between E11 and E11.25 (Bullejos and Koopman 2005; Kidokoro et al., 2005; Hiramatsu et al., 2009). Indeed, when Sry expression is artificially delayed by 6 hours, Sox9 expression is initiated, but not maintained, causing sex reversal (Hiramatsu et al., 2009).
SRY SOX9
dpc
Figure 8. Expression of SRY and SOX9 during sex determination in mice. A – In XY gonads, SRY expression peaks at 11.5 days post coitum (dpc) and SOX9 up-regulation begins around E11.5. After sex determination, cells expressing SOX9 organize into testis chords, as shown at 13.5 dpc (Adapted from Kashimada and Koopman 2010).
In contrast with mice, in which Sry is down-regulated in testes, Sry/SRY expression in most mammals remains at low levels, in even in post-natal testes (Payen et al., 1996; Parma et al., 1999; Salas-Cortes et al., 1999; Hanley et al., 2000; Diaz-Hernandez et al., 2008; Ross et al., 2009). Moreover, in mice, Sry expression is restricted to somatic cells in XY gonads (Sekido et al., 2004; Wilhelm et al., 2005; Jameson et al., 2012), whereas in humans and pigs,
SRY expression was found in germ cells (Salas-Cortes et al., 1999; Montazer-Torbati et al.,
2010).
Many genes or pathways have been implicated in activating Sry expression including the insulin receptors Ir (Insr), Irr, and Igf1r (Nef et al., 2003; Pitetti et al., 2013), Wt1 (Hammes et al., 2001), the phospho-relay GADD45γ-MAP3K4-MAP2K3/6-p38 MAPK pathway (Bogani et al., 2009; Gierl et al., 2012; Warr et al., 2012; Johnen et al., 2013; Warr et al., 2014; Warr et al., 2016), and Gata4 (Tevosian et al., 2002), as diagramed in Figure 1 (Nef et al., 2019). For all of these factors, loss-of-function mutations in XY mice caused reduced Sry levels and partial to complete sex reversal.
As mentioned in Chapter 6, insulin receptor signaling is required for proper development of the genital ridges of both XY and XX gonads. Consequently, in XY Insr
Igf1r double mutant gonads, Sry expression is delayed in XY gonads and testicular
development fails (Nef et al., 2003; Pitetti et al., 2013).
The first hints that WT1 regulates male sex determination came from humans with Frasier syndrome, a diseased caused by an improper balance between (+) and (-) lysine, threonine, and serine (KTS) isoforms (Barbaux et al., 1997; Klamt et al., 1998). This disease is characterized by early onset glomerular disease and XY sex reversal, which has been recapitulated in mice models (Hammes et al., 2001). Interestingly, mice lacking the +KTS isoform exhibited reduced Sry levels, indicating a regulatory role. Indeed, cell transfection studies confirmed WT1 with GATA4 can activate the SRY/Sry promoter, and that the +/- KTS isoforms of WT1 exhibit different activation capabilities (Hossain and Saunders 2001; Miyamoto et al., 2008).
With respect to GATA4, the phosphorylated form of this protein binds the Sry promoter (Gierl et al., 2012). Phosphorylation of GATA4 is mediated by the P38 mitogen activated kinase (Gierl et al., 2012; Warr et al., 2012), which is part of the GADD45γ-MAP3K4-MAP2K3/6-p38 MAPK signaling cascade.