Tracking neuronal development in the adult brain
Thèse
Karen Bakhshetyan
Doctorat en biophotonique
Philosophiæ doctor (Ph. D.)
Québec, Canada
© Karen Bakhshetyan, 2017
Tracking neuronal development in the adult brain
Thèse
Karen Bakhshetyan
Sous la direction de:
Armen Saghatelyan, directeur de recherche
Tigran Galstian, codirecteur de recherche
iii
Résumé
Les connaissances des voies moléculaires et cellulaires régulant le développement neuronal dans le cerveau adulte peuvent être utilisées pour mettre au point des stratégies efficaces de thérapie de remplacement cellulaire. L’étude de la dynamique et des mécanismes nécessaires à la neurogenèse adulte, requiert des techniques d’imagerie en temps réel. En outre, il est important de développer des méthodes d'imagerie sans marqueurs.
Mon travail vise, en partie, à relever ces défis. Les néo-neurones générés chez l’adulte migrent densément le long des vaisseaux sanguins et des tubes gliaux dans le courant de migration rostral (CMR). Cet alignement peut créer une anisotropie qui peut être détectée en lumière polarisée. J'ai d'abord essayé cette technique pour la détection sans marqueurs des cellules migratrices dans le CMR. Bien que l’imagerie avec la lumière polarisée suscite certaines espérances, elle a toutefois fait apparaître que l'anisotropie des cellules migratrices est très faible et que sa détection est entravée par des signaux de fortes intensités provenant des axones myélinisés se trouvant à proximité.
Ensuite, j'ai étudié la migration des neuroblastes marqués viralement pour élucider certains mécanismes nécessaires à leur migration. La signalisation GABAergique joue un rôle important dans la migration neuronale, déterminée par le gradient chlorique trans-membranaire. Ce dernier est, à son tour, contrôlé par KCC2, un co-transporteur responsable de l'extrusion de Cl-. Il est connu que KCC2 est exprimé dans les stades de développement
plus avancés. Toutefois, le rôle de KCC2 dans la migration neuronale est inconnu et mes expériences suggèrent que ce co-transporteur est impliqué dans la migration radiale, mais pas tangentielle, de neuroblastes.
Enfin, j'ai exploré in vivo comment la plasticité structurelle des néo-neurones générés chez l’adulte dans le bulbe olfactif (BO) est modulée par les odeurs. On ne sait pas comment le fonctionnement du BO s’ajuste à l’environnement olfactif de facon rapide lorsque de nouvelles synapses de néo-neurones ne sont pas encore formées. Mes données in vivo d'imagerie à deux photons complètent les travaux antérieurs de notre laboratoire, révélant une nouvelle forme de plasticité structurale dans le cerveau adulte.
iv
Ainsi, en utilisant diverses méthodes d'imagerie j'ai essayé de mieux comprendre la migration et la plasticité des nouveaux neurones dans le cerveau adulte.
v
Abstract
The knowledge about molecular and cellular pathways orchestrating neuronal development in the adult brain can be used to build up efficient strategies for cell replacement therapies. Adult neurogenesis is a very dynamic process, and it is crucial to monitor it directly to decipher mechanisms required for neuronal development. Furthermore, it is important to develop label-free imaging methods.
My work is, in part, aimed at addressing these challenges. Adult-born neurons migrate densely along blood vessels and glial tubes in the rostral migratory stream (RMS). This alignment may create anisotropy which can be detected in polarized light. I first tried this technique for label-free detection of migratory cells in the RMS. While this imaging may have some promises, it showed that anisotropy in migrating cells is quite low and its detection is hampered by large signals deriving from nearby myelinated axons.
I further studied the migration of virally labeled neuroblasts to elucidate some of the mechanisms required for their migration. GABAergic signaling plays an important role in neuronal migration and is defined by transmembrane Cl- gradient. This, in turn is controlled
by the Cl- extruding co-transporter KCC2, known to have a late developmental expression.
The role of KCC2 in neuronal migration is unknown and my experiments suggest that this co-transporter is involved in the radial, but not tangential migration of neuroblasts.
Finally, I explored in vivo the odor-related structural plasticity of adult-born neurons in the olfactory bulb (OB). It remains unknown how OB functioning is adjusted to rapidly changing odor environment when new synapses of adult-born neurons have not yet been formed. My in vivo two-photon imaging data complements the previous work in our lab, revealing altogether a new form of structural plasticity in the adult OB.
Thus, using diverse imaging methods I tried to better understand the migration and plasticity of new neurons in the adult brain.
vi
Table of Contents
Résumé ... iii Abstract ... v Table of Contents ... vi List of tables ... x List of figures ... xiList of abbreviations ... xiv
Acknowledgements ... xvii
Foreword ... xviii
1. Introduction ... 1
1.1. Adult Neurogenesis ... 1
1.1.1. Neuronal development in adult rodent brain ... 1
1.1.2. The olfactory system ... 4
1.1.3. Proliferation of neuronal precursors in the SVZ ... 9
1.1.4. Migration of neuroblasts in the adult brain ... 10
1.1.5. Maturation and integration of adult-born interneurons in the OB ... 12
1.1.6. Synaptic development of adult-born GCs ... 15
1.1.7. Adult neurogenesis as a form of structural plasticity to adjust OB network to changing environmental conditions ... 17
1.2. Overview of GABAergic signaling in the brain ... 19
1.2.1. The role of GABA in neuronal development during embryogenesis and in adulthood ... 20
1.2.2. Cation-chloride cotransporters ... 23
1.2.3. The role of KCC2 in brain development ... 24
1.2.4. The role of KCC2 and Cl- gradient in neuronal migration ... 27
1.3. Tools for imaging neuronal development ... 29
1.3.1. Fundamentals of optical microscopy... 29
1.3.2. Wide-field and two-photon imaging ... 33
1.3.3. Light polarization ... 36
1.3.4. Label-free imaging using polarized light ... 38
1.3.5. Polarized light imaging in biomedical applications ... 40
1.4. Neurodegenerative disorders. Parkinson’s disease. ... 41
vii
3. Results - Comparative study of myelinated fiber bundles with polarized light
imaging under normal and pathological conditions ... 45
3.1. Résumé ... 46
3.2. Abstract ... 47
3.3. Introduction ... 48
3.4. Materials and methods... 53
3.4.1. Animals ... 53
3.4.2. Preparation of human tissue ... 53
3.4.3. Myelin staining ... 54
3.4.4. Immunohistochemistry ... 54
3.4.5. Image acquisition and processing ... 54
3.4.6. Data analysis ... 55
3.5. Results ... 56
3.6. Discussion ... 58
3.7. References ... 60
3.8. Figures ... 81
4. Results - Activation of KCC2 affects radial but not tangential migration of neuronal precursors in the adult brain ... 87
4.1. Résumé ... 88
4.2. Abstract ... 89
4.3. Introduction ... 90
4.4. Materials and Methods ... 91
4.4.1. Animals ... 92
4.4.2. Stereotaxic injections ... 92
4.4.3. Immunohistochemistry ... 92
4.4.4. Slice preparation and time-lapse video-imaging ... 93
4.4.5. Statistical analysis ... 93
4.5. Results ... 94
4.5.1. KCC2 expression in the SVZ-OB pathway ... 94
4.5.2. KCC2 activation does not affect tangential migration in the RMS ... 94
4.5.3. KCC2 activation fosters radial migration of neuroblasts in the OB ... 95
4.6. Discussion ... 96
4.7. References ... 99
viii
5. Results - Principal cell activity induces spine relocation of adult-born interneurons
in the olfactory bulb ... 107
5.1. Résumé ... 109
5.2. Abstract ... 110
5.3. Introduction ... 111
5.4. Results ... 112
5.4.1. Dendritic spines of adult-born GC relocate in the OB network ... 112
5.4.2. Spine relocation is preceded by spine head filopodia growth ... 113
5.4.3. Relocated spines are maintained in the OB network ... 115
5.4.4. MC activity induces SHF directional growth and spine relocation ... 115
5.4.5. Glutamate released from MC controls the motility of SHF ... 117
5.4.6. MC-derived BDNF induces the spine relocation of adult-born GC ... 118
5.4.7. Spines with SHF are maintained after sensory deprivation ... 119
5.4.8. Spine relocation is involved in odor information processing ... 120
5.5. Discussion ... 121
5.6. Methods ... 123
5.6.1. Animals ... 123
5.6.2. Stereotaxic injection ... 124
5.6.3. Time-lapse two-photon imaging in vivo ... 124
5.6.4. Time-lapse two-photon imaging in vitro ... 125
5.6.5. Image analysis ... 126
5.6.6. Stimulation of mitral cells ... 128
5.6.7. Iontophoresis and puff application ... 129
5.6.8. Unilateral nostril occlusion ... 129
5.6.9. Immunohistochemistry ... 130 5.6.10. In situ hybridization ... 131 5.6.11. OB network model ... 131 5.6.12. Statistical analysis ... 132 5.7. References ... 132 5.8. Figures ... 136 5.9. Authors’ contributions ... 162 6. General conclusions ... 163 References ... 165
ANNEX A - Tracking neuronal migration in adult brain slices ... 185
ix
A2. Abstract ... 187
A3. Introduction ... 188
A4. Basic Protocol ... 189
A4.1. Time-lapse video-imaging of neuronal migration in adult acute brain slices .. 189
A4.2. Materials ... 189
A4.3. Protocol steps ... 190
A5. Support Protocol ... 192
A5.1. Stereotaxic injection of viral vectors into the SVZ of the adult mouse ... 192
A5.2. Materials ... 193
A5.3. Protocol steps ... 193
A5.4. Reagents and Solutions ... 194
A6. Commentary ... 194
A6.1 Background Information ... 194
A6.2. Critical Parameters and Troubleshooting ... 196
A6.3. Anticipated Results ... 197
A6.4. Time Considerations ... 197
A7. References... 198
x
List of tables
Chapter 3
xi
List of figures
Chapter 1
Figure 1. First histological evidence of adult neurogenesis in rodents.
Figure 2. A schematic drawing of the adult mouse forebrain and cells in the subventricular zone (SVZ), rostral migratory stream (RMS), and olfactory bulb (OB).
Figure 3. Diagram of olfactory system pathways.
Figure 4. Diagram of olfactory bulb neuronal elements, grouped into categories of (A) afferent fibers, (B) principal cells and (C) local interneurons.
Figure 5. Connectivity of olfactory bulb showing basic molecular, cellular and functional organization.
Figure 6. Diagram of morphological classes of adult-born granule cells and their positions in the olfactory bulb.
Figure 7. Electron micrographs of synapses in the EPL.
Figure 8. Regulation of KCC2 functions by transcriptional control, subcellular targeting and protein phosphorylation.
Figure 9. Basic configurations of a modern compound microscope with an infinity-corrected objective lens.
Figure 10. Light path through a compound microscope with transmission geometry. Figure 11. Schematic diagram of an epifluorescence microscope.
Figure 12. Simplified Jablonski diagram.
Figure 13. Optical layout of fluorescence microscopy techniques. Figure 14. Two-photon excitation microscopy.
Figure 15. Different states of light polarization. Figure 16. Polarized light imaging geometry.
Chapter 3
Figure 1. Schematic diagram of polarized light imaging.
Figure 2. Mouse brain dataset in polarized light, sagittal sections. Figure 3. Fiber orientation information obtained with polarized light.
xii
Figure 4. Comparison of polarized light imaging and TH immunostaining.
Figure 5. Comparative assessment of fiber intensity in polarized light between control and PD in internal capsule.
Figure 6. Comparison of PLI and Luxol Fast Blue staining for myelin, anterior limb of internal capsule.
Chapter 4
Figure 1. Tracking tangential and radial migration in adult brain slices.
Figure 2. Comparison of KCC2 immunohistochemical staining in RMS and OB.
Figure 3. Tangential migration parameters of adult-born neuroblasts in the RMS in response to KCC2 activation.
Figure 4. Tangential migration parameters of adult-born neuroblasts in the RMS-OB in response to KCC2 activation.
Figure 5. Radial migration parameters of adult-born neuroblasts in the RMS-OB in response to KCC2 activation.
Figure 6. Radial migration parameters of adult-born neuroblasts in the GCL in response to KCC2 activation.
Chapter 5
Figure 1. Relocation of mature spines of adult-born GC in the OB.
Figure 2. SHF determine the relocation of the spines of adult-born but not early-born GC. Figure 3. Relocated spines are stabilized in the OB network and are part of the
dendrodendritic synapses.
Figure 4. Olfactory sensory activity stabilizes SHF and promotes spine relocation. Figure 5. Glutamate released from MC stabilizes spine head filopodia.
Figure 6. BDNF application promotes spine relocation.
Figure 7. The activity of BDNF-lacking MC does not induce spine relocation. Figure 8. Spines with SHF are selectively maintained after sensory deprivation.
Figure 9. Spine relocation promotes fast synchronization of MC with functional consequences for odor information processing.
xiii
Supplementary Figure 2. SHF dynamic at different maturational stages.
Supplementary Figure 3. Random stimulation pattern of MC does not induce GC spine relocation.
Supplementary Figure 4. Activation of AMPARs is required for SHF motility.
ANNEX A
Figure 1. Tissue preparation.
xiv
List of abbreviations
AD Alzheimer’s disease
AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ANOVA Analysis of variance
AP Anterio-posterior α-SN α-synuclein
BDNF Brain derived neurotrophic factor
BL Baseline
BrdU 5-bromo-2-deoxyuridine BV blood vessel
CaMK Ca2+/calmodulin-dependent protein kinase CC Corpus callosum
CCC Cation-chloride cotransporter CCD Charge-coupled-device CNS Central nervous system CTD C-terminal domain DAB Diaminobenzidine
DAPI 4,6-Diamidino-2-phenylindole DCX Doublecortin
DG Dentate Gyrus
DIC Differential interference contrast DNA Deoxyribonucleic acid
DPI Days post-injection DTI Diffusion tensor imaging DTT Diffusion tensor tractography DV Dorso-ventral
EGF Epidermal growth factor EPL External plexiform layer
EPSC Excitatory post synaptic current GABA Gamma aminobutyric acid GAD Glutamic acid decarboxylase GC Granule cell
GCL Granule cell layer
GDNF Glial cell line-derived neurotrophic factor GFAP Glial fibrillary acidic protein
GFP Green fluorescent protein GL Glomerular layer
xv
HD Huntington’s disease
IPSC Inhibitory post synaptic current
IR Infrared
KCC K+/Cl- cotransporter
KO knockout
LB Lewy body
LN Lewy neurite
LOT Lateral olfactory tract LTP Long-term potentiation LV Lateral ventricle MC Mitral cell MCL Mitral cell layer
mEP Miniature excitatory post synaptic current mGluR2 metabotropic glutamate receptor 2
MIA Melanoma inhibitory activity
mIPS Miniature inhibitory post synaptic current ML Medio-lateral
MRI Magnetic resonance imaging NA Nucleus accumbens
NCAM Neural cell adhesion molecule NeuN Neuronal nuclei
NIR Near-infrared
NKCC Na+/K+/Cl- cotransporter NMDA N-Methyl-D-Aspartate NSC Neural stem cells NTD N-terminal domain OB Olfactory bulb
OGB Oregon Green BAPTA ONL Olfactory nerve layer OSN Olfactory sensory neurons Pax6 Paired box gene 6
PBS Phosphate-buffered saline PD Parkinson's disease
PET Positron emission tomography PFA Paraformaldehyde
PG Periglomerular cell PGL Periglomerular cell layer PLI Polarized light imaging
xvi
PSD95 Postsynaptic density protein 95 RI Refractive index
RMS Rostral migratory stream SD Standard deviation
SEM Standard error of the mean SGZ Subgranular zone
SHF Spine head filopodia SLC Solute carriers SN Substantia nigra
SPECT Single photon emission computerized tomography STIM Stimulation
SVZ Subventricular zone TCS Transcranial sonography TH Tyrosine hydroxylase
TrkB Tropomyosin related kinase B TTX Tetradotoxin
VEGF Vascular endothelial growth factor VBM Voxel based morphometry
xvii
Acknowledgements
Firstly, I want to express my deep gratitude and appreciation to my Director of Research, Dr. Armen Saghatelyan, for his tremendous support and for teaching me a host of techniques that will help me throughout my career. I am also grateful to Dr. Tigran Galstian for his guidance and for sharing his insights in physics and optics. Thanks to Dr. Marina Snapyan for her professionalism and for always being there to support and encourage. I also thank Dr. Daniel Côté who helped me a lot with the different imaging techniques. I am grateful to Dr. Gurgen Melkonyan, for developing the first prototypes of label-free imaging and for his sincere support that helped starting my project. I thank the members of Armen’s laboratory, past and present: Vincent, Archana, Delphine, Sarah, Arthur, Cedric, Marcos, Helia, Rodrigo, Lynda, Lusine, Jivan, Qian and Caroline, as well the members of the laboratories of Drs. Daniel Côté and Martin Parent and all my colleagues CRIUSMQ who made this journey more enjoyable.
Thank you all of my friends who are so close no matter how far. Thank you, Alik and Tsovik - you and I can share the silence, finding comfort together - the way old friends do. Thank you, Armina - with you to live the dreams we always had. Thank you, my darlings, Maya and Hayk, thanks for all the joy you're bringing. And last but not least, thank you, my mother and father for bringing me into this world and for all your love and care that surrounds me every single day of my life. With you forever, nothing really matters to me. Any way the wind blows…
xviii
Foreword
During the first part of my PhD work I focused on label-free imaging methods for tracking the development of migrating cells in the migratory stream of forebrain. I also studied the mechanisms controlling migration and the implication of mature adult-born neurons in the functioning of olfactory system. My thesis consists of one published paper where I am a coauthor, one paper that is in preparation and the third work which should be explored further.
First part of my results represents the paper which is preparation, where we did comparative study of myelinated fibers in human brain sections under normal and pathological conditions (Parkinson’s disease). We demonstrate significant differences of fiber intensities imaged in polarized light between these two groups. Our results highlight the importance of further multimodal imaging studies in the areas of the brain which are generally not known to be associated with neurodegenerative diseases. I am the main contributor in this paper; I co-developed the polarimetric imaging technique, conducted all experiments and data analysis. Dr. Gurgen Melkonyan has designed the first prototype of polarimeter and assisted in some experiments. Dr. Martin Parent has provided with human brain samples from healthy subjects and patients with Parkinson’s disease. Dr. Armen Saghatelyan and Dr. Tigran Galstian are my director and co-director respectively and they designed and supervised this project.
The second part of my results aims to unveil the role of KCC2 in different types of neuronal migration. Our preliminary results demonstrate that KCC2 activation fosters radial migration of neuroblasts in the rostral migratory stream and in the olfactory bulb. This work needs to be continued to reveal the exact mechanisms of KCC2 action on radially migrating neuroblasts. I had main contribution in this paper by conducting all experiments. Dr. Armen Saghatelyan has designed and supervised this project.
In third part of my thesis I present a research article published in Nature Communications in 2016 and titled “Principal cell activity induces spine relocation of adult-born interneurons in the olfactory bulb”. Here we reveal a new form of structural remodeling
xix
where mature spines of adult-born but not early-born neurons relocate in an activity-dependent manner. The publication of this paper was done thanks to important contribution from several co-authors. Vincent Breton-Provencher is the first author of the paper; he performed most of the experiments, analyzed the data, co-designed the study and co-wrote the paper with Armen Saghatelyan. I performed the majority of in vivo imaging experiments.
1
1.
Introduction
1.1.
Adult Neurogenesis
1.1.1. Neuronal development in adult rodent brain
In contrast to some lower vertebrates, known to regenerate whole regions in the central nervous system (CNS), traditionally it was assumed that all neurons in mammals are being produced only in embryonic and early postnatal stages of development. This dogma has been, however, challenged by discovery of persistent proliferation, migration and integration of neurons in the adult mammalian brain. First evidence of adult neurogenesis dates half a century ago, when Altman and Das have shown the presence of deoxyribonucleic acid (DNA) synthesis marker, 3H-thymidine in hippocampal neurons of adult rodent brains (Altman and Das, 1965). They further discovered another even larger neurogenic niche in the postnatal brain giving rise to granule and periglomerular cells of the olfactory bulb (OB) (Fig. 1; Altman, 1969). Despite continuous research in the field during next two decades, the results didn’t get proper acknowledgment by scientific community mainly due to the lack of evidence of adult neurogenesis in the primates at that time (Rakic, 1985).
The situation has changed only in 1990’s when adult-born cell proliferation in subventricular zone (SVZ) and further migration via rostral migratory stream (RMS) have been discovered (Lois and Alvarez-Buylla, 1993; Reynolds and Weiss, 1992). These important studies were followed by a series of publications revealing a physiological importance of adult neurogenesis by showing that environmental enrichment and learning modulates the level of production of new neurons in the adult rodent brain (Gould et al., 1999; Kempermann et al., 1997). Adult neurogenesis was also discovered in the dentate gyrus (DG) of hippocampus of primates and humans (Eriksson et al., 1998), further reinforcing the notion that specific regions of the adult brain produce new neurons throughout the life. Nowadays adult neurogenesis is a widely accepted fact, shown on practically all mammal species studied and the current research topics range from the molecular basis and functional implications of adult neurogenesis to its role in
2
neurodegenerative disorders and brain injury recovery. In mammals adult neurogenesis under normal, uninjured conditions is confined to two main regions. One is the subgranular zone (SGZ) of DG which supplies the hippocampus with new granule cells, and second one
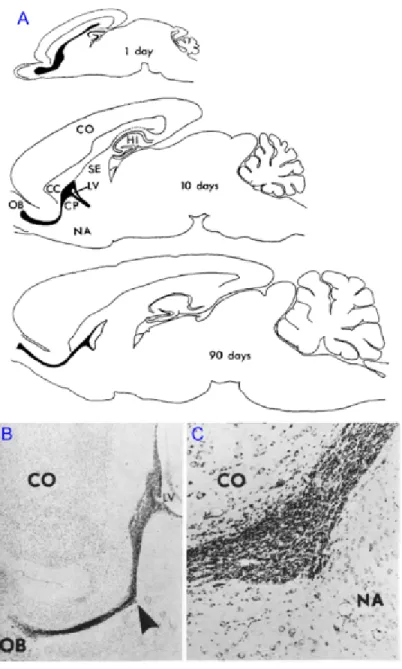
Figure 1. First histological evidence of adult neurogenesis in rodents (adapted from Altman, 1969).
A) Drawing of sagittal sections of the brain from rats of different ages showing the RMS (black). CC, corpus callosum; CO, cerebral cortex; CP, caudate-putamen; HI, hippocampus; LV, inferior horn of lateral ventricle; NA, nucleus accumbens; OB, olfactory bulb; SE, septum. B) Photomicrograph of the subependymal layer of the inferior horn of the lateral ventricle (LV) and the RMS (arrow) in sagittal section in a 21 day old rat. Cresyl violet, X16. C) The RMS at higher magnification.
3
is the SVZ bordering the lateral ventricles (LV) from where the new-born cells migrate via RMS to the OB to become interneurons in granule cell and periglomerular cell layers (GCL and PGL, respectively). The neurogenesis in the OB of rodents is much more pronounced with much higher cell turnover (~30 000 cells produced per day) and longer migration distances (several millimeters) (Lois and Alvarez-Buylla, 1994; Winner et al., 2002). The cells proliferate in SVZ, where a GFAP-positive subpopulation of stem cells gives rise to proliferating transit amplifying cells that, in turn, differentiate into migrating immature neurons (neuroblasts) (Fig. 2; Doetsch et al., 1999; Ming and Song, 2005).
These newly formed neuroblasts migrate tangentially in chains towards the OB via RMS in a tubular structure formed by glial cells (Lois et al., 1996). They also use blood vessels as a scaffold for migration and source of molecular factors (Snapyan et al., 2009). The migration of neuroblasts from the SVZ to the OB is orchestrated by various mechanisms.
Figure 2. A schematic drawing of the adult mouse forebrain and cells in the subventricular zone (SVZ), rostral migratory stream (RMS), and olfactory bulb (OB) (adapted from Gengatharan et al., 2016).
4
The chain migration of neuroblasts highly depends on polysialylated-neural cell adhesion molecule (PSA-NCAM) proteins on the surface of the migrating neuroblasts. Several other factors are also known to coordinate the motility of neuroblast migration in chains, such as netrin, ephrin-B2, integrin and γ-aminobutyric acid A receptor (GABAAR) activity (Bolteus
and Bordey, 2004; Conover et al., 2000; Hu et al., 1996; Murase and Horwitz, 2002). Netrin is also involved in the directionality of chain migration, as is the Slit/Robo signaling
(Murase and Horwitz, 2002; Nguyen-Ba-Charvet et al., 2004; Wu et al., 1999).
Having reached the core of OB, the neuroblasts drastically change the mode and directionality of migration. They detach from the chains and undertake individual radial migration into GCL and PGL. The process of detachment from chains is initiated by reelin (Hack et al., 2002) and tenascin-R, which then control the radial migration to their final destination in the OB (Saghatelyan et al., 2004). Whether the cells will stop in GCL or continue their radial migration to reach PGL, is influenced by transcription factor Pax6 (Hack et al., 2005; Kohwi et al., 2005). Once reached their target area, the cells get mature and integrate in the olfactory network as functional granule cells (GC) and periglomerular cells (PG) (Lledo and Saghatelyan, 2005). Both cell types are axonless interneurons making reciprocal dendrodendritic synapses with mitral or tufted cells (Shepherd, 2004).
1.1.2. The olfactory system
The olfactory system is essential for the survival of many animal species, providing vital information about food location and influencing social and sexual behaviours. In mammals, odor information is conveyed by olfactory sensory neurons (OSN) located in the olfactory epithelium into the OB where it is processed by bulbar principal cells and interneurons (Shepherd, 2004). From the OB, the information is conveyed to the piriform cortex, amygdala, hippocampus, and entorhinal cortex (Davis, 2004; Shepherd, 2004). Olfactory system is the only sensory system where information processing occurs without a thalamic relay. It is assumed that the OB “substitutes” thalamus in terms of processing odor information and relaying it to higher cortical areas (Kay and Sherman, 2007).
The OB consists of multiple layers each containing morphologically distinct cells (Fig. 3). The neurons in the OB are classified according to the layers in which their cell bodies
5
reside, such as PG in the glomerular layer (PGL), tufted cells in the external plexiform layer (EPL), mitral cells (MC) in the mitral cell layer (MCL), and GCs in the GCL (Fig. 4). The OB receives the sensory information from OSNs in the olfactory epithelium (Shepherd, 2004). The mammals have ~1000 different functional odorant receptors and, interestingly, each OSN expresses only a single odorant receptor. The axons from different OSNs expressing the same receptor come together into a spherical structure in the OB, called glomerulus. Therefore, the glomeruli are considered to be the functional units in the OB and together they represent spatial map of the different olfactory receptors located in the olfactory epithelium. The unique affinity profiles of odorant receptors together with specific arrangement of terminals in glomeruli gives rise to significant combinatorial complexity in odor processing, which allows to discriminate more than 1.7 trillion of different odors (Bushdid et al., 2014). While these data were nominated for “Breakthrough of the Year 2014” and heavily promoted by the popular press, they raised a lot of skepticism in the scientific community (Gerkin and Castro, 2015; Meister, 2015). In
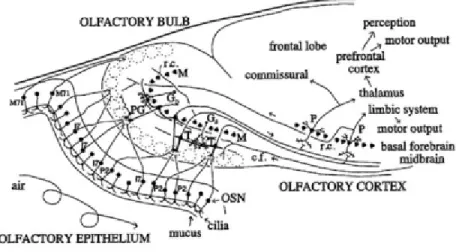
Figure 3. Diagram of olfactory system pathways (Shepherd, 2004).
Here some of the major connections in the mammalian olfactory bulb are shown. The olfactory sensory neurons in the epithelium send the input signal to olfactory bulb, which in turn projects it to the olfactory cortex. Olfactory epithelium populated with overlapping types of olfactory sensory neurons project to individual glomeruli. c.f., centrifugal fiber; G, granule cell; M, mitral cell; OSN, olfactory sensory neuron; P, pyramidal cell; PG, periglomerular cell; r.c., recurrent axon collateral; T, tufted cell.
6
particular, the authors used mathematical modeling of experimental data showing that humans can successfully discriminate 148 pairs of odors (Bushdid et al., 2014). However, several flaws in the analytical model were identified (Gerkin and Castro, 2015; Meister, 2015). Moreover, if the same method would have been applied to human color vision, one would estimate that humans can distinguish at least 1027 colors, which is in dramatic
conflict with experimental evidence (Meister, 2015). Therefore, the claim that humans can discriminate 1.7 trillions of odors is unjustified (Gerkin and Castro, 2015; Meister, 2015) and further studies are required to solve this issue.
In glomeruli, the terminals of OSN axons synapse with the primary dendrites of the principal neurons (mitral or tufted cells) that convey the sensory information from the periphery to the second-order neurons, forming thereby the basis of an odor processing column (Mori and Sakano, 2011; Ressler et al., 1994). The PG and short axon neurons in the GCL play an important modulatory role via inter- and intraglomerular excitatory and inhibitory connections to the principal neurons (Aungst et al., 2003). The PGs can be distinguished based on the expression of calretinin, calbindin, parvalbumin, glutamate and
γ-aminobutyric acid (GABA), with some of the latter co-expressing tyrosine hydroxylase (TH), an enzyme involved in dopamine synthesis (Bagley et al., 2007; Brill et al., 2009; Kosaka et al., 1995; Whitman and Greer, 2007a). This abundance of heterogeneous subpopulations of cells is not fully clear yet, but is apparently related to complexity of the functions in the first stages of odor processing.
Beneath the GL is the EPL where the lateral dendrites of principal output neurons make dendrodendritic synapses with the GCs. The EPL is also sparsely populated by somas of tufted cells, astrocytes and parvalbumin-expressing interneurons that make synapses to output neurons or other interneurons (Hamilton et al., 2005). Next follows a thin layer of MC somas from which this layer has got its name. The long lateral dendrites of mitral cells and tufted cells (further jointly referred as principal cells) reach the EPL where they form glutamatergic synaptic connections with the dendrites of interneurons (Fig. 4). The principal cells transfer the olfactory information with their axons to the olfactory cortex via the lateral olfactory tract (LOT), which, in turn, conveys the signals further to piriform cortex, amygdala, hippocampus (Davis, 2004; Igarashi et al., 2012; Shepherd, 2004).
7
Figure 4. Diagram of olfactory bulb neuronal elements, grouped into categories of (A) afferent fibers, (B) principal cells and (C) local interneurons (adapted from Shepherd, 2004).
ONL, olfactory nerve layer; GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; IPL, internal plexiform layer; GRL, granule cell layer. In A: ON, olfactory nerve fibers. Centrifugal afferents are from the contralateral anterior olfactory nucleus (cAON), ipsilateral anterior olfactory nucleus (iAON), tenia tecta (TT), olfactory cortex (OC), horizontal limb of the diagonal band (HDB), locus coeruleus (LC), and raphe nucleus (Ra). pE, pars externa of the AON; pM, pars medialis of the AON. In B: dendrites and axon collaterals of a mitral cell (M), and internal tufted cell (iT, or a displaced mitral cell, dM), a middle tufted cell (mT), and an external tufted cell (eT) are illustrated, a, axon; d, dendrite. In C: GI, GII, and GIII, three types of granule cells; PG, periglomerular cell; SA(S), superficial short-axon cell; SA(B), Blanes cell.
8
Deeper from MCL is the GCL consisting mostly of the cell bodies of GCs, as well as a sparse amount of short-axon cells. The GCs are notable for their lack of axon and they have characteristically large dendritic spines. The primary dendrite of each GC extends radially toward the outer layers of OB, giving rise to secondary branches (distal dendrites) and terminating in the EPL (Fig. 4c). The rest of GC processes are short basal dendrites serving
Figure 5. Connectivity of olfactory bulb showing basic molecular, cellular and functional organization (adapted from Shepherd, 2004).
Molecular components (left): OR - olfactory receptor, ON - olfactory nerve, AMPA - 2-amino-5-phosphonovaleric acid, NMDA - N-methyl-D-aspartate, M/T - mitral/tufted cell, PG - periglomerular cell, GluR - ionotropic glutamate receptor, GABA R - GABA receptor, DAR - dopamine receptor, NE - norepinephrine, aAR - alpha adrenoreceptor, mGluR2 - metabotropic glutamate receptor, GR - granule cell, Synaptic circuit (middle): ORN - olfactory receptor neuron, J, K - ORN subsets, e - excitatory, i - inhibitory. Structural-functional relations (right): top - overlapping responses of ORNs to a range of odors (1..n); middle - ORN subsets connectivity to the glomeruli; bottom - due to lateral inhibition, the response spectra of M/T cells have less overlap
9
as inputs from other brain regions. The GCL is comparatively wide and depending where the GC somas reside, their morphology and functional role might be different. The dendritic ramifications of superficial GCs reach EPL where they mainly contact the tufted cell dendrites, while the dendrites of deep GCs synapse to the MCs in deeper layers of EPL (Greer, 1987; Mori et al., 1983; Orona et al., 1983). Less heterogeneous than PGs, the GCs are nevertheless shown to have small subpopulations expressing calretinin, glycoprotein 5T4, metabotropic glutamate receptor 2 (mGluR2), Neurogranin and Ca2+
/calmodulin-dependent protein kinases IIα (CaMKIIα) and IV (CaMKIV) (Batista-Brito et al., 2008; Gribaudo et al., 2009; Imamura et al., 2006; Jacobowitz and Winsky, 1991; Liu, 2000; Néant-Fery et al., 2012; Zou et al., 2002).
The GCs provide recurrent and lateral inhibition to lateral dendrites of principal neurons (Arevian et al., 2008; Isaacson and Strowbridge, 1998; Schoppa and Urban, 2003; Tan et al., 2010; Urban, 2002). This inhibition is mediated through reciprocal synapses, where glutamate is released from the lateral dendrites of the mitral or tufted cells onto the spine of a GC, which in turn induces the release of GABA back onto the principal cell dendrites (Isaacson and Strowbridge, 1998; Price and Powell, 1970; Shepherd, 2004). The GCs and PGs play an important role in the rhythmic activity of the OB. PGs coordinate theta activity by regulating baseline and odor-evoked inhibition, whereas GCs are involved in the synchronization of MC activity and generation of gamma rhythm (Arevian et al., 2008; Fukunaga et al., 2014; Urban, 2002; Yokoi et al., 1995). An overview of synaptic connectivity of OB is presented in Fig. 5. A striking feature of adult OB is its ability to produce new GCs and PGs throughout the life of an animal. In the next parts, I will discuss different processes leading to the generation, migration, maturation and functional integration of adult-born interneurons and will discuss the current understanding of the role played by these cells in olfactory system.
1.1.3. Proliferation of neuronal precursors in the SVZ
Adult-born neurons in the OB are derived from stem cells located in the SVZ bordering lateral ventricles. From the SVZ neuronal precursors migrate several mm to reach the OB and integrate into its neuronal network (Fig. 2). The SVZ neurogenic niche contains various cell types, each having an important contribution in neurogenic activity: neural
10
stem cells (NSC), transit-amplifying cells, neuroblasts, astrocytes, endothelial and ependymal cells (Ming and Song, 2011). Adult neurogenesis comprises a complicated and interrelated chain of events which are orchestrated by timely involvement of molecular cues. The first step of these events is considered to be a division of slow dividing NSCs in the SVZ - the Type B cells, which are derived from radial glial cells producing the neurons during embryonic life (Fuentealba et al., 2015; Merkle et al., 2014; Young et al., 2007). The type B cells are astrocyte-like NSCs giving rise to transit-amplifying cells (Type C cells), which in turn divide rapidly to give rise to neuronal precursors (Type A cells) (Doetsch et al., 1999). NSCs are located in the specialized microenvironment where they need to integrate multiple signals to maintain their quiescence or get activated to generate neurons. They contact blood vessels via their basal processes and are exposed to a variety of factors from the cerebrospinal fluid via small apical process bearing a non-motile primary cilium (Fig. 2; Doetsch et al., 1999). The non-neuronal cells in SVZ such as the ependymal and endothelial cells modulate adult neurogenesis by providing molecular factors.
1.1.4. Migration of neuroblasts in the adult brain
The migration of neuroblasts in the SVZ-OB pathway consists of succession of migratory and stationary phases (Snapyan et al., 2009). Typically the initial periods of extension of leading process and exploration are followed by the displacement of the cell body towards the place of the leading process (Wichterle et al., 1997). These processes are regulated by interplay of different intracellular mechanisms (Lalli, 2014). For instance, the stabilization of the leading process is attributed to doublecortin (DCX), a microtubule-associated protein (Belvindrah et al., 2011; Koizumi et al., 2006), while the movement of nucleus in the cytoplasm is supported by Lis1/Ndel1 complex (Hippenmeyer et al., 2010).
In the SVZ, neuroblasts assemble together in tightly packed chains and begin to migrate towards the OB through a special environment in the RMS along topographically aligned blood vessels (Saghatelyan, 2009; Snapyan et al., 2009; Whitman and Greer, 2009). It has been proposed that cilia beating of ependymal cells creates a gradient of chemorepellent molecules secreted from the septum and choroid plexus, such as Slit (Sawamoto et al., 2006; Wu et al., 1999), that pushes away neuroblasts from the SVZ (Sawamoto et al., 2006). While this gradient and ependymal cells cilia beating play an important role in the
11
neuroblasts migration in the SVZ, it is difficult to imagine how the unidirectional vectorial gradient of a single molecule might explain the complex-shaped curvature migration of newborn cells in the RMS. It would be intuitive to suggest that the further migration of neuroblasts is somehow controlled by attractive factors coming from OB. However surgical removal of the OB did not affect neuroblasts migration in the RMS (Jankovski et al., 1998; Kirschenbaum et al., 1999), questioning the role of the OB in the directional guidance of neuronal precursors. The work from our lab has revealed that blood vessels play an important role in the neuroblasts migration (Snapyan et al., 2009). They topographically outline the migratory stream and neuroblasts use these long, parallel running blood vessels as a substrate for their migration. In the context of vasculature-associated neuronal migration model, no attractive molecules secreted from the OB are needed and a single repellent molecule might be sufficient to push cells away from the posterior parts of the brain (SVZ), with vasculature providing local cues to keep migrating precursors in the RMS and guide them towards the OB (Snapyan et al., 2009). Therefore, the migration of neuroblasts in the RMS would be controlled by the local factors and in line with this, myriads of local factors derived either from neuroblasts, or astrocytes and endothelial cells have been shown to regulate neuronal migration.
Astrocytes ensheath the chains of neuroblasts and their processes undergo rapid structural changes in response to neuroblasts-secreted repulsive protein Slit1 (Kaneko et al., 2010). Slit1 repels astrocytic processes via Robo receptor and leads to the formation of structurally permissive glial tunnels enabling neuronal migration (Kaneko et al., 2010). Astrocytes may also release soluble factors that modulate neuroblasts migration and/or survival. For example, astrocytes may release glutamate or melanoma inhibitory activity (MIA) protein, which are required for neuroblasts migration (Mason et al., 2001; Platel et al., 2010). Astrocytes also express high affinity GABA transporters and inhibition of GABA uptake reduces neuroblasts migration (Bolteus and Bordey, 2004).
Brain derived neurotrophic factor (BDNF) released from the endothelial cells promotes migration via low affinity BDNF receptor, p75NTR present on neuroblasts (Snapyan et al., 2009). Interestingly, migrating neuroblasts control their own migration by regulating the amount of free extracellular BDNF. GABA released from neuronal precursors induces
12
Ca2+-dependent insertion of high-affinity TrkB receptors into the plasma membrane of
astrocytes that ensheath migrating neuroblasts and contact blood vessels. This leads to trapping of extracellular BDNF and, therefore, to the entry of migrating cells to the stationary phase. Through this mechanism, neuronal precursors regulate availability of BDNF required for their own migration (Snapyan et al., 2009).
A number of other molecular factors also affect neuronal migration in the adult brain. These include ephrin-B (Conover et al., 2000), and integrin families (Belvindrah et al., 2007; Murase and Horwitz, 2002); the ErbB4 (Anton et al., 2004) and prokineticin 2 (Ng et al., 2005) receptors; as well as various growth factors such as glial cell line-derived neurotrophic factor (GDNF; Paratcha et al., 2006), and vascular endothelial growth factor (VEGF; Bozoyan et al., 2012; Wittko et al., 2009).
Tangential chain-arranged migration continues until the neuroblasts reach the OB. Here they detach from chains and start individual radial migrations along blood vessels to outer layers of OB (Bovetti et al., 2007; Lledo and Saghatelyan, 2005; Snapyan et al., 2009). While many migration related mechanisms here are similar to the ones operating during tangential migration, there are however some specific differences. The processes of separation of neuroblasts from chains are associated with the expression of extracellular matrix glycoprotein tenascin-R in the RMS-OB (David et al., 2013; Saghatelyan et al., 2004). Also the glycoprotein reelin released by the principal cells of the OB has a role in the transition to radial migration and in the final placement of adult-born neuroblasts in the OB (Hack et al., 2002; Kim et al., 2002).
1.1.5. Maturation and integration of adult-born interneurons in the OB
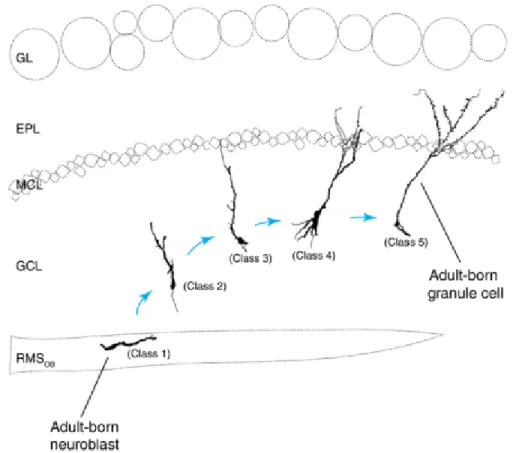
When the precursor cells find their final position in the OB, they differentiate and mature to become fully functional OB interneurons. The neuroblasts differentiate into two types of interneurons - the GCs and the PGs and while doing so their morphology evolves from bipolar cells with short processes into a fully developed neurons with dendritic branches extending hundreds of microns (Carleton et al., 2003; Lledo et al., 2006; Lledo and Saghatelyan, 2005; Petreanu and Alvarez-Buylla, 2002). The maturation stages of GCs are divided into 5 classes (Fig. 6; Carleton et al., 2003; Petreanu and Alvarez-Buylla, 2002).
13
According to this classification, the class 1 cells are the tangentially migrating neuroblasts of RMS-OB. It takes 5 days for the neuronal precursors to get there from the SVZ. The neuroblasts detaching from the chains and starting to migrate radially are considered class 2 cells. Then within 9 to 15 days from their birth, the growing primary dendrites of class 3 cells start to reach the EPL, while not yet contacting the MC. Starting from approximately 11 days from their generation, yet immature GCs of class 4 begin to develop their secondary dendrites that are spineless at this stage. Eventually, near the 20th-30th day, the
GCs reach the morphological class 5 by developing multiple dendritic spines and become synaptically integrated in OB.
Class 5 adult-born GCs are morphologically similar to the mature GCs produced at embryonic and neonatal age (thereafter called early-born neurons), however they tend to
Figure 6. Diagram of morphological classes of adult-born granule cells and their positions in the olfactory bulb (adapted from Petreanu and Alvarez-Buylla, 2002).
EPL - External plexiform layer; GL - glomeruli layer; MCL - mitral cell layer; GCL - granule cell layer; RMS - rostral migratory stream.
14
occupy deeper areas in the GCL (Imayoshi et al., 2008), while early-born GCs are more concentrated in the superficial parts of the GCL (Lemasson et al., 2005). There is a correlation between the position of GC in GCL and the area of EPL where their dendrites reach, meaning that adult-born and early-born GCs may preferentially target the dendrites of mitral or tufted cell, respectively (Greer, 1987; Shepherd et al., 2004). This evidence hints on the differences in functional roles of adult-born and early-born GC populations.
There are less studies of adult-born PGs and classification of morphological changes of adult born PGs has not been done (Belluzzi et al., 2003; Mizrahi, 2007). The migration of PG neuroblasts seems to be faster than for GCs, despite the longer distance (Hack et al., 2005), but dendritogenesis and synaptogenesis take longer time as compared to GC development (Mizrahi, 2007; Whitman and Greer, 2007a).
Apart from differences in morphology, the evidence of electrophysiological distinctions (such as spiking activity and sodium conductance) between early-born and adult-born GCs further supports the idea of distinct functional role of these two subpopulations (Belluzzi et al., 2003; Carleton et al., 2003). Both tangentially and radially migrating neuroblasts have no synaptic currents, but their ionotropic AMPA and GABA receptors are already functional (Carleton et al., 2003) The radially migrating cells express NMDA receptors, however their presence in earlier stages is a matter of controversy (Carleton et al., 2003; Platel et al., 2010).
The post-synaptic currents start to appear together with the growth of the primary dendrite of adult-born GCs. The inhibitory synaptic activity prevails at this stage, probably due to presence of GABA (Carleton et al., 2003; Panzanelli et al., 2009). With the development of secondary dendrites and spines, excitatory post-synaptic activity appears (Carleton et al., 2003) and coincidentally the cells begin to give rise to sodium currents (Carleton et al., 2003). Na+ current become more prominent with further growth of GC distal dendrites in
the EPL (Carleton et al., 2003). The sodium currents and action potentials of adult-born GC interneurons develop at a later maturational phase than those of early-born GCs (Belluzzi et al., 2003; Carleton et al., 2003; Kelsch et al., 2008). Interestingly, compared to their neonatal counterparts, the synaptically integrated adult-born GCs and PGs have higher
15
sodium currents, their conductance-voltage ratio is more steep and the activation threshold is more negative (Belluzzi et al., 2003; Carleton et al., 2003; Saghatelyan et al., 2005). 1.1.6. Synaptic development of adult-born GCs
GCs are axonless neurons that have basal and apical dendrites. The apical dendrite is composed of an unbranched proximal segment and highly branched distal segment with numerous spines. The spines of GCs are constituents of dendrodendritic synapses that these interneurons form with the lateral dendrites of MCs. In dendrodendritic reciprocal synapses glutamate is released from the lateral dendrites of the MC onto the spine of a GC, which in turn induces the release of GABA back onto the principal cell dendrites (Isaacson and Strowbridge, 1998). The dendrodendritic synapses are the only output synapses of GCs in the OB (Shepherd, 2004) and are responsible for recurrent and lateral inhibition of principal neurons (Isaacson and Strowbridge, 1998; Schoppa and Urban, 2003).
During the development of adult-born GCs, they first receive input synapses on the basal dendrites and on the proximal part of the primary dendrite, before forming the output ones on the distal dendrites (Kelsch et al., 2008; Pallotto et al., 2012; Panzanelli et al., 2009; Whitman and Greer, 2007b). This contrasts with development of early-born interneurons that receive input and output synapses simultaneously (Kelsch et al., 2008). Early in the development of the adult-born GCs, there are more GABAergic than glutamatergic synapses, which is assumed to be an important factor for the proper formation of dendrites and spines in the EPL (Pallotto et al., 2012; Panzanelli et al., 2009). Soon after, the output dendrodendritic synapses between distal dendrites of adult-born cells and lateral dendrite of MC or tufted cells begin to develop (Kelsch et al., 2008; Panzanelli et al., 2009). Electronic microscopy study confirmed the presence of dendrodendritic synapses between adult-born GCs and MC lateral dendrites (Fig. 7; Whitman and Greer, 2007b).
It is thought that adult-born neurons by first receiving input synapses before forming the output ones, silently integrate into the OB without disturbing already functional neuronal network (Kelsch et al., 2008; Lledo and Saghatelyan, 2005). Optogenetic targeted-stimulation of adult-born cells has demonstrated their GABAergic outputs onto the MCs (Alonso et al., 2012; Bardy et al., 2010; Valley et al., 2013). While no glutamatergic inputs
16
has been yet recorded from the adult-born GC following optogenetic or electrical stimulation of MC, the synapses between GC and MC have clear dendrodendritic architecture (Fig. 7), high presence of PSD95 puncta in the spine of GCs (Panzanelli et al., 2009; Whitman and Greer, 2007b) and express AMPA and NMDA receptors (Breton-Provencher et al., 2014). All this suggest that adult-born GC spines receive glutamatergic input from bulbar principal neurons. The details and the mechanism of reciprocal synapse development on the distal dendrites of adult-born GCs are not yet fully known. What we know so far is that initially when the cells integrate in the OB, their spine density increases and later with maturation it decreases again (Pallotto et al., 2012; Whitman and Greer,
Figure 7. Electron micrographs of synapses in the EPL ( from Whitman and Greer, 2007b)
A - Dendrodendritic synapse in the EPL between a mitral cell dendrite and a granule cell spine, unlabeled. B, C - Examples of mitral to granule excitatory synapses on labeled spines at 42 dpi. Virally labeled cells were marked by GFP immunohistochemistry and DAB; the spines of new granule cells are darkly stained. D - An example of a bidirectional dendrodendritic synapse on the same spine head. Md - Mitral cell dendrite; Gr - granule cell spine.
17
2007b). This can be explained by increased search of synaptic partners during early maturational periods, followed by synaptic pruning that may lead to the decreased spine density at later maturational stages (Panzanelli et al., 2009; Whitman and Greer, 2007b). The work from our laboratory has shown that MC activity orchestrates the initial spinogenesis and formation of contact between GC and MC dendrites (Breton-Provencher et al., 2014). Using time-lapse two-photon imaging of adult-born GCs at their different maturational stages in acute slices of the OB, our lab has demonstrated that principal cell activity regulates the filopodia dynamic during the early but not the late maturational stages through the activation of NMDA receptors (NMDARs) on adult-born GC dendrites (Breton-Provencher et al., 2014). Recent in vivo data suggested that GCs have high synaptic turnover even when they are fully mature (Sailor et al., 2016). The mechanisms leading to stabilization of some spines and disappearance of others are not clear, but it is plausible that these mechanisms are activity-dependent.
1.1.7. Adult neurogenesis as a form of structural plasticity to adjust OB network to changing environmental conditions
It is estimated that tens of thousands new neurons are added to the OB every day. This cell addition is accompanied by apoptosis of GCs in order to maintain OB volume. Interestingly, the largest amount of dying cells belongs to immature population of adult-born neurons (Kuhn et al., 2005; Petreanu and Alvarez-Buylla, 2002). In fact, since the sites of progenitor generation (SVZ) and adult-born cells integration (OB) are located several mm apart, it is though that adult brain produces an excess of new neurons with more than half destined for programmed cell death. However, this overproduction allows for having large number of immature cells in the OB at any moment during the life of animal which leads to up- or downregulation of the number of integrating adult-born neurons in response to changing odor environment. Indeed, the number of adult-born cells can be modulated by odor enrichment (Rochefort et al., 2002), odor deprivation (Petreanu and Alvarez-Buylla, 2002; Saghatelyan et al., 2005; Yamaguchi and Mori, 2005), pheromones (Mak et al., 2007; Shingo et al., 2003), and associative tasks based on olfaction (Mouret et al., 2008; Sultan et al., 2010). In contrast, early-born neurons persist over time in the OB (Lemasson et al., 2005).
18
Several studies have shown that when adult-born neurons integrate into functional bulbar network, they are more active as compared to their early-born counterparts. For example, odor stimulation induces higher activation of adult-born as compared to early-born interneurons (Carlén et al., 2002) and blocking adult neurogenesis only for 1 month results in about 50% reduction in the inhibitory activity received by principal cells (Breton-Provencher et al., 2009; Saghatelyan et al., 2005). Furthermore, the constant arrival of adult-born interneurons and, consequently, the persistent formation and elimination of dendrodendritic synapses formed by these cells with the principal neurons allows the OB circuitry to maintain and modulate the spatio-temporal coding of sensory information and facilitate odor learning and memory (Alonso et al., 2012; Breton-Provencher et al., 2009; Mak et al., 2007; Sultan et al., 2010).
Why OB needs such an elaborated process for promoting plasticity? The main reason for this could be because of the nature of output synapse in the OB. The main output synapse is dendrodendritic reciprocal synapse between interneurons and bulbar principal cells. It is therefore difficult to imagine other forms of plasticity in these synapses, such as long-term potentiation (LTP) or long-term depression that induce changes the efficacy of the synapse and allow adjustment of neuronal network in other brain regions. Because of the reciprocal nature of dendrodendritic synapse, any changes in the efficacy of glutamatergic part will be immediately accompanied by counterbalancing changes in the inhibitory part of the same synapse. In line with this, no LTP was found in dendrodendritic OB synapses (Dietz and Murthy, 2005; Gao and Strowbridge, 2009) and therefore other forms of structural plasticity should exist to adjust bulbar network functioning.
The continuous supply of new neurons provides the OB with a reservoir of plastic cells and it is thought to constantly sculpt the bulbar network in response to changing environmental conditions (Sailor et al., 2016). The plasticity brought about by adult-born neurons depends on the formation, retraction and/or stabilization of new synaptic contacts (Breton-Provencher et al., 2014; Livneh and Mizrahi, 2012; Mizrahi, 2007) and growing evidence suggests that new adult-born neurons in the OB are involved in the different types of behavior, including short- (Breton-Provencher et al., 2009; Rochefort et al., 2002) and long-term odor memory (Alonso et al., 2012; Sultan et al., 2010), odor discrimination
19
(Enwere et al., 2004), parental (Mak and Weiss, 2010) and social (Mak et al., 2007) behavior. However, there is an important conceptual problem since environmental changes can be very rapid, whereas the synaptogenesis of adult-born neurons occurs over a longer time scale. It remains thus completely unknown how bulbar network functions when rapid
and persistent changes in environmental conditions occur, even though new synapses have not yet been formed.
In the third objective of my thesis, I undertook in vivo imaging of adult-born neurons with a relatively rapid acquisition rate (once every 5 min), to understand how these cells react to rapid changes in the odor environment and if there are other forms of structural plasticity in the bulbar network that adapt its functioning to rapid and persistent changes in the environmental conditions.
1.2.
Overview of GABAergic signaling in the brain
During the formation of the nervous system, the cells undergo several crucial developmental changes which are regulated by many molecular cues. Being the main inhibitory neurotransmitter in the adult CNS (Watanabe et al., 2002), GABA is known to play an excitatory role during early neuronal development (Rivera et al., 1999). GABAergic synapses develop before the appearance of glutamatergic ones (Chen et al., 1995; Del Rio et al., 1992) and the shift from the excitatory to inhibitory actions of GABA is regulated by neuronal activity and expression of cation-chloride cotransporters (Ben-Ari et al., 1997; 2002).
GABA binds to three types of neurotransmitter receptors: ligand-gated ion-channels such as GABAA and GABAC (also called GABAA-rho) receptors; and G protein-coupled
metabotropic GABAB receptors. Both GABAA and GABAC are ligand-gated Cl- channels,
with GABAC being bicuculline insensitive and entirely composed of rho (ρ) subunits
(Johnston, 2013). GABAC has slow onset and offset kinetics (Johnston, 2013) and is
predominantly expressed in the retina (Qian and Ripps, 2001). GABAB receptors are
metabotropic G protein-coupled receptors that modulate neuronal functions via their coupling to K+ and Ca2+ channels. Activation of K+ channels, and particularly Kir3.1
20
hyperpolarize the membrane and inhibit neuronal activity by shunting excitatory transmission (Lüscher and Slesinger, 2010). Pre- and postsynaptic GABAB receptors also
modulate voltage-gated Ca2+ channels, which affects synaptic transmission and plasticity
(Chalifoux and Carter, 2011; Pérez-Garci et al., 2006).
GABAA receptors are responsible for the most of the physiological actions of GABA in
CNS. They are pentameric receptors composed from different subunits surrounding a central chloride ion-selective channel (Sigel and Steinmann, 2012). The differences in subunit composition determine the functional characteristics of GABAA receptors (Farrant
and Nusser, 2005). In the mature brain GABAA receptors located in the postsynaptic
membrane mediate rapid (millisecond range) neuronal inhibition, whereas those located extrasynaptically respond to ambient GABA and induce longer inhibition (Sigel and Steinmann, 2012). In the developing brain, GABAA-mediated depolarization activates
voltage-gated Ca2+ channels that releases Mg2+ block from NMDA channels and induces
further increase in the Ca2+ entry (Ben-Ari, 2002). This synergistic action of GABA A and
NMDA receptors in immature neurons underlies so called Giant Depolarizing Potentials (GDP), a pattern of synchronized large oscillations in the intracellular Ca2+, which is
required for the activity-dependent maturation and establishment of neuronal network (Ben-Ari, 2002; Wu and Sun, 2015).
Not only GABA regulates maturation of neuronal networks, but it also modulates proliferation of neuronal precursors, their migration, differentiation, axonal and dendritic growth and synaptogenesis. Below, I briefly discuss the role of GABA in all these developmental processes.
1.2.1. The role of GABA in neuronal development during embryogenesis and in adulthood
1.2.1.1. Neuronal proliferation
GABAergic neurons first appeared at embryonic day 12 (E12) are localized in the subventricular and ventricular zones, the areas of extensive neuronal proliferation (Del Rio et al., 1992). At this stage, GABA plays an excitatory role and it has been shown that it
21
inhibits DNA synthesis and thus proliferation of cortical progenitors (Jovanovic and Thomson, 2011; Wang and Kriegstein, 2009). From mechanistic point of view, this inhibition is likely due to depolarization-induced Ca2+ fluxes that affect the cell cycle
length (LoTurco et al., 1995). Interestingly, the effect of GABA on cell proliferation is cell type- and context-specific. For example, GABA increases the proliferation of neuronal progenitors in the embryonic ventricular zone, but conversely decreases proliferation of progenitors located in the SVZ (Haydar et al., 2000). While the reasons for such differential effects are still not clear, the findings indicate that the depolarizing effect of GABA on neuronal proliferation is cell type specific.
GABA also affects proliferation of neuronal progenitors located in the adult SVZ and SGZ of DG, two neurogenic regions in the adult brain. In the DG, GABA is released from parvalbumin-expressing interneurons and inhibits proliferation of stem cells-like radial glia cells through activation of the γ2-subunit-containing GABAA receptor (Song et al., 2012).
In the SVZ, GABA also depolarizes neuronal progenitors, but the mechanism of GABA release is different (Liu et al., 2005; Wang et al., 2003). Migrating neuroblasts are GABAergic cells (Wang et al., 2003; Snapyan et al., 2009) and release GABA in a non-synaptic, non-vesicular fashion (Liu et al., 2005). This induces tonic activation of GABAA
receptors in the neuronal progenitor cells and decreases their proliferation (Fernando et al., 2011; Liu et al., 2005; Nguyen et al., 2003). Both in the embryonic and adult brains GABAA receptor activation induces phosphorylation of the histone proteins, likely via Ca2+
signaling, which in turn mediates the inhibitory effect of GABA on the cell cycle (Fernando et al., 2011).
1.2.1.2. Neuronal migration
GABA also affects migration of neuronal precursors in the embryonic and adult brain. Both GABAA and GABAB receptors may be involved in the migratory effects observed after
GABA application (Behar et al., 1998; López-Bendito et al., 2003; Snapyan et al., 2009; Wang et al., 2003). Intriguingly, at higher concentration GABA promotes migration of interneuronal precursors via activation of GABAB receptors and G-protein coupled
mechanisms, whereas at low concentration GABA increases migration of progenitors for excitatory neurons via GABAA-mediated mechanism (Behar et al., 1998). Blocking the
22
depolarizing action of GABA affects neuronal migration and circuit formation (Cancedda et al., 2007; Manent et al., 2005; Wang and Kriegstein, 2011). It has been also shown that GABAA receptors may have a dual role on radially migrating neurons by acting as a
migration-promoting signal in lower layers and as a STOP signal in upper cortical layer, cortical plate (Luhmann et al., 2015).
In the adult, GABAA receptors are expressed by the neuronal progenitors in both
neurogenic regions: SGZ of the DG and SVZ-OB pathway. The role of GABAA receptors
on the migration of neuronal precursors in the SGZ has not been yet explored, mainly because of very short migration distance that these cells propagate radially, along the processes of radial glia (Bond et al., 2015; Bordey, 2007). Interestingly, it has been recently revealed that these cells migrate much larger distances tangentially, along the blood vessels (Sun et al., 2015). Since GABAergic signaling plays an important role in the vasculature-mediated migration of neuronal precursors in the SVZ-OB pathway (Snapyan et al., 2009), it is conceivable that GABA may also modulate tangential migration of neuronal precursors in the adult SGZ. In the adult SVZ-OB pathway, activation of GABAA receptors on the
migrating neuroblasts reduces the speed of cell migration (Bolteus and Bordey, 2004; Snapyan et al., 2009). GABAA receptors are also expressed by the astrocytes that ensheath
the chains of migrating neuroblasts (Lois et al., 1996; Luskin, 1998). Activation of GABAA
receptors on astrocytes induces insertion of TrkB, a high affinity BDNF receptors, on the plasma membrane which traps vasculature-derived BDNF and leads to the entry of migratory cells into the stationary phase (Snapyan et al., 2009).
1.2.1.3. Neuronal differentiation and maturation
GABAergic signaling controls cell differentiation by regulating expression of several key proneuronal genes (Bertrand et al., 2002; Hevner et al., 2006). For example, application of GABA increases expression of NeuroD (a basic helix-loop-helix transcriptional factor) which is a downstream regulator of neuronal differentiation (Schwab et al., 2000). In addition to promoting differentiation towards neuronal phenotype, GABAergic signaling, which operates before the glutamatergic one, regulates neurite outgrowth and the level of dendritic arborization (Khazipov et al., 2001; Tyzio et al., 1999). In adults, GABA also promotes dendritic development and synaptic integration of adult-born neuronal