Publisher’s version / Version de l'éditeur:
Highway Research Board Bulletin, 275, pp. 18-31, 1961-08-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Characteristics of Kingston carbonate rock reaction
Swenson, E. G.; Gillott, J. E.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=c6df3ea9-34f8-40de-b506-fe7aab0401ba https://publications-cnrc.canada.ca/fra/voir/objet/?id=c6df3ea9-34f8-40de-b506-fe7aab0401ba
Ser
TH1
N21r
2
no. 130
c.
2
NATIONAL
RESEARCH
COUNCIL
C A N A D A
DIVISION O F BUILDING RESEARCH
CHARACTERISTICS OF KINGSTON CARBONATE ROCK REACTION
BY
E. G. SWENSON AND J. E. GILLOTT
REPRINTED FROM
HIGHWAY RESEARCH BOARD BULLETIN NO. 2 7 5 . 1 9 6 0 , P. 1 8 - 3 1 DIV R E S E A R C H PAPER N O O F T H E 'ISION O F BUILDING OTTAWA AUGUST 1961 P R I C E 2 5 C E N T S N R C 5 5 8 3
T h i s p u b l i c a t i o n i s b e i n g d i s t r i b u t e d by the Di'vision of Building R e s e a r c h of the National R e s e a r c h Council. It should not be r e p r o d u c e d i n whole o r i n p a r t , without p e r m i s
-
s i o n of t h e o r i g i n a l p u b l i s h e r . T h e D i v i s i o n would be glad to be of a s s i s t a n c e i n obtaining s u c h p e r m i s s i o n .P u b l i c a t i o n s of the D i v i s i o n of Building R e s e a r c h m a y be o b t a i n e d by m a i l i n g the a p p r o p r i a t e r e m i t t a n c e , ( a Bank, E x p r e s s , o r P o s t Office Maney O r d e r o r a cheque m a d e pay- able a t p a r in O t t a w a , to the R e c e i v e r G e n e r a l of C a n a d a , c r e d i t National R e s e a r c h Council) to the National R e s e a r c h Council, Ottawa. S t a m p s a r e not a c c e p t a b l e .
A coupon s y s t e m h a s b e e n i n t r o d u c e d to m a k e pay- m e n t s f o r p u b l i c a t i o n s r e l a t i v e l y s i m p l e . C ~ u p o n s a r e a v a i l - able i n d e n o m i n a t i o n s of
5,
2 5 a n d 50 c e n t s , and m a y b e ob- t a i n e d by m a k i n g a r e m i t t a n c e a s i n d i c a t e d above. T h e s e coupons m a y be u s e d f o r the p u r c h a s e of a l l National R e s e a r c h Council publications including s p e c i f i c a t i o n s of the Canadian G o v e r n m e n t S p e c i f i c a t i o n s B o a r d .Characteristics of Kingston Carbonate
Rock Reaction
E. G. SWENSON and J. E. GILLOTT R e s e a r c h Officers, Building Materials Section
Division of m i l d i n g R e s e a r c h
National R e s e a r c h Council, Ottawa, Canada
Reprinted from Bulletin 275 (1960)
Highway R e s e a r c h Board, Washington, D. C., USA
Characteristics of Kingston Carbonate
Rock Reaction
E.G. SWENSON*, and J . E . GILLOTT, Research Officers, Building Materials Section, Division of Building Research, National Research Council, Ottawa, Canada
THE EXCESSIVE expansion and cracking of concrete in which argillaceous dolomitic limestones from Kingston, Ontario a r e used a s aggregates, has certain unusual fea- t u r e s when compared with the effects of alkali-silica reactivity normally associated with alkali-aggregate reaction. Since the publication of preliminary studies of this case ( I ) , further r e s e a r c h in this laboratory, together with reports of another apparent- ly r e l a e d phenomenon (2, - - 3 ) has indicated that the mechanism of this reaction may possibly be different from other previously described cement-aggregate reactions.
The use of cement with sufficiently low alkali content a p p e a r s to have provided a satisfactory solution to the field problem in the Kingston a r e a . Although standard ASTM methods of test for potential alkali-aggregate reactivity failed to r e v e a l this be- havior, i t may be detected by measuring expansion of concrete prisms exposed to a highly humid atmosphere (1).
In this paper l a b o r a t o r y r e s u l t s a r e presented that show the marked differences and similarities between the characteristics of this phenomenon and those of the well-known alkali-silica reaction. Much of the data were obtained on concrete t e s t specimens and a r e , therefore, considered to be of practical value also.
GENERAL MANIFESTATIONS OF THE REACTION
The phenomenon i s characterized by excessive expansion and cracking of concrete when a high alkali cement i s used and the concrete i s exposed to a moist environment. In sidewalks and floor slabs, where the bottom side is exposed to a continuously humid atmosphere and the top side i s exposed to the weather, differential movement has pro- duced a characteristic pattern cracking (Fig. 1). In the field these cracks penetrate about two-thirds of the depth of an average slab. The a r e a s surrounded by the cracks, from 2 to 4 in. a c r o s s , appear to be relatively sound. Spalling does not appear to be characteristic of this reaction and edges a t the cracks a r e s t i l l sharp in c a s e s more than 20 y e a r s old. There i s no megascopic evidence on the surface or in t h e cracks of old o r new affected concretes of any material o r formations that a r e foreign to normal concrete.
Great variability in both r a t e and degree of expansion and cracking has occurred in field concrete where Kingston carbonate rock has been used a s coarse aggregate. In laboratory concrete, kept continuously in the curing chamber, expansion h a s reached 0.1 percent within s i x weeks. Cracking occurs at expansions of about 0.05 t o 0.10 percent. In extreme c a s e s in the field, cracking has occurred within two t o three months of placing.
Using the same reactive aggregate and a cement with low alkali content, no exces- sive expansion has occurred to date in laboratory concretes a f t e r nearly 4 y e a r s in a highly humid environment and in field concrete a f t e r two y e a r s exposure.
RESPONSE OF VARIOUS METHODS OF TEST
The failure of the standard ASTM t e s t methods to show potential deleterious alkali- reactivity in the Kingston carbonate rock has been previously reported (1).
-
Consider-able additional work with these t e s t s and certain modifications of them confirmed the e a r l i e r findings.
Mortar Bar Test
In the mortar b a r test (ASTM Designa- tion C227-52T) the Kingston rock produc- ed abnormal expansion but, on the basis of accepted c r i t e r i a for alkali-silica r e - activity (0.05 percent a t 3 months and 0.10 percent a t 6 months), this rock would be classified a s not deleteriously reactive t o cement alkalies. Typical ex- pansions a t 3 and 6 months a r e given in Table 1 for the "very reactive" and the "least reactive" from each of two major quarries. Expansions of the concrete p r i s m s , in which the rock is in the form of coarse aggregate (maximum s i z e ,
'/4
in. ) a r e much g r e a t e r in both rate and de-gree. It shouli be noted that the mortar Figure 1. Map cracking in a sidewalk in
b a r s a r e conditioned at 100 F whereas Kingston.
the concrete p r i s m s were conditioned a t 73 F and 100 percent relative humidity.
The low expansions of both m o r t a r b a r s and concrete p r i s m s a r e of the s a m e o r d e r when a low alkali cement is used.
Mortar b a r s containing very reactive aggregate were continued in the t e s t and a t the end of 39 months showed an expansion of 0.060 percent. The corresponding b a r s made with the low alkali cement had expanded 0.035 percent at this a g e . Rate and degree of expansion of the mortar b a r s were not reduced when a lower water-cement r a t i o was used. In this respect the phenomenon differs from the alkali-silica type. The very active calcined shale, when substituted f o r 25 percent by weight of the cement, reduced expansion in the m o r t a r b a r test by about the same amount a s the low alkali cement (1). It i s c l e a r that the mortar b a r t e s t will give an indication of this phenomenon but c d i f f e r e n t criterion would have to be established from that used for alkali-silica type reactions.
Quick Chemical T e s t
The quick chemical test (ASTM Designation C289-54T) was found to be inapplicable a s a method for detecting the adverse behavior of the Kingston dolomitic limestone (1). The average silica release f o r the most reactive rock was 18.4 millimoles per l i t e r ; average reduction in alkalinity was 144 millimoles per l i t e r . These values would lead one t o conclude that the aggregate i s f r e e fromdeleterious alkali-reactivity. The corresponding values for the least reactive rocks were 8 . 3 and 323 millimoles per liter. The inadequacy of the present t e s t where dolomitic limestones a r e concerned has beennotedand possible modifications suggested both in procedure and interpretation ( 1 , 4 , - - - 5). One such modified procedure, in which the determinations were made on the acid insoluble residue, gave values of silica r e - lease and reduction inalkalinity of 49.1 and 23.6 millimoles per l i t e r , respectively, when recalculation on the basis of the original rock. These values could be interpreted a s indicat- ing potentially deleterious alkali-silica reactivity. Similar values were, however, obtained for the "nonreactive" rock.
Conrow Test
In the Conrow test (ASTM Designation C342-55T) the very reactive Kingston rock gave expansions that were not significantly g r e a t e r than those of nonreactive rocks. At 12 months the values for the very reactive rock when made with high and low alkali cements, were 0.029 and 0.018 percent, respectively. The corresponding values for the "nonreactive" Kingston rocks were 0.029 and 0.040 percent and for the nonreactive control rock, 0.012 and 0.021 percent.
TABLE 1
EXPANSIONS IN STANDARD ASTM MORTAR BAR T E S T COMPARED W m H THOSE O F CONCRETE PRISMS CONDITIONED A T 100 PERCENT
RELATIVE HUMIDITY AND 73 F
P e r c e n t Linear Expansion (percent) Q u a r r y Cement 3 Months 6 Months
Level Alkali, M o r t a r Concrete M o r t a r Concrete Carbonate Rock (feet) a s Nan0 Bar T e s t P r i s m s B a r T e s t P r i s m s Q u a r r y A V e r y r e a c t i v e 0-24 1.19 0.031 0.175 0.043 0.235 Very r e a c t i v e 0-24 0 . 4 5 0.016 0.018 0.024 0.023 L e a s t r e a c t i v e 24-30 1.19 0.005 0.016 0.006 0.018 Q u a r r v B Very r e a c t i v e 0- 12 1 . 1 9 0.025 0.063 0.033 0.092 L e a s t r e a c t i v e 12- 1 3 1 . 1 9 0.004 0.021 0.009 0.030 Control - - . . . - - - Nonreactive
-
1 . 1 9 0.004 0.003 0.004 0.004 Petrographic ExaminationPetrographic examination of cut and polished sections of affected field and laboratory concrete a t a g e s up t o 3 y e a r s showed that the reaction had s o m e c h a r a c t e r i s t i c s v e r y s i m i l a r t o the alkali-silica reaction. It lacked c e r t a i n other f e a t u r e s , however, that have been established a s e s s e n t i a l c h a r a c t e r i s t i c s of s u c h r e a c t i o n s by study of many s u c h c a s e s . The following observations a r e b a s e d on examinations made by authorities on alkali-aggregate reaction a t the r e q u e s t of the a u t h o r s (6,
- -
7) and on t h e findings of t h i s laboratory.Many of the carbonate rock p a r t i c l e s exhibit "reaction r i m s " : s o m e a r e n a r r o w and d a r k and o t h e r s a r e wider and l e s s well-defined. The dominant silica m i n e r a l is
q u a r t z which is not known t o be deleteriously r e a c t i v e t o c e m e n t alkali.
F r a c t u r i n g is abundant and p e n e t r a t e s the carbonate rock p a r t i c l e s a s w e l l a s the cement m o r t a r , but only a v e r y s m a l l quantity of alkali s i l i c a - g e l can be detected. Considerable m i c r o - f r a c t u r i n g w a s observed a n d the f r e s h l y broken m o r t a r s u r f a c e s had a slightly chalky appearance. A significant number of contact s u r f a c e s between the cement paste and aggregate p a r t i c l e s had been disrupted by l a r g e f r a c t u r e s a n d micro- f r a c t u r e s , and many of the s o c k e t s f r o m which the p a r t i c l e s w e r e removed showed slight brownish s t a i n s . Deposited on the f r a c t u r e s u r f a c e s w e r e varying amounts of calcium carbonate and calcium sulphoaluminate, the l a t t e r in somewhat l a r g e r amount than n o r m a l . T h e r e appeared t o be s o m e s i m i l a r i t y between t h e s e affected concretes and c o n c r e t e s that have responded t o the Scholer t e s t .
Where low-alkali c e m e n t s had been used and no excessive expansion had occurred, t h e r e was a l s o r i m formation, the r i m s generally being b r o a d e r and about as pre- valent a s in affected c o n c r e t e s made with a high-alkali cement.
Concrete P r i s m T e s t
T h e concrete p r i s m t e s t w a s the only method found t o be r e l i a b l e in detecting the type of alkali-reactivity p r e s e n t in the Kingston dolomitic limestone. It consisted of measuring length change of c o n c r e t e p r i s m s continuously conditioned a t n e a r 100 per- cent r e l a t i v e humidity and 73 F. The r a t e and degree of expansion w e r e compared f o r c o n c r e t e s made with the Kingston limestones a n d low- and high-alkali c e m e n t s , and s i m i l a r control s p e c i m e n s made with nonreactive limestones. The method h a s the p r a c t i c a l advantage of being a d i r e c t t e s t on concrete. Careful control p e r m i t t e d detection of reactivity through a b n o r m a l expansion a f t e r only t h r e e o r four weeks for
the very reactive rocks, but s e v e r a l months, o r even a year or more, may be required for l e s s reactive rock o r f o r evaluating the effectiveness of low-alkali cement in reducing expansion to a safe value.
Expansion of concrete p r i s m s accele- rated slightly when subjected to higher temperatures or t o a wetting-drying cycl- ing action such a s a modified Scholer test (8). The advantage i s more than offset, h;wever, by the more complicated appa- ratus required and the greater difficulty of control. Comparable r e s u l t s of these t e s t s a r e given later.
The concrete p r i s m s used in these t e s t s were 3- by 4- by 16-in. or 3- by 3- by 10-in., with reference studs inset a t each end during fabrication. The moulds were lined with vinyl sheeting to avoid a coat- ing of grease. The comparator (Fig. 2) was s o designed that the p r i s m rested on a f i r m base; the measuring plug a t the bottom was adjusted to the measuring stud on the p r i s m by means of a counter- weight system. The micrometer a t the top measured to the n e a r e s t 0.001 in.
Concrete mixtures were a l l in the range of normal job concretes with control mix- t u r e s made a t the same time and in exact- ly the s a m e way a s the t e s t mixtures. Cement-sand ratios were 1 :2 and 1~:21/4; ce-
ment-stone r a t i o s v a r i e d f r o m 1:2% to l:3%,
.
Compaiator assembly f o rmeasu-
depending on the maximum s i z e stone. i n g l e n g t h change of concrete prisms. Water-cement ratios ranged from 0.44 to
0.60 by weight with slumps from l/z in. to 3 in. f o r all except special cases.
CHARACTERISTICS OF THE REACTION IN CONCRETE Influence of Composition
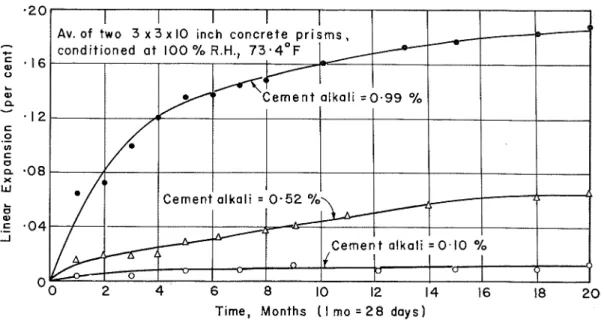
Cement Alkali. -The direct influence of the alkali content of the cement on the ab- normal expansion of concretes made with the reactive dolomitic limestone i s shown in Figure 3. Concrete mixture proportions were 1:21/4: 21/3, with a %-in. maximum size stone and a water-cement ratio of 0.47. Each curve r e p r e s e n t s the average linear change of two 3- by 3- by 10-in. p r i s m s maintained continuously a t near 100 percent relative humidity and 73 F. The total percent cement alkali (calculated a s sodium oxide) with the sodium and potassium oxide values for each (given in brackets) were: 0.99 (0.96, 0.05); 0.52 (0.46, 0.09); and 0.10 (0.01, 0.14).
Cracking of the affected concretes became visible a t expansions of 0.06 to 0.10 per- cent. The control concrete made with a nonreactive reference limestone and a high- alkali cement showed negligible expansion of the s a m e order a s the bottom curve of Figure 3.
The increase in the r a t e and degree of expansion with increasing alkali content was found to hold whether a l l the alkali derived f r o m the cement o r whether p a r t of the alkali was added. The effect of added alkalies i s shown in Figure 4. Mixture propor- tions were 1:2:31/4, with a maximum size aggregate of
3/4
in. and a water-cement ratio of 0.475. The low alkali cement used contained 0.31 percent total alkali calculated a s-
C5
* I 6 0 L al a e m e n t alkali ~ 0 . 9 9 Ol0-
- 1 2 C 0 .- In C I , Cement a l k a l i = 0 - 5 2 % Li C e m e n t a l k a l i = 0 . 1 0 % I O ( I 16 18 2 0 Time, Months ( I mo = 2 8 d a y s ) -Figure 3. Effect of cement a l k a l i content on expansion of concrete containing reactive Kingston carbonate rock a s coarse aggregate.
Figure
4.
Concrete expansion with r e a c t i v e carbonate rock and low a l k a l i cement with added a l k a l i s .sodium oxide (0.14 percent sodium oxide and 0.26 percent potassium oxide). Incre- ments of sodium and potassium hydroxide were added to the mixing water in equi- molecular quanitites to give total alkali contents of 0.71 and 1.11 percent. The plots a r e derived from the average expansions of duplicate 3- by 4- by 16-in. prisms con- ditioned continuously a t 100 percent relative humidity and 73 F. Results similar to those in Figure 4 were obtained with a low-alkali Type V cement when sodium hydro- xide was added to the mixing water.
With higher alkali contents, the sodium form of the alkali was more aggressive than the potassium form. This characteristic of the reaction is similar to that for the alkali- silica type (9), and was observed in other tests using different cements. These results indicated t h a the alkali was the only component of the cement that acted a s the reac- tant o r catalyst in the reaction of the carbonate rock. This conclusion was supported by expansion results involving a total of 15 cements varying in composition and fineness.
From a practical point of view, these and many other experimental results indicated that the generally accepted limit of 0.6 percent on total cement alkali where an alkali- reactive aggregate is to be used, i s too high f o r the Kingston rock. It i s suggested that a limit of 0.45 o r 0.40 percent be accepted for this case.
Potential Inhibitors. -Partial replacement of the high-alkali cement with pozzolanic materials, an effective method of inhibiting alkali-silica reactions, appeared t o be effective for a limited period but not a t later ages for concretes made with the Kingston rock. This i s illustrated in Figure 5, where expansions of concretes with no replace- ment a r e compared with concretes in which 25 percent of the cement, by weight, was replaced by an active calcined shale and by a fly ash (cement alkali = 1.19 percent, calculated a s sodium oxide). This limited influence of a pozzolan does not necessarily indicate a difference in behavior of this reaction from the alkali-silica type; rather it
may be due to the degree of reactivity present. Nevertheless, it was found that a 1 percent addition of lithium chloride, an effective inhibitor of alkali-silica reactions (10)
-
had no retarding influence on this reaction at early or at late ages.Ten pozzolanic materials were tried, some in 25 percent replacement of cement by weight, and others in 25 percent replacement by volume. Some had no effect, others a small early influence, taking into account the reduction in alkali a s a consequence of reduction in cement.
It should be remembered that the calcined shale appeared t o have a definite inhibit- ing influence in the mortar bar test.
Aggregate Size. -The rate and degree of expansion of concretes made with the Kingston rock and a high alkali cement were found to decrease with decreasing maximum size of aggre ate. This is shown in Figure 6, where the expansion of the concrete made with
3
F
/8 to /&in. rock is greater than that for the concrete made with
%
to 3/8-in. rock. Thedotted line shows the expansion of the mortar b a r s made with the rock (maximum size
'/4 in. ),.
Other Variables in Concrete. -The rate and degree of expansion of concretes made with the reactive Kingston limestone and a high alkali cement was found not to be signi- ficantly influenced by varying the water-cement ratio between 0.44 and 0.70. The lower water-cement ratios actually gave slightly higher expansions both for the cements and the mortar bars. In this respect the reaction differs from the alkali-silica type.
The moisture condition of the aggregate prior to incorporation in the concrete also had no significant influence in the expansion; however, saturated aggregate produced slightly higher expansion than aggregate exposed to laboratory drying o r to 100 per- cent relative humidity conditions.
The type, grading, and proportion of siliceous sand had no detectable influence when used with the reactive-rock coarse aggregate. No measurable increase in expansion was obtained when the siliceous sand was replaced by sand made from the reactive limestone.
Entrained a i r did not appear to have any influence on the expansion of concretes containing the reactive combinations. Variation in method and degree of compaction and in finishing techniques also had no observable effect.
Variations in amounts of each of the "reactants" (high alkali cement and reactive r o c k ) p r o d u c e d t h e corresponding propor- tional changes in rate and degree of expan- sion of the concretes.
Variation in the Carbonate Rock. -Stra- tigraphically the rocks in the two quarries a r e of Ordovician age and belong to the Black River formation. The rock from the s e v e r a l operating o r natural horizons in
16 20 24 each of the two q u a r r i e s showed, on the
T i m e . Months ( I in0 = 28 d a y s I b a s i s of expansion of concrete p r i s m s , variable degrees of reactivity. - ~ h e s e dif- Figure
5 .
I n f l u e n c e on expansive concreteof r e d u c t i o n i n cement and of p a r t i a l r e - ferences a r e compared in Table 2 with val- placement by pozzolans. ues f o r corresponding samples made with
a low alkali cement. The t e s t s a m ~ l e s were continuously exposed t o 100 percent relative humidity and 73 F.
The most reactive rock is found in the top 24 ft of Quarry A . It has been used ex- tensively as c o a r s e aggregate for concrete in the Kingston a r e a , and was used in most of the studies of the characteristics of the reaction. PhysicaJ.ly it was found t o be satisfactory a s a coarse aggregate when evaluated by conventional acceptance tests.
This rock is a fine-grained calcareous dolomite o r dolomitic limestone with an acid insoluble content of 5 to 1 5 percent and containing approximately equal proportions of calcite and dolomite. Bulk specific gravity w a s 2.70 and absorption 0.7 percent. Pore volume, a s measured by the mercury vacuum p r e s s u r e method, was found t o be very small.
h the acid insoluble portion, clay minerals present a r e illite and l e s s e r amounts of chlorite together with a s m a l l proportion of finely divided quartz, feldspar, and graph- itic material. In the more highly calcitic carbonate rock used as a nonreactive refer- ence, the acid insoluble material, though somewhat s m a l l e r in amount, was found t o have a very s i m i l a r composition.
The bed a t a depth of 24 to 30 ft produced a relatively s m a l l expansion of the concrete. Its dolomite content is much higher than the calcite content. It is a slightly green rock with an acid insoluble content of about 40 percent. The acid insoluble constituents a r e similar to those in the upper horizons except that this bed contains, in addition, a con- siderable quantity of silt-size and very fine sand-size particles of quartz. L e s s than 5 percent of this rock was considered
physically unsound. Pore size was also
very small but absorption was relatively 2 0 high, about 3 percent.
The 3C- to 36-ft layer in the same
-
.-
quarry produced even l e s s expansion of O . 1 6 concrete than the "green" rock. It is a La still more dolomitic and has a higher ab-
-
.12sorption, 6 percent. The acid insoluble C
0
portion is about 30 percent. C a The rocks from the other horizons in 2 . o e
W
the two q u a r r i e s varied in colnposition ,- from dolomitic limestones t o calcitic .- a
e
. 0 4dolomites with no clear-cut relation be- _I
tween reactivity and con~position. The
relatively r a r e occurrence in nature of 0
0 4 8 12 16 20 2 4
carbonate rocks having roughly equal T i m e , Months ( I mo = 2 8 d o y s )
proportions of calcite and dolomite
(11)
suggests a constitutional instability which::
2:
~ G e s ~ ~:Z~o~~r::; ~ ~ : C ~ ~ ~ ~ ~ ~
alkalies. In certain of the rocks soaked in alkaline solution a reduction in dolomite content was observed on X-ray films. In the c a s e of a composite sample of the 0- to 24-ft s e r i e s the strong dolomite lines have almost completely disappeared while three new lines, attributed to brucite, have appeared on the pattern. In the 1072- to 12-ft bed the dolomite lines a r e weakened a f t e r the alkaline attack on the rock (Fig. 7). The highly dolomitic rock a t Kingston produced only a s m a l l abnormal expansion, and the highly calcitic reference rocks showed no reactivity. The X-ray patterns show no ap- parent change before o r a f t e r alkaline attack.
The rocks that produced only s m a l l expansion of concrete (24 to 30 ft layer) w e r e combined with various proportions of the nonreactive reference rock, both a s c o a r s e aggregate and a s fine aggregate. In no case was a pessimum obtained. Combinations of the very reactive rock and the least reactive rock produced dilution effects only.
Extensive studies a r e being continued by the Division in an attempt to determine the mechanism of the reaction.
Influence of Environment
Moisture. -Laboratory and field observations showed that the Kingston reaction was dependent on a moist environment a s a r e alkali-silica reactions. In the concrete prism t e s t maximum r a t e and degree of expansion was obtained by continuous exposure to near 100 percent relative humidity. Continuous immersion in water a t the s a m e tem- perature (73 F ) gave similar expansion values for concrete p r i s m s . This dependency on moisture is shown by the expansion values plotted in Figure 8. Duplicate 3- by 4-
by 16-in. concrete p r i s m s were made with the reactive rock and with a nonreactive limestone rock a s a reference, each with a high alkali (1.19 percent) and a low alkali
TABLE 2
EXPANSION OF CONCRETE MADE WlTH ROCK FROM DIFFERENT LEVELS IN THE TWO QUARRIES
P r i s m s , 3 by 4 by 16 in. Mix = 1:2:31/4
Condition: 100 percent relative Max. s i z e rock =
'/4
in. humidity and 73 F Slump = 1 in.Percent
Cement Linear Expansion (percent) Quarry Level Alkali
(ft)
a s Nan0 6 months 12 months 18 months 24 months Quarrv AA B R U C I T E L I N E S P H I L I P S P O W D E R C A M E R A 1 1 4 . 6 MM D l A . I 0
-
2 4 'd
-
2 4 ' l 0 $ 1 2 ' S E R I E S S E R I E S B E D B E D A F T E R C O M P O S I T E A F T E R S O A K I N G S O A K I N G I N A L K A L I IN ALKALI D O U B L E - B E A M R E C O R D I N G M I C R O O E N S I T O M E T E R T R A C E O F S T R O N G E S T D O L O M I T E A N D C A L C I T E L I N E . J O Y C E , L O E B L B CO. L T D . M O D E L E 12 M K I l l W A L K E R D E S I G N E X P A N S I O N O R R E C O R D I N G R A T I O 1 : 5 O B J E C T I V E 0 . 2 8 N . A . 16 M M .Time, Months (.I mo = 28 d a y s )
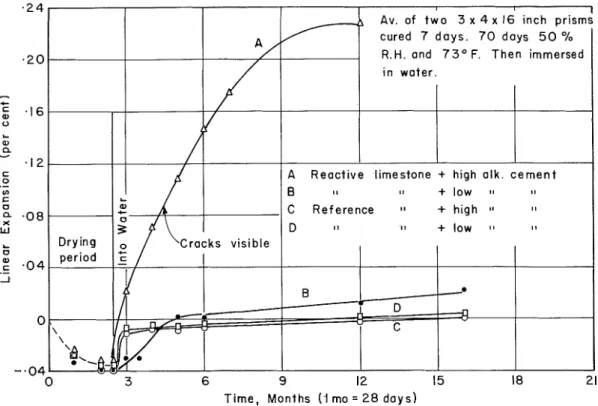
Figure 8. Expansion of t e s t c o n c r e t e s following i n i t i a l drying p e r i o d . cement (0.36 percent). After curing 7 days the p r i s m s were conditioned a t 50 percent relative humidity and 73 F f o r 63 days, a f t e r which they were immersed in water. All the specimens contracted normally and by the s a m e amounts during the drying period. Once immersed in water, however, the concrete made with the reactive Kingston rock and the high alkali cement began to expand rapidly while the one with the low alkali cement showed the usual slight expansion. The initial drying period appeared t o have had no permanent effect on the subsequent reaction under moist conditions.
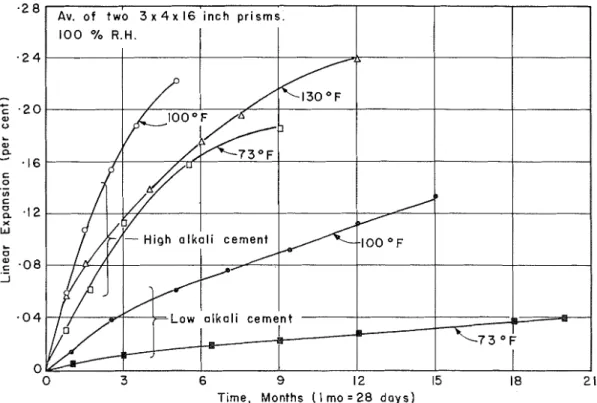
Temperature. -The influence of temperature on the reaction is shown in Figure 9. Duplicate concrete p r i s m s made with the reactive carbonate rock and with high and low alkali cements were conditioned a t near 100 percent relative humidity a t temperatures of 73, 100, and 130 F. With the high alkali cement (1.19 percent) the increase in the r a t e and degree of expansion with increase in temperature f r o m 73 to 100 F , an-a the apparent reversion a t 130 F indicate that the temperature effect in the reaction is re- markably s i m i l a r to that of the alkali-silica type of reaction (12). With the low alkali cement (0.31 percent) the expansion became excessive a t the higher temperature, suggesting that, in the field, f o r a combination of a high temperature and a moist environment, the cement alkali must be very low t o prevent excessive expansion and cracking.
Cycling Involving Moisture and Temperature.
-
The r a t e and degree of expansion of concrete containing Kingston limestone a s c o a r s e aggregate have been compared for different exposure conditions (1). A modified Scholer t e s t (wetting for 6 hours a t room temperature and drying for 6 hours a t 130 F ) produced a somewhat high- e r expansion than exposure to 100 percent relative humidity a t 73 F. The expan- sions of corresponding specimens subjected t o slow freez-thaw cycling (freezing in a i r ) and to outside exposure were much lower. In the wetting-drying t e s t , the expected retardation of the reaction due to drying was apparently more than offset by the high drying temperature. In the freeze-thaw t e s t the observed retardation was apparently caused by the lower temperature.0 3 6 9 12 15 18 2 1
Time, Months ( 1 mo = 28 d o y s )
Figure 9. E f f e c t of temperature on expansion o f concrete made with r e a c t i v e carbonate rock.
The n o r m a l Scholer t e s t (16 hours wetting a t room t e m p e r a t u r e and 8 h o u r s drying a t 130 F) w a s c a r r i e d out on non-air-entrained concretes made with low and high alkali cements and c o a r s e aggregate f r o m each of the operating horizons in t h e two quarries. The expansions a t v a r i o u s a g e s a r e shown in Table 3. T h e s e values a r e comparable with those exposed to 100 p e r c e n t relative humidity and 73 F in Table 2. T h e s e two
methods of t e s t a p p e a r , t h e r e f o r e , to r e v e a l reactivity of the rock through expansion of concrete.
Companion specimens subjected t o freeze-thaw cycling (6 hours freezing a t 18 F a n d 6 hours thawing a t room t e m p e r a t u r e ) showed the usual lower r a t e of expansion f o r the concretes made with the r e a c t i v e r o c k s and the high alkali cements. With low alkali cements, only the 30- t o 36-ft bed in Q u a r r y A showed low durability. Air-entrained concrete made with the v e r y r e a c t i v e rock and a low alkali cement showed n o sign of disintegration a f t e r m o r e than 1,000 cycles.
EXPANSION O F CARBONATE ROCK IN ALKALINE SOLUTION
The p r e s e n c e of a considerable clay fraction in the r e a c t i v e Kingston limestone sug- gested the possibility that relatively weak clay s e a m s w e r e opened up t o t h e strong al- kali solution p r e s e n t in concrete made with high alkali cement, and that the expansion of the concrete may t h e r e f o r e have resulted f r o m cracking of the rock itself r a t h e r than f r o m an inherent tendency t o expand.
Samples of the Kingston r o c k and of r e f e r e n c e carbonate r o c k were i m m e r s e d in 2-molar alkaline solution, made up of n e a r l y equal p a r t s of sodium and potassium hy- droxide and containing 0 . 0 1 equivalents of calcium ion and 0.15 equivalents of sulfate ion.
In one experiment 500 g m of the reactive crushed r o c k
('/z
to3/4
in. ) w a s vacuum s a t u r a t e d with the above solution and compared with a c o n t r o l specimen of inactive carbonate rock t r e a t e d in t h e s a m e way. The volume changes of the solutions were measured and, by subtracting the volume change of the c o n t r o l specimen f r o m the t e s tTABLE 3
RESULTS O F SCHOLER TEST ON ROCKS FROM SELECTED LEVELS IN THE QUARRIES
Mix: 1:2:3', C u r e before cycli:?:: Slump = 2 to 3 in. 7 d a y s moist r o o m
Max s i z e =
3/4
in. 21 days drying, 50 percent relative humidity and 73 F . P e r c e n tCement Linear Expansion (percent) Quarry Level Alkali
(ft) a s NazO 3 months 6 months 12 months 18 months Q u a r r v A
Q u a r r y B
0-12 1 . 1 9 0.043 0.051 0.070 0.074
0.36 0.016 0.020 0.026 0.028
specimen a t each reading, s m a l l fluctuations due t o t e m p e r a t u r e change and r e s i d u a l absorption w e r e c o n s i d e r e d a s cancelling out. The net i n c r e a s e in volume exhibited by the reactive rock w a s remarkably s i m i l a r to the expansion c u r v e s for the affected con- c r e t e s previously described. Net expansions w e r e : 0.06 a t 1 month, 0.10 a t 2 months, 0.15 a t 4 months, and 0.22 a t 8 months. An e s t i m a t e d one-sixth of the rock had c r a c k e d up but t h i s should not have affected the tot11 volume of solid plus solution.
Rock p r i s m s , measuring 1% by S3/4 in., w e r e c u t from s e v e r a l selected beds in
Q u a r r y A and f r o m reference carbonate rocks. The ends w e r e planed and polished to p e r m i t the fitting of special plates f o r measuring length changes in a comparator. P r i s m s were i m m e r s e d continuously in 2-, 0.44-, and 0.06-molar alkaline solutions and in water. The s m a l l volume change due to wetting was corrected for by taking a s z e r o expansion the readings a f t e r one-day i m m e r s i o n .
Although many of the reactive p r i s m s cracked, mainly along bedding planes, a f t e r only a week o r two inthe 2-molar solution, s o m e p r i s m s remained uncracked a n d volume changes were measured up to one y e a r of a g e . Typical plots a r e shown in Figure 10 f o r the 6- t o 7-ft bed in Quarry A.
The expansion of the rock in the 2-molar alkaline solution w a s remarkably s i m i l a r in r a t e and degree to the expansion of concretes made with the reactive rock a n d a high alkali cement. The 0.44-molar solution had no effect but did show a slight tendency to produce expansion a f t e r 9 months. This solution did produce a considerable expansion of a m o r e r e a c t i v e rock, the 1072- t o 12-ft bed. T h e reference rock, not shown, yielded values closely corresponding t o the lowest c u r v e s of F i g u r e 10.
T h e s e r e s u l t s would suggest that the alkali, although producing cracking a t planes of weakness, a l s o produces an expansion of the r e a c t i v e rock i t s e l f . This method would a p p e a r to be a means of determining the type of reactivity exhibited by the Kingston dolomitic lime stone.
I Legend:
-
H prism, 2 m o l a r sol'n. M V"
, 2 I 1 1 1 M V"
, 0.44 II V II,
0.06 Id S a m ~ l e cut f r o mt
- V I',
w a t e r7 - 1
prismrdN0crai+
S e e n o t e /4H = Bedding planes p e r p e n d i c u l a r to length
1
0 I 2 3 4 5 6 7 8 9 10
Time, Months ( I mo = 28 d a y s )
Figure 10. Expansion of limestone prisms i n a l k a l i n e s o l u t i o n s , 6
-
7 ' bed, Quarry A . SUMMARY AND CONCLUSIONSThe alkali-reactivity of the Kingston carbonate rock was found to be s i m i l a r in c e r - tain important r e s p e c t s t o the alkali-silica type of reaction. Certain c h a r a c t e r i s t i c s w e r e , however, markedly different.
The points of similarity w e r e : (1) the specific influence of alkali content, whether it
d e r i v e s f r o m t h e cement o r i s added, (2) the more a g g r e s s i v e influence of t h e sodium than the potassium hydroxide, (3) the influence of t e m p e r a t u r e , with evidence of a n optimum, (4) the d i r e c t influence of moisture, and (5) the abnormal expansion of concrete, followedby map-cracking where moisture conditions vary a t two surfaces.
C h a r a c t e r i s t i c s that show s o m e limited d e g r e e of s i m i l a r i t y w e r e : (1) r a t e and de- g r e e of expansion w e r e apparently higher in t h i s c a s e ; (2) d e c r e a s e in expansion with d e c r e a s e in particle s i z e of aggregate, apparently due to break-up a t planes of weakness
as well a s to i n c r e a s e in s u r f a c e a r e a f o r a f i x e d amount of alkali; (3) r i m formation p r e s e n t but different in appearance f r o m the alkali-silica type; (4) microscopic evidence of fracturing of paste and aggregate but d i f f e r e n c e in appearance of affected a r e a s .
Distinguishing features w e r e : (1) absence of significant quantities of gel; pore volume was extremely s m a l l , and amount of s i l i c a v e r y s m a l l , hence i t is not likely that failure to detect gel is due to i t s distribution throughout the pores; (2) absence of minerals o r rock types known to r e a c t deleteriously with cement alkali; (3) failure of alkali-silica reaction inhibitors to control t h i s alkali-carbonate rock reaction; (4) i n c r e a s e in water- cement r a t i o apparently does not i n c r e a s e r a t e of reaction; (5) uncracked p a r t s of the affected concrete appear to r e m a i n intact, even a f t e r many y e a r s .
The Kingston carbonate reaction is not detectable by the standard ASTM tests. It may be detected by exposing concrete p r i s m s made with the suspected r o c k and a high alkali cement t o n e a r 100 p e r c e n t relative humidity and 73 F conditions, o r t o the Scholer t e s t . It may a l s o be detected by measuring expansions of the rock in alkaline solution.
A s a tentative conclusion r o c k s composed of n e a r equal proportions by weight of dolomite and calcite may be regarded as suspect and it s e e m s possible t h a t a connection e x i s t s between the expansive reactivity and t h e dedolomitization reaction (replacement of dolomite by calcite and brucite).
Work i s continuing t o e s t a b l i s h whether the reactivity with a l k a l i is controlled by the composition, t e x t u r e , o r s t r u c t u r e of the rock.
REFERENCES
Swenson, E . G . , "A Canadian Reactive Aggregate Undetected by ASTM T e s t s .
"
A m e r . Soc. f o r Testing M a t e r i a l s , Bulletin No. 226 (Dec. 1957).Lemish, J . , Rush, F . E . , and Hiltrop, G. L . , "Relationship of Physical P r o p e r - t i e s of Some Iowa Carbonate Aggregate t o the Durability of Concrete." HRB Bull. 196, pp. 1-16 (1958).
Bisque, R. E . , and Lemish, J . , "Chemical C h a r a c t e r i s t i c s of Some Carbonate Aggregates a s Related to the Durability of Concrete." HRB Bull. 196, pp. 29- 45 (1958).
Mielenz, R. C.
,
Greene, K. T .,
and Benton, E. J . , "Chemical T e s t f o r Reactivity of Aggregate with Cement Alkalis; Chemical P r o c e s s e s in Cement-Aggregate Reaction." P r o c . , A m e r . Concrete I n s t . , Vol. 44, pp. 193-221 (1948). Chaiken, B., and Halstead, W. J . , "Correlation between Chemical and M o r t a r BarT e s t s f o r Potential Alkali Reactivity of C o n c r e t e Aggregates." HRB Bull. 239, pp. 24-40 (1960).
Mielenz, R. C. P r i v a t e communication. Mather, Bryant. P r i v a t e communication.
Scholer, C. H., "A Wetting and Drying T e s t f o r Predicting Cement-Aggregate Reaction. ' I K a n s a s State College Bulletin, C i r c u l a r No. 2 (Aug. 15, 1950).
Davis, C. E. S., "Studies in Cement-Aggregate Reaction, XXVI Comparison of Effect of Soda and Potash on Expansion.
"
A u s t r a l i a , J o u r . of Applied Science, Vol. 9, NO. 1, pp. 52-62 (1958).McCoy, W. J., and Caldwell, A. G., "New Approach t o Inhibiting Alkali-Aggregate Reaction." P r o c . , A m e r . Concrete I n s t . , Vol. 47, p. 693 (1951).
Pettijohn, F. J.
,
"Sedimentary Rocks. " H a r p e r B r o s . , New York, second ed. (1957).L e r c h , W . , "Chemical Reactions. Concrete Aggregates. " A m e r . Soc. f o r T e s t - ing M a t e r i a l s , Special Technical Bulletin No. 169, pp. 334-45 (1956).