HAL Id: hal-00701810
https://hal-brgm.archives-ouvertes.fr/hal-00701810
Submitted on 26 May 2012HAL is a multi-disciplinary open access
archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Thermodynamics of hydration of MX80-Na: an
experimental study of the hydration energies
Hélène Gailhanou, Philippe Blanc, Arnault Lassin, Philippe Vieillard, R.
Denoyel, E. Bloch, Stéphane Gaboreau, Eric Giffaut
To cite this version:
Hélène Gailhanou, Philippe Blanc, Arnault Lassin, Philippe Vieillard, R. Denoyel, et al.. Thermo-dynamics of hydration of MX80-Na: an experimental study of the hydration energies. International meeting ”Clays in Natural and Engineered Barriers for Radioactive Waste Confinement”, Oct 2012, Montpellier, France. �hal-00701810�
Thermodynamics of hydration of MX80-Na: an
experimental study of the hydration energies.
H. Gailhanou1, P. Blanc1, A. Lassin1, P. Vieillard2, R. Denoyel 3, E. Bloch3, S. Gaboreau1 and E. Giffaut4
1. BRGM, 3 Avenue Claude Guillemin, BP 6009, F-45060 Orléans Cedex 2 (h.gailhanou@brgm.fr)
2. CNRS-IC2MP-UMR7285, HydrASA, 5 Avenue Albert Turpain, F-86022 Poitiers Cedex (philippe.vieillard@univ-poitiers.fr)
3. MADIREL, Aix-Marseille Université, CNRS-UMR 7246, F-13397 Marseille Cedex 20 (emily.bloch@univ-provence.fr)
4. ANDRA – Parc de la Croix Blanche, 1-7 rue Jean Monnet, 92298 Châtenay-Malabry Cedex 2, France
Hydration properties of swelling clay minerals may be very variable depending on the chemical composition of the clay, on the nature of the interlayer cations and on the interlayer charge (Berend et al., 1995; Vieillard et al., 2011). The Wyoming smectite has been largely studied, notably for assessing its hydration behavior as a function of the interlayer cations, in connection with its structural characteristics (Ferrage et al., 2005; Salles et al., 2007). In the present work, carried out as part of a collaborative Andra/BRGM/HydrASA research program for ThermoChimie project, we propose an original experimental study, based on adsorption and desorption isotherms performed on MX80 clay samples. The goal is to determine energetic contributions to the reactions of hydration, which have been revealed to be non-negligible with respect to the stability of the clay minerals (Gailhanou et al., submitted). In particular, the present work addresses the problems of the hysteresis loop between adsorption and desorption isotherms and of the irreversibility of hydration reactions. This is directly related to the application of classical thermodynamics to the hydration reactions of clay minerals.
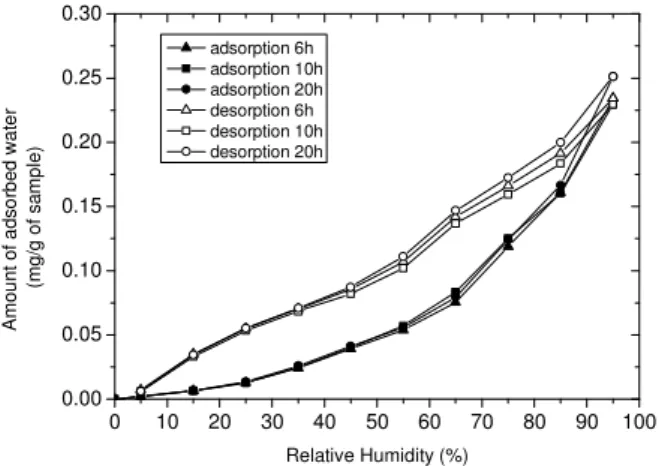
In a first stage, an experimental study is dedicated to better understand the origin of the hysteresis loop which is systematically observed for the adsorption-desorption isotherms at 25°C. The development of the hysteresis loop has been studied by considering several kinetically related parameters: stabilization periods, temperatures (from 25°C to 60°C) and hydration steps (Figure 1). No sensible change was observed in the hysteresis loop. Therefore, the amount of adsorbed water depends on the followed reaction pathway (adsorption or desorption). The variations in microstructures and in the distribution of hydration layers (0/1/2 water layers; Ferrage et al., 2005) as a function of relative humidity (RH) could provide a possible explanation for this phenomenon.
0 10 20 30 40 50 60 70 80 90 100 0.00 0.05 0.10 0.15 0.20 0.25 0.30 adsorption 6h adsorption 10h adsorption 20h desorption 6h desorption 10h desorption 20h A m o u n t o f a d s o rb e d w a te r ( m g /g o f s a m p le ) Relative Humidity (%)
Figure 1. Adsorption/desorption isotherms at 25°C on Na-saturated MX-80. Influence of the stabilization period between two injections of water, on the hysteresis loop.
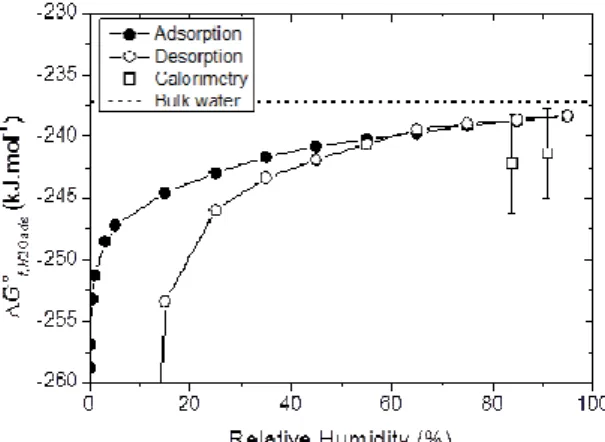
Apparent Gibbs free energies of hydration have been calculated from both the adsorption and desorption isotherms (Tardy and Duplay, 1992). Figure 2 presents the Gibbs free energies of formation of adsorbed water ∆G°f,H2Oads, obtained by considering the reaction H2O(l) = H2O(ads), calculated from the adsorption and the desorption isotherms. The calculation method has been optimized at very low RH in order to improve the accuracy of the results. The integration of P/P0 values has been performed on both the adsorption and desorption branches following the corresponding reaction pathway.
Figure 2. Apparent Gibbs free energies of formation of water adsorbed in Na-saturated MX-80, at 25°C, calculated from adsorption (open circles) and desorption (black circles) isotherms and from calorimetric measurements (squares).
The plots of ∆G°f,H2Oads from the adsorption and desorption isotherms diverge from each other at low
RH, because of the irreversibility of the hydration reaction. However, surprisingly, they are very similar for RH> 55%. This implies that, for this RH range, the Gibbs free energy of hydration is independent of the reaction pathway, and consequently, may be used for classical thermodynamic calculations.
However, comparison between the data obtained from calorimetric measurements at two highly hydrated states (squares in Fig. 2; obtained from solution calorimetry for enthalpy and low temperature adiabatic calorimetry for entropy, Gailhanou et al., submitted) and the aforementioned values at the corresponding RH, shows a non-negligible discrepancy, despite a rather large uncertainty associated with the calorimetric values (Figure 2). This discrepancy is not yet explained, but could originate partly from the contribution of energies of dilution in the calorimetric measurements.
References:
Berend I., Cases J-M., François M., Uriot J-P., Michot L., Masion A., Thomas F. (1995) Mechanism of adsorption and desorption of water vapor by homoionic montmorillonites : 2. The Li+, Na+, K+, Rb+ and Cs+ -exchanged forms. Clays and Clay Minerals, 43, 3, 324-336.
Ferrage, E., Lanson, B., Sakharov, B. A., and Drits, V. A. (2005) Investigation of smectite hydration properties by modeling of X-ray diffraction profi les. Part 1. Montmorillonite hydration properties. Amer. Miner., 90, 1358–1374.
Gailhanou, H., Blanc, P., Rogez, J., Mikaelian, G., Kawaji, H., Olives, J., Amouric, M., Denoyel, R., S., B., Montouillout, V., Vieillard, P., Fialips, C. I., Giffaut, E., Michau, N., and Gaucher, E. C., (submitted). Thermodynamic properties of illite IMt-2, smectite MX-80 and beidellite SBld-1 by calorimetric methods: Enthalpies of formation, heat capacities , entropies and Gibbs free energies of formation.
Geochim. Cosmochim. Acta .
Salles F., Beurroies I., Bildstein O., Jullien M ., Raynal J ., Denoyel R., Van Damme H. (2008) A calorimetric study of mesoscopic swelling and hydration sequence in solid Na-montmorillonite. Applied Clay Sc. 39, 186-201.
Tardy Y. and Duplay J. (1992) A method of estimating the Gibbs free energies of formation of hydrated and dehydrated clay minerals. Geochim. Cosmochim. Acta 56, 3007–3029.
Vieillard, P., Blanc, P., Fialips, C. I., Gailhanou, H., and Gaboreau, S., 2011. Hydration thermodynamics of the SWy-1 montmorillonite saturated with alkali and alkaline-earth cations: a predictive model. Geochimica