DNA polymerase beta inhibitor pamoic acid:
toxicity to metakaryotic human cancer stem cells
(HT-29)
by
Tushar Vinod Kamath
S.B., Massachusetts Institute of Technology (2015)
MASSACHUSETTS INSTITUTE
JUN
08
2016
LIBRARIES
Submitted to the Department of Biological Engineering
in partial fulfillment of the requirements for the degree of
Masters of Engineering in Biological Engineering
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
June 2016
@
Massachusetts Institute of Technology 2016. All rights reserved.
Author...Signature
redacted
Department of Biological Engineering
/
May 20, 2016
Signature redacted
Certified by
...
William G. Thilly
Professor of Genetics, Toxicology, and Biological Engineering
Accepted by...
~-, iiThesis Supervisor
AySignature redacted
Forest White
Chair, Graduate Program Committee in Biological Engineering,
Professor of Biological Engineering
DNA polymerase beta inhibitor pamoic acid: toxicity to
metakaryotic human cancer stem cells (HT-29)
by
Tushar Vinod Kamath
Submitted to the Department of Biological Engineering on May 20, 2016, in partial fulfillment of the
requirements for the degree of
Masters of Engineering in Biological Engineering
Abstract
Amitotic cells with large, hollow bell-shaped nuclei, or metakaryotic stem cells, are the post-embryonic stem cells of the fetal organs from about the fourth week post conception through physical maturity. These metakaryotic stem cells, after acquiring necessary genetic, and possibly other events, are also the stem cells of precancerous, cancerous and metastatic lesions of carcinogenesis. Furthermore, our lab has discov-ered that metakaryotic stem cells, both in fetal development and tumor growth, use a peculiar mode of DNA synthesis and segregation that involves inter alia expression of large amounts of RNA polymerase beta during DNA synthesis. It was hypothesized that an inhibitor of DNA polymerase beta would be toxic to metakaryotic stem cells at concentrations lower than necessary to kill eukaryotic non stem cells.
The polymerase beta small-molecule inhibitor chosen for this study was pamoic acid, a napthoic acid derivative. With it we determined the relative sensitivity of metakaryotes and eukaryotic cells in the human colorectal cancer cell culture, HT-29mes, that expresses characteristics expected of colorectal cancer metastases. We conclude that, at 300 pM and above pamoic acid does not selectively kill metakary-otes in cell culture below that concentration which kills eukarymetakary-otes. Rather, pamoic acid acts in a similar fashion as X-rays: eukaryotic non-stem cells are killed at lower doses than those that kill metakaryotic stem cells. Treatment of pamoic acid with these concentrations causes concomitant declines in colony-formation potential for both metakaryotes and eukaryotes alike. At lower overall survival levels, surviving colonies appear to have arisen from metakaryotic cells, not eukaryotic cells as evi-denced by the presence of visible metakaryotic cells in most colonies and the ability of such colonies to support continuous growth upon passaging. We conclude with the possibility that this specific polymerase beta inhibitor is not an effective metakary-ocide in culture, insofar as they are not selectively toxic for these stem cells in the HT-29mes colorectal cancer cell line.
Thesis Supervisor: William G. Thilly
Acknowledgments
While this short acknowledgement cannot adequately cover all the people that have immensely contributed to my growth as a scientist and person, I hope to spend some ink giving those most important to me credit they deserve.
To my whole family, but especially my mother, father, and brother. I can never appreciate enough all the sacrifices that my mother and father made to provide me with the education of a lifetime; constant support in whatever I do; and endless, unconditional love. My brother has always been a steadfast rock of positive energy and I thank him for all these years being by my side.
To my friends at MIT who have helped me through the rollercoaster ride that was these past four years. Late night philosophical discussions, walks along the esplanade, Spongebob jokes, warm cookies, and their unyielding camaraderie are just a few of the things I've cherished and will remember for a lifetime.
To Prof. Sam Wilson at the NIEHS, who provided me with the initial knowledge of pamoic acid and polymerase beta and the pamoic acid derivative compounds.
To the members of this lab and all my predecessors, especially Kailin Duan and Lo-hith Kini. Kailin was there by my side when I first encountered rigors of benchwork, helping me get through my first, most egregious errors in the lab. Lohith's heartfelt words of advice and mentorship have pushed me to strive further in my professional career.
To Rita DeMeo, for being in the office day-in and day-out, always there to give me an extra hand and words of happiness and support. To Dr. Elena Gostjeva, for teaching me these methods in metakaryotic biology that allowed me to pursue this line of work and her encouragement in my development as a scientist. Finally, to my advisor, Prof. Thilly, for equipping me with Occam's razor; exposing me to the true life of a scientist; and instilling in me the drive to finish shoveling the chicken coop, no matter the mess.
Contents
1 Introduction 15
1.1 Metakaryotes: the stem cells of organogenesis and carcinogenesis . . 16
1.2 Metakaryotic stem cells make use of a double-stranded RNA/DNA intermediate, circular chromosomes, and exclude Hoechst 33342 . . . 18
1.3 Fetal/juvenile metakaryotic stem cells drive adult colorectal carcino-genesis . . . . 20
1.4 X-ray resistance of metakaryotes . . . . 21
1.5 The functional role of polymerase beta . . . . 21
1.6 The link between polymerase beta and cancer . . . . 24
1.7 Pamoic acid: a small-molecule polymerase beta inhibitor . . . . 25
1.8 Current technologies and assays for metakaryotic stem cells . . . . 26
2 Materials and Methods 29 2.1 HT-29 as a model for colon carcinomas and cell culture . . . . 29
2.2 HT-29 maintenance and passaging . . . . 30
2.3 Pamoic acid treatment protocol . . . . 31
2.4 Colchicine treatment protocol . . . . 31
2.5 Hoechst staining for identification of metakaryotic nuclei . . . . 32
2.6 Imaging and microscopy . . . . 33
2.7 Image analysis and colony-formation assay . . . . 33
2.8 Statistical calculations . . . . 33
3.1 Colchicine acts as a mitocide and enriches metakaryotes within the HT-29mes cancer cell line . . . . 35 3.2 Pamoic acid acts like X-rays in toxicity assays against the HT-29mes
colon cancer cell line . . . . 37 3.3 Re-passaging of metakaryotic colonies after treatment with pamoic acid 39
4 Discussion and Conclusion 41
A Tables 45
List of Figures
B-1 European-American Males (EAM) coincidence-corrected age-specific colon cancer mortality rate per 100,000 for the birth cohorts
1800-09;...;2000-09 vs. age interval of death, t = 0.5, 1, 2, 3, 4, 5-9;...;100-104. 48
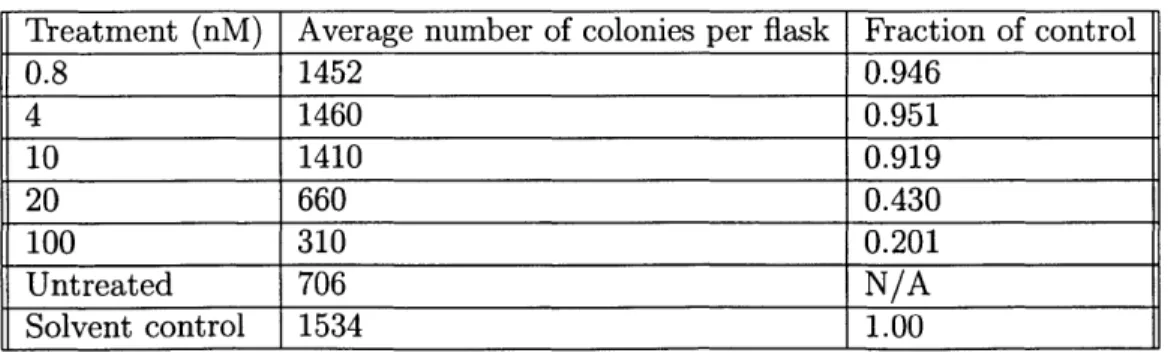
B-2 Phase contrast microscopy images of 38-hour colchicine treatment three days after seeding reveals enrichment of metakaryotic stem cell-derived colonies. Phase contrast microscopy at 200x magnification reveals colony formation after treatment with colchicine at varying concen-trations. Cells were treated as (a) normal control; (b) 0.8 nM; (c) 4 nM; (d) 10 nM; (e) 20 nM; and (f) 100 nM. Notice, pointed to with red arrows, in (e) and (f) the presence of bell-shaped nuclei forming from cytoplasmic bodies that cap the cell nucleus. . . . . 49 B-3 Phase contrast microscopy images of HT-29mes reveals continued
se-lective killing of eukaryotes in culture. In the same experiment as figure
2, cells were treated with colchicine at varying concentrations for 38
hours. Normal control (undosed) (a) and 0.8 nM-dosed (b) cells showed stable growth four days after treatment. Enrichment of metakaryotic colonies were found in 20 nM (c) and 100 nM (d) trials. . . . . 50
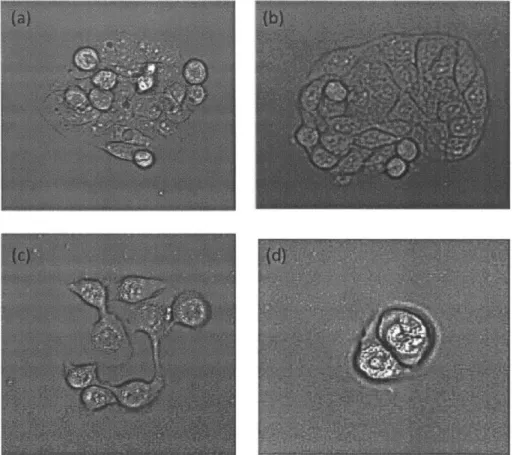
B-4 Phase contrast microscopy images of HT-29mes colonies five days after seeding. Cells were treated with colchicine for 38 hours at varying concentrations. The normal control (undosed) (a) and 0.8 nM (b) cell populations showed similar distributions of eukaryotic/metakaryotic colonies. In the 20 nM (c) and 100 nM (d) doses, the fraction of eukaryotes was far lower than the control case. . . . . 51
B-5 Fluorescent and phase contrast images of HT-29mes nuclei after Hoechst 33342 staining. HT-29mes cells were imaged 15 days after initially plat-ing and treatment with colchicine for 38-hours at varyplat-ing concentra-tions. In (a) and (b), HT-29mes were untreated; (c) and (d) are phase contrast images at 200x magnification of 38-hour 100 nM treated
HT-29 cells; (e) and (f) are fluorescent images of Hoechst-stained 38-hour
colchicine-treated HT-29mes cells. . . . . 52
B-6 Survival curve of HT-29mes following 38-hour exposure to colchicine in 25 cm-squared flasks shows a drop-off and plateau around 20 % of fraction of untreated colonies. The bracketed data point indicates an estimated survival as determined by previous work from Dr. Deborah Moshinsky (CAI, Beverly, MA) . . . . 53 B-7 Pamoic acid treated HT-29mes colonies show significant growth
inhibi-tion at high concentrainhibi-tions of the drug within days of treatment. Phase contrast images of untreated control (a), 300 pM (b), 500 pM (c), and
700 pM (d) trials 24 hours after treatment with pamoic acid. Phase
contrast images of untreated control (e), 300 pM (f), 500 pM (g), and
700 pM (h) trials after 48 hours of pamoic acid treatment. Notably the 700 pM case shows significant retardation of growth. Phase contrast
images of untreated control (i), 300 pM (j), 500 pM (k) three days
after treatment with pamoic acid. All of the colonies in the 700 pM were killed at this time point. By two weeks, all colonies in the 500
pM are also all killed. . . . . 54 B-8 Phase contrast microscopy images of untreated controls (a), 333 pM
treated (b), 300 pM treated (c), 266 pM treated (d), 233 pM treated (e), 200 pM treated (f), 166 pM treated (g), 133 pM treated (h), and
100 pM treated (i) HT-29mes colonies. The growth levels associated
with each of these indicates that up to 300 pM there is no statistically significant difference in the levels of metakaryotes . . . . 55
B-9 Phase contrast microscopy images of untreated colonies (a), 500 PM treated (b), 466 pM treated (c), 433 taM treated (d), 400 puM treated (e), and 366 pM treated (f) HT-29mes colonies. Complete colony growth inhibition was present in all the trial flasks from 366 to 500 [tM. 56
B-10 All subfigures are epifluorescent/phase contrast overlaid images of Hoechst-stained HT29mes colonies after treatment with 300 puM of pamoic acid for six days. In (a), one can see bell-shaped nuceli taking the form of a 'thecal'-like cell. (b) contains a distribution of eukaryotes and
m etakaryotes . . . . 57
B-11 Survival curve of HT-29mes colonies after two week exposure to pamoic acid at varying concentrations. Note, the initial drop-off of colonies starts around 333 pIM. At 400 pM and greater, all colonies are dead after two weeks. Metakaryotes are visible on the declining part of the curve (300 and 333 pM). The bracketed data point indicates an estimated survival based on the population initially seeded into the treated flask. The shape of this curve is very similar to X-ray treatment as seen in figure 13, suggesting pamoic acid acts like X-rays on HT-29m es cells . . . . 58
B-12 Phase contrast images of re-passaged HT-29mes colonies after two weeks of exposure to pamoic acid at 300 pM. Initially seeded sin-gle cells were mono-disperse and healthy-looking on the plate in (a) and (b). After one week, cells grew out to form large, metakaryote-containing colonies in (c) and (d) . . . . 59
B-13 Results of X-ray exposure of triplicate 25 cm-squared flasks seeded at 2000 cells 24 hours prior to treatment (100 rads/min). Total num-ber of colonies were counted five weeks after treatment. Estimates of metakaryotes and eukaryotes were derived based on evidence that about 9 % of large colonies formed are derived from metakaryotes. From this, an estimate was made of the survival curves of the eukary-otic (green triangles) and metakaryeukary-otic (purple crosses) fractions as a function of x-ray treatment. . . . . 60
List of Tables
A. 1 Colchicine-treated HT-29mes colony levels . . . . 45
Chapter 1
Introduction
Effective therapy for cancers remains a standing challenge in the field of biomedical research. While there exist some notable success stories for rational drug develop-ment strategies (i.e. imatinib for chronic myelogenous leukemia, CML), far and away the most applied therapies still rely on largely non-specific mechanisms of action, in-cluding chemotherapy targeted at rapidly proliferating cells or DNA-damaging agents. These therapies create significant toxic side effects and, in fact, sometimes provide lit-tle recourse for patients as relapse inevitably ensues along with increased metastasis. Such a profound lack of progress is evident in the nearly flat age-adjusted mortality rates of cancer of the lower GI tract present with European-American males (EAM) (Figure 1). The need, therefore, for more targeted treatments aimed at the underlying cells at risk for cancer is pressing and necessary to advance treatments in the clinic.
One significant hypothesis in the field of cancer research that can explain the fail-ure of common therapy is that of the cancer stem cell, or those cells that drive the growth of tumors and metastases that are constitutively resistant to X-rays and stan-dard chemotherapeutic agents. The field of cancer stem cells, however, is riddled with complexity insofar as reliable markers for tumor cell "stemness" remain unavailable and the hierarchy by which a tumor is organized varies between and within organ-specific cancers. In other words, no one has been able to "see" or image these stem cells. In 2003, however, a new phenomenon was observed by Dr. Elena V. Gostjeva of MIT's Biological Engineering department that indicate the cells at risk for
trans-formation into cancer stem cells are "metakaryotes." With little precedent, these metakaryotes exhibit unique morphological and functional phenotypes, including the ability to self-renew and asymmetrically differentiate. These metakaryotes also use a unique double-stranded RNA/DNA intermediate for nuclear genome replication and make use of DNA polymerase beta during replication. Herein, we investigate the metakaryote by way of interrogation with multiple small-molecule inhibitors in order to rationally determine some of its unique properties against a backdrop of eukaryotic cells (Thilly et al., 2014).
In this thesis, I determined the toxicity of pamoic acid, a well-known polymerase beta inhibitor, against the heterogeneous HT-29mes colon cancer cell line popula-tion, an easily manipulated in vitro system containing a fraction of metakaryotes. Pamoic acid, a naphthoic acid derivative, has been shown to exhibit polymerase beta inhibition in vitro and in cell culture in the micromolar range. Using time- and dose-dependent studies, we concluded that pamoic acid acts in a similar fashion as X-ray, DNA-damaging agents that concomitantly act on both eukaryotic and metakaryotic populations.
1.1
Metakaryotes: the stem cells of organogenesis
and carcinogenesis
Metakaryotic stem cells have been shown to drive the processes of organogenesis, car-cinogenesis, and wound-healing. Exhibiting a peculiar bell-shaped nuclei, these cells act as the precursors to human organs, the meta-organs, and thus were dubbed the metakaryotes (Gostjeva et al., 2005). These metakaryotes are neither prokaryotic nor eukaryotic in nature. Instead, metakaryotes show, unlike eukaryotes, a phenomenon of nuclear organization and replication, making use of a double-stranded RNA/DNA replicative intermediate (Thilly et al., 2014); maintaining a ring-like chromosomal structure through paired telomere-joined chromatids (Gruhl et al., 2010); and un-dergo an amitotic form of nuclear division (Gostjeva et al., 2006). Contributing to
the hypothesis of the metakaryotic stem cell, these cells are posited to exhibit a mu-tator/hypermutable phenotype that would lead to the requisite mutation rates that would lead to a frank carcinoma in the colon and rectal tissue (Kini et al., 2013). Briefly, we will examine the literature up to this date on the metakaryote and its relation to carcinogenesis, especially in colorectal adenocarcinomas.
The metakaryotic, bell-shaped nuclei were first identified by Dr. Elena Gostjeva in 2003 in surgical discards of human fetal colon and colon adenoma/adenocarcinoma samples. These cells exhibited both symmetric and asymmetrical division, the shib-boleth of a stem cell, and gave rise to differentiated cells further up in the colonic crypt. The metakaryotic stem cell was found at far higher frequencies amongst colonic adenocarcinomas, up to 1 % of all dividing cells, as compared to regular human fetal gut samples, indicating that these cells were present at higher proportions during carcinogenesis as compared to organogenesis. Of note, these metakaryotes seemed to show a growth rate within adenocarcinomas at a similar level as the fetal gut at around the 2 0-2 4th week of development, consistent with a 1 9th century idea that a tu-mor could perhaps derive from a dysregulation of organ developmental programming (Gostjeva et al., 2006). Such an idea is not novel insofar as Cohnheim (Cohnheim,
1875) proposed that tumors were an outgrowth of malfunctioning embryonic/fetal
stem cells.
Observations from multiple tissue samples of fetuses during 5 to 16 weeks of gesta-tion revealed presence of metakaryotes during development. Metakaryotes arise from the embryonic cell precursor through symmetric amitosis and form a tubular syncytia which contains increasing numbers of metakaryotes until the 1 2-14th week of
gesta-tion. These metakaryotes are organized as either "kissing" bells or in a "stacked" set of cups within the syncytia. The syncytial nuclei are then distributed throughout the developing fetus as mononuclear cells and form the meta-organs through asymmetric divisions that form eukaryotic nuclei. Some of these eukaryotic nuclei go on to form solid tissues as they increase by mitotic divisions to develop organized epithelium (Thilly et al., 2014). These nuclear forms remained undiscovered until recently due to instability of the phenotype insofar as the degeneration of the bell-shaped nuclei
phenotype 15 minutes after surgical removal even when fixed fast and held in cold medium (Gostjeva et al., 2009). The presence of metakaryotes in higher fractions in adenomas and adenocarcinomas of the colon as compared to normal adult ep-ithelial tissue pointed towards the hypothesis that these cells could give rise to solid tumors. Further, these asymmetric amitotic phenomenon arising from the metakary-otic stem cells that ultimately create the tumor parenchyma lend itself to the idea of a slowly-dividing, mutator/hypermutable cell "at risk" for initiation, giving rise to a preneoplasm in a solid organ.
1.2
Metakaryotic stem cells make use of a
double-stranded RNA/DNA intermediate, circular
chro-mosomes, and exclude Hoechst 33342
Metakaryotes undergo a particularly unique form of genome replication, making use of a nuclear pan-genomic double-stranded RNA/DNA (dsRNA/DNA) intermediate previously not found in other human cells (Thilly et al., 2014). This dsRNA/DNA intermediate was first identified in the HT-29 colorectal cancer cell line culture la-beled by antibody immunofluorescence during replication. Once the metakaryotic cell division occurred, the daughters reformed and the genome became the canoni-cal double-stranded DNA stage found in eukaryotes. Thilly and colleagues further showed that this dsRNA/DNA intermediate localized in the rim of the bell nuclei of the metakaryotes and continued further down during the process of DNA repli-cation; notably, no presence of such an intermediate was found within eukaryotes in the same culture. Such a discovery, however, begs the question of the significance of such an intermediate during development. One hypothesis offered in Thilly et al. is the concept of a sequential switch, insofar as the segregation of anti-polar DNA strands would predict a binary change. The dsRNA/DNA intermediate phenotype identified in the metakaryote could also be accompanied by an RNase H1, an enzyme involved in exonucleolytic activity, that would process the intermediate back into its
double-stranded DNA state. Such evidence is corroborated by the fact that RNase HI knockout mice are arrested in early stages of development, leading to the hypothesis that RNase HI could be a necessary endonuclease for the subsequent metakaryotic divisions that establish a mammalian organ. What's more, both polymerase beta and zeta were found to be localized in the metakaryotic stem cells at levels above 100,000 molecules per nuclei. Differentially expressed polymerases could indicate a preference of a more error-prone replicative enzyme that would lead to endogenous sources of the mutator phenotype.
Our lab has also uncovered structural differences in the chromosomal arrange-ments of metakaryotic cells compared to their eukaryotic counterparts. Metakaryotes exhibit a ring-shaped chromosomes wherein the telomeres of sister-chromatids are end-joined across the whole genome (Gruhl et al., 2010). Imaging fluorescence in-situ hybridization of cells derived from fetal tissue of the hindgut revealed that mononu-clear metakaryotes contained 23 telomeric regions. Syncytial bell-shaped nuclei during weeks 5-12 of development showed 23 centromeric and 23 or 46 telomeric fluorescent regions, indicating a switch between the ring-shaped structure and a regular chro-mosomal structure. Indeed, the ring-shaped chrochro-mosomal structure first recognized in spontaneous and x-ray treated pollen of Zea mays. The ring-shaped chromosomes were thought to possibly undergo change during plant development, a possibly ex-planation for clonal variation due to change in stem cell lineage (Gruhl et al., 2010 and McClintock, 1932). These BFB cycles, however, seem to be primarily present in adult tissue, whereas metakaryotic ring structures are evident in fetal/juvenile tis-sue and epithelial cancerous lesions. Such ring chromosome structures found in the metakaryote could also portend a deeper mechanistic role, for example acting as a genetic switch that could lead to the directed fate development found in organogen-esis. Similar genetic switches could also produce the developmental states found in solid tumors.
Within colon cancer cell lines, observations from our lab have identified a Hoechst-negative population that could likely be a side population of stem cells or simply a barrier to entry for small-molecules, affecting the reactivity of chemotherapeutics.
When the HT-29mes cancer cell line is stained with the vital Hoechst 33342, ob-servations such as those in Figure 5 indicate an exclusionary mesenchymal sac that prevents entry of many small-molecule drugs, a possible mechanism of resistance. This phenotype of Hoechst exclusion portends challenges in effective delivery of ther-apies into the heart of cancer colonies; such an issue could be resolved with enzymatic degradation of the physical barrier that excludes Hoechst. Current studies are under-way to determine if hyaluronidase, a potent enzyme that degrades hyaluronic acid, in combination with metakaryocides would deplete colonies in vitro.
Yet, others had interpreted that the Hoechst-negative side population within the tumor makes use of efflux pumps to acquire resistance to small-molecule drugs (Had-nagy et al., 2006 and Scharenberg et al., 2002). Previous work identified hematopoi-etic stem cells that overexpress small molecule transporters, such as the ABCG2 transporter, that efficiently effluxes Hoechst 33342 (Scharenberg et al., 2002). How-ever, this mechanistic explanation fails to explain the constitutive radiation resistance of cancer stem cells. No study has yet to combine radiation resistance and the hy-peractive efflux pump, relating them to the putative cancer stem cell.
1.3
Fetal/juvenile metakaryotic stem cells drive adult
colorectal carcinogenesis
In addition to the aforementioned phenotypes of metakaryotes, these stem cells ex-hibit unique fetal/juvenile mutator/hypermutable phenotypes that give rise to adult neoplasms. Previous work has advanced the basic two-step Armitage and Doll model to include stratification of risk in a heterogeneous population (Kini et al., 2013). This model also incorporates the idea that metakaryotic stem cells are mutator/hypermutable with a relatively high gene-inactivating mutation rate (2 - 5* 10 5 per stem cell dou-bling). These cells at risk contribute to the initiation period during the fetal/juvenile period of development. Further efforts are being investigated to determine how to further stratify this algebraic model to include variations in the mutation rate and
size of the individual in order to quantify risk for cancer. These initial hypotheses have been applied to the age-specific colon cancer rates for European-American males born 1890-99 with remarkable success (Kini et al., 2013).
1.4
X-ray resistance of metakaryotes
Metakaryotes have been shown to be far more resistant to X-rays and radiation ther-apy than regular eukaryotes, predicting the resistance phenotype many cancers have that portends low patient survival rates. Observations from pancreatic ductal adeno-carcinoma retrieved by surgeons at the Medical College of Wisconsin using the Whip-ple procedure demonstrated a lack of damaged metakaryotic cells. Further treatment of tumors with both common chemotherapeutics and radiation therapy revealed no morphological change in the metakaryotes found in primary human tissue samples.
Further, in cell culture, the HT-29 cancer cell line was treated with X-rays at
1600 rads, a treatment far higher than given to patients with cancer, to determine
the effects of radiation therapy on metakaryotes. These large doses of x-rays did not seem to perturb the nuclei of metakaryotes and, rather, selectively killed eukaryotes.
Of the initial colonies plated, 5 % of those survived all had metakaryotes. Figure
13 shows colony survival after treatment with radiation doses, including estimated
surviving eukaryotic and metakaryotic fractions. This observation could be a direct, simple explanation as to why some tumors are consistently insensitive to radiation therapy and chemotherapeutics that act via a DNA-damaging mechanism of action.
1.5
The functional role of polymerase beta
Errors in polymerase regulation or processivity have been associated with multiple cancerous and lesion-producing phenotypes (Lange et al., 2011). Broadly, eukary-otic polymerases can be subdivided into four families: A, B, X, and Y. Each of the
15 human polymerases has a specified function that can be classified into:
polymerases belong to those of the replicative subgroup, including polymerases al-pha, delta, and epsilon, primarily involved in DNA replication, nucleotide excision repair (NER), and mismatch repair (MMR). These three, along with polymerase zeta, belong to the B family of enzymes based on their amino acid sequence similarity.
Relevant to this study is the family X of polymerases that are primarily involved in base excision repair (BER) and include the polymerases: beta, lambda, mu, and terminal deoxynucleotide transfer (TDT). Polymerase beta, the smallest eukaryotic polymerase (at 39 kDa), has been implicated in producing a number of cancerous lesions. Polymerase beta consists of two domains, a smaller 8-kDa lyase domain, that acts primarily by 5- deoxyribosephosphate (dRP) lyase activity, and a larger 31-kDa polymerase domain that synthesizes DNA via a gap-filling mechanism.
The functionality of polymerase beta spans a wide variety of biological processes: from meiosis to recombination to base excision repair (BER). Primarily, in normal tissue, polymerase beta functions in DNA repair, specifically in base excision repair,
by catalyzing a polymerization step. Base excision repair, briefly, occurs when small
base lesions that do not distort the DNA helix structure significantly are corrected by a well-characterized set of proteins that include polymerase beta. This polymerase functions especially during short-patch (few nucleotides need to be replaced) BER and generally synthesizes one nucleotide during single-stranded breaks (SSB), though evidence has been shown with polymerase beta acting in long-patch (multi-nucleotide replacement) BER (Krokan and Bjoras, 2013).
Notably, polymerase beta seemed to be the only polymerase that functions in BER as reconstitution with other polymerases (alpha, epsilon, and delta) did not rescue the phenotype of homozygous null polymerase beta fibroblasts (Sobol et al., 1996). Further, based on antibody localization experiments, Sweasy and colleagues deter-mined that polymerase beta, highly expressed in mice testis, plays roles in synapsis and recombination during meiosis (Plug et al., 1997). Further, developmental studies in mice have shown an increased level of polymerase beta mRNA during spermatoge-nesis and the development of testis, while a decrease in transcript levels of polymerase beta in other organs, such as the brain and lungs, during development. The increased
levels of polymerase beta during the pachytene stage of sperm generation suggested a strong link between recombination events during meiosis and the over-expression of polymerase beta, indicating a functional role of polymerase beta in homologous repair (Nowak et al., 1990).
While some prior studies have concluded that homozygous null polymerase beta knockout mice are not embryonic lethal (Sugo et al., 2000), further efforts have con-firmed that embryonic lethality is indeed induced in true knockout mice (unpublished results from Drs. Elena Gostjeva, MIT and Kanjiro Asagoshi, Chiome Biosciences, Saitama, Japan).
Initial studies in polymerase beta knockouts in mouse embryonic fibroblasts showed increased sensitivity of these cells to alkylating agents, inducing apoptosis and chro-mosomal breakage. These studies showed that treatment of polymerase beta de-ficient fibroblasts with standard alkylating agents, including
N-methyl-N-nitro-N-nitrosoguanidine (MNNG) and methyl methanosulfate (MMS), displayed hypersensi-tivity and, presumably, DNA-damage induced apoptosis. Reconstituting polymerase beta into these cells rescued the hypersensitive phenotype and, combined with experi-ments showing UV radiation did not sensitize these cells, confirmed that base excision repair deficiency was the primary cause of the MMS/MNNG sensitivity (Ochs et al.,
1999 and Ochs et al., 2002). Whether, however, these fibroblast cell lines contain
metakaryotes is uncertain, thereby begging the question whether such studies cap-ture the underlying etiology of human cancers.
Further, expression levels of polymerase beta has been shown to determine the sen-sitivity of human tumor cells to ionizing radiation. Two carcinoma cell lines, A549 and
SQD9, were transfected with dominant negative polymerase beta that lacked
function-ality in its polymerase domain but still held to the DNA substrate. This transfection increased the radio sensitivity of these two epithelial carcinoma cell lines by a fac-tor of 1.5-1.7 (Vens et al., 2002). Similar to the above-mentioned fibroblast studies, however, these cell lines have not been confirmed to contain metakaryotes, indicating uncertainty that these results recapitulate the development of metakaryotic-driven human tumors.
In the course of examining literature of human polymerases, it is interesting to note the functional similarity between DNA polymerase gamma and polymerase beta.
DNA polymerase gamma, while belonging to a wholly separate family of polymerases (A family), maintains a catalytically slow dRP lyase mechanism that is similar to the
functionality of the 8-kDa domain of DNA polymerase beta (Pinz and Bogenhagen, 2000). Of note, polymerase gamma is the sole polymerase that functions to replicate the mitochondrial genome. Importantly, the mitochondrial genome has been shown to use an double-stranded RNA/DNA replicative intermediate, similar to those found in metakaryotic asymmetric fission (Xu et al., 1996), perhaps indicating that small-molecules targeted at polymerase beta could also be used in inhibiting the function of gamma. Notably, polymerase gamma-induced mutations make up the primary source of errors associated with mitochondrial genome mutations, indicating another endogenous source of genetic change (Zhang et al., 2006). Insofar as most of the current drugs available for targeting metakaryotes inhibit mitochondrial function to some effect,polymerase gamma could be an alternative viable therapeutic target for selective inhibition of metakaryotic growth
1.6
The link between polymerase beta and cancer
The primary evidence for the link between polymerase beta and human cancer comes from direct observations of over-expression of this enzyme in dividing nuclei of hu-man metakaryotic stem cells by Drs. Elena Gostjeva and Vera Koledova. Using
in-situ hybridization, prior results have shown localization of over 100,000 molecules
of polymerase beta per metakaryotic nucleus, along with polymerase zeta and RNase H1. Such a high concentration of polymerase beta could theoretically allow this error-prone polymerase to outcompete standard eukaryotic replication enzymes. The error-prone replication of polymerase beta, then, would lead to endogenous sources of mutations that would create the genetic or other events that lead to the development of cancer. Further in vitro efforts support the claim that polymerase beta could be the endogenous source of mutations for familial adenomatous polyposis coli (FAPC).
In this lab, Muniappan and Thilly had earlier reported the mutational spectrum of polymerase beta within the gene adenomatous polyposis coli (APC) gene. Surpris-ingly the in vitro polymerase replication errors generated by a wild-type polymerase beta matched well with in vivo mutations generated in human tumors that lead to colon cancer by the double-knockout of APC. Specifically, three mutational hotspots found in the exon 15 of APC matched with three hotspots found in human colon cancers, that make up 54 % of all reported in vivo APC hotspot mutations. The probability of this occurring was concluded, based on a hypergeometric distribution, to be significant indicating that the low-fidelity replicative errors of polymerase beta could be on the pathway to the development of cancer through the generation of
APC mutations (Muniappan and Thilly, 2002). Notably, the conclusion of this study
suggests that polymerase beta, in its wild type, could generate one pathway of can-cer. This, however, does not substantiate the claim that polymerase beta variants or overexpression of polymerase beta (that could outcompete standard processive poly-merases) is the sole cause of cancer. Rather, the mutator phenotype of polymerase beta could be one endogenous source of risk of acquiring cancer, combined with other environmental or familial risks on the metakaryotic cancer stem cell could bring about a neoplasm.
1.7
Pamoic acid: a small-molecule polymerase beta
inhibitor
Pamoic acid 1, also known as embonic acid, is a naphthoic acid derivative and well-characterized inhibitor of polymerase beta, with a relatively low equilibrium dissocia-tion constant (Kd = 10 pM). In its lyophilized form, pamoic acid is a yellow-colored
dry powder and can be reconstituted in tris-buffered saline (TBS) by modulating the
pH to create a pamoate ion that dissolves well in aqueous solutions.
Pamoic acid was first identified as a strong candidate for polymerase beta
inhi-1
IUPAC name: 4-[(3-Carboxy-2-hydroxynaphthalen-1-yl) methyl]-3-hydroxynaphthelene-2-carboxylic acid
bition by small molecule nuclear magnetic resonance (NMR) shift mapping (Hu et al., 2004). In a screen of 10 compounds that were structurally related to the natural product polymerase beta inhibitor, koetjapic acid, pamoic acid was identified as the strongest and most specific effector on polymerase beta. Further characterization of pamoic acid concluded that a 300 pM treatment of this compound sensitized wild-type mouse embryonic fibroblasts to a 1-hour mM dose of MMS; the same treatment did not further sensitize polymerase beta deficient fibroblasts. In addition, through in
vitro assays the authors concluded that pamoic acid works to inhibit both the 8-kDa
dRP lyase domain and the polymerase domain (Hu et al., 2004) in a concentration-dependent manner. Further studies have validated the chemical shift mapping ap-proach using docking simulations showing pamoic acid binding to the 8-kDa dRP lyase domain in a detailed three-dimensional model (Hazan et al., 2008).
Given the ease of access of pamoic acid and its relatively strong and selective affin-ity for polymerase beta, we attempted to use this small molecule as our toxicology candidate. While pamoic acid does not have a very strong binding affinity to poly-merase beta (less than 10 pM equilibrium dissociation constant), we predicted that micromolar concentrations would be sufficient to induce a growth phenotype change in cell culture.
1.8
Current technologies and assays for
metakary-otic stem cells
The relatively low fraction of metakaryotes present in regular HT-29mes colonies and in clinical tumor samples presents difficulties when trying to determine morphological or phenotypic behavior of only the metakaryotic cancer stem cells. Prior efforts using microwell and single-cell monodisperse methods have improved our capabilities of determining colony growth and tumor-initiating potential. Herein, I will describe some recent assays used to determine features of metakaryotic stem cells.
mi-crowells or tissue treated cell culture flasks (25 or 75 centimeter-squared). Single cells are seeded in a monodisperse fashion into either the flasks or in each well of a microwell plate. The monodisperse single cell resolution is achieved using a novel dissociation method developed by Kailin Duan in our lab (Gostjeva et al., 2016 in
press). Briefly, 0.25 % trypsin with EDTA is placed in a 37 'C incubator overnight
af-ter thawing from a 4 'C refrigerator. The trypsin is then refrozen at 4 C until needed for dissociation from a tissue treated flask. After cells are pelleted via a centrifuge, the suspension is triturated 40-50 times with a P10000 pipette (10 mL pipette tip).
This single-cell suspension can be confirmed by phase contrast microscopy.
Growing colonies from single cells allows one to better understand the concept of turnover units in human cell culture. Given a set of 1000 cells plated in either a flask or microwell plate, only a fraction (between 2-20 %) of these cells are metakaryotes and thus can propagate indefinitely, growing to large colonies. Yet other cells in the population will grow till some terminal stage wherein they will lose their ability to divide further: the cells that initiate these colonies are necessarily eukaryotes. Thus, using this simple technique, one can identify metakaryotes based on their growth kinetics.
Further confirmation of presence of metakaryotes can be obtained by live Hoechst imaging. Hoechst 33342, a vital stain, binds to double-stranded DNA within nuclei of living cells; at low enough concentrations, Hoechst does not kill the cell. The staining with Hoechst can elucidate the general morphology of the nuclei of cells. Insofar as metakaryotes maintain typically bell-shaped nuclei, these can be visualized on an epifluorescent microscope.
Chapter 2
Materials and Methods
2.1
HT-29 as a model for colon carcinomas and
cell culture
HT-29 cells were obtained from American Type Culture Collection (American Type Culture Collection, Manassas, VA). The HT-29 cell line was originally derived from the primary colonic adenocarcinoma of a 44-year old Caucasian female by the late Dr.
J. Fogh (Memorial Sloan-Kettering) in 1964. The cell line was passaged from passage
number 212 to 244 over the course of all of the experimentation. Over the course of passaging for years in the Thilly lab, Dr. Gostjeva has noticed that the HT-29 population, when grown under careful conditions contained some mesenchymal cells, including a diffuse layer of cells and can grow out with a Hoechst-negative colony center. A cell line derived from this Hoechst-negative colony is hereafter denoted as HT-29mes to indicate some mesenchymal phenotypes in the population. Further this cell line has been shown to contain metakaryotes from previous work (Gostjeva et al.,
2006 (Cancer Genetics/Cytogenetics)), at concentrations approximately 2 to 20 % of all colony-forming cells (Gostjeva et al., 2016 in press). When not being subjected passaging or experimentation, the HT-29mes cell lines were maintained in a 37 C incubator supplemented with no CO2 in Falcon tissue culture treated flasks (Fisher
Scientific, Waltham, MA) with 9 % fetal bovine serum (FBS, Gibco, Waltham, MA), free of antibiotics.
2.2
HT-29 maintenance and passaging
HT-29 or HT-29mes cells were passaged by trypsin treatment every 7 days during the course of the experimentation and re-seeded in 75 cm-squared tissue culture treated flasks (Fisher Scientific, Waltham, MA). Briefly, the previous media in flasks were aspirated and cells were washed with phosphate buffered saline (PBS, Thermo Fisher Scientific, Waltham, MA). To the flasks, 8mL of 0.05 % trypsin with EDTA (Sigma Aldrich) were added and cells were incubated for approximately 10 minutes at 37 C. Once cells were dissociated and relatively mono-disperse in the solution, the result-ing trypsin-treated cell solution was added to modified essential medium with 9 % v/v fetal bovine serum to bring the resulting trypsin concentration to approximately
0.025 % v/v in a 15-mL Falcon tube (Thermo Fisher Scientific, Waltham, MA). The
resulting solution was then centrifuged for 5 minutes at 20 rpm for pelleting. The resulting excess solution was aspirated off, leaving the pellet intact. Approximately 10mL of MEM with 9 % FBS was added to the pellet and triturated 40 to 50 times with a P10000 pipette to create a mono-disperse single-cell solution. To ensure a mono-disperse and single cell solution, approximately 1iOuL of the resulting cell so-lution was then added onto a microscope glass slide and imaged on the black/white channel of a Zeiss AxioVision D1 microscope at 200x magnification under transmit-ting light. If the resultransmit-ting solution was not at an appreciable level of mono-dispersion, the solution was triturated 10-20 more times and re-imaged.
Cells were then counted using a Z1 Coulter Counter (Beckman Coulter, Danvers, MA) using standard protocol. Briefly, the Coulter Counter was flushed with 10mL of Isoton II Diluent (Beckman Coulter, Danvers, MA). To each of three Coulter Counter containers, 9.8 mL of Isoton II Diluent was added along with 200uL of the cell sus-pension and mixed gently with a P2000. Cells were then counted on the Coulter Counter and the resulting number was multiplied by 10,000 to determine the total
number of cells in 10mL of the original solution. The HT-29mes cells, at a single-cell suspension, were then seeded into 4 T75 cm-squared flasks at 16,000 cells per flask along with 15mL MEM with 9 % FBS.
2.3
Pamoic acid treatment protocol
The primary experiment conducted in this thesis used pamoic acid as a polymerase beta inhibitor against HT-29mes metakaryotic stem cells in culture. Dried, powdered pamoic acid (Sigma Aldrich) was first measured out to 0.058 g. To create a stock solution of 10mM pamoic acid, the powder was re-suspended in 15 mL of Tris-buffered saline (TBS HCl, pH = 7.4, Sigma Aldrich). At this concentration, pamoic acid does not dissolve in TBS readily due to the pH differences. Thus, to the un-dissolved pamoic acid solution, 400 uL of 1 N NaOH was added in order to generate the pamoate ion that readily interacts with water. The pH was regularly measured by indicator strips during the course of NaOH treatment; once no yellow powder was present in the solution, 1OOuL (0.01 % v/v) of 1 N HCl was added to the solution to bring the pH back down to 7-7.4. The resulting 10mM pamoic acid was stored at 4
C until dosing. For dosing, cells were placed in suspension in media according to the
passaging/maintenance protocol above. Once in suspension, the cells were counted via the Z1 Coulter Counter and 2,000 cells were seeded into each flask along with
10 mL of MEM and 9 % FBS in a T25 cm-squared flask. The HT-29mes cells were
incubated for 24 hours before pamoic acid was added into the flask by pipetting. The control and trial flasks were then incubated and imaged every 24 hours to assay for visual colony formation.
2.4
Colchicine treatment protocol
Similar to the pamoic acid experiment, colchicine was used to determine the relative levels of metakaryotes in culture. Colchicine was purchased in lyophilized form from Sigma Aldrich at a mass of 500 mg. To create a 10uM stock solution of colchicine,
approximately 2.0 * 104 g of colchicine was weighed out and dissolved in 50 mL
of pure, de-ionized water (Sigma Aldrich, Natick, MA). The resulting solution was stored in a refrigerator at 4 C. For dosing, cells were placed in suspension in media according to the passaging/maintenance protocol above. Once in suspension, the cells were counted via the Z1 Coulter Counter (Beckman Coulter) and approximately 2,000 cells were seeded into each flask along with 10 mL MEM and 9 % FBS in a
25 cm-squared tissue treated culture flask. The HT-29mes cells were incubated for
24 hours before the appropriate concentration of colchicine was added per flask. The control and trial flasks were incubated and imaged every 24 hours to visualize colony formation.
2.5
Hoechst staining for identification of
metakary-otic nuclei
Hoechst 33342 was used to visualize bell-shaped nuclei phenotype associated with metakaryotic stem cells. A sterile working solution was prepared by dissolving Hoechst 33342 in Modified Essential Medium (MEM) to reach a concentration of 1microgram per mL (2.2 micromolar). To viably stain the HT-29mes cells with the Hoechst stain-ing solution, first the medium in the T25 flasks was aspirated. The flasks were three times washed with approximately 5mL of phosphate-buffered saline (PBS) and 5mL of Hoechst working solution was then added into the flasks. The cells were incubated at 37 C for 25 minutes. Once incubated, the cells were imaged under a DAPI (4',6-diamidino-2-phenylindole) filter on the Zeiss AxioObserver D1 microscope. Images were acquired using the AxioVision software with the "multidimensional acquisition" setting with multiple images being taken in both the regular phase-contrast setting using the black-and-white camera and fluorescent images with the color camera. Af-ter imaging and counting, the Hoechst working solution was aspirated and the cells were washed three times with PBS. The cells were replenished with MEM and 9 % FBS and incubated at 37 'C.
2.6
Imaging and microscopy
All images were taken with the Zeiss AxioObserver D1 cameras either under
fluores-cence or transmitting light in the black-white or color channel. The AxioObserver microscope includes a black-white CCD camera, a desktop computer with the Axio-Vision software, and a manual adjustment platform. Most images were taken using either the 20x or 100x magnifying All images were prepared by saving as an un-compressed TIFF and cropped in ImageJ (version 64, National Institutes of Health). Fluorescent channels were saved in their own images and saved as uncompressed TIFF formatted images. Using the batch convert process, ImageJ was used to convert the TIFF-formatted images into JPEG.
2.7
Image analysis and colony-formation assay
All image analyses were conducted in ImageJ. To determine the presence of
metakary-otes, the Hoechst protocol was used and images were taken to visualize bell-shaped nuclei in the colonies. Bell-shaped nuclei were identified post-experiment. To count cells, a 1mmx1mm grid was placed under each flask and cells were counted in each square, similar to a hemocytometer. For each flask, six 1cmxlcm squares were in-dependently counted and used to extrapolate the number of colonies formed in each flask.
2.8
Statistical calculations
To determine the statistical significance of these colony counts, a normal distribution was used to calculate the number of counts necessary to determine whether a drop-off would be within a 95 % confidence interval range. We test the statistical significance
by testing the null hypothesis (Ho) stated as: there is no significant difference in
colony forming potential between the control, untreated and treated population of HT-29mcs colon cancer cells. Numerically, if we define the mean number of colonies in six untreated flasks as PA and the mean number of colonies in six treated flasks as
PBr then our formula is as follows:
IPA - PBI - 2. (JA UB) > 0
Herein, the standard deviation of population A and B are UA and O-B, respectively.
If this inequality holds, we must reject the null hypothesis. If we consider a plate of
cells seeded with 4000 single, mono-disperse cells, we should see approximately half of these cells form large colonies. Thus, to determine whether there is a 90 % drop-off in colony formation due to a certain metakaryocide, we expect the following calculation:
(2000 - 1800) - 2 - (2000 + 1800)1/2 > 0
This value determines that if a 90 % decrease in colony count is witnessed in a plate initially seeded at 4000 cells.
Chapter 3
Results
3.1
Colchicine acts as a mitocide and enriches
metakary-otes within the HT-29mes cancer cell line
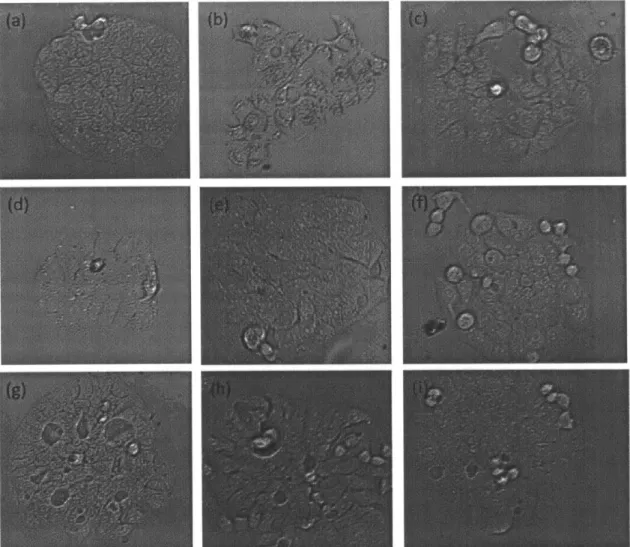
The HT-29mes cell line was subjected to varying concentrations of colchicine, a known mitocide, in order to discover if it would enrich metakaryotic stem cells among the surviving population. Previous results have shown that nanomolar (nM) concentra-tions of colchicine allow for the enrichment of metakaryotes, insofar as the resulting percentage of viable colonies as a percentage of untreated controls was 7.7 % in a 75 cm-squared tissue culture treated flask (Gostjeva et al., 2016). Further confirmation of this experiment provided that each of the colonies that grew back showed visible metakaryotes and formed colonies independently and rapidly.
I attempted to replicate the 20 nM dosages that would cause complete suppression
of eukaryotic growth. To 25 cm-squared tissue culture treated flasks (Sigma Aldrich), varying doses of colchicine (0 as a control, 0.8, 4, 20, and 100 nM) dissolved in water were added to 2000 seeded HT-29 cells. These cells were mono-disperse and plated as single cells to ensure that metakaryotes were not possible shielded by their eukaryotic neighbors when dosing occurred. A de-ionized water and normal control were both also plated at the same concentration of cells.
ap-proximately every 24 hours to determine their response. The colchicine was removed
by refreshing media after 38 hours. Initially, all cells at the single cell level divided
and formed doublets. Many of these cells exhibited a metakaryotic bell-shaped nu-cleus and would continue to grow out into their own colonies as seen in Figure 2. Small colonies formed for about half of all plated cells which then died off; the other half of the cells on the plate would go on to form larger colonies. Thus for the 2000 cells seeded, approximately 1000 would form large colonies.
After day four, some colonies containing between four and eight cells (either eu-karyotic or metaeu-karyotic) underwent apoptosis. At 20nM of colchicine dosed into the flasks, all cells were presumed to be metakaryotic in nature, exhibiting bell-shaped nuclei and rapidly reforming colonies once replenished with media. Below 20nM of colchicine dosed in, the percent viability was far higher than 20 % (around 43
% of untreated control HT-29 colony counts) indicating that these cells were not
all metakaryotic in nature and formed eukaryotic colonies. No previous results have shown metakaryote fractions higher than 20 % of the colonies found in a flask prior to enrichment and thus we assume the colchicine did not completely remove all metakary-otes at this concentration. Above 20 nM of colchicine, very few cells were colony form-ing, close to zero percent of the initial untreated colonies (data not shown in figure); these are most likely colchicine-resistant metakaryotic stem cells. Prior experiments of HT-29mes treated for 38 hours with 100 nM showed 3.8 % of untreated colonies forming large colonies of HT-29mes containing metakaryotes. To best enrich the pop-ulation of metakaryotes in the HT-29mes cell line, however, a lower concentration of around 20 nM is necessary in order to obtain an adequate number of cells. Thus, we find that 20 nM of colchicine treatment at 38 hours of exposure (24 hours after plating) allowed for enrichment of metakaryotes and displayed a viability of 20.2 % of the untreated controls in the same experiment. This value, 20 % of the untreated colonies, is in accordance with previous observations that metakaryotes make up 2-20
% of cancer cells in culture.
After seven days, the 20nM, 38-hour treated HT-29mes were treated with Hoechst 33342, a viable marker that binds to the minor groove of double-stranded DNA,
used to identify bell-shaped nuclei in a population of metakaryotes and eukaryotes. Notably, bell-shaped nuclei were present in almost every colony formed from single cells present after treatment with 20 nM of colchicine. Some colonies formed did not contain any visible metakaryotes, indicating that some eukaryotic colonies could have formed as outgrowths of the metakaryotic colonies. Side-by-side images of Hoechst stain (in the DAPI channel) and phase contrast microscopy are shown in Figure 5.
Notably, this general method provides a means for enriching metakaryotes out of the HT-29mes cell population. Once isolated, these unique stem cells can be further characterized through mass spectrometry or single-cell RNA sequencing to identify underlying proteomic or genomic differences between metakaryotic cells and eukaryotes. Current studies are underway using this method to identify the molecular mechanisms of resistance to most common chemotherapeutics in metakaryotic stem cells.
3.2
Pamoic acid acts like X-rays in toxicity assays
against the HT-29mes colon cancer cell line
Pamoic acid, a well-known small-molecule polymerase beta inhibitor, was used to treat the HT-29mes cell culture to determine its toxicity on metakaryotes and eu-karyotes. Pamoic acid, in its lyophilized form is a yellowish colored powder, was first tested at concentrations near its dissociation constant (Kd) with the 8-kDa domain of polymerase beta, approximately 13 pM, ranging above and below by an order of mag-nitude. The pamoic acid was dosed into the cell culture and not washed out before being imaged and counted. These concentrations, however, proved to be insufficient to yield a significant change in colony-formation ability of the HT-29mes colonies. As seen in Figure 8, concentrations of pamoic acid up through 266AM for multiple days did not reduce the viability or colony-formation potential of the HT-29mes cells, even without removing the pamoic acid from culture up to two weeks. This indicates that both metakaryotic and eukaryotic cells were unaffected at such low
concentra-tions of pamoic acid. Once cells were removed from the pamoic acid condiconcentra-tions, the HT-29mes colonies regrew with similar growth kinetics. Further images of these cells revealed that many colonies contained metakaryotes. Thus, at low concentrations, near the equilibrium dissociation constant, of pamoic acid no selective toxicity was induced due to differential expression in polymerase beta between metakaryotes and eukaryotes. The inadequate dosage could be due to a number of factors including: insufficient delivery into the HT-29mes cells or an excess of polymerase beta existing in both eukaryotes and metakaryotes.
The HT-29mes colonies were then subjected to far higher levels of pamoic acid, up through 1000 [M, or approximately 100 times the dissociation constant value with polymerase beta. At these higher concentrations, the HT-29mes colonies lost a significant fraction of their colony-forming potential. As one can see on the survival
curve in Figure 11, the fraction of HT-29mes large colonies decreasing linearly with increasing concentrations of pamoic acid. At concentrations above 366 AM, no cells survive, indicating an excessive toxicity that destroys both metakaryotes and eukary-otes. Below 366 pM, however, the levels of colony formation decreased linearly with the amount of concentration added. Pamoic acid was dosed in at intervals of 33
pM from 100 to 300 pM and 300 to 500 taM in separate experiments. Between 300
and 500 pM, the levels of HT-29mes colonies decreased linearly with the increasing amount of pamoic acid added. After two weeks, all cells were dead in the 500 and
700 pM treatments (Figure 7 and 9).
There was, however, a steep drop off in the viability at any value above 366
pM. Above this concentration, all cells were dead and re-passaging cells from the
previously treated flask yielded no new colonies (Figure 9). Thus at or above 366
pM, all eukaryotes and metakaryotes were killed, indicating that the pamoic acid
was non-specific with its action on both types of cells due to either an insufficient dosage or a lack of difference in concentration of polymerase beta within the cells (Figure 11).
At levels below 366 pM, pamoic acid seems to have acted like X-rays on the heterogeneous population of cells. Rather than selectively killing metakaryotes in
culture and thus creating a plateau in the survival curve, increasing levels of pamoic acid linearly decreased the number of total colonies found in the flask (Figure 11). Evidence of metakaryotes in colonies treated with 300 pM of pamoic acid is shown from Hoechst 33342 staining (Figure 10). Thus, just like doses of X-rays, the action of pamoic acid is not sensitive to the type of cell and rather affects both eukaryotes and metakaryotes at the same dose and exposure time; the levels of eukaryotes, however, decreased appreciably and more rapidly than the metakaryote population.
3.3
Re-passaging of metakaryotic colonies after
treat-ment with pamoic acid
While metakaryotes were clearly visible in the HT-29mes colonies after two weeks of exposure to pamoic acid, we attempted to discover if these metakaryotes were still viable and could produce their own colonies after treatment with pamoic acid.
To ensure that pamoic acid was neither cytostatic nor cytotoxic to metakaryotes, cells initially treated at 300 pM and 333 pM for one week were re-passaged at 4000 cells per flask into new 25 cm-squared tissue treated culture flasks in a single-cell, mono-disperse fashion. After seven days in the untreated condition, cells treated with 300 bpM grew out to form visible colonies again at levels similar to those found in regularly passaged HT-29mes cells (Figure 12). This indicates that removal of pamoic acid reverted the HT-29mes cells back to untreated state. Thus, I concluded that, once the treatment ceased, pamoic acid did not continue to have significant long-term effects on the growth rate or colony-forming potential of the HT-29mes cell line.
Notably, the colonies exposed to 300 pM of pamoic acid also contained metakary-otes and metakaryotic colonies were visible at appreciable levels, far higher than those present within regularly passaged HT-29mes colonies. At even higher concentrations (above 333 pLM), very few colonies survived treatment; however, more than half of these colonies would give rise to metakaryotes after repassaging in the 300 and 333 pM
treatment conditions. The actual proportion of metakaryote-derived colonies could far exceed 50 %, however, as some metakaryotic colonies can become eukaryotic, and the metakaryotic phenotype was present infrequently.