Published in: Clinica Chimica Acta (2020), vol. 502, pp. 84–90 DOI:https://doi.org/10.1016/j.cca.2019.12.008
Status : Postprint (Author’s version)
SCLEROSTIN
WITHIN
THE
CHRONIC
KIDNEY
DISEASE
SPECTRUM
Antoine Bouquegneaua,*, Peter Evenepoelb, Francois Paquota, Olivier Malaisec, Etienne Cavalierd, Pierre Delanayea
a Department of Nephrology-Dialysis-Transplantation, University Hospital of Liège (ULg CHU), Liège,
Belgium
b Department of Nephrology and Renal Transplantation, University Hospitals Leuven, Leuven, Belgium c Department of Rheumatology, University Hospital of Liège (ULg CHU), Liège, Belgium
d Department of Clinical Chemistry, University Hospital of Liège (ULg CHU), Liège, Belgium
KEYWORDS: Sclerostin, Chronic Kidney Disease, Bone metabolism ABSTRACT
Sclerostin is sometimes presented as a promising biomarker in assessing bone health both in the general population and chronic kidney disease patients. However, it is still unclear whether it has any true added value compared to existing bone biomarkers in predicting bone turnover and/or bone density in chronic kidney disease patients. A wealth of papers has been published to evaluate the association between sclerostin and vascular calcifications development or even as prognostic biomarker for mortality, but often with conflicting results. Standardization and harmonization of analytical techniques is a prerequisite to advance clinical knowledge in sclerostin.
Pathophysiology
BIOLOGICAL PATHWAYS OF SCLEROSTIN
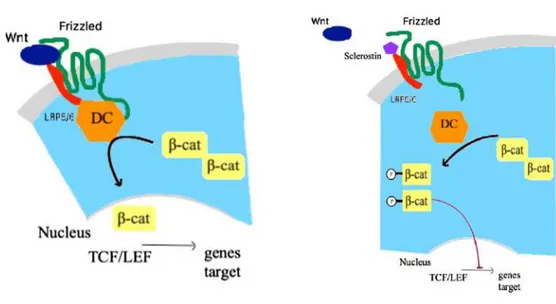
Sclerostin (scl) is a 22-kD osteocyte-specific glycoprotein and the gene product of SOST [1]. Scl acts as an inhibitor of the wingless-type mouse mammary tumor virus integration site (Wnt) -catenin pathway [2]. Wnt-β-catenin signaling participates in multiple physiologic pro-cesses crucial to embryonic development and tissue homeostasis [2]. It is increasingly acknowledged that the Wnt-β-catenin signaling pathway plays an important role in skeletal development and maintenance of bone mass [3].
Wnt-β-catenin signaling is initiated by binding of Wnt ligands to the dual receptor complex including frizzled protein and the low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6). This interaction results in inactivation of the multiprotein β-catenin “destruction complex”, thus relieving the central signaling mediator β-catenin from its constitutive proteosomal degradation. β-catenin subsequently accumulates in the cytoplasm and translocates into the nucleus, where it associates with transcription factors to control target
Published in: Clinica Chimica Acta (2020), vol. 502, pp. 84–90 DOI:https://doi.org/10.1016/j.cca.2019.12.008
Status : Postprint (Author’s version)
gene transcription [4]. Wnt-β-catenin signaling pathway is tightly regulated by several inhibitors, among which Dickkopf-related protein 1 (DKK1) and scl. Scl specifically inhibits Wnt-β-catenin pathway by binding to LRP5/6 [5]. In an animal model with a loss-of-function mutations of LRP5/6, a decrease in bone mineral density (BMD) is observed, whereas gain-of function mutations of LRP5 lead to increased BMD [3,4].
Scl is produced by osteocytes [6]. Osteocytes are former osteoblasts that have been buried in the bone matrix. Osteocytes are able to sense mechanical loading and secrete local factors that cause bone resorption and formation [7]. In this context, the role of scl on bone metabolism is nicely described in animal models with unloaded bone. In such models, scl concentrations are dramatically increased. Scl, secreted by osteocytes, is thus presented as the missing link between the absence of mechanical stress and the impaired bone formation
[8]. At the opposite, when osteocytes detect mechanical stress, the secretion of scl is de-creased, releasing inhibition on the precursors to osteoblasts, and thus will initiate new bone formation [8]. In the same view, injection of scl antibodies in rats increased trabecular and cortical bone mass and induced a strong increase in bone formation rate [9]. The impact of scl on bone is also described by its action in other pathways: interaction with bone morphogenic protein (BMP) [10] and the osteoprotegerin - receptor activator of nuclear factor k β - receptor activator of nuclear factor k p ligand (OPG-RANK-RANKL) system [11,12].
DETERMINANTS OF SCLEROSTIN
Several clinical and biological factors are identified as determinants of scl secretion. Table 1 lists major factors affecting either scl expression in bone and/or plasma scl concentrations. The role of renal function will be discussed in details in a dedicated paragraph. Briefly, age and body mass index (BMI) increase scl production [13,14]. Hormones also regulate scl production: BMP stimulates secretion, whereas parathyroid hormone (PTH) [15] suppresses the expression of scl [16]. Fibroblast growth factor 23 (FGF23) impacts the production of scl as well as calcitriol (1,25-dihydroxyvitamin D) and serum phosphate levels [17,18]. Scl is modulated also by clinical conditions. An upregulation of its concentration is seen in diabetic patients and after parathyroidectomy [19]. Circulating Wnt signaling inhibitors may also be modulated by glucocorticosteroids (GCs) [3] or estrogens level [20].
Table 1. Determinants of sclerostin expression.
Determina
nt Impact evidenceExperimental evidenceClinical Mechanisms
Age Positive [8] [13,14] • Unknown but might be an age-related
reduction of daily activity, and results in muscles generating fewer stimuli and lower mechanical stress on bone
• Alternatively, it may result from a decreased bone mass, c.q. decreased osteocyte number
PTH Negative [15,21,22] [16,23,24] • Direct action of PTH promoting
Wnt-β-catenin pathway
• Direct impact on osteoblast
differentiation Phosphoru
s Positive [25,26] [27] •• Through increase of FGF23Changes in SOST expression through a PTH-independent mechanism
Calcitriol Negative [18,28,29] [30]
• Decreased activation of 1α-hydroxylase
Published in: Clinica Chimica Acta (2020), vol. 502, pp. 84–90 DOI:https://doi.org/10.1016/j.cca.2019.12.008
Status : Postprint (Author’s version)
Estrogens Negative [31] [13]
• Cytokine TNFα (from T cells under an estrogen deficient condition) increased sclerostin
Diabetes Positive [32] [33] • Wnt-β-catenin pathway implicated in
pancreatic islet development and the production of incretin hormone
• Role in Wnt signaling in hepatic glucosemetabolism by acting through the same pathways
BMI: Body Mass Index; PTH: Parathyroid Hormone; FGF23: Fibroblast Growth Factor 23; TNF: Tumor Necrosis Factor; Wnt: wingless-type mouse mammary tumor virus integration site.
Sclerostin: Human data
SCLEROSTIN AND BONE METABOLISM
Mutations of the SOST gene lead to rare genetic diseases characterized by high bone mass, such as Sclerosteosis or Van Buchem disease [34]. The fundamental role of scl in humans is well illustrated by the clinical effect of anti-scl antibody (Romosozumab) in large randomized clinical trials including osteoporotic women where this therapy leads to benefit in terms of BMD and even reduction of fractures [35-37].
The potential role of scl as a plasma biomarker to assess BMD is more difficult to apprehend. In the general or osteoporotic population, serum scl levels is positively correlated with BMD in most studies [13,28-42] but not in all [14,43]. Beyond discrepancies between studies, the positive association between scl and BMD is counterintuitive. Different hypotheses are found in the literature. Increased scl concentrations could reflect the number of osteocytes (higher number when BMD is higher), or a high scl concentration may be associated with low bone turnover, which is repeatedly associated with increased BMD, partly as a consequence of accentuated secondary mineralization. Finally, the “brake” hypothesis can be mentioned: when BMD is low (for any possible reason), the concentration of scl is lower than when BMD is “normal” because its inhibitory effect on bone formation is not required. All these explanations remain still speculative. In the general and osteoporotic populations, the role of scl as a bone biomarker is still not fully understood, and thus actually not recommended in clinical practice. (see Fig. 1)
Sclerostin within the chronic kidney disease
spectrum
SCLEROSTIN AND CHRONIC KIDNEY DISEASE
The interpretation of plasma scl concentrations in chronic kidney disease (CKD) patients is still more challenging. It is demonstrated that scl concentrations increase as glomerular filtration rate (GFR) decreases [27]. Whether this is due to reduced renal clearance, increased skeletal production, or even an extraskeletal production is still a subject of debate [44]. In end stage renal disease (ESRD) patients, dialysis modality may also influence serum scl level [45].
A bunch of work attempts to demonstrate the potential role of Wnt signaling in Chronic Kidney Disease and Mineral Bone disorder (CKD- MBD) process. CKD-MBD defined the overall 1°) skeletal alteration (either abnormalities in bone turnover (T), mineralization (M), or bone volume or strength (V)), 2°) mineral disorders and 3°) soft tissue or vascular calcifications (VCs) appearance with the progression of CKD [46]. Those abnormalities are critical contributors of the high cardiovascular (CV) morbidity and mortality and fracture rate observation in this population.
The impact of scl in CKD-MBD development is well described in the jck mouse model, a genetic model of polycystic kidney disease that exhibits progressive renal failure. An increase in bone scl expression is observed in the early stages of renal disease [47]. In another CKD an-imal model of adynamic bone disease (ABD), high dietary phosphate intake is associated with a high osteocyte expression of SOST [25]. Moe et al. [22] observed improved bone properties
in an animal model of progressive CKD treated with anti-scl antibodies, however, only when the PTH levels is low.
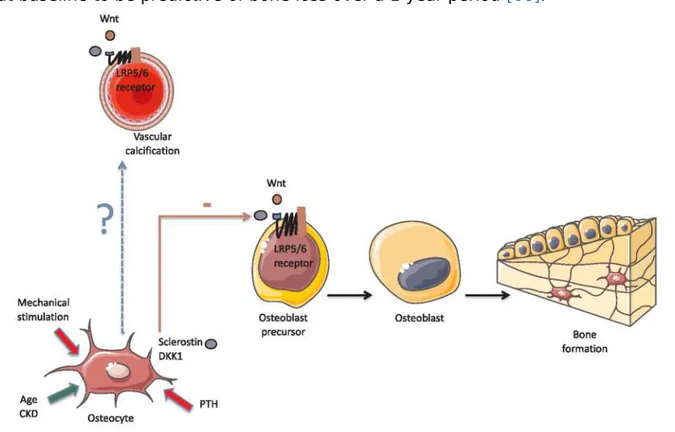
In CKD patients, circulating scl levels are negatively correlated with serum PTH [48-50]. PTH downregulates scl expression in osteocytes, and this interaction represents an important aspect of how PTH stimulates bone metabolism [51]. It is suggested that scl could contribute to the well-known PTH resistance in CKD [52], which could cause and/ or aggravate ABD [53]. Therefore, blocking scl is a valuable research target in treatment of low-bone turnover [4]. In vivo data have implicated the Wnt signaling pathway in the development of VCs especially in CKD models. Indeed, an upregulation of sclerostin mRNA is show in calcified aortic valve tissue of haemodialysis (HD) patients [54]. It is hypothesized that scl is involved in the pathogenesis of the calcification paradox which refers to disease states characterized by the coincidence of skeletal demineralization and vascular mineralization. This calcification paradox is one of the characteristic features of CKD-MBD [55]. Fig. 2shows the possible effect of scl into the VC process. Some controversy exists over the role of scl into the progression of atherosclerosis and in VC development, either in the general [56] or in the CKD population
[57].
SCLEROSTIN AND BONE METABOLISM IN CKD/ESRD PATIENTS
Regarding the role of scl as a biomarker of bone turnover, results are somewhat discordant, and few data are available with bone turnover assessed by the recognized gold standard, i.e. the bone biopsy. In a study of 60 HD patients, Cejka et al. demonstrated that scl levels is inversely correlated with bone formation rate, but the ability of scl to make the distinction between low and high bone turnover is actually low [48]. A similar finding was also made in a cohort of peritoneal dialysis (PD) patients, where both serum and bone scl correlate negatively with the histomorphometric parameters of bone turnover and osteoblastic number. However, scl do not outperform bone alkaline phosphatase (bALP) as a predictor of bone turnover [19]. Into the whole range of CKD stages and with bone biopsies analyses, Lima et al. demonstrated that levels of scl correlate with bone formation rate and osteoid surface but not with osteoblast surface [58].
action. β-cat: Beta-catenin; DC: destruction complex; LEF: Lymphoid enhancing factor; LRP5/6: Low density lipoprotein receptor related protein 5/6; TCF: T-cell factor; Wnt: Wingless-type mouse mammary tumor virus integration site family member.
Many observational and transversal studies, without bone biopsies, demonstrate a negative correlation between scl concentrations and PTH [38,59,60] or other biomarkers of bone formation [49,61]. However, in a longitudinal study analyzing the variation of PTH and bone biomarkers in dialysis patients over a 1-year period, no correlations between scl variations and PTH or bone biomarkers variations are found [62]. The real role of scl as a biomarker of bone turnover in CKD patients remains unclear.
However, by its mechanism of action, scl could be more a biomarker of bone density rather than a biomarker of bone turnover. For instance, in a transversal French study of HD patients, serum scl concentration is higher in the higher BMD tertile [63]. In a 181 Japanese HD patients cohort, scl is also positively associated with BMD [61]. A study on 89 PD patients demonstrates a positive association in multiple regression analysis, between serum scl levels and BMD, but the marginal association between scl and PTH is surprising in this study [64]. In another transversal study including 518 ESRD patients, the same positive correlation is observed between scl and BMD [65]. To the best of our knowledge, only one cohort studied scl in a longitudinal design, with eighty-one incident HD patients, and demonstrates an high scl levels at baseline to be predictive of bone loss over a 1-year period [66].
Fig. 2. Probable effect of sclerostin into the vascular calcification process. CKD: Chronic
Kidney Disease; DKK1: Dickkopf-related protein 1; LRP5/6: Low density lipoprotein receptor related protein 5/6; PTH: Parathyroid Hormone; Wnt: Wingless-type mouse mammary tumor virus integration site family member. Green arrow: Promotion of sclerostin production by osteocytes. Red solid line: Inhibition of sclerostin secretion by osteocytes.
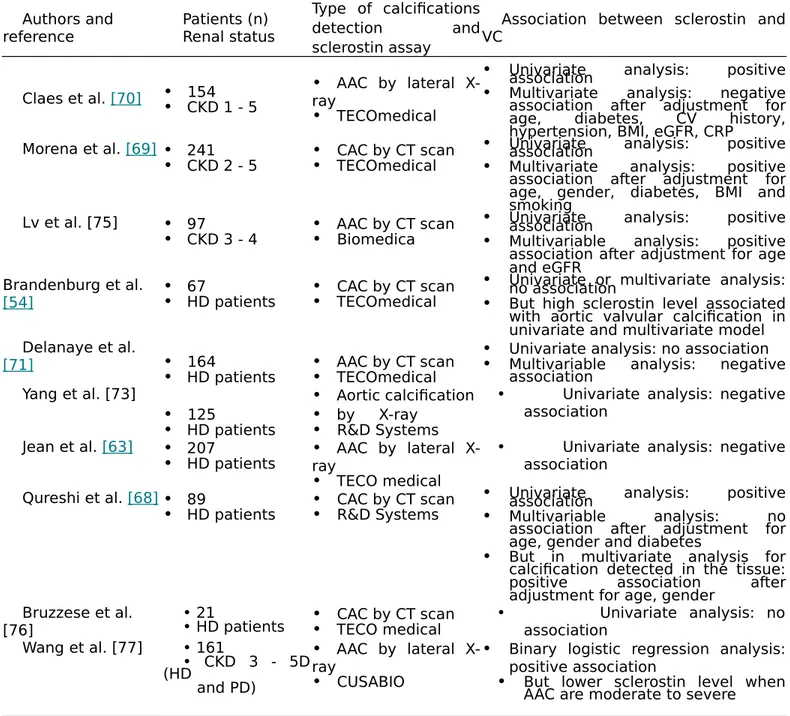
Table 2. Studies on correlation between sclerostin and vascular calcifications development. Authors and reference Patients (n) Renal status Type of calcifications detection and sclerostin assay
Association between sclerostin and VC
Claes et al. [70] •• 154CKD 1 - 5 •rayAAC by lateral
X-• TECOmedical
• Univariate analysis: positiveassociation
• Multivariate analysis: negative association after adjustment for age, diabetes, CV history, hypertension, BMI, eGFR, CRP
Morena et al. [69] • 241
• CKD 2 - 5 •• CAC by CT scanTECOmedical
• Univariate analysis: positiveassociation
• Multivariate analysis: positive association after adjustment for age, gender, diabetes, BMI and smoking
Lv et al. [75] • 97
• CKD 3 - 4 •• AAC by CT scanBiomedica
• Univariate analysis: positiveassociation
• Multivariable analysis: positive association after adjustment for age and eGFR
Brandenburg et al.
[54] •• 67HD patients •• CAC by CT scanTECOmedical
• Univariate or multivariate analysis:no association
• But high sclerostin level associated with aortic valvular calcification in univariate and multivariate model Delanaye et al.
[71] • 164
• HD patients •• AAC by CT scanTECOmedical
• Univariate analysis: no association
• Multivariable analysis: negative association Yang et al. [73] • 125 • HD patients • Aortic calcification • by X-ray • R&D Systems
• Univariate analysis: negative association
Jean et al. [63] • 207
• HD patients •rayAAC by lateral
X-• TECO medical
• Univariate analysis: negative association
Qureshi et al. [68]• 89
• HD patients •• CAC by CT scanR&D Systems
• Univariate analysis: positiveassociation
• Multivariable analysis: no
association after adjustment for age, gender and diabetes
• But in multivariate analysis for calcification detected in the tissue:
positive association after
adjustment for age, gender Bruzzese et al.
[76] • 21• HD patients •• CAC by CT scanTECO medical • Univariate analysis: noassociation Wang et al. [77] • 161 • CKD 3 - 5D (HD and PD) • AAC by lateral X-ray • CUSABIO
• Binary logistic regression analysis: positive association
• But lower sclerostin level when AAC are moderate to severe
AAC: Abdominal Aortic Calcification; BMI: Body Mass Index; CAC: Coronary Artery Calcification; CI: Confidence Interval; CRP: C-Reactive Protein; CT: Computer Tomography; CV: Cardio Vascular; CVD: Cardio Vascular Disease; eGFR: estimated Glomerular Filtration Rate; OR: Odds Ratio; PTH: Parathyroid Hormone; RR: Relative Risk; VC: vascular calcification.
In non-dialysis CKD patients, high circulating scl levels correlates with higher BMD and better bone microarchitecture [67]. A study including CKD stage 3b and 4 patients demonstrates that scl is positively associated with BMD at the hip and at the lumbar spine [67]. The po-tential role of scl as a biomarker of BMD should be confirmed by future analyses.
SCLEROSTIN AND VASCULAR CALCIFICATIONS IN CKD/ESRD PATIENTS
The potential role of scl as a biomarker of VCs is difficult to establish. Indeed, results in the literature are quite discrepant: some authors found positive [45,68,69] or negative associations [70-74], such association could vary in the same study when univariate or multivariate analyses are considered. The table 2 summarized the data on the association between scl and VCs. In addition, carotid intima-media thickness (CIMT) measurement, could detect early atherosclerosis development compared to the late assessment of VC through CT scan. A study on CKD population demonstrates that serum scl level is not correlate with CIMT, arguing that scl is rather link to the formation of calcification in CKD patients rather than atherosclerotic development [57]. Further studies are needed to confirm this hypothesis.
SCLEROSTIN AND SURVIVAL OUTCOMES IN CKD/ESRD PATIENTS
Studies evaluating the association between circulating scl concentrations and mortality in CKD have yielded inconsistent results as well. Indeed, some investigators report high circulating scl levels to be associated with better survival [49,50] whereas others report an inverse [76,79]
or no [71] association. These conflicting data may be explained by case-mix, use of different assays, and different competing factors used in multivariate analysis. Most studies on scl and survival outcomes are summarized in Table 3.
A meta-analysis has been performed regarding the association between scl concentrations and CV or all-cause mortality. The authors concluded that scl was not associated with mortality, but the number of studies included was low, and the analysis revealed a high heterogeneity, meaning that the results have to be taken with caution [80].
Analytical methods for sclerostin measurement
Analytical determination of scl is challenging, especially in case of decreasing renal function. This is a well-known story in the CKD context, where fragments of proteins can theoretically accumulate. Lack of specificity of assays can lead to spurious and different results according to the assay used, as it is well known with PTH determination [84,85]. Delanaye and colleagues compare four different assays (BM (Biomedical), TE (TECOmedical), R&D (RD) and MesoScaleDiscovery (MSD) in a population of non-dialysis and dialysis patients. The goal of the study is to determine if the choice of the assay could affect the correlation between circulating scl and clinical or biochemical determinants [86]. Median scl concentrations shows impressive differences between assays and the agreement between assays remained quite poor, both in the non-dialysis or dialysis group. A significant negative correlation is found between GFR (measured by a reference method) and scl measured by BM and TE, but not when MSD and RD assays are considered [86]. The presumed association between GFR and scl could be an artefact due to lack of specificity of the assays. Associations between scl and age, gender, weight or PTH were also different according to the assay considered in the group of non-dialysis patients. With the TE assay, PTH and GFR are identified as significant variables determinant the scl concentration above age and weight. With the RD assay, scl concentration is independently associated with age, gender, PTH and GFR. With the MSD assay, no variable was found to be associated with circulating scl.
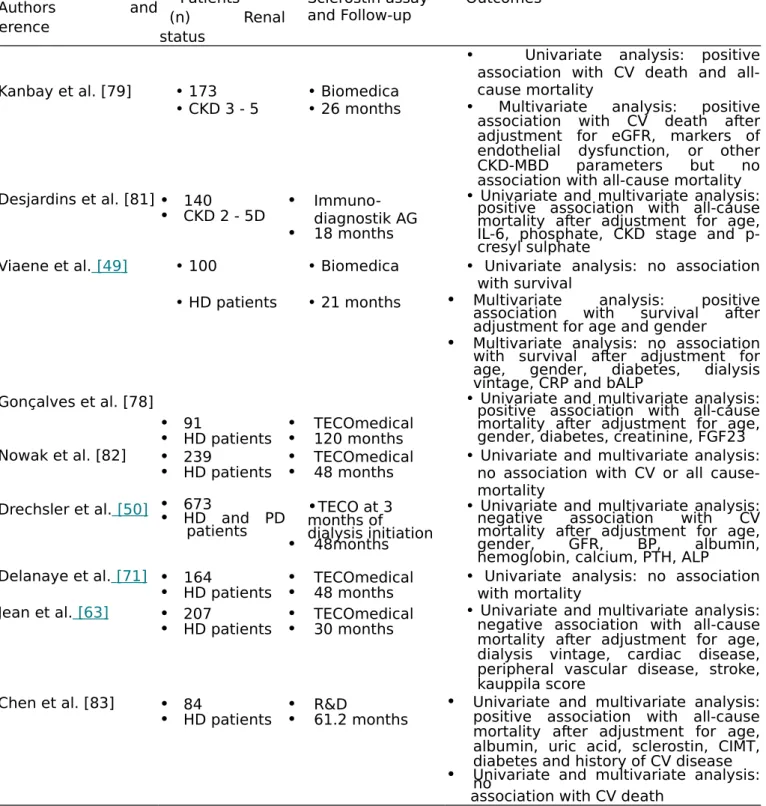
Table 3. Sclerostin ' studies associated with cardio-vascular or all-cause mortality outcomes. Authors and reference Patients (n) Renal status Sclerostin assay
and Follow-up Outcomes
Kanbay et al. [79] • 173 • Biomedica
• Univariate analysis: positive association with CV death and all-cause mortality
• CKD 3 - 5 • 26 months • Multivariate analysis: positive
association with CV death after adjustment for eGFR, markers of endothelial dysfunction, or other CKD-MBD parameters but no association with all-cause mortality Desjardins et al. [81]• 140
• CKD 2 - 5D • Immuno-diagnostik AG
• 18 months
• Univariate and multivariate analysis: positive association with all-cause mortality after adjustment for age, IL-6, phosphate, CKD stage and p-cresyl sulphate
Viaene et al. [49] • 100 • Biomedica • Univariate analysis: no association with survival
• HD patients • 21 months • Multivariate analysis: positive
association with survival after adjustment for age and gender
• Multivariate analysis: no association with survival after adjustment for age, gender, diabetes, dialysis vintage, CRP and bALP
Gonçalves et al. [78]
• 91
• HD patients •• TECOmedical120 months
• Univariate and multivariate analysis: positive association with all-cause mortality after adjustment for age, gender, diabetes, creatinine, FGF23 Nowak et al. [82] • 239
• HD patients •• TECOmedical48 months • Univariate and multivariate analysis:no association with CV or all cause-mortality
Drechsler et al. [50] •• 673HD and PD
patients
•TECO at 3 months of dialysis initiation
• 48months
• Univariate and multivariate analysis: negative association with CV mortality after adjustment for age, gender, GFR, BP, albumin, hemoglobin, calcium, PTH, ALP
Delanaye et al. [71] • 164
• HD patients •• TECOmedical48 months • Univariate analysis: no associationwith mortality Jean et al. [63] • 207
• HD patients •• TECOmedical30 months
• Univariate and multivariate analysis: negative association with all-cause mortality after adjustment for age, dialysis vintage, cardiac disease, peripheral vascular disease, stroke, kauppila score
Chen et al. [83] • 84
• HD patients •• R&D61.2 months •
Univariate and multivariate analysis: positive association with all-cause mortality after adjustment for age, albumin, uric acid, sclerostin, CIMT, diabetes and history of CV disease
• Univariate and multivariate analysis:no association with CV death
bALP: bone Alkaline Phosphatase; BP: Blood Pressure; CI: Confidence Interval; CIMT: Carotid Intima-Media Thickness; CKD-MBD: Chronic Kidney Disease and Bone and Mineral Disorder; CRP: C-reactive Protein; CV: Cardio-Vascular; eGFR: estimated Glomerular Filtration Rate; ESRD: End Stage Renal Disease; FGF23: Fibroblast Growth Factor 23; HD: Haemodialysis; HR: Hazard ratio; IL: Interleukin; PTH: Parathyroid Hormone.
versus HDF on scl concentrations. Indeed, HDF is associated with a greater scl decline after dialysis session when measured by TE and BM assays, but this decline was not observed when scl is measured with the RD or MSD assays. Our clinical knowledge about the role of scl in CKD and dialysis patients is obviously influenced by the assay used to measure it. We actually do not know which assay is preferable to be used, waiting for a reference method like mass spectrometry. None of the assays used can currently be considered as a reference method, and thus all results have to be interpreted with caution.
Conclusions
Scl could be a promising biomarker in assessing bone health in CKD patients, but it is not clear whether it has any true added value compared with existing bone biomarkers in predicting bone turnover and/ or bone density. Its clinical utility for predicting hard clinical end points is still unknown. Measuring serum scl in clinical routine remains difficult and its interpretation is still challenging, and thus up-to-now not recommended. Further studies, both analytical and clinical, should help to better interpret the scl results.
Credit authorship contribution statement
Antoine Bouquegneau: Conceptualization, Validation, Supervision, Writing original draft, Writing review & editing. Peter Evenepoel: Supervision, Writing -original draft, Writing - review & editing. François Paquot: Writing - review & editing. Olivier Malaise: Writing review & editing. Etienne Cavalier: Writing -review & editing. Pierre Delanaye: Conceptualization, Validation, Supervision, Writing - original draft, Writing - review & editing.
References
[1]W. Balemans, M. Ebeling, N. Patel, E. Van Hul, P. Olson, M. Dioszegi, et al., Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST), Hum. Mol. Genet. 10 (5) (2001) 537-543.
[2]W. Kim, M. Kim, E. Jho, Wnt/β-catenin signalling: from plasma membrane to nu
cleus, Biochem. J. 450 (1) (2013) 9-21.
[3]H.Z. Ke, W.G. Richards, X. Li, M.S. Ominsky, Sclerostin and Dickkopf-1 as ther - apeutic targets in bone diseases, Endocr. Rev. 33 (5) (2012) 747-783.
[4]R. Baron, M. Kneissel, WNT signaling in bone homeostasis and disease: from humanmutations to treatments, Nat. Med. 19 (2) (2013) 179-192.
[5]D.M. Joiner, J. Ke, Z. Zhong, H.E. Xu, B.O. Williams, LRP5 and LRP6 in develop - ment and disease, Trends Endocrinol. Metab. 24 (1) (2013) 31-39 .
C.W. Lowik, et al., Sclerostin is a delayed secreted product of osteocytes that in
hibits bone formation, Fed. Am. Soc. Exp. Biol. 19 (13) (2005) 1842-1844. [7]S.L. Dallas, M. Prideaux, L.F. Bonewald, The osteocyte: an endocrine cell and
more,Endocr. Rev. 34 (5) (2013) 658-690 .
[8]A.G. Robling, P.J. Niziolek, L.A. Baldridge, K.W. Condon, M.R. Allen, I. Alam, et al., Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/ sclerostin, J. Biol. Chem. 283 (9) (2008) 5866-5875.
[9]M.S. Ominsky, D.L. Brown, G. Van, D. Cordover, E. Pacheco, E. Frazier, et al., Differential temporal effects of sclerostin antibody and parathyroid hormone on cancellous and cortical bone and quantitative differences in effects on the osteoblast lineage in young intact rats, Bone 81 (2015) 380-391.
[10] N. Kamiya, T. Kobayashi, Y. Mochida, P.B. Yu, M. Yamauchi, H.M. Kronenberg, et al., Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts, J. Bone Miner. Res. 25 (2) (2010) 200-210.
[11] D.A. Glass, P. Bialek, J.D. Ahn, M. Starbuck, M.S. Patel, H. Clevers, et al., Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation, Dev Cell. 8 (5) (2005) 751-764.
[12] R. Sapir-Koren, G. Livshits, Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles? Osteoporos Int. 25 (12) (2014) 2685-2700.
[13] U.I. M ö dder, K.A. Hoey, S. Amin, L.K. McCready, S.J. Achenbach, B.L. Riggs, et al., Relation of age, gender, and bone mass to circulating sclerostin levels in women andmen, J. Bone Miner. Res. 26 (2) (2011) 373-379.
[14] M.-S.M. Ardawi, H.A. Al-Kadi, A.A. Rouzi, M.H. Qari, Determinants of serum sclerostin in healthy pre- and postmenopausal women, J. Bone Miner. Res. 26 (12)(2011) 2812-2822 .
[15] I. Kramer, G.G. Loots, A. Studer, H. Keller, M. Kneissel, Parathyroid hormone (PTH)- induced bone gain is blunted in SOST overexpressing and deficient mice, J. BoneMiner. Res. 25 (2) (2010) 178-189.
[16] M.-S.M. Ardawi, A.M. Al-Sibiany, T.M. Bakhsh, A.A. Rouzi, M.H. Qari, Decreased serum sclerostin levels in patients with primary hyperparathyroidism: a cross-sec tional and a longitudinal study, Osteoporos. Int. 23 (6) (2012) 1789-1797 .
[17] W. He, Y.S. Kang, C. Dai, Y. Liu, Blockade ofWnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury, J. Am. Soc. Nephrol. 22 (1) (2011)90-103.
[18] Z.C. Ryan, H. Ketha, M.S. McNulty, M. McGee-Lawrence, T.A. Craig, J.P. Grande, et al., Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium, Proc.
Natl. Acad. Sci. USA 110 (15) (2013) 6199-6204.
[19] R.A. de Oliveira, F.C. Barreto, M. Mendes, L.M. dos Reis, J.H. Castro, Z.M.L. Britto, et al., Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamicbone disease, Kidney Int. 87 (5) (2015) 1039-1045 .
[20] K. Fujita, M.M. Roforth, S. Demaray, U. McGregor, S. Kirmani, L.K. McCready, et al., Effects of estrogen on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in postmenopausal women, J. Clin. Endocrinol. Metab. 99 (1) (2014) E81-E88 .
[21] M. Wan, C. Yang, J. Li, X. Wu, H. Yuan, H. Ma, et al., Parathyroid hormone sig
naling through low-density lipoprotein-related protein 6, Genes Dev. 22 (21) (2008)2968-2979.
[22] S.M. Moe, N.X. Chen, C.L. Newman, J.M. Organ, M. Kneissel, I. Kramer, et al., Anti- sclerostin antibody treatment in a rat model of progressive renal osteodystrophy, J Bone Miner Res. 30 (3) (2015) 499-509 .
[23] V.M. Brandenburg, J. Floege, Adynamic bone disease-bone and beyond, NDT Plus. 1(3) (2008) 135-147.
[24] H. Keller, M. Kneissel, SOST is a target gene for PTH in bone, Bone 37 (2) (2005) 148-158.
[25] J.C. Ferreira, G.O. Ferrari, K.R. Neves, R.T. Cavallari, W.V. Dominguez, L.M. Dos Reis, et al., Effects of dietary phosphate on adynamic bone disease in rats with chronic kidney disease-role of sclerostin? PloS One 8 (11) (2013) .
[26] X. Zhou, Y. Cui, X. Zhou, J. Han, Phosphate/pyrophosphate and MV-related pro
teins in mineralisation: discoveries from mouse models, Int. J. Biol. Sci. 8 (6) (2012) 778-790.
[27] S. Pelletier, L. Dubourg, M.-C. Carlier, A. Hadj-Aissa, D. Fouque, The relation be
tween renal function and serum sclerostin in adult patients with CKD, Clin. J. Am.Soc. Nephrol. 8 (5) (2013) 819-823.
[28] N. Ito, D.M. Findlay, P.H. Anderson, L.F. Bonewald, G.J. Atkins, Extracellular phosphate modulates the effect of 1α,25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells, J. Steroid. Biochem. Mol. Biol. 136 (2013) 183-186 .
[29] M.K. Sutherland, J.C. Geoghegan, C. Yu, D.G. Winkler, J.A. Latham, Unique reg
ulation of SOST, the sclerosteosis gene, by BMPs and steroid hormones in humanosteoblasts, Bone 35 (2) (2004) 448-454.
[30] P. Evenepoel, K. Claes, E. Cavalier, B. Meijers, P. Stenvinkel, G. Behets, et al., Adistinct bone phenotype in ADPKD patients with end-stage renal disease, Kidney Int. 95 (2) (2019) 412-419.
[31] B.-J. Kim, S.J. Bae, S.-Y. Lee, Y.-S. Lee, J.-E. Baek, S.-Y. Park, et al., TNF-α mediates the stimulation of sclerostin expression in an estrogen-deficient condition, Biochem.Biophys. Res. Commun. 424 (1) (2012) 170-175.
[32] T. Jin, The WNT signalling pathway and diabetes mellitus, Diabetologia 51 (10)(2008) 1771-1780 .
[33] S. Morales-Santana, B. Garc í a-Fontana, A. Garc í a-Mart í n, P. Rozas-Moreno, J.A. Garc í a-Salcedo, R. Reyes-Garc í a, et al., Atherosclerotic disease in type 2 dia - betes is associated with an increase in sclerostin levels, Diabetes Care 36 (6) (2013) 1667 -1674 .
[34] W. Van Hul, W. Balemans, E. Van Hul, F.G. Dikkers, H. Obee, R.J. Stokroos, et al., Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21, Am. J. Hum. Genet. 62 (2) (1998) 391-399.
[35] M.R. McClung, A. Grauer, S. Boonen, M.A. Bolognese, J.P. Brown, A. Diez-Perez, et al., Romosozumab in postmenopausal women with low bone mineral density, N.Engl. J. Med. 370 (5) (2014) 412-420.
[36] F. Cosman, D.B. Crittenden, J.D. Adachi, N. Binkley, E. Czerwinski, S. Ferrari, etal., Romosozumab treatment in postmenopausal women with osteoporosis, N. Engl. J. Med. 375 (16) (2016) 1532-1543.
[37] K.G. Saag, J. Petersen, M.L. Brandi, A.C. Karaplis, M. Lorentzon, T. Thomas, et al., Romosozumab or alendronate for fracture prevention in women with osteoporosis, N. Engl. J. Med. 377 (15) (2017) 1417-1427.
[38] P. Garnero, E. Sornay-Rendu, F. Munoz, O. Borel, R.D. Chapurlat, Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroidhormones, and fracture risk in postmenopausal women: the OFELY study,Osteoporos. Int. 24 (2) (2013) 489-494.
[39] A. Arasu, P.M. Cawthon, L.-Y. Lui, T.P. Do, P.S. Arora, J.A. Cauley, et al., Serum sclerostin and risk of hip fracture in older Caucasian women, J. Clin. Endocrinol. Metab. 97 (6) (2012) 2027-2032.
[40] Z. Sheng, D. Tong, Y. Ou, H. Zhang, Z. Zhang, S. Li, et al., Serum sclerostin levels were positively correlated with fat mass and bone mineral density in central south Chinese postmenopausal women, Clin. Endocrinol. (Oxf). 76 (6) (2012) 797-801.
[41] K. Amrein, S. Amrein, C. Drexler, H.P. Dimai, H. Dobnig, K. Pfeifer, et al., Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults, J. Clin. Endocrinol. Metab. 97 (1) (2012)148-154.
[42] P. Szulc, S. Boutroy, N. Vilayphiou, M. Schoppet, M. Rauner, R. Chapurlat, et al., Correlates of bone microarchitectural parameters and serum sclerostin levels in men: the STRAMBO study, J. Bone Miner. Res. 28 (8) (2013) 1760-1770.
[43] M.-S.M. Ardawi, A.A. Rouzi, S.A. Al-Sibiani, N.S. Al-Senani, M.H. Qari, S.A. Mousa,High serum sclerostin predicts the occurrence of osteoporotic fractures in post menopausal women: the Center of Excellence for Osteoporosis Research Study, J. Am. Soc. Bone Miner. Res. 27 (12) (2012 Dec) 2592-2602. [44] P. Evenepoel, P. D'Haese, V. Brandenburg, Sclerostin and DKK1: new
players inrenal bone and vascular disease, Kidney Int. 88 (2) (2015) 235-240. [45] L. Lips, de Roij, C.L.M. van Zuijdewijn, P.M. Ter Wee, M.L. Bots, P.J. Blankestijn, M.A. van den Dorpel, Serum sclerostin: relation with mortality and
impact of hemodiafiltration, Nephrol. Dial Transplant. 32 (7) (2017) 1217-1223. [46] M. Ketteler, G.A. Block, P. Evenepoel, M. Fukagawa, C.A. Herzog, L. McCann,
et al., Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters, Kidney Int. 92 (1) (2017) 26-36.
[47] Y. Sabbagh, F.G. Graciolli, S. O'Brien, W. Tang, L.M. dos Reis, S. Ryan, et al., Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy, J. Bone Miner. Res. 27 (8) (2012) 1757-1772.
[48] D. Cejka, J. Herberth, A.J. Branscum, D.W. Fardo, M.-C. Monier-Faugere, D. Diarra, et al., Sclerostin and Dickkopf-1 in renal osteodystrophy, Clin. J. Am. Soc. Nephrol.6 (4) (2011) 877-882.
[49] L. Viaene, G.J. Behets, K. Claes, B. Meijers, F. Blocki, V. Brandenburg, et al., Sclerostin: another bone-related protein related to all-cause mortality in haemo dialysis? Nephrol. Dial Transplant. 28 (12) (2013) 3024-3030.
[50] C. Drechsler, P. Evenepoel, M.G. Vervloet, C. Wanner, M. Ketteler, N. Marx, et al., High levels of circulating sclerostin are associated with better cardiovascular sur vival in incident dialysis patients: results from the NECOSAD study, Nephrol. Dial Transplant. 30 (2) (2015) 288-293.
[51] T. Bellido, V. Saini, P.D. Pajevic, Effects of PTH on osteocyte function, Bone 54 (2)(2013) 250-257.
[52] F.G. Graciolli, K.R. Neves, F. Barreto, D.V. Barreto, L.M. Dos Reis, M.E. Canziani, et al., The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease, Kidney Int. 91 (6) (2017) 1436-1446.
[53] Z. Massy, T. Drueke, Adynamic bone disease is a predominant bone pattern in earlystages of chronic kidney disease, J. Nephrol. 30 (5) (2017) 629-634. [54] V.M. Brandenburg, R. Kramann, R. Koos, T. Kruger, L. Schurgers, G.
Muhlenbruch, et al., Relationship between sclerostin and cardiovascular calcification in hemo dialysis patients: a cross-sectional study, BMC Nephrol. 10 (14) (2013) 219.
[55] M. Cozzolino, P. Ure ñ a-Torres, M.G. Vervloet, V. Brandenburg, J. Bover, D. Goldsmith, et al., Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol. Dial Transplant. 29 (10) (2014) 1815-1820 .
[56] A. Gaudio, V. Fiore, R. Rapisarda, M.H. Sidoti, A. Xourafa, A. Catalano, et al., Sclerostin is a possible candidate marker of arterial stiffness: Results from a cohort study in Catania, Mol. Med. Rep. 15 (5) (2017) 3420-3424.
[57] A. Figurek, G. Spasovski, Is serum sclerostin a marker of atherosclerosis in patients with chronic kidney disease-mineral and bone disorder? Int. Urol. Nephrol. 50 (10) (2018) 1863-1870.
[58] F. Lima, H. Mawad, A.A. El-Husseini, D.L. Davenport, H.H. Malluche, Serum bone markers in ROD patients across the spectrum of decreases in GFR: Activin A in creases before all other markers, Clin. Nephrol. 91 (4) (2019)
222-230.
[59] A.H. van Lierop, J.E. Witteveen, N.a.T. Hamdy, S.E. Papapoulos, Patients with primary hyperparathyroidism have lower circulating sclerostin levels than eu- parathyroid controls, Eur. J. Endocrinol. 163 (5) (2010) 833-837.
[60] C. Durosier, A. van Lierop, S. Ferrari, T. Chevalley, S. Papapoulos, R. Rizzoli, Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women, J. Clin. Endocrinol. Metab. 98 (9) (2013) 3873-3883.
[61] E. Ishimura, S. Okuno, M. Ichii, K. Norimine, T. Yamakawa, S. Shoji, et al., Relationship between serum sclerostin, bone metabolism markers, and bone mi
neral density in maintenance hemodialysis patients, J. Clin. Endocrinol. Metab. 99(11) (2014) 4315-4320.
[62] F. Paquot, P. Delanaye, X. Warling, M. Moonen, N. Smelten, F. Jouret, et al., Variations of sclerostin with other bone biomarkers over a one-year period in he
modialysis patients, Clin. Chim. Acta 486 (2018) 183-184.
[63] G. Jean, C. Chazot, E. Bresson, E. Zaoui, E. Cavalier, High serum sclerostin levels are associated with a better outcome in haemodialysis patients, Nephron 132 (3)(2016) 181-190.
[64] T.-H. Kuo, W.-H. Lin, J.-Y. Chao, A.-B. Wu, C.-C. Tseng, Y.-T. Chang, et al., Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: a cross-sectional study, BMC Nephrol. 20 (1) (2019) 266.
[65] P. Evenepoel, K. Claes, B. Meijers, M.R. Laurent, B. Bammens, M. Naesens, et al., Bone mineral density, bone turnover markers, and incident fractures in de novo kidney transplant recipients, Kidney Int. 95 (6) (2019) 1461-1470. [66] H.H. Malluche, D.L. Davenport, T. Cantor, M.-C. Monier-Faugere, Bone
mineraldensity and serum biochemical predictors of bone loss in patients with CKD ondialysis, Clin. J. Am. Soc. Nephrol. 9 (7) (2014) 1254-1262.
[67] S. Thambiah, R. Roplekar, P. Manghat, I. Fogelman, W.D. Fraser, D. Goldsmith, et al., Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness, Calcif. Tissue Int. 90 (6) (2012) 473-480.
[68] A.R. Qureshi, H. Olauson, A. Witasp, M. Haarhaus, V. Brandenburg, A. Wernerson, et al., Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification, Kidney Int. 88 (6) (2015) 1356-1364 .
[69] M. Morena, I. Jaussent, A.-M. Dupuy, A.-S. Bargnoux, N. Kuster, L. Chenine, et al., Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: potential partners in vascular calcifications, Nephrol. Dial Transplant. 30 (8) (2015)1345 - 1356.
[70] K.J. Claes, L. Viaene, S. Heye, B. Meijers, P. d'Haese, P. Evenepoel, Sclerostin: Another vascular calcification inhibitor? J. Clin. Endocrinol. Metab.
98 (8) (2013)3221-3228.
[71] P. Delanaye, J.-M. Krzesinski, X. Warling, M. Moonen, N. Smelten, L. Medart, et al., Clinical and biological determinants of sclerostin plasma concentration in hemo dialysis patients, Nephron. Clin. Pract. 128 (1-2) (2014) 127-134. [72] A. Kirkpantur, M. Balci, A. Turkvatan, B. Afsar, Serum sclerostin levels, arteriovenous fistula calcification and 2-years all-cause mortality in prevalent he - modialysis patients, Nefrologia 36 (1) (2016) 24-32.
[73] C.-Y. Yang, Z.-F. Chang, Y.-P. Chau, A. Chen, W.-C. Yang, A.-H. Yang, et al., Circulating Wnt/β-catenin signalling inhibitors and uraemic vascular calcifications, Nephrol. Dial Transplant. 30 (8) (2015) 1356-1363.
[74] P. Evenepoel, E. Goffin, B. Meijers, N. Kanaan, B. Bammens, E. Coche, et al., Sclerostin serum levels and vascular calcification progression in prevalent renal transplant recipients, J. Clin. Endocrinol. Metab. 100 (12) (2015) 4669-4676.
[75] W. Lv, L. Guan, Y. Zhang, S. Yu, B. Cao, Y. Ji, Sclerostin as a new key factor in vascular calcification in chronic kidney disease stages 3 and 4, Int. Urol. Nephrol.48 (12) (2016) 2043-2050.
[76] A. Bruzzese, A. Lacquaniti, V. Cernaro, C.A. Ricciardi, S. Loddo, A. Romeo, et al., Sclerostin levels in uremic patients: a link between bone and vascular disease, Ren Fail. 38 (5) (2016) 759-764.
[77] X.-R. Wang, L. Yuan, J.-J. Zhang, L. Hao, D.-G. Wang, Serum sclerostin values are associated with abdominal aortic calcification and predict cardiovascular events in patients with chronic kidney disease stages 3-5D, Nephrol. Carlton. Vic. 22 (4) (2017 Apr) 286-292.
[78] F.L.C. Gon ç alves, R.M. Elias, L.M. dos Reis, F.G. Graciolli, F.G. Zampieri, R.B. Oliveira, et al., Serum sclerostin is an independent predictor of mortality in hemodialysis patients, BMC Nephrol. 2 (15) (2014) 190.
[79] M. Kanbay, D. Siriopol, M. Saglam, Y.G. Kurt, M. Gok, H. Cetinkaya, et al., Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients, J. Clin. Endocrinol. Metab. 99 (10) (2014) E1854-E1861.
[80] M. Kanbay, Y. Solak, D. Siriopol, G. Aslan, B. Afsar, D. Yazici, et al., Sclerostin, cardiovascular disease and mortality: a systematic review and meta-analysis, Int. Urol. Nephrol. 48 (12) (2016 Dec) 2029-2042.
[81] L. Desjardins, S. Liabeuf, R.B. Oliveira, L. Louvet, S. Kamel, H.-D. Lemke, et al., Uremic toxicity and sclerostin in chronic kidney disease patients, Nephrol. Ther. 10 (6) (2014) 463-470.
[82] A. Nowak, F. Artunc, A.L. Serra, E. Pollock, P.-A. Krayenb ü hl, C. Muller, et al., Sclerostin quo vadis? - is this a useful long-term mortality parameter in prevalent hemodialysis patients? Kidney Blood Press Res. 40 (3) (2015) 266-276.
sclerostin with carotid artery atherosclerosis and all-cause mortality in Chinese patients un dergoing maintenance hemodialysis, BMC Nephrol. 19 (1) (2018) 264.
[84] E. Cavalier, P. Delanaye, L. Vranken, A.-C. Bekaert, A. Carlisi, J.-P. Chapelle, et al., Interpretation of serum PTH concentrations with different kits in dialysis patients according to the KDIGO guidelines: importance of the reference (normal) values,Nephrol. Dial Transplant. 27 (5) (2012) 1950-1956.
[85] J.-C.P. Souberbielle, H. Roth, D.P. Fouque, Parathyroid hormone measurement in CKD, Kidney Int. 77 (2) (2010) 93-100.
[86] P. Delanaye, F. Paquot, A. Bouquegneau, F. Blocki, J.-M. Krzesinski, P. Evenepoel, et al., Sclerostin and chronic kidney disease: the assay impacts what we (thought to) know, Nephrol. Dial Transplant. 33 (8) (2018) 1404-1410 .