HAL Id: hal-01740067
https://hal.sorbonne-universite.fr/hal-01740067
Submitted on 21 Mar 2018HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Cosmic origin of the chemical elements rarety in nuclear
astrophysics
Elisabeth Vangioni, Michel Cassé
To cite this version:
Elisabeth Vangioni, Michel Cassé. Cosmic origin of the chemical elements rarety in nu-clear astrophysics. Frontiers in Life Science , Taylor and Francis, 2017, 10 (1), pp.84-97. �10.1080/21553769.2017.1411838�. �hal-01740067�
Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=tfls20
Frontiers in Life Science
ISSN: 2155-3769 (Print) 2155-3777 (Online) Journal homepage: http://www.tandfonline.com/loi/tfls20
Cosmic origin of the chemical elements rarety in
nuclear astrophysics
Elisabeth Vangioni & Michel Cassé
To cite this article: Elisabeth Vangioni & Michel Cassé (2017) Cosmic origin of the chemical elements rarety in nuclear astrophysics, Frontiers in Life Science, 10:1, 84-97, DOI:
10.1080/21553769.2017.1411838
To link to this article: https://doi.org/10.1080/21553769.2017.1411838
© 2018 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Published online: 10 Jan 2018.
Submit your article to this journal
Article views: 96
View related articles
VOL. 10, NO. 1, 84–97
https://doi.org/10.1080/21553769.2017.1411838
Cosmic origin of the chemical elements rarety in nuclear astrophysics
Elisabeth Vangioniaand Michel CassébaInstitut d’Astrophysique de Paris, Université Pierre & Marie Curie – Paris VI, CNRS-UMR 7095, Paris, France;bSorbonne Universités,
Institut Lagrange de Paris, Paris, France
ABSTRACT
We perceive a world of great diversity but numerous things are composed of about a hundred dif-ferent chemical elements, among them are hydrogen, carbon, nitrogen, oxygen, iron, and uranium. These elements are combined with one another in a multitude of ways to produce complexity of all objects. However, there are only three nucleosynthetic astrophysical sites: (i) big bang nucleosynthe-sis, where hydrogen and helium are produced; (ii) stars, where all elements from carbon to uranium are synthesized and (iii) interstellar medium in galaxies where lithium (a part of), beryllium and boron are made by non-thermal collisions between cosmic rays and interstellar matter. The origin of the atoms is now well understood. It is one of the greatest astrophysical discovery in the twentieth cen-tury. All the elements in the Mendeleev table, and specifically the atoms of life: carbon, nitrogen and oxygen, come from the work of all generations of stars in galaxies. Presently, after 13.8 Gyr, atomic matter in the universe is composed of 70% hydrogen, 28% helium and only about 2% by mass, of all the other elements. Complex (and also some specific light) atoms are rare in the Universe.
ARTICLE HISTORY Received 7 August 2017 Accepted 23 November 2017 KEYWORDS Astrophysics; nucleosynthesis; cosmology; chemical evolution
1. Matter in the universe
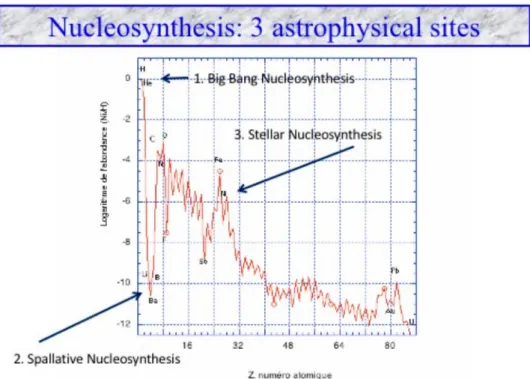
From matter of the chemists to matter of the astrophysi-cists. Three sites of nucleosynthesis (synthesis of the nuclei of atoms).
The well-known Mendeleev table of elements is dis-played in Figure1. It exhibits the diversity of atoms. In addition, Figure2shows quantitatively the abundance of elements in the solar system with respect to their atomic number (number of protons).
In general, the more complex is an atom, the less abundant it is. Moreover, nuclei with even number of protons are more abundant than odd ones. More gen-erally, even–even nuclei (with even number of protons and even number of neutrons) are more frequent than even–odd elements that are in turn more common than odd–odd ones. This is due to the additional sta-bility offered by pairing of protons on the one side and neutrons on the other side.
Indeed, a lone nucleon, affected by a lack of bond-ing, destabilizes its environment. This is reflected by the binding energy per nucleon which is higher for even–even nuclei. The spikes in Figure2show clearly
CONTACT Elisabeth Vangioni vangioni@iap.fr Institut d’Astrophysique de Paris, Université Pierre & Marie Curie - Paris VI, CNRS-UMR 7095, 98 bis, Bd
Arago, 75014 Paris, France
this trend. Note that iron nucleus has the highest bind-ing energy per nucleon, this is why it is relatively abun-dant (peak in Figure2). Based on this trend, we will describe the origin and evolution of atomic matter in the Universe.
In Sections 2and3, we present a brief overview of the big bang theory and baryogenesis (genesis of pro-tons and neutrons), Sections4and5will be devoted to the big bang nucleosynthesis (BBN) and non-thermal nucleosynthesis, respectively. In Section6, we describe the nucleosynthesis in stars and the cosmic evolution of matter in the Universe. We conclude in Section7.
2. The big bang theory
Atoms are rare in the Universe, ordinary matter repre-sents only 5% of the total substance of the Universe. 95% (dark matter and dark energy) content of the cosmos is unknown.
There are presently three observational evidence (pillars) in favor of the big bang model: (i) the expan-sion of the Universe, the galaxies fly apart according to
© 2018 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure 1.The well-known Mendeleev table corresponds to the matter of chemists. All chemical elements are included in this table, from hydrogen to heavy elements such as uranium. Heavier elements that are also shown have been synthesized in the laboratory. A box defines an element, it is characterized by the number of protons in the nucleus of the atom, Z. (Copyright, Ministère de la Recherche, France).
Figure 2.Astrophysical matter in the Universe. The abundances (normalized to hydrogen) are displayed with respect to the atomic number Z (number of protons).There are three astrophysical sites for nucleosynthesis (arrows); hydrogen and helium from big bang, carbon to uranium from stars and lithium, beryllium and boron from collisional processes between cosmic rays and interstellar matter, so-called spallative nucleosynthesis. Uranium is the least abundant element in the solar system.
Figure 3.The law of Hubble is the first pillar of the big bang theory. It corresponds to the fact that relative velocities between galaxies are proportional to their distance. This is interpreted as a direct observation of the expansion of the Universe.
the Hubble law, H is the Hubble constant (67.5 km/s and by Megaparsec, a parsec corresponding to 3.08× 1013km): the expansion speed v is proportional to the distance between galaxies, D (v = H × D) (Figure3), (ii) the Cosmic Microwave Background (CMB) radi-ation, the fossil photons are set free when the Uni-verse became transparent to its own light. They are present in the whole Universe but, because of the expansion, have cooled down to a temperature of 2.73 K. These two pillars are observational. (iii) Primor-dial or BBN is a theoretical one but firmly established, which reproduces the abundance of cosmological light chemical elements over 9 orders of magnitude (Coc et al.2014,2015).
The study of the beginning and the evolution of the Universe is one of the most fascinating subjects in modern cosmology. This section provides a brief overview of the early Universe.
The Universe was born in a ‘big bang’ at a moment when the density and the temperature were (almost) infinite, in the most symmetric state. At that time, all fundamental physical forces such as gravitational, electromagnetic, strong and weak ones were united. Due to the rapid expansion of the Universe (the first pillar), the temperature dropped quickly, and the fun-damental forces were separated. At about 10−36 sec-ond after the big bang, the Universe went through a very short and rapid expansion called inflation, during the time range, 10−35 to 10−30 second, the dimen-sion of the Universe was multiplied by an enormous
factor of at least 1026, even to 10100, due to probably the separation of the strong interaction. Cosmic infla-tion is thought to be responsible for the remarkable degree of homogeneity seen in the present Universe at a large scale, and at the same time of the structure formation in the Universe. On the one hand, infla-tion stretched space and eliminated its defects and on the other hand, the quantum fluctuation of the infla-tion (the field that drives inflainfla-tion) led to the seeds of galaxies .
A few minutes later, atomic nuclei started to form. Then protons and neutrons began to combine into atomic nuclei producing hydrogen, helium and a trace of lithium. BBN lasted until the temperature and den-sity of baryons (baryons correspond to normal matter, protons and neutrons) became too low for further nucleosynthesis. The elements necessary for life, such as carbon and oxygen, had not been formed at this moment.
After inflation and primordial nucleosynthesis, not much change occurred for the next hundred thou-sand years or so. The Universe continued to expand, gradually cooling off until its temperature fell to a few thousand Kelvin. At that point, its density was only 10−21g/cm3, on average.
At that time the electrons were captured by hydro-gen and helium nuclei, forming a gas of electrically neutral atoms. Prior to this moment, the Universe was a plasma. A plasma is a physical state of matter where protons, photons, and electrons are not formed as atoms but are strongly interacting.
So, about 380, 000 years after the big bang, the Universe cooled below 3000–4000 K. The photons decoupled from matter and streamed freely. This radi-ation, the CMB, was first detected by A. Penzias and R. Wilson in 1964. They won the Nobel Price in 1978. In 1989, the COBE mission had observed CMB in great details (Figure 4) and another Nobel Prize in 2006 was awarded to J. Mather and G. Smoot (Smoot2000) for the discovery of the anisotropy of the CMB radiation.
More recently, observational CMB results from the two satellites Wilkinson Microwave Anisotropy Probe (WMAP) and Planck confirm inflationary cosmol-ogy and determined the cosmological parameters with
an unprecedented precision (Ade et al. 2016;
Hin-shaw et al.2013). The small temperature/density vari-ations detected on the sky map are the ‘seeds’ that will grow into the galaxies and galaxy clusters seen
Figure 4.This is the CMB pillar of the big bang theory. Data points come from the COBE satellite.The black body curve predicted by the big bang theory (curve) and that observed in the CMB (points) are perfectly superposable .
in the present Universe. These successful experiments lead to conclude that the Universe contains about only 4.9% of baryons (atoms), 26.6% of dark mat-ter and 68.4% of dark energy. Dark matmat-ter and dark energy have opposed gravitational effects. Dark mat-ter has an attractive gravitational effect, whereas dark
energy has a repulsive (anti-gravitational) one. The precise proportions of the different cosmic ingredi-ents are shown in Figure 5. The intrinsic properties of dark matter and dark energy remain poorly under-stood. Anyway, inflationary models, the expansion of the Universe, the CMB and BBN are the foundation of modern cosmology. The history of the Universe from the inflation up to now is depicted in Figure6.
The Universe then entered the cosmic dark ages because there were no stars and no light from stars. Only hydrogen and helium clouds were present at that time. A few hundred million years after the big bang, matter collapsed into minihalos which became the birth sites for the first stars since they provided gravi-tational wells that retained gas to form stars. The light from the first stars ended the dark ages. These first stars forged the first complex nuclei as carbon and oxygen. Thus, they play a crucial role in the global evolution of the Universe.
To summarize, at the beginning, all space and time, all energy and matter emerged from symmetric condi-tions that we can guess today only in abstract equa-tions. The history of the Universe from this earliest instant has been a saga of ever-growing asymmetry and
Figure 5.Content of the Universe. Critical density (ρc) of the Universe corresponds to the value predicted by the theory of inflation. Atoms represent only 4.9% of the Universe content. Among them the visible atoms (gas and galaxies) are even more rare (0.003). Most of baryons (hydrogen and helium) stay in the intergalactic medium. Fossil photons (CMB) represent a tiny fraction of the energy density of the Universe though being abundant.
Figure 6.Cosmic timeline. Evolution of cosmic structure from the big bang up to now. After the dark ages, the first stars appeared about 400 millions years after the big bang and then galaxies started to develop. Recent observations suggest that the expansion of the Universe is accelerating due to dark energy. (Credit: NASA/WMAP Science Team).
increasing complexity. As space expanded and Uni-verse cooled, particles began aggregating and struc-tures started forming from this ultrahot plasma. Eventually clusters and galaxies, stars, planets and even life itself emerged. In at least one corner of the cosmos where conditions were ideal, intelligent beings evolved to the point where they could begin to comprehend these fantastic origins. Above all, from the big bang on, the Universe is continuously evolving.
3. Baryogenesis
Due to matter/antimatter asymmetry (1+ 109 protons
compared to 109antiprotons), only one proton for 109
photons remained after annihilation.
The theoretical prediction of antimatter made by Paul Dirac in 1931 is one of the most impressive discover-ies (Dirac1934). Antimatter is made of antiparticles that have the same (e.g. mass) or opposite (e.g. electric charge) characteristics but that annihilate with parti-cles, leaving out at the end mostly photons. A symme-try between matter and antimatter led him to suggest that ‘maybe there exists a completely new Universe made of antimatter’. Now we know that antimatter exists but that there are very few antiparticles in the Universe. So, antiprotons (an antiproton is a proton but
with a negative electric charge) are too rare to make any macroscopic objects.
In this context, the challenge is to explain why anti-matter is so rare (almost absent) in the observable Universe.
Baryogenesis (i.e. the generation of protons and neutrons AND the elimination of their corresponding antiparticles) implying the emergence of the hydrogen nuclei is central to cosmology. Unfortunately, the prob-lem is essentially unsolved and only general conditions of baryogenesis were well posed by A. Sakharov a long time ago (Sakharov1979).
Baryogenesis requires at least departure from ther-mal equilibrium, and the breaking of some fun-damental symmetries, leading to a strong observed matter–antimatter asymmetry at the level of 1 proton per 1 billion of photons.
Mechanisms for the generation of the matter–anti matter strongly depend on the reheating tempera-ture at the end of inflation, the maximal temper-ature reached in the early Universe. Forthcoming results from the Large Hadronic Collisionner (LHC) at CERN in Geneva, BABAR collaboration, astrophys-ical observations and the Planck satellite mission will significantly constrain baryogenesis and thereby pro-vide valuable information about the very early hot Universe.
4. Big bang nucleosynthesis
There are a few atoms per unit volume in the Universe, only a few 10−31g/cm3 on average. Moreover there are
only five atoms of lithium-7 synthesized for 1010atoms
of hydrogen.
In the standard big bang cosmology, none of the chemical elements existed from the very beginning. Three were synthesized during the first minutes by nuclear reactions. About one second after the big bang, the Universe had expanded and cooled to the point where nuclear physics plays its role. The indi-vidual protons and neutrons in the primordial soup started sticking together to make heavier, more com-plex nuclei. Before that moment it was just too hot, with a temperature that exceeded 10 billion Kelvin, about a million times hotter than the surface of the Sun today.
In a short period called the ‘Era of Nucleosynthesis’, the Universe became a thermonuclear reactor where nuclei of the lightest elements did form. All present hydrogen and deuterium, almost all present helium and a very few lithium (but a significant fraction of the present day ones) were created. The remnants of these light elements that are detectable today in the cosmos give us an important information about those early conditions and prove that our Universe indeed began in a very hot, condensed state. They can also be used to make a good estimate of the average density of normal matter today.
Just before to the Era of Nucleosynthesis and after the baryogenesis phase, the Universe was a hot soup of mostly electrons and positrons, photons and neutri-nos. However, for every proton or neutron, there were at least a billion photons. The conditions of the Uni-verse, say the kinetic energies of particles, during this epoch was not too different from those studied here on Earth by nuclear physicists, so its primordial evolution is well understood. We have the greatest confidence that we can make valid statements about the cosmol-ogy of this epoch based on our knowledge of nuclear physics.
Let us give a closer look.
A key to understand the physics of this period is the concept of thermal equilibrium. This phenomenon represents a state of balance between opposing nuclear reactions. When a system is in thermal equilibrium, its temperature alone determines the relative quantities of the different interacting species, here nuclei, that are
present. When the Universe was in thermal equilib-rium its temperature governed the ratio of neutrons to protons, n/p.
Before one second, the temperature was above ten billions kelvin, the number of neutrons and protons were roughly equal, because these particles of about the same mass were easily converted into each other. After ten seconds, the temperature had fallen to three billions kelvin, and the reactions producing neutrons from protons were slowing down and the ratio n/p had fallen to 1/6. As the Universe kept expanding and cool-ing, n/p continued to drop because of neutron decay. So after about two hundred seconds, it was only 1/7.
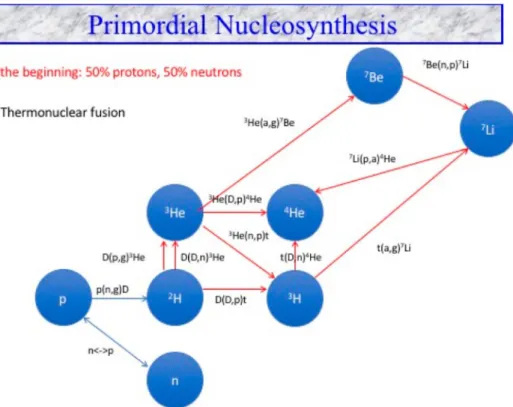
This is when the temperature of the Universe had dropped to the point where proton and neutron merged together to form a deuterium nucleus D, which is the heavy isotope of hydrogen, the starting point of further nucleosynthesis.
Then, two neutrons plus a proton made a nucleus of tritium, two protons plus a neutron made3He or two protons and two neutrons made4He, the most stable of nucleus of all the light element is4He.
Figure 7 presents the nuclear network of BBN.
There are 12 nuclear reactions which are responsible for the production of these elements via thermonu-clear fusion.
At the end of BBN, essentially all the baryons in the Universe existed either freely as single protons or were trapped inside of 4He nuclei. Some tiny residues of deuterium D, tritium and3He remained, along with a trace of7Li, which has three protons and four neutrons in its nucleus (for a review, see Fields et al.2016).
Figure 8 displays the evolution of the different
species (from H to Li) and temperature as a function of time. One sees that the abundances are stabilized after 10,000 seconds or below 108Kelvin.
The final primordial abundances, normalized to hydrogen, H, are the following (Coc et al.2015):
D/H = 2.45 ± 0.05 × 10−5, Li/H = 5.61 ± 0.26 × 10−10.
Traditionally helium is expressed in mass fraction, so,
Yp= 0.2484 ± 0.0002.
On the observational side, to derive the most primi-tive abundances of these elements, one has to extract
them from observations of astrophysical sites which are thought to be nonevolved and primitive.
Deuterium is a very fragile isotope (since it is an odd–odd nucleus), easily destroyed in stars. Its most primitive abundance is determined from the observa-tion of clouds present on the line of sight of distant quasars (very distant primeval galaxies) (see Cooke et al.2014,2016,2017). Deuterium is only produced during the BBN. Consequently, it is a very good cos-mological tracer.4
He is produced in BBN and by stars. Its prim-itive abundance is deduced from observations in ionized hydrogen regions of compact blue galax-ies, rather primitive. 4He mass fraction is obtained from the extrapolation to zero metallicity (in astro-physics, metallicity represents all elements heavier than helium) (see Aver et al.2013,2015).
The primitive lithium abundance is deduced from observations of low metallicity stars in the halo of our Galaxy where the lithium abundance is almost inde-pendent of metallicity (Spite and Spite1982). This Li abundance is interpreted as corresponding to the BBN
7Li yield.
So, astronomical observations lead to: D/H =
(2.527 ± 0.03) × 10−5 (Cooke et al. 2017), Li/H =
(1.58 ± 0.31) × 10−10(Sbordone et al.2010). Helium, in mass fraction, Yp= 0.2449 ± 0.0040 (Aver et al.
2015)
There is a global agreement over a range of nine orders of magnitude between spectroscopic observa-tions and theoretical values deduced from CMB data. However, regarding lithium (Figures9and10), there
is a discrepancy by of a factor of ∼ 3–5. The
the-oretical abundance is larger than the observational one. Many studies are been devoted to this so-called ‘lithium problem’. At the present time, this discrepancy is not explained.
Even though the direct detection of primordial CNO isotopes seems highly unlikely with the present observational techniques, it is important to better esti-mate their BBN production. The value of the initial
CNO abundance is estimated to be as low as(0.5 −
3) × 10−15(in number of atoms relative to hydrogen, CNO/H) (Coc et al. 2015, 2012). This is very low. The astrophysical production site of these elements is clearly stars.
To summarize, BBN predicts the primordial abun-dances of the light cosmological elements:4He, D,3He
and 7Li that are produced during the first 20 min
after the big bang when the Universe was dense and
Figure 7.Network of nuclear reactions involved in BBN. There are 12 nuclear reactions responsible for the production of hydrogen, helium and lithium.
10-12 10-11 10-10 10-9 10-8 10-7 10-6 10-5 10-4 10-3 10-2 10-1 1 102 103 104 105 10 -12 10 -11 10 -10 10 -9 10 -8 10 -7 10 -6 10 -5 10 -4 10 -3 10 -2 10 -1 1 102 103 104 105 Time (s) Mass fraction 7Li 7Be 3 He 4 He 3 H 2 H n 1 H
Figure 8.Evolution of the mass fraction of light isotopes ( hydro-gen, deuterium, helium and lithium as a function of time (in sec-onds). The synthesis of these isotopes stops at about 104seconds. Lithium is extremely rare, 1 for 10 billions compared to hydrogen, (from Coc et al.2015).
hot enough for nuclear reactions to take place. There is an overall agreement between the calculated and observed abundances, except for the7Li.
Thanks to this BBN calculation we determined the total quantity of atoms in the Universe: only 4.9% of the total cosmological substance. Presently, primordial nucleosynthesis remains an invaluable tool for probing the physics of the early Universe.
5. Collisional nucleosynthesis: origin and evolution of Lithium-Beryllium-Boron
Lithium, Beryllium and Boron are extremely rare in the Universe; only one lithium, one beryllium and one boron atom for about 109, 1011and 109hydrogen atoms
respectively.
The origin and evolution of the rare elements – Lithium–Beryllium–Boron (LiBeB) – are a crossing point between different astrophysical fields: spectr-oscopy, non-thermal nucleosynthesis, big bang and
0.22 0.24 0.26 WMAP Ωb h2 Mass fraction 4
He
10-2 10-6 10-5 10-4 10-3 3 He/H, D/HD
3He
10-10 10-9 1 10 7 Li/H 7Li
Planck 1010Figure 9.Abundances of the cosmological elements as a func-tion of the ratio baryons/photons,η, for three neutrino fami-lies. The vertical areas correspond (i) to the CMB baryonic den-sity observations coming from satellites WMAP (dot) and Planck (solid). The horizontal areas (hatched and dotted lines) repre-sent the different adopted observational abundances. The dash-dotted lines correspond to4He calculated with a different value of the number of neutrino families derived from the Planck mission (from Coc et al.2014).
stellar nucleosynthesis and finally theory of galactic evolution.
Indeed, they are rare because they are fragile and they are destroyed in stars.
A glance to the abundance curve (Figure2) suffices to capture the essence of the problem: a gap separates He and C. At the bottom of this precipice rests the trio LiBeB. They are characterized by the simplicity of their nuclear structure (6–11 nucleons) and their scarcity in the Universe.
Due to the expansion of the Universe, the BBN
has stopped at A= 7, and primordial thermonuclear
fusion has been unable to proceed efficiently beyond lithium and the calculated primordial 6Li, Be and B abundances are negligibly small (Figure11).
Figure 10.Li/H of old stars in the galactic halo as a function of [Fe/H] (bottom scale, which is referred to the solar abundance, 0 corresponds to the solar value,−3 corresponds to 1/1000 of the solar value etc., astrophysical conventions : [Fe/H] = log(Fe/H) of the star – log(Fe/H) of sun). The time scale is also shown, note that these stars are very old (about 12–13 Gyrs old). Primitive lithium abundance is deduced from observations of low metal-licity stars (black points) in the halo of our Galaxy. There is a dis-crepancy (about a factor 3 to 5) between the value deduced from these observed spectroscopic abundances and the one calculated (horizontal line).
The production and evolution of LiBeB are due to the interaction of Cosmic Rays (CR are composed essentially of very rapid protons which diffuse in the galactic disk) with the interstellar medium (ISM, gas between stars; gas and stars compose a galaxy).
The result of a nuclear collision depends on the composition of the beam (CR) and the target (for example, carbon and oxygen in the ISM), and also on the relative velocity of the projectile and the tar-get nuclei (as it can be the reverse CR vs ISM). Stellar nucleosynthesis implies low energies and high par-ticle densities. In contrast, spallative nucleosynthesis implies high energies (CR) and very low densities (ISM).
The energy required to sustain the CR energy den-sity is mainly supplied by supernovae (SN, exploding massive stars) which are not necessarily the sources of CR nuclei, but their acceleration agents.
Two main LiBeB producers emerge, (i) fast protons interacting with carbon, nitrogen and oxygen (CNO) in the ISM at rest and (ii) fragmentation of CO nuclei in flight on H and He in the ISM. Massive stars are
10-22 10-21 10-20 10-19 10-18 10-17 10-16 10-15 10-14 10-13 1 2 3 4 5 6 7 8 9 10 10 -22 10 -21 10 -20 10 -19 10 -18 10 -17 10 -16 10 -15 10 -14 10 -13 1 2 3 4 5 6 7 8 9 10 6 Li 9 Be 10 B 11 B CNO WMAP 2009 1010 N(X)/N(H)
Figure 11.Abundances by number of atoms relative to H for
6Li,9Be,10B,11B and CNO (and uncertainties) as a function ofη,
baryons/photons ratio. We see that these isotopes are extremely rare at the end of BBN (from Coc et al.2012).
able to furnish freshly synthesized C and O and accel-erate them via the shock waves they induce in their surroundings. This mechanism is related to superbub-bles (excavated by the winds and explosion of massive stars).
These different mechanisms are included in a global evolutionary model to follow the whole evolution of each element, (for a review see Vangioni2012).
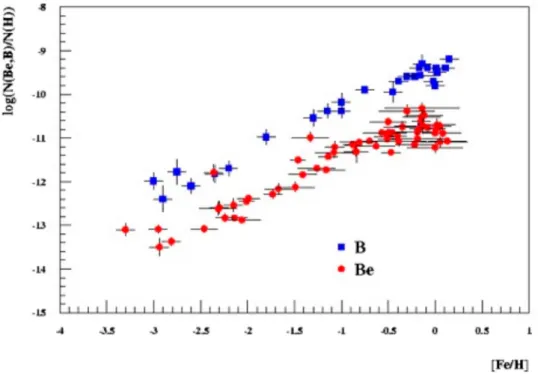
Observations of BeB are shown in Figure12taking the iron abundance in stars as an evolutionary index ([Fe/H] = 0 corresponding to the iron abundance of the solar system).
To summarize, LiBeB are exceptional since they are both simple and rare. Typically, in the Solar System:
Li/H =2× 10−9, B/H =7× 10−10, Be/H = 2.5 × 10−11
whereas carbon and oxygen are C/H = 3.5 × 10−4
and O/H = 8.5 × 10−4. As shown in the following, stellar nucleosynthesis forge the whole variety of nuclei from carbon to uranium.
Figure 12.Same as Figure10, but for Be and B. We note the very low abundance of these elements compared to hydrogen (between 10−14to 10−9).
6. Nucleosynthesis in stars and cosmic evolution
Stars are the agents of complexification of matter. After 13.8 Gyr and many successive generations of stars in galaxies, complex elements (from carbon to uranium) represent only about 2 % of atoms by mass.
Another frontier of modern cosmology is understand-ing the end of the cosmic dark ages, when the first luminous objects (stars and galaxies) reshaped the primordial Universe into the current Universe. The advancement of supercomputing power in the last decade has allowed us to start investigating the forma-tion of the first stars by modelling the relevant physical processes. The first star formation scenarios suggest that they could have been massive, with a typical mass
range of about 40–100 solar masses (1 solarmass=
2× 1033 g). Some of them died in energetic SN explo-sions. These first SN have dumped considerable energy and spread some complex elements to the interstellar medium.
The formation of the first stars transformed the sim-ple early Universe into a highly complicated one. The first stars made from the hydrogen and helium left from the big bang are called the Population III (Pop III) stars. They are rare, massive, with a short lifetime (a few millions years old).
At present time, there is no direct detection of Pop III stars. Nevertheless, the observation of present-day stars may provide us hints to study the Pop III star formation in the past (Vangioni-Flam et al.2000).
The standard cosmological model offers a fun-damental theory for the large-scale formation and suggests that the cosmic structure has formed in a hierarchical manner. So, the first generation of small galaxies was likely well in place 400 millions years after the big bang. Galaxies began then a phase of merg-ing and coalescence with other galaxies, whereby they built up from masses of several millions solar masses to hundred billions of solar masses.
Complexification of matter takes place in stars, gen-erations after gengen-erations.
So, all the heavy elements – as carbon, nitrogen, oxygen, silicon, aluminum, copper and iron – were formed afterwards by thermonuclear fusion in the core of stars.
In a galaxy 90% of stars are like our Sun or less mas-sive, the other 10% being more massive. Low mass stars like our Sun cook hydrogen nuclei to make helium nuclei and then transform helium nuclei into C and N. This elaborated matter is dispersed into the ISM through the so-called planetary nebula phase.
The more massive and less numerous ones, hav-ing exhausted their hydrogen, burn helium to make
Figure 13.Supernova SN87a in the Large Magellanic Cloud (a satellite galaxy of the Milky Way located at 163 000 light years) has been observed in 1987 with all available detectors and telescopes (bottom before explosion, above after explosion). This is really the modern time supernova. This observation, in agreement with the-oretical models of explosive nucleosynthesis, has strengthened our confidence in them, specifically concerning the synthesis of iron. (Copyright Anglo Australian Observatory).
carbon, oxygen and a host of heavier elements. These elements are spewed into space by stellar explosions, supernovae, like SN1987a that appeared in the Large Magellanic Cloud on 23 February 1987 (Figure13).
Note that the most heavy elements (with atomic numbers higher than iron) are the rarest (see Figure1). To synthesize these elements, we identify three pro-cesses and three categories. (For a review see Arnould et al.2007.) Indeed, three different mechanisms s, r, and p processes are called for to account for the pro-duction of these three types of stable elements, rapid capture of neutrons (r) slow capture of neutrons (s) and capture of protons (p). The first process corresponds to rapid neutron captures by heavy nuclei (after iron). These captures must be rapid to undergo radioactive decay. This process occurs in high density of free neu-trons as for example a binary neutron star merger. On the other hand, the s process is the other predomi-nant mechanism for the production of heavy elements, it occurs within intermediate mass stars, where the neutron flux is sufficient to cause reactions. Taken together, the r and s processes account for the major-ity of elements heavier than iron. The third p process can occur in supernova explosions and is proposed to explain the origin of heavy proton-rich elements.
Yet, all the elements heavier than helium make up less than 2% of the visible matter in the Uni-verse, complex nuclei are rare. The other 98% is the
Figure 14.The nuclear chain leading to carbon formation.
primordial hydrogen and helium left over after the BBN phase.
Origin of carbon: three helium nuclei merge to syn-thesize one carbon nucleus.
The assumption that carbon is built inside stars through thermonuclear fusion of three helium (called
α nuclei, 2 protons plus 2 neutrons) nuclei has
been proposed by Edwin Salpeter in the early 1950 (Salpeter 1952). The triple alpha reaction (3α giv-ing12C) is through to operate in red giant stars at a temperature of about 100 millions Kelvin.
Edwin Salpeter showed that a resonant metastable state of 8Be nucleus enables red giant stars to burn helium to carbon at rather low central temperatures, 2× 108Kelvin. Fred Hoyle followed this way with the insight that a new, previously unknown resonance in
12C would further increase the amount of carbon from 8Be+ α reaction and, in particular, allows carbon to
be produced at the still lower temperature 108Kelvin, estimated to be that of red giant stars (Figure14).
As shown in Figure15, the excited level of12C at 7.65 MeV just above the 7.3667 MeV energy of8Be+
α, allows rapid enough reactions for carbon to form
before the unstable8Be decays (with a the lifetime of 10−16second).
On the other hand, the level of16O at 7.1187 MeV lies just below that of12C+ α at 7.1616 MeV; if it were
Figure 15.Nuclear levels of carbon and beryllium nuclei and the nuclear chain of carbon synthesis.C∗is the excited level of carbon (from Ekstrom et al.2010).
higher by just 0.043 MeV, carbon would be quickly destroyed to oxygen.
This episode marked an important milestone in the early development of nuclear astrophysics. Since carbon and oxygen are so important to living
Figure 16.Cosmic evolution of atoms in the Universe. Primitive clouds of gas are composed of hydrogen and helium coming from big bang. Inside them, gravitational contraction induces star for-mation; the synthesized complex elements are rejected into the interstellar medium through essentially SN explosions.
molecules, this system is often cited as an example of anthropic fine tuning.
To summarize, freshly synthesized He nuclei com-ing from hydrogen burncom-ing, fuse with protons or other helium nuclei and helium burning can start. A signifi-cant amount of8Be builds up in spite of the very short lifetime allowing the addition of a thirdα particle to produce a stable12C nucleus.
Consequently, these so-called nuclear resonances increase considerably the combination probability of anα particle with a8Be to form a carbon. In this con-text, a fine tuning nuclear situation has permitted the existence of carbon and later of life.
7. Conclusion
Global economy in the cosmos: only 5% of the content of the Universe are atoms, and among them, only 2% are more complex than hydrogen and helium.
During the evolution of galaxies, nucleosynthesis takes place mainly in massive stars which release mat-ter enriched in heavy elements into the inmat-terstellar medium when they explode as supernovae (Figure16). Accordingly, the abundances of heavy elements in the gas increase with time. The observed abundance
of metals in stars (formed in the ISM) is an indication of their age: the older, the lower the metallicity.
To conclude, we started from a symmetric state of the Universe, which evolved though symmetry break-ing and subsequent complexification. At the startbreak-ing point, particles and antiparticles were of equal num-ber. Then, due to a slight asymmetry not completely understood, matter exceeded antimatter at the level of one over one billion. At that time, the Universe was bathed with protons, neutrons, electrons, photons and neutrinos.
Then, primordial nucleosynthesis occurred pro-ducing mainly hydrogen and helium with a trace of lithium. A long time after (about 400 millions yrs) the first massive stars were born producing the first CNO atoms in small quantities via SN explosions.
The following SN all along the galactic evolution accelerate nuclei which collide with the ISM nuclei and produce the very rare light nuclei LiBeB particularly fragile due to their nuclear structure. It is clear that nuclear physics is the key to the understanding of the evolution of ordinary matter in the Universe.
Carbon is a fascinating nucleus since it is the basis of life and it is the product of an extraordinary energy coincidence at the level of nuclei.
It is stricking that the process of complexification of matter has to overcome these different obstacles; one sees that the result is very inefficient since the present mass fraction of atoms heavier than helium is only about 2% of about 5% of the baryonic matter in the Universe. This shows the extraordinary quality of rarety regarding the ordinary matter.
Even rarer is life since, at least on the Earth, the biomass is only of the order of 1.5× 10−10the mass of our planet!!
Acknowledgments
The authors thank warmly the referee for his/her useful improvements.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work made in the ILP LABEX (under reference ANR-10-LABX-63) was supported by French state funds managed by the
ANR within the Investissements d’Avenir programme under reference ANR-11-IDEX-0004-02 and by the ANR VACOUL, ANR-10-BLAN-0510.
References
Ade PAR, Aghanim N, Arnaud M, Ashdown M, Aumont J, Bac-cigalupi C, Banday AJ, Barreiro RB, Bartlett JG, Bartolo N, et al.2016. [Planck Collaboration], Planck 2015 results. XVI. Cosmological parameters. Astron Astrophys. 594:A13. Arnould M, Goriely S, Takahashi K. 2007. The r-process of
stellar nucleosynthesis: astrophysics and nuclear physics achievements and mysteries. Phys Rep. 450:97– 213. Aver E, Olive KA, Porter RL, Skillman ED.2013. The
primor-dial helium abundance from updated emissivities. J Cosmol Astropart Phys. 11:17.
Aver E, Olive KA, Porter RL, Skillman ED.2015. The effects of He I λ 10830 on helium abundance determinations. J Cosmol Astropart Phys. 07:11.
Coc A, Goriely S, Saimpert M, Vangioni E.2012. Standard big bang nucleosynthesis up to CNO with an improved extended nuclear network. Astrophys J. 744:158.
Coc A, Petitjean P, Uzan JP, Vangioni E, Descouvemont P, Illiadis C, Longland R. 2015. New reaction rates for improved primordial D/H calculation and the cosmic evo-lution of deuterium. Phys Rev D. 92:123526.
Coc A, Uzan JP, Vangioni E.2014. Standard big bang nucle-osynthesis and primordial CNO abundances after Planck. J Cosmol Astropart Phys. 10:50.
Cooke RJ, Pettini M, Jorgenson RA, Murphy MT, Steidel CC.
2014. Precision measures of the primordial abundance of deuterium. Astrophys J. 781:31.
Cooke RJ, Pettini M, Nollett KM, Jorgenson RA.2016. The primordial deuterium abundance of the most metal-poor Damped Lyman-? System. Astrophys J. 830:148.
Cooke R, Pettini M, Steidel CC.2017. A one percent deter-mination of the primordial deuterium abundance. arXiv: 1710.11129.
Dirac PA,1934. Discussion of the infinite distribution of elec-trons in the theory of the positron. Proc Cambridge Philos Soc. 30:150.
Ekstrom S , Coc A, Descouvemont P, Meynet G, Olive KA, Uzan J-P, Vangioni E.2010. Effects of the variation of funda-mental constants on Population III stellar evolution. Astron Astrophys. 514:A62.
Fields BD, Molaro P, Sarkar P.2016. Big bang nucleosynthesis, in online version [Particle Data Group Collaboration]. Chin Phys C. 40:100001.
Hinshaw G, Larson D, Komatsu E, Spergel DN, Bennett CL, Dunkley J, Nolta MR, Halpern M, Hill RS, Odegard N, et al.
2013. Nine-year Wilkinson Microwave Anisotropy Probe (WMAP) observations: cosmological parameter results. Astrophys J Suppl Ser. 208:19.
Sakharov AD.1979. Baryon asymmetry of the universe. Zhur-nal Eksperimental’noi i Teoreticheskoi Fiziki. 76:1172–1181. Salpeter EE.1952. Nuclear reactions in stars without hydrogen.
Sbordone L, Bonifacio P, Caffau E, Ludwig H-G, Behara NT, González Hernández JI, Steffen M, Cayrel R, Freytag B, Van’t Veer C, et al.2010. The metal-poor end of the Spite plateau. I. Stellar parameters, metallicities, and lithium abundances. Astron Astroph. 522:A26.
Smoot GF. 2000. CMB anisotropy experiments. Phys Rep. 333–334:269–308.
Spite F, Spite M.1982. Abundance of lithium in unevolved halo stars and old disk stars: interpretation and consequences. Astron Astrophys. 115:357.
Vangioni-Flam E, Cassé M, Audouze J.2000. Lithium-bery-llium-boron: origin and evolution. Phys Rep. 333:365–387. Vangioni E.2012. Cosmic chemical evolution with




![Figure 10. Li/H of old stars in the galactic halo as a function of [Fe/H] (bottom scale, which is referred to the solar abundance, 0 corresponds to the solar value, − 3 corresponds to 1/1000 of the solar value etc., astrophysical conventions : [Fe / H] = l](https://thumb-eu.123doks.com/thumbv2/123doknet/14800723.606109/11.914.485.811.72.499/galactic-function-referred-abundance-corresponds-corresponds-astrophysical-conventions.webp)