Publisher’s version / Version de l'éditeur:
Technical Note (National Research Council of Canada. Division of Building Research), 1958-12-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20359168
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Assessment of Possible Materials for Use in the Calibration of a Neutron Moisture Meter
Burn, K. N.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC: https://nrc-publications.canada.ca/eng/view/object/?id=96fb3175-9b08-484d-b6e2-ff8b466854ed https://publications-cnrc.canada.ca/fra/voir/objet/?id=96fb3175-9b08-484d-b6e2-ff8b466854ed
·
,NATIONAL RESEARCH COUNCIL OF CANADA
94
'if
eGセウ[Zセ
[Zセg
reZaセセe
No.
269
NOT FOR PUBLICATION
FOR INTERNAL USE
PREPARED BY PREPARED FOR K.N. Burn Disoussion CHECKED BY CBC APPROVED BY NBH DATE Deoember
1958
SUBJECT Assessment of Possible Materials for Usein the Calibration of a Neutron Moisture Meter
Several methods have been developed for determining the moisture oontent of soils and other similar materials by means
of probes which scatter neutrons and deteot the moderating effect of moisture in a medium upon the neutron flux. The principle is the same for a11. They differ only in the means used for detecting the moderated neutron flux.
The Principle of the nセオエイッョ Meter
The predominant process which occurs when high energy neutrons are scattered into a medium, is a loss of velocity of
eaoh neutron 'as it collides with the nuclei of atoms of the medium. Eventually, through loss of kinetic energy, the velocities are
reduoed to those encountered in gases at normal temperatures and pressures (thermal neutrons).
The rate 。セ which this slowing down process occurs depends upon:
(i) The mass of the nucleus in collision with the neutron, and
(ii) The probability that the two will collide.
The mass of the hydrogen nucleus is nearly equal to the neutron mass. Collision with hydrogen atoms, therefore, reduces the kinetic energy and hence the velocity of neutrons more quickly than col11sion with heavier nuclei. The large difference between the masses of hydrogen atoms and those normally encountered in soils means that the relative effeotiveness of hydrogen atoms in slowing down neutrons is very pronounced.
The probability that a neutron will collide with the nucleus of an atom is dependent on its scattering cross-section. For most elements the value is lOW, inoreasing slightly with a
.
,2
-decrease in neutron energy. The increase is about twenty-fold for hydrogen, however, as neutrons decrease from emission
velocities to thermal velocities.
Hydrogen, when these two processes are combined, becomes the most effective medium for reducing the velocity of emission neutrons. If a detector of thermal or low velocity neutrons is placed near a neutron souroe in a medium containing hydrogen, the activity registered is due almost entirely to neutrons slowed
down by hydrogen atoms. The other atoms present in media suoh as soils playa negligible part in this prooess. In natural soils hydrogen may be present in several forms but, with some exoeptions, it oocurs prinoipally in the wa ter held by the soil particle s.
Therefore, the slow neutron aotivity registered by a suitable
deteotor oan be read as a measure of the conoentration of water in a soil medium once this has been determined by calibration.
Attempts to caloulate a calibration of activity vs ooncentration in moisture density have had limited suocess beoause of one or two unknown parameters.
Field Calibration
Although the ultimate aim in constructing a neutron moisture meter is to measure the water contents of natural soils, oalibration
in situ faces many diffioulties. The determination of moisture oontent from field borings is not sufficiently accurate to use as a standard against whioh to calibrate. There is no control over moisture content in the field and it is impossible, therefore, to reproduce results. Moisture oontents vary so randomly with depth
in natural soils that it would be extremely diffioult to state what moisture oontent was being "sampled" by a neutron moisture meter. Sinoe complete oontrol of all parameters is neoessary for accurate calibration it must be undertaken in the laboratory.
Laboratory Calibration
(a) Boundary oonditions
Workers in this field have found it possible to calibrate the neutron meter in the laboratory by using a suitable medium in oil drums of standard 4S-gal oapacity, キィゥセィ measure about
23
in. diameter and are 36 in. high. Experiments have shown that for the usual range of bulk densities of soils, the diameter of sample aotually involved in the action desoribed depends only upon the hydrogen density. The maximum diameter "sampled" by a neutron... measuring device at the lowest moisture oontents generallyto
15
in. with only a slight increase in moisture content. Thus, an oil drum of this size, filled with soil serves as a sample ofinfinite extent so far as this measuring technique is concerned. (b) Use of natural soil material
Natural soils were the first to be considered since they are most readily available. For calibration purposes, however,
they have several disadvantages. Uniform mixing of soil and water is very difficult, especially with clays. The use of sand makes mixing easier but very high moisture contents cannot be attained before movement of water under gravity occurs. Even if the mixture of soils and water could be accomplished in a satisfactory manner, difficulties in placing and in control of density present problems. Total weights and volumes might be reproduced.within certain limits but,densities of layers may vary appreciably.
(c) Requirements of artificial material
Fortunately, the neutron technique measures the relative occurrence of hydrogen atoms, not of water molecules, so for calibration purposes hydrogen atoms in some other form can be substituted for hydrogen atoms in water. In fact, even the solid portion of the soil and the oxygen atoms associated with the
hydrogen atoms in the water molecules can be replaced by any other material provided the scatter geometry of neutrons is not affected. The scatter geometry will not be greatly affected so long as the bulk density of the medium used is in the range characteristic of soils. Some elements, however, do not act only as targets for the elastic rebound of neutrons, but capture and absorb neutrons to become induoed radioaotive isotopes. The presence of suoh elements takes a oertain percentage of neutrons out of play causing an erroneously low reading of thermal-neutron activity. Among the elements to be avoided are boron, iron, and the abundant Halogen group. Since atoms of small mass are more effeotive in
slowing down neutrons than those of large mass, their use should also be avoided. Those elements having atomio weights between
that of hydrogen and those of the lightest elements usually enoountered in soils (i.e. oxygen, aluminum Bnd silioon) should not be used.
(d) Use of saseous mixtures
After initial consideration a mixture of hydrogenous and non-hydrogenous gases appeared to be an ideal way of prOViding
uniform and adjustable hydrogen densities. The problems of plaoing, homogeneity, and density control would not exist. Further
."
4
-gases were unacceptable because of their low densities. Calcu-lations also showed that for even the very driest end of the range the required hydrogen density could not be obtained by the use of gaseous compounds without resorting to excessively high pressures.
(e) Use of liquids
Recognition of the density requirement led next to consideration of the possibility of finding a non-hydrogenous
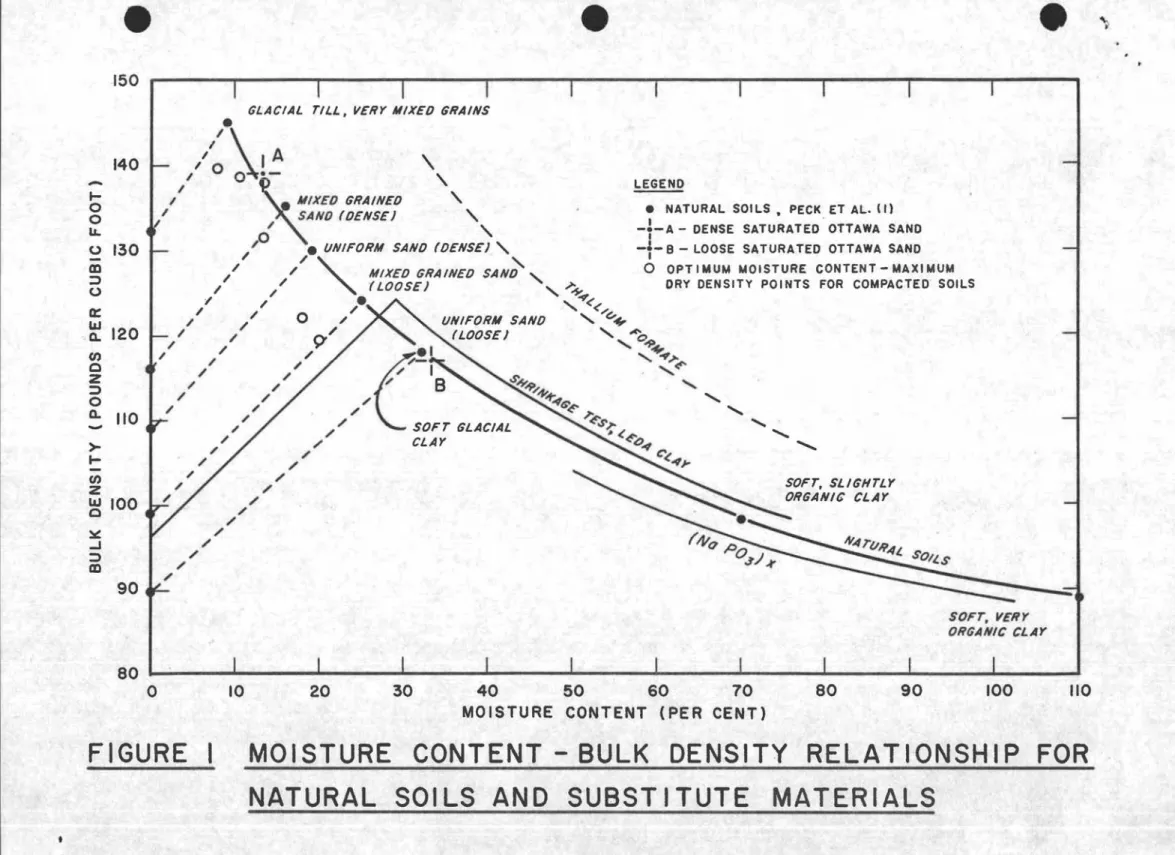
liquid (or one containing only a very small percentage of hydrogen atoms) having a density of about 2 gm/cc. To this a hydrogenous liquid 」ッセャ、 be added having a density of about 1 gm/cc since there are many available in this range. With this combination it is easy to follow the normal density-moisture content relationship of natural soils (Fig. 1) by selecting two liquids and mixing them in various proportions (1).
Heavy liquids ウオセィ as are used in the laboratory for separation. of ores by flotation were investigated but were found to be prohibitive in cost; their densities were too high and they had the added disadvantages of containing some Halogen element or of being toxio (Fig. 1). The tables of physical constants of organic and inorganic compounds in the Handbook of Chemistry and Physics were searched but it was found that the liquids having the required high density contalned either a
Halogen or some other unsuitable element, and a combination with the appropriate density range could not be found.
Fluids comprised only of organic compounds such as petroleum products were also considered, but these were all of low-bulk densities and had a high concentration of hydrogen densitios, making them unsuitable for the desired range.
(f) Use of solids
Solids with low melting temperatures would be suitable for calibration media since the material could be poured into place before solidifying. This type of medium would be ideal in that it could easily be kept for constant use as a calibration standard without loss of hydrogen content or change in bulk density. However, the bulk densities of these materials are about 60 lb/cu ft and the hydrogen concentration about
three-quarters that of water giving relationship' considerably removed from those of natural soila.
(g) Use of laminated solids
Interlayering of hydrogenous and non-hydrogenous films was considered. The cutting and stacking of thin discs in different combinations to produce various hydrogen densities seemed a tedious arrangement which might better be accomplished
# •
5
-by rolling different film combinations onto an access tube. Many plastic films are now available and metal foils such as aluminum could be used to provide the required bulk density. This would set up a directional geometry in the medium, however, and the idea was dismissed since the neutron flux might be
distorted and therefore not representative of natural soils. (h) Use of solid mixtures
The next step was to consider mixtures of hydrogenous and
セッョMィケ、イッァ・ョッオウ solids, even though this introduced the accompanying problems of mixing, placing and density control. Uniform mixing of different particles is possible only through prolonged agitation of a mix, but this presents no great difficulty, requiring only time and suitable equipment. However, when a material comprised of particles of different density and size is removed from where it was mixed, then is weighed and placed in some other container, segregation will always occur to some degree. Spherical particles of uniform size could be used to minLmize this effect, and from this the maximum range in densities could be calculated.
Several possibilities of using sand-size particles were considered. Large sizes were thought to be unsuitable because they might have the effect of ooncentrating hydrogenous particles and non-hydrogenous particles in pockets, introducing a scatter geometry different from that in natural soilse With this size stipulation the following combinations were considered:
i) Mixtures of hydrogenous spheres with non-hydrogenous spheres to give a fairly
complete range of hydrogen densities ii) A medium consisting of non-hydrogenous
spheres coated uniformly with some hydrogenous materials. Film thickness could be modified to vary hydrogen density over the lower
range of natural soil moisture densities
セゥャI Spheres of material of lower hydrogen (concentration) than that of water, at various compacted densities for the higher range of natural soil moisture
densities
(iv) A combination of the above 'methods to give required equivalent moisture densities.
Non-hydrogenous Ispheres (approximately) of the correct
density are available in the form of sorted Ottawa sand. (Glass spheres are also available but because the material 6enerally contains small proportions of boron it 1s unsuitable). Suitable
6
-hydrogenous spheres are not readily available. Coatine the spheres with a hydrogenous material might require the develop-ment of elaborate equipdevelop-ment and techniques. The first two possible combinations were therefore discarded.
The third was used by the writer in the calibration of a foil-type probe. Various mixtures of well-graded sand and powdered dextrose were used with very small quantities of water. Considerable difficulty was experienced in mixing and ーャ。」ゥョァセ
and tmiformity of density was probably not attained since compaction was done with a hand tamper.
(i) Use of solid-liquid ウセウー・ョウゥッョウ
Another possibility of preparing a suitable calibration medium is to combine a solid and a liquid. With proper agitation and circulation the problem of homogeneity would be solved but this operation would involve ancillary equipment which might better be avoided.
Two suspensions used in the commercial separations of ores by flotation are known. One is unsuitable because it contains iron which has a high absorption cross-section for neutrons; the other contains galena (lead sulphide) giving a range of densities much higher than those in natural soils for the same moisture
contents.
(j) Use of solid-liquid solutions
Several possibilities of mixing soluble solids and liquids were investigated. No combination was found to give a complete range of equivalent moisture contents, and many compounds of the right specific gravity were soluble only in very small proportions. The most suitable solute that could be found was sodium hexameta-phosphate (Napo
3)x commercially available as Calgon, which permitted
a maximum concentration equivalent to a minimum moisture content of
50
per cent, a point at about the centre of the required range (Fig.U.(k) Use of saturated solid-liquid mixtures
An obvious combination for the lower end of the range is a saturated mixture of well-defined sand and water. This may almost be considered a natural soil medium but is actually a special case in which saturation will prevent redistribution of water. The difficulty of placing and homogeneity are problems which are hoped to be overcome by sedimenting wetted sand into water in a given manner and vibrating when required to densify the medium.
7
-For spherioal partioles of uniform size the ratios of void and solid volumes are known for maximum and minimum packing. Maximum or olose paoking results in a void spaoe equal to
26
per oent of the total volume. Using Ottawa sand as the solid partioles this would result in a moisture oontent of
13.2
per oent of weight. Loose or oubical packing-results in a void ratioof 47 per oent or a moisture oontent of
33.3
per cent. Since the sand particles will not all be exactly the same size if a standard29-30
fraction is used, the void ratios will probably be somewhat lower than the theoretioal figures. Therefore the moisture contents will also be slightly lower. A denser paokingoould be obtained with a mixture of well-graded Ottawa sand than with a uniform fraotion. This would result 1n a lower equivalent
soil moisture content, perhaps less than 10 per oent • .,
Proposal for Full Range Laboratory Calibration
It is now proposed to calibrate the neutron meter using a medium of sodium hexametaphosphate and water for equivalent moisture oontents of
50
and 70 per oent, and water saturated Ottawa sand for moisture oontents of 30 and 10 per cent even though some preliminary investigation of density control キゥャセ be required for the latter medium.It is disappointing that a more simple and positive method could not be found for the low moisture content range. In spite
of an extensive search for suitable materials for this calibration, an obvious simple solution may have been overlooked. Therefore, any comments on or suggestions about this note wi11 be welcomed.
Reference
1. Peck, R.B. et ale Foundation Engineering. John Wiley and Sons Inc., New York,
1954.
p.2l, <4l0p.).e
e
-
...
150 , i i 1 • I J i i i i ,
GLACIAL TILL, VERY MIXED GRAINS
120
-SOFT, SLIGHTLY ORGANIC CLAY
• NATURAL SOl LS • PECK ET AL. II) -T-A - DENSE SATURATED - OTTAWA SAND
-t-a -
LOOSE SATURATED OTTAWA SANDo
OPTIMUM MOISTURE CONTENT-MAXIMUM DRY DENSITY POINTS FOR COMPACTEO- SOILS LEGEND / / / / /iᄋセ
I I I A \ / 0 0 . - , I Iセmixed
GRAINED , I SAND (DENSE) , I ,,0 • lINIFORM SAND (DENSE) ,
/ .I"
"-I' / MIXED GRAINED SAND
"-I / (LOOSE) " .;-+ I I セ\ I I . " </. I I 0 1 / lINIFORM SAND "G-+
/ .II
9'1' (LOOSE)BセoセK
I I / . 1 "- セN[M I I I(/-1
...
4!'" I / / B S-S--9. "-I / セセセ ... I I / セgGセ ... I I / BNセ ... I I I' SOFT GLACIAL sセ \セ ... セ / CLAY \Iセ ... ' / セ I / Gセセ I' / I' I / 140 90 _ 110 I-o o LL o 130 CD :;) o a:: &&J Q. UJ o Z :;) o Q. >- I-UJ Z &&J 100 o セ -I :;) CD SOFT, VERY ORGANIC CLAY /10 100 90 80 40 50 60 70MOISTURE CONTENT (PER CENT)
30 20
10
80 I ' I , I I I , ! I ! ,